Abstract
Background:
Automated insulin delivery (AID) systems offer promise in improving glycemic outcomes for individuals with type 1 diabetes. However, data on those who struggle with suboptimal glycemic levels despite insulin pump and continuous glucose monitoring (CGM) are limited. We conducted a randomized controlled trial to assess the effects of an AID system in this population.
Methods:
Participants with hemoglobin A1c (HbA1c) ≥ 58 mmol/mol (7.5%) were allocated 1:1 to 14 weeks of treatment with the MiniMed 780G system (AID) or continuation of usual care (UC). The primary endpoint was change in time in range (TIR: 3·9-10·0 mmol/L) from baseline to week 14. After this trial period, the UC group switched to AID treatment while the AID group continued using the system. Both groups were monitored for a total of 28 weeks.
Results:
Forty adults (mean ± SD: age 52 ± 11 years, HbA1c 67 ± 7 mmol/mol [8.3% ± 0.6%], diabetes duration 29 ±13 years) were included. After 14 weeks, TIR increased by 18.7% (95% confidence interval [CI] = 14.5, 22.9%) in the AID group and remained unchanged in the UC group (P < .0001). Hemoglobin A1c decreased by 10.0 mmol/mol (95% CI = 7.0, 13.0 mmol/mol) (0.9% [95% CI = 0.6%, 1.2%]) in the AID group but remained unchanged in the UC group (P < .0001). The glycemic benefits of AID treatment were reproduced after the 14-week extension phase. There were no episodes of severe hypoglycemia or diabetic ketoacidosis during the study.
Conclusions:
For adults with type 1 diabetes not meeting glycemic targets despite use of insulin pump and CGM, transitioning to an AID system confers considerable glycemic benefits.
Keywords: automated insulin delivery, continuous glucose monitoring, glycemic control, hybrid closed-loop, insulin pump, time in range, type 1 diabetes
Introduction
Maintaining hemoglobin A1c (HbA1c) ≤ 53 mmol/mol (7%) is crucial in preventing or delaying complications associated with type 1 diabetes. 1 Nevertheless, despite the use of insulin pumps and continuous glucose monitoring (CGM), many struggle to achieve and maintain optimal glycemic levels. 2 Observational studies have indicated that only 21% to 30% of insulin pump users meet the recommended glycemic targets.2,3 This challenge has prompted the development of automated insulin delivery (AID) systems that offer promise in improving glycemic outcomes compared with insulin pump and CGM systems without automatic insulin dosing capabilities. 4 However, the initial pivotal trials and clinical experience with the use of AID systems have mainly included participants with good or fair glycemic outcomes.5,6 Moreover, there exists significant variability among study cohorts, with many trials including mixed groups of participants with different pre-AID treatment modalities (insulin pump or multiple injections, with/without CGM). 7
There are several potential factors that could hinder insulin pump and CGM users in meeting glycemic targets, such as missed or delayed meal boluses and/or insufficient bolus dosing due to concerns about hypoglycemia.8,9 However, with the advancements in insulin delivery automation and its positive impact on individuals with reasonable glycemic outcomes, we hypothesize that AID systems could also enhance glycemic outcomes in individuals with less-than-optimal blood glucose (BG) management. Accordingly, we aimed to investigate the effects of the MiniMed 780G system in individuals with type 1 diabetes and HbA1c ≥ 58 mmol/mol (7.5%) despite treatment with insulin pump and CGM or intermittently scanned CGM (isCGM).
Methods
Study Design
We conducted a 14-week open-label, single-center, randomized, controlled trial with a 14-week extension period. After the initial screening visit, participants were allocated to treatment with the MiniMed 780G system (AID group) or to continue their usual care (UC) with insulin pump and CGM/isCGM (UC group) for 14 weeks. After this first trial period, the UC group switched to treatment with the AID system and the AID group continued their AID treatment. Both groups were monitored for another 14 weeks (Supplemental Figure S1). The study was carried out according to the Helsinki declaration and was approved by the local ethics committee (H-20077186) and the Danish Data Protection Agency (P-2021-169). The study is registered at clinicaltrials.gov (NCT04914910).
Participants
Participants were recruited from the outpatient clinic at Steno Diabetes Center Copenhagen, Denmark. Eligibility criteria were age 18 to 75 years; type 1 diabetes ≥ 2 years, HbA1c ≥ 58 mmol/mol (7.5%), insulin pump treatment ≥ 12 months, CGM/isCGM ≥ 6 months, use of insulin aspart ≥ 1 week, use of insulin bolus calculator for most meals and snacks, and carbohydrate intake ≥ 80 g per day. Exclusion criteria included pregnancy or breast-feeding; use of drugs (other than insulin) known to affect glucose metabolism; use of hybrid closed-loop systems and severe cardiac disease or retinopathy contraindicating HbA1c < 53 mmol/mol (7%). All participants gave written informed consent before inclusion in the trial.
Randomization
Eligible participants were randomized after the initial screening visit and allocated 1:1 stratified by baseline HbA1c (< 64 mmol/mol or ≥ 64 mmol/mol [8%]). An allocation table with blocks of different sizes generated by sealedenvelope.com was uploaded by a person not otherwise involved in the study to RedCap, a secure web-based data management program, which performed the randomization. Study enrolment and group assignment were done by the investigators based on the electronic randomization. Investigators and participants were not masked to arm assignment due to the nature of the intervention.
Procedures
After the initial screening visit, participants wore a CGM (guardian 3 link/guardian 4) for 2 weeks. After completing the CGM monitoring period, participants allocated to the AID group were transitioned to treatment with the AID system and attended a 3-hour training course in groups. The training included general information regarding AID systems, setting up the 780G system with individual pump settings and practical advises for everyday use of the pump (including carb entry, exercise, sick day rules, etc) (Supplemental Table S1). As all the study participants had a high HbA1c at baseline, the initial glucose target was set to 6.1 mmol/L (110 mg/dL) and reduced to 5.5 mmol/L (100 mg/dL) within two weeks. Active insulin time was initially set to 3 hours and reduced to 2.0 to 2.5 hours within two weeks unless there were concerns of high frequency of hypoglycemia. All other pump settings were used unchanged from their current insulin pump. Five follow-up contacts were scheduled during the first 14-week trial period comprising three physical visits (weeks 2, 12, and 14) and two telephone consultations (weeks 4 and 8). The UC group attended the same amount of follow-up visits. In both groups, pump settings were adjusted at each visit according to the investigator’s judgment. All participants completed another two-week CGM monitoring period at weeks 14 and 28. After completion of the primary study period, all participants entered the 14-week extension period. The UC group was transitioned to the AID system at week 14 and followed an identical education program. During the extension period the AID group only had two visits at weeks 26 and 28 (Supplemental Figure S2). Hemoglobin A1c was monitored at baseline, weeks 14 and 28. At the same timepoints, patient-reported outcomes (PROs) were assessed by the following questionnaires: Diabetes Treatment Satisfaction Questionnaire, 10 Hypoglycemia Fear Survey (worry subscale), 11 Diabetes Distress Scale, 12 and Insulin Dosing Systems: Perceptions, Ideas, Reflections, and Expectations (INSPIRE measures). 13 The participants also wore an accelerometer (ActiGraph GT3X, Pensacola, FL, USA) on the non-dominant wrist for one week at baseline, weeks 14 and 28.
Outcomes
The primary outcome was the difference between treatment groups in the change in time in range (TIR; 3.9-10.0 mmol/l) from baseline to week 14 assessed by two weeks of CGM data. The secondary outcomes were the difference in the change in time above range (TAR; > 10.0 mmol/L, > 13.9 mmol/L), time below range (TBR; < 3.9 mmol/L, < 3.0 mmol/L), mean sensor glucose (SG), standard deviation (SD) of mean SG, coefficient of variation (CV), HbA1c; body weight and total daily insulin dose (TDD). Time in ranges, mean SG, SD, and CV were also analyzed for the following timepoints: 06:00 to 23:59 (wake) and 24:00 to 05:59 (sleep). Ancillary endpoints included total carbohydrate intake per day, time spent in SmartGuard mode, PROs, physical activity level, severe hypoglycemia events, diabetic ketoacidosis events, and within-group changes from weeks 14 to 28 in TIR, TAR, TBR, HbA1c, CV, and body weight.
Statistical Analyses
The sample size calculations were based on the primary outcome of the study. To detect a clinically significant difference in TIR of 5% (corresponding to 75 minutes per day) between treatment arms (expected standard deviation of 5%; alpha = 0.05; beta = 0.2; and two-sided unpaired t test), a sample size of 17 persons per treatment group was required. We accounted for a drop-out rate of 15% thus aimed to include 40 persons in the study. All outcome analyses comparing the AID system with UC were conducted using a linear mixed effect model with treatment, visit and treatment × visit as fixed effects and participant’s intercept as random effect for normally distributed data. A compound symmetry covariance structure was applied. Wilcoxon signed-rank test was used when data were not normally distributed. All analyses were performed as intention-to-treat on all randomized participants. All statistical analyses were done using SAS Enterprise guide version 8.3. Data are presented as median (interquartile range) or mean ± SD, unless otherwise stated. A P value of .05 was considered statistically significant.
Results
Between June 8, 2021 and September 5, 2022, 40 participants were enrolled and randomly assigned to the AID group (n = 20) or UC group (n = 20). All 40 participants completed the first 14-week trial period; one participant from the AID group was lost to follow-up during the 14-week extension period (Figure 1). At baseline, the mean (SD) age was 52 (11) years, diabetes duration was 29 (13) years, HbA1c was 67 (7) mmol/mol (8.3 [0.6]%) and body mass index (BMI) was 27.1 (4.3) kg/m2 (Table 1).
Figure 1.
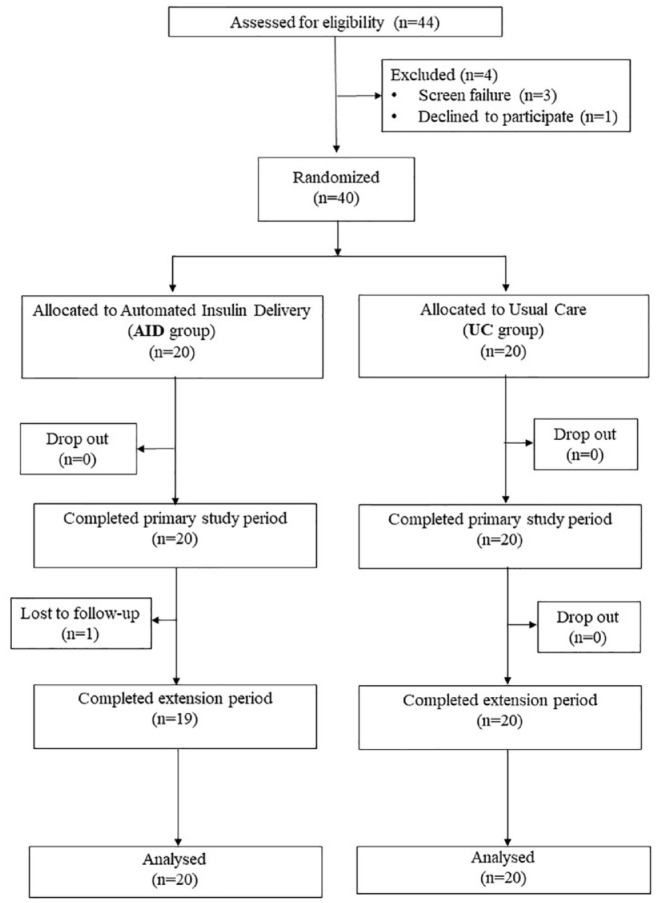
Trial profile.
Table 1.
Baseline Characteristics.
| AID group (n = 20) | UC group (n = 20) | |
|---|---|---|
| Men | 9 (45) | 9 (45) |
| Age (years) | 54.9 (10.0) | 49.2 (12.2) |
| Diabetes duration (years) | 31.5 (13.4) | 27.7 (13.1) |
| HbA1c (mmol/mol) | 66.8 (6.8) | 67.7 (7.3) |
| HbA1c (%) | 8.3 (0.6) | 8.3 (0.7) |
| BMI (kg/m2) | 27.1 (4.1) | 26.9 (4.4) |
| Duration of pump therapy (years) | 10.6 (4.5) | 11.5 (5.0) |
| Total daily insulin dose (U/day) | 43.1 (17.0) | 48.3 (24.0) |
| Complications | ||
| • Retinopathy | 11 (55) | 12 (60) |
| • Albuminuria | 5 (25) | 2 (10) |
| • Nephropathy | 6 (30) | 6 (30) |
| • Cardiovascular disease | 4 (20) | 0 (0) |
| Pre-AID insulin pump treatment | ||
| • Sensor augmented pump | 17 (85) | 10 (50) |
| • Pump with stand-alone sensor | 3 (15) | 10 (50) |
Data are presented as mean (SD) or n (%).
Abbreviations: AID, automated insulin delivery; UC, usual care.
The mean baseline TIR was 56.3 (9.0) % in the AID group and 56.5 (8.2) % in the UC group.
At week 14, TIR increased with 18.7% (95% confidence interval [CI] = 14.5%, 22.9%) in the AID group compared with –2.1% (95% CI = −6.5, 1.8) for the UC group (between-group difference, P < .0001). TIR > 70% was achieved by 80% of participants in the AID group and by 10% in the UC group (P < .0001). The mean TAR level 1 (SG > 10 mmol/L) decreased by 11.2% (95% CI = −14.0, −8.4) in the AID group and remained unchanged in the UC group (between-group difference, P < .0001). Time in extreme hyperglycemia (TAR level 2, SG > 13.9 mmol/L) also decreased significantly more in the AID group than in the UC group (between-group difference, P < .0001). Time below range decreased in both groups without any significant inter-group difference (P = .63) (Table 2). At baseline, mean SG was 9.5 (0.7) mmol/L in both groups. After 14 weeks, mean SG had decreased to 8.2 (0.4) mmol/L in the AID group and was 9.9 (1.2) mmol/L in the UC group (between-group difference, P < .0001). The reduction in SD of mean SG was significant greater in the AID group than in the UC group (P = .002). There was no significant difference in change in CV between groups (Table 2).
Table 2.
Glycemic and Metabolic Outcomes for the Primary Study Period.
| AID group (n = 20) |
UC group (n = 20) |
P value (AID vs UC) |
|||
|---|---|---|---|---|---|
| Baseline | Week 14 | Baseline | Week 14 | Δ baseline-week 14 | |
| Distribution of glucose values (% time in ranges) a | |||||
| < 3.0 mmol/l | 0.7 (0.9) | 0.4 (0.4) | 0.9 (1.4) | 0.4 (0.5) | P = .64 |
| < 3.9 mmol/l | 2.2 (1.5) | 1.7 (1.0) | 2.6 (1.6) | 1.7 (1.1) | P = .63 |
| 3.9-10.0 mmol/l | 56.3 (9.0) | 75.1 (5.4) | 56.5 (8.2) | 54.1 (12.8) | P < .0001 |
| > 10 mmol/l | 29.6 (4.5) | 18.4 (4.0) | 28.5 (5.5) | 28.9 (7.8) | P < .0001 |
| > 13.9 mmol/l | 11.1 (5.9) | 4.5 (2.5) | 11.6 (4.4) | 14.9 (8.5) | P < .0001 |
| Mean glucose and glycemic variability a | |||||
| Mean SG (mmol/l) | 9.5 (0.7) | 8.2 (0.4) | 9.5 (0.6) | 9.9 (1.2) | P < .0001 |
| SD (mmol/l) | 3.4 (0.6) | 2.9 (0.4) | 3.6 (0.4) | 3.6 (0.6) | P = .002 |
| CV (%) | 35.8 (5.0) | 35.0 (4.5) | 37.8 (4.6) | 35.6 (4.9) | P = .26 |
| Distribution of glucose values during sleep (24:00-05:59) a | |||||
| < 3.0 mmol/l | 0.8 (1.3) | 0.5 (0.8) | 0.7 (1.3) | 0.3 (0.5) | P = .89 |
| < 3.9 mmol/l | 2.9 (2.5) | 1.5 (1.4) | 2.9 (2.9) | 1.1 (1.5) | P = .85 |
| 3.9-10.0 mmol/l | 60.8 (15.6) | 86.0 (10.3) | 58.6 (14.7) | 54.8 (19.2) | P < .0001 |
| > 10 mmol/l | 26.5 (11.2) | 10.1 (8.9) | 28.5 (10.7) | 30.4 (13.2) | P < .0001 |
| > 13.9 mmol/l | 8.9 (7.1) | 1.9 (2.7) | 9.2 (7.2) | 13.3 (10.4) | P = .0006 |
| Body weight and total daily insulin dose | |||||
| Body weight (kg) | 82.7 (14.3) | 83.6 (14.5) | 84.3 (18.5) | 84.8 (18.6) | P = .91 |
| TDD (U/day) | 42.8 (16.6) | 48.7 (22.8) | 48.3 (24.0) | 49.7 (25.7) | P = .84 |
Data are presented as mean (SD).
Abbreviations: AID, automated insulin delivery; CV, coefficient of variation; SD, standard deviation; SG, sensor glucose; TDD, total daily dose; UC, usual care.
Assessed by 2-week CGM.
During sleep (24:00-05:59), the mean TIR in the AID group increased by 25.2% (95% CI = 16.9, 33.6) while there was no significant change in the UC group (between-group difference, P < .0001). Time below range during sleep decreased in both groups without any between-group difference.
In the AID group, HbA1c decreased from 67 (7) to 57 (4) mmol/mol (8.3 [0.6]%-7.3 [0.4]%) and from 68 (7) to 67 (7) mmol/mol (8.3 [0.4]%-8.3 [0.4]%) in the UC group (between-group difference, P < .0001).
The mean TDD at baseline was 42.8 (16.6) units/day for the AID group and increased to 48.7 (22.8) units/day at week 14. In the UC group, baseline TDD was 48.3 (24.0) units/day and 49.7 (26.4) units/day at week 14 (between-group difference in change, P = .32).
The mean carbohydrate entry in the pump increased significantly more in the AID group than in the UC group, AID group: 31.9 g (95% CI = 16.9, 47.5 g); UC group: 1.1 g (95% CI = −16.7, 14.5); between-group difference P < .0001.
The mean body weight at baseline was 82.7 (14.3) kg and increased by 0.82 (2.9) kg after 14 weeks for the AID group. The mean baseline body weight for the participants in the UC group was 84.3 (18.6) kg and increased by 0.50 (1.3) kg (between-group difference, P = .64). There was no difference in change in total daily step count measured by accelerometer between groups (P = .49).
There were no episodes of severe hypoglycemia during the study. One participant in the AID group was hospitalized with hyperglycemia (no ketoacidosis) probably due to injection set failure and concomitant urinary tract infection.
At week 14, the mean total Diabetes Treatment Satisfaction Questionnaire (DTSQs) score increased in the AID group by 7.6 (95% CI = 5.34, 9.86) and in the UC group by 2.0 (95% CI = −0.3, 4.29) (between-group difference, P = .0012). Perceived frequency of hyperglycemia decreased significantly more in the AID group than in the UC group (between-group difference, P < .0001), but there was no difference between groups in perceived frequency of hypoglycemia. Fear of hypoglycemia decreased significantly more in the AID group than in the UC group, that is, the mean total Hypoglycemia Fear Survey (HFS) score decreased by 7.45 (95% CI = −10.15, −4.75) in the AID group and by 3.14 (95% CI = −5.98, −0.31) in the UC group (between-group difference, P = .03). The number of people with hypoglycemia unawareness did not change from baseline to week 14 in any of the groups. There was no difference in the change in mean total Diabetes Distress Score (DDS) between groups (P = .08). However, when analyzing the seven DDS subscales, the AID group had a bigger decrease in subscale “Management of distress” than the UC group (management, P = .002). The participants had relatively high expectations of the AID system at baseline (mean INSPIRE score = 80.8 [9.6]). At week 14, the actual appraisal of the AID was slightly lower (mean INSPIRE score = 77.3 [12.0] for the AID group).
After the 14-week extension period (at week 28), TIR increased to 74.1 (6.5)% in the UC group and remained stable in the AID group (74.0 [6.9]%). Hemoglobin A1c in the UC group decreased to 57 (4) mmol/mol (7.3 [0.4]%) but was unchanged in the AID group (Figure 2). The mean change in body weight from baseline to week 28 was 1.4 (4.1) kg for the AID group and 1.6 (2.0) kg for the UC group (between-group difference, P = .63). The mean change in TDD after 28 weeks was 6.6 (8.3) units/day for the AID group and 2.7 (6.3) units/day for the UC group (between-group difference, P = .29).
Figure 2.
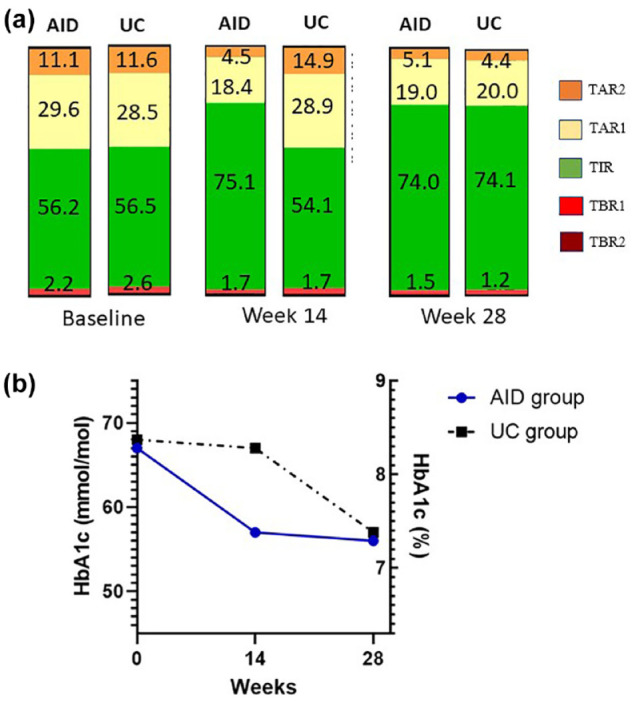
Glycemic outcomes after 28 weeks. (a) Mean time in ranges at baseline, weeks 14 and 28. TIR, time in range (3.9-10 mmol/L); TAR1, time above range, level 1 (10.1-13.9 mmol/L); TAR2, time above range, level 2 (> 13.9 mmol/L); TBR1, time below range, level 1 (3.0-3.8 mmol/L); TBR2, time below range, level 2 (< 3.0 mmol/L). (b) Mean HbA1c at baseline, ***P > .0001, weeks 14 and 28.
Abbreviations: AID, automated insulin delivery; UC, usual care.
At the end of the extension period, 35 (89.7%) participants used a BG target of 5.5 mmol/L and four (10.3%) used a glucose target of 6.1 mmol/L. Active insulin time was 2 to 2.5 hours in 36 (92.3%) participants and 2.75 hours in three (7.7%) participants. At study end, the participants spent 97.8 % of time in auto mode (SmartGuard mode).
Discussion
This randomized, controlled trial evaluated the effects of an AID system in adults with type 1 diabetes treated with insulin pump and CGM/isCGM, with HbA1c ≥ 58 mmol/mol (7.5%). To our knowledge, this is the first randomized trial investigating the MiniMed 780G system in a type 1 diabetes population with long-standing diabetes duration, a high frequency of late diabetes complications, and a HbA1c well above target level despite treatment with insulin pump and CGM/isCGM. When compared with UC, the use of the AID system substantially improved glycemic outcomes, with an 18.7% increase in TIR and a 10 mmol/mol decrease in HbA1c without a concurrent increase in hypoglycemia after 14 weeks of treatment. A 14-week extension phase confirmed sustained benefits, even with minimal trial oversight. Transitioning the UC group to the AID system replicated these benefits. Notably, 80% of the AID users reached TIR > 70%; hence, our study indicates that it is possible to reach glycemic targets for the vast majority of AID users, despite a high baseline HbA1c.
Though more pronounced than the results by others, the glycemic benefits of AID treatment in our study are in line with previous research groups.6,14,15 The participants were included based on high HbA1c levels, and therefore had a relatively low baseline TIR (= 56%). This baseline characteristic may have widened the scope to observe pronounced improvements. Indeed, work by Schoelwer et al demonstrated that new AID users with lower baseline TIR experienced greater increases in TIR. 16 The participants in our study spent 97.8% of time in auto mode at study end, indicating a high acceptance and use of the system. Switching to AID treatment reduced TBR, but there was no significant difference between groups. None of the participants experienced episodes of severe hypoglycemia during the study. These findings confirm results from other studies showing no change in the rate of hypoglycemia despite improvements in TIR with AID systems.6,15 Fear of hypoglycemia decreased more in the AID group than in the UC group, despite no between-group difference was found in change in TBR. This might reflect that AID users have a higher degree of trust that the AID system will handle pending hypoglycemia. Furthermore, we found that carbohydrate entry in the AID system was 30 g higher than at baseline, which could reflect more confidence in entering the entire amount of ingested carbohydrates without the resultant fear of hypoglycemia. However, alternative explanations for the higher carbohydrate entry into the pump may include entering of “fake” carbohydrates to compensate for the inability to do manual correction bolus within the system or simply that some people have adopted less restrictive dietary habits due to the AID of the system.
The AID group demonstrated a decrease in both mean SG and SD of mean SG. However, CV remained unchanged, likely attributable to the concurrent reduction in mean SG and SD. In a study of 10 404 AID users, CV was found to be incongruous in demonstrating improvements in glycemic control beyond reductions in mean SG. 17
Participants in the AID group had a higher increase in treatment satisfaction, than the UC group along with a greater decrease in perceived frequency of hyperglycemia with AID treatment. These findings are in line with other studies that also found a high degree of treatment satisfaction with AID systems.18,19 The mean total DDS score at baseline was > 2 for all participants indicating moderate diabetes distress. Diabetes distress is common among people with type 1 diabetes and is associated with reduced quality of life, high HbA1c, and an increased risk of complications.20,21 After 14 weeks, the mean total DDS score had decreased in the AID group, but the difference between groups were not significantly different. However, when looking into the seven subscales of the DDS, 20 the AID group significantly improved scores in the area of management of distress compared with the UC group. This could indicate that AID systems can help to ease the burden of diabetes management due to the AID and frequent autocorrections, which might also relieve the stress around accurate carbohydrate counting and risk of insufficient bolusing.
The study stands out from previous research on AID systems due to its focus on a specific patient group. Prior to the study, the participants had used insulin pumps for an average of 11 years, attended regular visits at Steno Diabetes Center Copenhagen, and received care from experienced healthcare professionals specialized in insulin pump treatment. Despite this high level of care, the participants’ HbA1c levels remained above 58 mmol/mol (7.5%). Accordingly, the study’s findings strongly demonstrate that AID systems are the optimal solution for addressing the needs of this particular patient group.
The strengths of the study include the randomized controlled study design with participants’ representative of the general population of people with type 1 diabetes with long diabetes duration using insulin pump and CGM. Another strength is inclusion of an extension period, which demonstrates the sustainability of the glycemic outcomes found with AID treatment in this specific group. The study also has several limitations. The trial period was relatively short with an inter-group comparative period of 14 weeks. This may have been too short to detect rare adverse events. Another limitation is the sparse ethnic diversity, which may make the results less generalizable. Moreover, we only included participants who used the bolus calculator daily. It would have been interesting to test whether the glycemic improvements could be achieved in individuals not using the bolus calculator daily, that is, omitting meal boluses daily. This issue may be addressed in future studies.
Conclusions
The findings of the present study demonstrate substantial glycemic benefits of switching to AID treatment in people with type 1 diabetes and high HbA1c despite treatment with pump and CGM/isCGM. These findings support integrating AID use as standard of care in adults with type 1 diabetes struggling to reach glycemic targets regardless of prior technology use.
Supplemental Material
Supplemental material, sj-docx-1-dst-10.1177_19322968241242399 for Automated Insulin Delivery in Adults With Type 1 Diabetes and Suboptimal HbA1c During Prior Use of Insulin Pump and Continuous Glucose Monitoring: A Randomized Controlled Trial by Merete B. Christensen, Ajenthen G. Ranjan, Karen Rytter, Olivia M. McCarthy, Signe Schmidt and Kirsten Nørgaard in Journal of Diabetes Science and Technology
Acknowledgments
The authors thank the trial participants and staff at Steno Diabetes Center Copenhagen who were involved in the conduct of the study.
Footnotes
Abbreviations: AID, Automated insulin delivery; CGM, Continuous glucose monitoring; isCGM, intermittently scanned continuous glucose monitoring; PRO; patient-reported outcome; SG, sensor glucose; TAR, time above range; TBR, time below range; TDD, total daily insulin dose; TIR, time in range; UC, usual care.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: MBC has received speaker honorarium from Sanofi and Novo Nordisk. KN holds shares in Novo Nordisk; has been a paid consultant for Novo Nordisk and Medtronic; has received speaker honorarium and honorarium for Advisory Board to her institution from Medtronic, Novo Nordisk, Convatec, and her institution has received research funding from Zealand Pharma, Novo Nordisk, Medtronic, and Dexcom. SS was an employee at Novo Nordisk A/S during study conduct and has received speaker fees from Novo Nordisk. KR own shares in Novo Nordisk and has received speaker’s honorarium from Medtronic. AGR and OMC have nothing to disclose.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was an investigator-initiated study and was partly funded by an unrestricted grant from Medtronic A/S. The funders of the study had no role in study design, data collection, data analyses, data interpretation, or writing of the manuscript, and did not impose any restrictions regarding the publication of the manuscript.
Prior Presentation: Parts of the study were accepted as an abstract at the 59th annual meeting of the European Association for the Study of Diabetes (EASD), Hamburg, Germany, October 5, 2023.
ORCID iDs: Merete B. Christensen  https://orcid.org/0000-0002-0095-5427
https://orcid.org/0000-0002-0095-5427
Signe Schmidt  https://orcid.org/0000-0002-6968-6675
https://orcid.org/0000-0002-6968-6675
Kirsten Nørgaard  https://orcid.org/0000-0003-1620-8271
https://orcid.org/0000-0003-1620-8271
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Holt RIG, DeVries JH, Hess-Fischl A, et al. The management of type 1 diabetes in adults. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2021;44:2589-2625. [DOI] [PubMed] [Google Scholar]
- 2. Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016-2018. Diabetes Technol Ther. 2019;21(2):66-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rytter K, Madsen KP, Andersen HU, et al. Insulin pump treatment in adults with type 1 diabetes in the capital region of Denmark: design and cohort characteristics of the steno tech survey. Diabetes Ther. 2022;13(1):113-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fang Z, Liu M, Tao J, Li C, Zou F, Zhang W. Efficacy and safety of closed-loop insulin delivery versus sensor-augmented pump in the treatment of adults with type 1 diabetes: a systematic review and meta-analysis of randomized-controlled trials. J Endocrinol Invest. 2022;45(3):471-481. [DOI] [PubMed] [Google Scholar]
- 5. Collyns OJ, Meier RA, Betts ZL, et al. Improved glycemic outcomes with Medtronic MiniMed advanced hybrid closed-loop delivery: results from a randomized crossover trial comparing automated insulin delivery with predictive low glucose suspend in people with type 1 diabetes. Diabetes Care. 2021;44(4):969-975. [DOI] [PubMed] [Google Scholar]
- 6. Matejko B, Juza A, Kieć-Wilk B, et al. Transitioning of people with type 1 diabetes from multiple daily injections and self-monitoring of blood glucose directly to MiniMed 780G advanced hybrid closed-loop system: a two-center, randomized, controlled study. Diabetes Care. 2022;45:2628-2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brown SA, Kovatchev BP, Raghinaru D, et al. Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med. 2019;381:1707-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rytter K, Madsen KP, Andersen HU, et al. Associations between insulin pump self-management and HbA1c in type 1 diabetes. Diabet Med. 2023;40(6):e15068. [DOI] [PubMed] [Google Scholar]
- 9. Spaans E, van Hateren KJJ, Groenier KH, Bilo HJG, Kleefstra N, Brand PLP. Mealtime insulin bolus adherence and glycemic control in adolescents on insulin pump therapy. Eur J Pediatr. 2018;177:1831-1836. [DOI] [PubMed] [Google Scholar]
- 10. Bradley C. Handbook of Psychology and Diabetes: A Guide to Psychological Measurement in Diabetes Research and Practice. Harwood Academic Publishers; 1994. [Google Scholar]
- 11. Cox DJ, Irvine A, Gonder-Frederick L, Nowacek G, Butterfield J. Fear of hypoglycemia: quantification, validation, and utilization. Diabetes Care. 1987;10(5):617-621. [DOI] [PubMed] [Google Scholar]
- 12. Polonsky WH, Fisher L, Earles J, et al. Assessing psychosocial distress in diabetes: development of the Diabetes Distress Scale. Diabetes Care. 2005;28(3):626-631. [DOI] [PubMed] [Google Scholar]
- 13. Weissberg-Benchell J, Shapiro JB, Hood K, et al. Assessing patient-reported outcomes for automated insulin delivery systems: the psychometric properties of the INSPIRE measures. Diabet Med. 2019;36(5):644-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bergenstal RM, Nimri R, Beck RW, et al. A comparison of two hybrid closed-loop systems in adolescents and young adults with type 1 diabetes (FLAIR): a multicentre, randomised, crossover trial. Lancet. 2021;397:208-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Choudhary P, Kolassa R, Keuthage W, et al. Advanced hybrid closed loop therapy versus conventional treatment in adults with type 1 diabetes (ADAPT): a randomised controlled study. Lancet Diabetes Endocrinol. 2022;10(10):720-731. [DOI] [PubMed] [Google Scholar]
- 16. Schoelwer MJ, Kanapka LG, Wadwa RP, et al. Predictors of time-in-range (70-180 mg/dL) achieved using a closed-loop control system. Diabetes Technol Ther. 2021;23(7):475-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Castañeda J, Arrieta A, van den Heuvel T, Cohen O. The significance of coefficient of variation as a measure of hypoglycaemia risk and glycaemic control in real world users of the automated insulin delivery MiniMed 780G system. Diabetes Obes Metab. 2023;25(9):2545-2552. [DOI] [PubMed] [Google Scholar]
- 18. Polonsky WH, Hood KK, Levy CJ, et al. How introduction of automated insulin delivery systems may influence psychosocial outcomes in adults with type 1 diabetes: findings from the first investigation with the Omnipod® 5 System. Diabetes Res Clin Pract. 2022;190:109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pinsker JE, Müller L, Constantin A, et al. Real-world patient-reported outcomes and glycemic results with initiation of control-IQ technology. Diabetes Technol Ther. 2021;23(2):120-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fisher L, Polonsky WH, Hessler DM, et al. Understanding the sources of diabetes distress in adults with type 1 diabetes. J Diabetes Complications. 2015;29(4):572-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hessler DM, Fisher L, Polonsky WH, et al. Diabetes distress is linked with worsening diabetes management over time in adults with Type 1 diabetes. Diabet Med. 2017;34(9):1228-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-dst-10.1177_19322968241242399 for Automated Insulin Delivery in Adults With Type 1 Diabetes and Suboptimal HbA1c During Prior Use of Insulin Pump and Continuous Glucose Monitoring: A Randomized Controlled Trial by Merete B. Christensen, Ajenthen G. Ranjan, Karen Rytter, Olivia M. McCarthy, Signe Schmidt and Kirsten Nørgaard in Journal of Diabetes Science and Technology


