Abstract
Background
Chronic insomnia increases the risk of various health problems and mental illness. Existing research suggests promise for both transcranial direct current stimulation (tDCS) and repetitive transcranial magnetic stimulation (rTMS) in treating chronic insomnia individually. However, the combined effects of tDCS and rTMS on this condition remain unclear. This study aimed to verify the efficacy and safety of tDCS combined with rTMS for the treatment of adult patients with chronic insomnia.
Methods
This was a randomised double-blind parallel-group controlled study. Overall, 157 participants with chronic insomnia were randomly assigned to one of three neurotherapy regimens: tDCS + rTMS, sham tDCS + rTMS, or tDCS + sham rTMS. All groups received 20 treatment sessions over 4 consecutive weeks. The primary outcome was the change in patients’ sleep as assessed by the Pittsburgh Sleep Quality Index (PSQI) at 2 weeks, 4 weeks, and 3 months of follow-up. The secondary outcome was the assessment of different dimensions of depression and anxiety in patients through the Hamilton Depression Scale (HAMD) and Hamilton Anxiety Scale (HAMA), as well as the occurrence of adverse events.
Results
Throughout the intervention and after the 3-month follow-up, the tDCS + rTMS group had significantly reduced total PSQI scores compared with the other two groups [tDCS + rTMS, 9.21 vs. sham tDCS + rTMS, 10.03; difference − 1.10; 95% confidence interval (CI), − 1.82 to − 0.38; p = 0.003; tDCS + rTMS, 9.21 vs. tDCS + sham rTMS, 10.76; difference − 2.14; 95% CI, − 2.90 to − 1.38; p < 0.001; sham tDCS + rTMS, 10.03 vs. tDCS + sham rTMS, 10.76; difference − 1.04; 95% CI, − 1.82 to − 0.26; p = 0.010), indicating improved overall sleep quality. Total HAMD and insomnia factor scores were significantly lower in the tDCS + rTMS group than in the other two groups after treatment (p < 0.05). Notably, no adverse events or serious adverse reactions were observed during the study period.
Conclusions
Combining tDCS with rTMS effectively relieved insomnia symptoms, achieving a significant therapeutic effect after 2-week of intervention, and demonstrating the persistence of treatment effects in later follow-up, emphasising the advantages of combination therapy in improving treatment stability and long-term benefits, reflecting the rapid and effective augmentation of combination therapy. This combined therapy may serve as a safe and effective treatment for adults with chronic insomnia.
Trial registration
This study was registered as a clinical trial with the China Clinical Trial Registration Center (ChiCTR2100052681).
Supplementary Information
The online version contains supplementary material available at 10.1186/s12916-024-03751-y.
Keywords: Chronic insomnia, Transcranial direct current stimulation, Repetitive transcranial magnetic stimulation, Combined therapy, Clinical trial
Background
Chronic insomnia is one of the most common sleep disorders. It is characterised by several key symptoms: difficulty falling asleep, maintaining sleep, early awakening with an inability to return to sleep, dissatisfaction with sleep quality, and daytime functioning impairment. The course of the disease lasts 3 months or more [1]. According to epidemiological surveys, the prevalence rate of insomnia disorders is approximately 10–20%. Among the affected individuals, approximately 50% have a history of chronic disease, showing a trend of chronicity [2, 3]. This significantly affects an individual’s physical and mental health, reduces the work efficiency and level of alertness, and may even lead to accidents, aggravating the economic burden on both the individual and society [4–6]. Pharmacological and non-pharmacological treatments are the main methods of treating insomnia. Among these, the most used drugs are sedative-hypnotic drugs, which aim to improve the sleep state. However, they often come with varying degrees of adverse reactions and can easily lead to withdrawal syndrome upon cessation of intake [7, 8]. Consequently, there is a pressing need for safe and effective non-pharmacological treatment programmes for insomnia. One such non-pharmacological treatment receiving increasing attention is neurotherapy. Specifically, techniques such as transcranial direct current stimulation (tDCS) and repetitive transcranial magnetic stimulation (rTMS) are widely used in clinical practice, both domestically and internationally, because of their non-invasive, penetrating, and easy-to-operate characteristics.
As a non-invasive brain stimulation method, rTMS uses time-varying magnetic signals to pass through the skull without attenuation. These signals generate induced currents in specific brain regions, inducing a depolarisation process and activating neurogenesis. This, in turn, can lead to a series of electrophysiological and functional changes [9]. Research has shown that rTMS can induce long-lasting changes in cerebral cortical excitability and modulate functional synaptic plasticity in the cortex through mechanisms such as long-term potentiation (LTP) or long-term depression (LTD) [10, 11]. The therapeutic effect is determined by multiple factors, such as stimulus intensity, stimulus frequency, number of transmitted pulses, position and type of coil, and the duration of the stimulation. Concerning the impact of rTMS on insomnia, a systematic evaluation showed that rTMS treatment could significantly increase slow-wave sleep and rapid eye movement sleep phases, thereby improving sleep quality [12]. Other studies have shown that rTMS treatment can significantly reduce the Pittsburgh Sleep Quality Index (PSQI) scores and shorten the time to insomnia symptom relief, suggesting that rTMS may have a positive impact on the treatment of patients with chronic insomnia [13, 14].
tDCS regulates cortical activity by applying a constant low-intensity current to the scalp through cathodal and anodal electrodes. Normally, anodal stimulation triggers neurone depolarisation, increasing cortical excitability, while cathodal stimulation triggers neurone hyperpolarisation, decreasing cortical excitability [15, 16]. Research has shown that weak constant currents can traverse the cranium, affecting the magnitude and direction of the intracranial electric field, thus causing excitatory changes in the cortex [17]. This regulation of brain discharge activity can lead to various effects, including ion activity, neurotransmitter release, and brain oscillatory activity. Compared with rTMS, the current generated by tDCS is not sufficient to directly induce neuronal action potentials; rather, it modulates neuronal resting membrane potentials through microcurrents, exerting subthreshold modulation that alters neuronal activity [18, 19]. Multiple studies have shown that tDCS treatment of insomnia leads to improvements in PSQI scores, indicating subjective measures of insomnia. In a 12-week study, significant improvements in sleep quality among patients were observed [20]. Additionally, another study explored the combined application of tDCS and electroencephalography, showing that applying slow-wave oscillatory stimulation in patients with insomnia can synchronise their brain waves with the sleep’s slow-wave frequency, potentially achieving sleep stabilisation [21].
Both tDCS and rTMS have shown positive effects in treating insomnia, with high safety and sustained efficacy reported. While both have their advantages, the combination of neurotherapy and medication is also a clinical option. The effect of combining tDCS and rTMS for chronic insomnia remains unclear. Specifically, whether this combination is superior to a single-modality neurotherapy or medication use requires further investigation. Therefore, we performed a double-blind, randomised, parallel-group, controlled trial using a treatment regimen combined with tDCS. This study aimed to examine the efficacy and enhancement of combining tDCS with rTMS in adult patients with chronic insomnia. We assessed response rate, remission rate, and different dimensions of insomnia, as measured by the PSQI, along with the HAMD and HAMA to evaluate patients’ depression, anxiety, and potential adverse events at the end of the 4 weeks of the intervention period. Our findings are anticipated to significantly advance the field of sleep medicine by offering a more effective and safe treatment option for chronic insomnia through the combination of tDCS and rTMS, potentially establishing a new treatment protocol and guiding clinicians in selecting the most efficacious strategies compared to traditional single-modality therapies.
Methods
Trial design
To investigate the efficacy of combining tDCS and rTMS for treating chronic insomnia, a 4-week randomised, double-blind, parallel-controlled trial was conducted with three groups: tDCS + rTMS, sham tDCS + rTMS, and tDCS + sham rTMS. Primary outcome indicators were evaluated at baseline, the end of the 2-week intervention, the end of the 4-week intervention, and 3 months after intervention. This study recruited and enrolled participants in Sleep Clinic Centre. All neuromodulation treatments and outcome measurements at different times were conducted at the Physical Therapy Centre to avoid measurement bias that may be introduced by different locations. Each measurement was performed according to a standardised procedure to ensure the reliability and validity of the data.
All procedures were approved by the Clinical Research Ethics Committee of Ningbo Kangning Hospital (No. NBKNYY-2019-LC-20), ensuring compliance with the ethical standards and regulations of the Declaration of Helsinki on Human Research. This study was registered as a clinical trial with the China Clinical Trial Registration Center (ChiCTR2100052681).
Participants
Between December 2021 and December 2022, 200 individuals suffering from chronic insomnia were recruited from Ningbo Kangning Hospital and randomly assigned to three groups. A total of 157 individuals completed the treatment and assessment. All participants were fully informed of the study procedure and voluntarily signed a written informed consent form. Prospective participants were pre-screened through a brief interview. Individuals who met the inclusion criteria were then further screened onsite by trained and licenced psychiatrists using the Mini-International Neuropsychiatric Interview (M.I.N.I.) [22] to verify the presence of co-morbid psychiatric disorders, which were included as an exclusion criterion in the study.
Inclusion criteria were (1) age 18–65 years, (2) diagnosis of chronic primary insomnia according to the International Classification of Diseases-11 (ICD-11) [23], (3) baseline PSQI total score > 5 [24], (4) administration of zopiclone, which is used for the treatment of insomnia, before the baseline visit as well as throughout the treatment programme, and (5) agreement to abstain from medications or other non-pharmacological treatments throughout the trial period.
Exclusion criteria were (1) serious physical illness, (2) history of epilepsy, (3) co-morbid psychiatric disorders according to the M.I.N.I., (4) neurologic disorders, (5) history of electroconvulsive therapy, (6) cochlear implants, cardiac pacemakers, implanted devices (i.e., deep brain stimulation), or metals in the brain, (7) pregnancy or breastfeeding, (8) history of drug or alcohol abuse/dependence, (9) presence of other sleep disorders, including sleep apnoea, periodic limb movements, narcolepsy, and (10) participation in concurrent clinical trials.
The criteria for study termination were as follows: (1) occurrence of a serious adverse event; (2) pregnancy; (3) two consecutive missed treatments; and (4) withdrawal of consent.
Intervention
The study participants were randomly assigned to one of three neurotherapy regimens, with a 30-min interval between tDCS and rTMS in all groups. The subjects received 20 treatments on weekdays for 4 consecutive weeks.
The tDCS + rTMS (combined) group was first treated with tDCS using a tDCS instrument. The stimulator model was tDCS-20A (Keyue Co., Ltd., Xi’an, China), which provided 2 mA of stable direct current power through a battery. Two sponges (5 × 5 cm2) were placed on the rubber electrodes for stimulation. The stimulation time was 20 min. The anode and cathode electrodes were placed on the left (F3 in the International 10–20 EEG Electrode Distribution System) and right dorsolateral prefrontal cortex (DLPFC) (F4 in the International 10–20 EEG Electrode Distribution System) [25, 26], respectively. The start and end of the stimulation each had a 30-s phase of slowly increasing and decreasing current intensity to avoid the physical discomfort caused by the sudden current change. Then, rTMS treatment was performed with an rTMS instrument (Magstim Ltd, Oxford, UK), targeting the right DLPFC with 1 Hz low-frequency transcranial magnetic stimulation. The stimulation intensity was 100% motor threshold, the stimulation time was 6 s, the inter-stimulus interval was 4 s, and the total number of pulses was 1800.
The sham tDCS + rTMS group underwent initial treatment with pseudo-stimulation using tDCS, and the stimulation electrode position, stimulation area, and stimulation time were consistent with those of the combined group. The pseudo-stimulation only outputs a current of up to 2 mA at the beginning and end of 10 s so that the participants could experience a feeling similar to that of the real stimulation. The instrument did not output electrical stimulation signals in the middle of 20 min. The rTMS treatment was then performed, and the stimulation position and stimulation time were consistent with those used for the combined group.
The tDCS + sham rTMS group was first treated with tDCS; the positions of the stimulation electrode, stimulation area, and stimulation time were consistent with those used for the combined group. Transcranial magnetic pseudostimulation treatment was delivered using a TMS instrument with a special pseudostimulation coil. Stimulation position, stimulation time, and other parameters were consistent with those used for the combined group. The pseudostimulation special coil can only emit sound but not output pulse stimulation.
Outcomes
During screening, all participants were evaluated using the Chinese version of the M.I.N.I. [22] to rule out psychiatric disorders. Chronic insomnia disorder was diagnosed according to ICD-11. At baseline, both demographic and clinical data were collected from participants. Demographic data included age, sex, marital status, education level, and occupation. Clinical data included body mass index, duration of insomnia, and personal history of alcohol consumption, smoking, and hypnotic medication use were collected.
At baseline, the end of the 2-week intervention, the end of the 4-week intervention, and 3 months after intervention, the total PSQI score and factor scores were assessed. At the 3-month post-intervention, the research team obtained follow-up outcomes through a structured telephone assessment (with video calls used if necessary) based on PSQI. The PSQI is a 19-item questionnaire containing 7 components: sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbance, sleep medication, and daytime dysfunction. Each component is scored on a 0–3 scale, with the total score ranging from 0 to 21, with higher values indicating poorer sleep quality. The PSQI is commonly used to evaluate the subjective sleep quality of participants within the past month and it is highly correlated with the Insomnia Severity Index [27, 28].
Meanwhile, at baseline and at the end of the 4-week intervention, patients’ depression and anxiety were assessed by HAMD and HAMA total scores, respectively, as well by each factor score. A 17-item version of the HAMD was used, with each item involving different aspects of depression symptoms, including four components: core symptoms (items 1–3), insomnia (items 4–6), anxiety (items 9–13,15), and somatic factors (items 7,8,14,16,17). Each item is scored on a 0–4 scale, and individual items are scored on a 0–2 scale. A total score ≤ 7 is considered to indicate no depressive symptoms. The HAMD assesses the severity of depressive symptoms in participants over the past 1–2 weeks, with items 4–6 examining sleep quality [29, 30]. The HAMA scale includes 14 items, each of which involves different aspects of anxiety symptoms, including two components: psychic anxiety (items 1–6, 14) and somatic anxiety (items 7–13). All items are scored on a 0–4 scale, with a total score ≤ 7 indicating no anxiety symptoms. The HAMA can assess the severity of anxiety symptoms in participants over the past 1–2 weeks [31].
At the end of the 4-week intervention, patients were asked, “Did you have any discomfort, epileptic seizures, or abnormal feelings during the study?” to assess the adverse events.
Sample size
We calculated the sample size required to estimate the change in the differences in total PSQI score among three groups, using a medium effect size (0.25), which is based on Cohen’s classification criteria for effect size [32]. The power was set to 90% and the two-tailed α level to 5%, choosing an F test, a repeated-measures analysis of variance (ANOVA), and between-factor model. G*Power software was used to calculate the sample size, and the minimum sample size was 141.
Randomisation to treatment and blinding
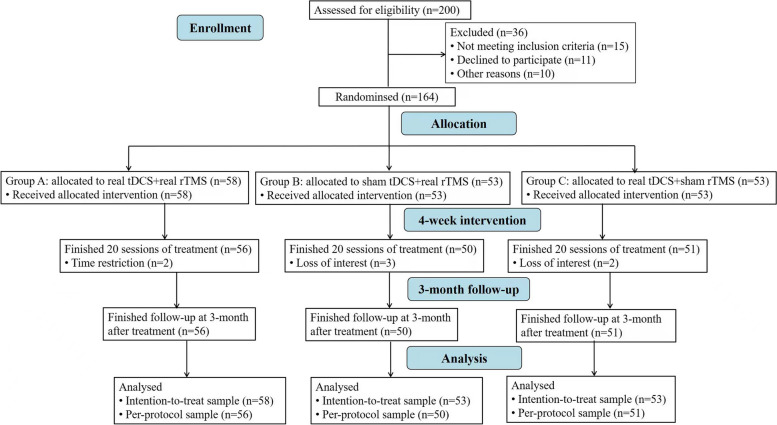
The CONSORT chart for this clinical trial is shown in Fig. 1. Patients were randomly assigned to one of three groups by an impartial third party using a computer-generated randomisation list (group A: real tDCS + real rTMS, group B: sham tDCS + real rTMS, group C: real tDCS + sham rTMS). In the three treatment groups, taking integers from 1 to 3, before the first intervention, the nurse assigned a number to each participant by opening a sealed opaque envelope to determine instrument selection. Four rTMS instruments and four tDCS instruments were used in this trial, including two sham-stimulation instruments and two true-stimulation instruments, all of which had the same size, appearance, weight, and odour. To maintain double-blinding, we coded all devices uniformly by the experimental designer. Throughout the intervention, participants were randomly selected and assigned to the same instrument. To assess the integrity of the blinding method in the trail, we asked participants to guess their assigned intervention after treatment. Blinding could be broken in case of an emergency. Neither the assessor nor the patient knew which treatment regimen they had been assigned. Treatments were carried out by trained staff who were not related to the study. Data were analysed by a separate researcher blinded to treatment allocation. After enrolment, patients received stable zopiclone medication. The medications of the patients in each group are detailed in Table 1, with no significant difference between groups.
Fig. 1.
Journal article reporting standards flowchart. tDCS, transcranial direct current stimulation. rTMS, repetitive transcranial magnetic stimulation
Table 1.
Demographic and clinical characteristics of the patients
| Characteristic | tDCS + rTMS Group (n = 56) | sham tDCS + rTMS Group (n = 50) | tDCS + sham rTMS Group (n = 51) | F/χ2 | p value |
|---|---|---|---|---|---|
| Gender | 2.546 | 0.280 | |||
| Male | 24 | 14 | 18 | ||
| Female | 32 | 36 | 33 | ||
| Age (years) | 44.59 ± 13.49 | 47.08 ± 14.18 | 42.47 ± 12.55 | 1.502 | 0.226 |
| Body mass index | 22.56 ± 3.30 | 21.82 ± 3.14 | 22.06 ± 2.97 | 0.768 | 0.466 |
| Marital status | 2.391 | 0.303 | |||
| Not married | 11 | 6 | 5 | ||
| Married | 45 | 44 | 46 | ||
| Occupation | 4.697 | 0.789 | |||
| Farmer | 3 | 4 | 5 | ||
| Student | 5 | 5 | 6 | ||
| Worker | 29 | 18 | 23 | ||
| Retired | 8 | 13 | 9 | ||
| Unemployed | 11 | 10 | 8 | ||
| Education level | 4.299 | 0.367 | |||
| Junior high school and lower | 21 | 26 | 28 | ||
| Senior high school | 14 | 11 | 8 | ||
| College and above | 21 | 13 | 15 | ||
| Smoking | 1.297 | 0.523 | |||
| No | 49 | 47 | 46 | ||
| Yes | 7 | 3 | 5 | ||
| Drinking | 1.308 | 0.520 | |||
| No | 52 | 48 | 46 | ||
| Yes | 4 | 2 | 5 | ||
| Disease duration (months) | 75.81 ± 90.62 | 79.19 ± 100.00 | 83.47 ± 30.82 | 0.122 | 0.885 |
| Zopiclone (mg/day) | 6.96 ± 2.42 | 7.50 ± 2.40 | 7.21 ± 2.58 | 0.624 | 0.537 |
| PSQI total score | 16.20 ± 2.74 | 15.90 ± 2.73 | 15.78 ± 2.17 | 0.371 | 0.691 |
| HAM-D total score | 21.09 ± 7.26 | 20.96 ± 6.16 | 21.47 ± 7.13 | 0.076 | 0.927 |
| HAM-A total score | 22.52 ± 9.56 | 23.48 ± 7.63 | 23.37 ± 6.82 | 0.226 | 0.798 |
F/χ2 and p for group effects
Significant results are highlighted (p < 0.05) in bold
PSQI Pittsburgh Sleep Quality Index, HAMA Hamilton Anxiety Scale, HAMD Hamilton Depression Scale
Statistical analysis
Statistical analysis was performed using SPSS 25.0 (IBM, Armonk, NY). Continuous variables were presented as mean ± standard deviation, whereas categorical variables were described using examples or percentages. The primary outcome was the change in patients’ sleep as assessed by the PSQI at the end of the 2-week treatment, the end of the 4-week treatment, and after 3 months. A response rate was defined as at least a 50% reduction in insomnia symptoms from baseline, as measured by the PSQI. The remission rate was defined as a total PSQI score < 5. The secondary outcome is the assessment of different dimensions of depression and anxiety in patients through the Hamilton Depression Scale (HAMD) and Hamilton Anxiety Scale (HAMA), as well as the occurrence of adverse events. One-way ANOVA was used to evaluate the between-group differences among the three groups in continuous variables at baseline, mainly including clinical symptoms. Chi-squared analysis was used to evaluate the between-group differences among the three groups in categorical variables at baseline, including gender, marital status, occupation, education level, smoking, and drinking. To analyse treatment effects, we conducted a repeated-measures ANOVA. This analysis explored the changes in clinical symptom scores across different treatment groups and timepoints. Post hoc tests were then used to compare differences between groups. The results were corrected using the Bonferroni correction. A random-effects mixed-model analysis was used to model each outcome variable as a linear function of treatment (i.e., tDCS + rTMS treatment group, sham tDCS + rTMS treatment group, or sham tDCS + rTMS treatment group) and period (i.e., baseline, 2 weeks, 4 weeks, 4 months), with “participant” included as a random variable. Results were considered significant if the two-tailed p-value was < 0.05.
Results
Baseline data
A total of 200 participants were enrolled, of whom 15 were excluded, 11 refused to participate, and 10 were unable to participate for other reasons. Therefore, 164 patients were enrolled and randomly assigned to one of the three neurotherapy regimens. Finally, a total of 157 patients completed the trial. At the end of the 4-week intervention period, the retention rate was 95.7% (157/164) (Fig. 1). At baseline, there were no significant differences among the three groups all aspects, including demographic and clinical information (Table 1).
Primary outcomes
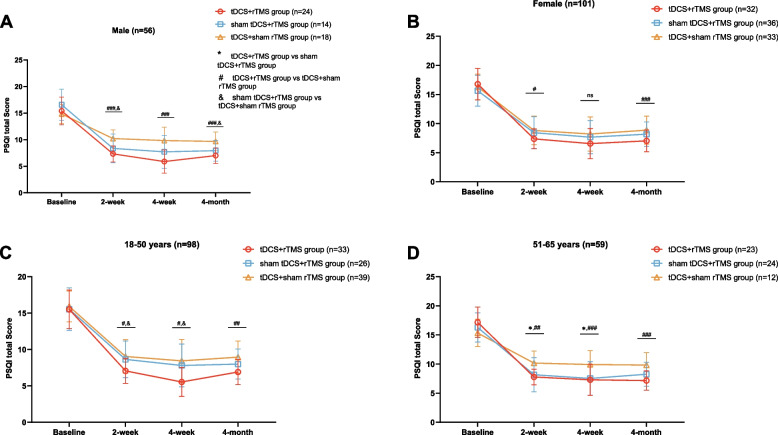
Based on the analysis of the total PSQI score and sex grouping, the results showed a significant difference in the total PSQI score among the three groups of males at 2 weeks, 4 weeks, and 3 months after treatment (p < 0.05), and the tDCS + rTMS group had the lowest PSQI total score (Fig. 2A, B). Further pairwise comparison showed that there was a significant difference in the total PSQI score between the tDCS + rTMS and tDCS + sham rTMS groups at 2 weeks, 4 weeks, and 3 months after treatment (p < 0.001). At 2 weeks of treatment and 3 months after treatment, there was a significant difference in the total PSQI score between the sham tDCS + rTMS and tDCS + sham rTMS groups (p < 0.05). There was a significant difference in the total PSQI score among the three groups of females after 2 weeks of treatment and 3 months after treatment (p < 0.05), and the tDCS + rTMS group had the lowest total PSQI score. At 4 weeks of treatment, there was no significant difference in the total PSQI score among the three groups. Further pairwise comparisons showed that there was a significant difference in the total PSQI score between the tDCS + rTMS and tDCS + sham rTMS groups at 2 weeks of treatment and 3 months after treatment (p < 0.05).
Fig. 2.
Repeated-measures analysis of variance and post hoc tests comparing PSQI scores at 2 weeks, 4 weeks, and 3 months after treatment for different neuromodulation groups stratified by gender (A, B) and age (C, D). * P < 0.05, ** P < 0.01, *** P < 0.001, tDCS + rTMS group vs sham tDCS + rTMS group; # P < 0.05, ## P < 0.01, ### P < 0.001, tDCS + rTMS group vs tDCS + sham rTMS group; & P < 0.05, && P < 0.01, &&& P < 0.001, sham tDCS + rTMS group vs tDCS + sham rTMS group; ns, no significant difference. PSQI, Pittsburgh Sleep Quality Index
Based on the analysis of total PSQI score and age groups (Fig. 2C, D), the results showed that there were significant differences in total PSQI score among those aged 18–50 years at 2 weeks, 4 weeks, and 3 months after treatment (p < 0.05), with the tDCS + rTMS group having the lowest total PSQI score. Further two-by-two comparisons showed that there was a significant difference in the total PSQI score between the tDCS + rTMS and tDCS + sham rTMS groups at 2 weeks, 4 weeks, and 3 months after treatment (p < 0.01). At 2 and 4 weeks of treatment, there was a significant difference in the total PSQI score between the tDCS + rTMS and sham tDCS + rTMS groups (p < 0.05). In those aged 51–65 years, there was a significant difference in the total PSQI score among the three groups at 2 weeks and 4 weeks of treatment (p < 0.05), and the tDCS + rTMS group had the lowest total PSQI score. Further two-by-two comparisons showed that there was a significant difference in the total PSQI score between the tDCS + rTMS and tDCS + sham rTMS groups at 2 weeks, 4 weeks, and 3 months after treatment (p < 0.05). At 2 and 4 weeks of treatment, there was a significant difference in the total PSQI score between the sham tDCS + rTMS and tDCS + sham rTMS groups (p < 0.05).
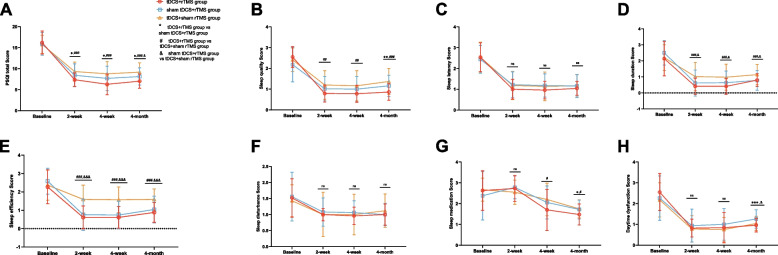
Longitudinal changes in insomnia severity among the three groups were revealed through the total PSQI score and factor scores at 4 weeks of intervention and 3 months of follow-up (Table 2). Repeated-measures ANOVA was conducted on the total and sub-item scores PSQI in the three treatment groups at different time periods, which showed that the total PSQI score, sleep quality, sleep duration, sleep efficiency, and sleep medication in the three groups differed, with a group × time interaction. A significant main effect of time was observed for the total and sub-item PSQI scores of the three groups. Additionally, the main effects of the group were significant for the total PSQI score, sleep quality, sleep duration, and sleep efficiency in the three groups. Further two-by-two comparisons (Fig. 3) showed that at 2 weeks of treatment, the post-treatment scores of total PSQI score, sleep quality, sleep duration, and sleep efficiency in the tDCS + rTMS group were significantly lower than those in the tDCS + sham rTMS group (p < 0.05). The post-treatment total PSQI scores in the tDCS + rTMS group were significantly lower than those in the sham tDCS + rTMS group (p < 0.05), and the post-treatment scores of sleep duration and sleep efficiency in the sham tDCS + rTMS group were significantly lower than those in the tDCS + sham rTMS group (p < 0.05). At 4 weeks of treatment, the total PSQI score, sleep quality, sleep duration, sleep efficiency, and sleep medication in the tDCS + rTMS group were significantly lower than those in the tDCS + sham rTMS group (p < 0.05). The total PSQI score in the tDCS + rTMS group was significantly lower than that in the sham tDCS + rTMS group after treatment (p < 0.05), and the post-treatment scores of sleep duration and sleep efficiency in the sham tDCS + rTMS group were significantly lower than those in the tDCS + sham rTMS group (p < 0.05). At the 3-month follow-up after treatment, the total PSQI score, sleep quality, sleep duration, sleep efficiency, and sleep medication in the tDCS + rTMS group were significantly lower than those in the tDCS + sham rTMS group (p < 0.05). The total PSQI score, sleep medication, and daytime dysfunction in the tDCS + rTMS group were significantly lower than those in the sham tDCS + rTMS group (p < 0.05). The total PSQI score, sleep duration, sleep efficiency, and daytime dysfunction in the sham tDCS + rTMS group were significantly lower than those in the tDCS + sham rTMS group (p < 0.05).
Table 2.
PSQI scores and comparison at baseline, 2 weeks, 4 weeks and 4 months in three groups
| Primary outcomes | Baseline | 2 weeks | 4 weeks | 4 months | Group F (p value) | Time F (p value) | Group *Time (p value) |
|---|---|---|---|---|---|---|---|
| PSQI total score | 9.10 (0.000) | 853.07 (0.000) | 8.18 (0.000) | ||||
| tDCS + rTMS | 16.20 ± 2.74 | 7.36 ± 1.61 | 6.27 ± 2.44 | 7.02 ± 1.70 | |||
| sham tDCS + rTMS | 15.90 ± 2.73 | 8.42 ± 2.70 | 7.68 ± 2.89 | 8.12 ± 2.04 | |||
| tDCS + sham rTMS | 15.78 ± 2.17 | 9.31 ± 2.28 | 8.78 ± 2.87 | 9.16 ± 2.21 | |||
| Sleep quality | 5.92 (0.003) | 364.67 (0.000) | 8.54 (0.000) | ||||
| tDCS + rTMS | 2.55 ± 0.50 | 0.79 ± 0.41 | 0.78 ± 0.41 | 0.86 ± 0.40 | |||
| sham tDCS + rTMS | 2.18 ± 0.83 | 1.02 ± 0.59 | 1.00 ± 0.61 | 1.16 ± 0.51 | |||
| tDCS + sham rTMS | 2.41 ± 0.57 | 1.20 ± 0.69 | 1.16 ± 0.73 | 1.37 ± 0.63 | |||
| Sleep latency | 1.35 (0.263) | 339.10 (0.000) | 1.32 (0.269) | ||||
| tDCS + rTMS | 2.54 ± 0.69 | 1.00 ± 0.47 | 0.96 ± 0.42 | 1.04 ± 0.33 | |||
| sham tDCS + rTMS | 2.44 ± 0.67 | 1.22 ± 0.62 | 1.18 ± 0.56 | 1.16 ± 0.55 | |||
| tDCS + sham rTMS | 2.53 ± 0.73 | 1.18 ± 0.68 | 1.14 ± 0.69 | 1.16 ± 0.54 | |||
| Sleep duration | 6.96 (0.001) | 292.79 (0.000) | 5.48 (0.001) | ||||
| tDCS + rTMS | 2.14 ± 1.07 | 0.41 ± 0.50 | 0.41 ± 0.49 | 0.80 ± 0.40 | |||
| sham tDCS + rTMS | 2.50 ± 0.76 | 0.62 ± 0.81 | 0.64 ± 0.72 | 0.78 ± 0.62 | |||
| tDCS + sham rTMS | 2.20 ± 0.80 | 1.02 ± 0.88 | 0.98 ± 0.81 | 1.14 ± 0.63 | |||
| Sleep efficiency | 23.47 (0.000) | 234.17 (0.000) | 11.12 (0.000) | ||||
| tDCS + rTMS | 2.27 ± 0.92 | 0.61 ± 0.62 | 0.61 ± 0.59 | 0.88 ± 0.54 | |||
| sham tDCS + rTMS | 2.58 ± 0.70 | 0.76 ± 0.85 | 0.74 ± 0.75 | 1.04 ± 0.73 | |||
| tDCS + sham rTMS | 2.37 ± 0.82 | 1.59 ± 0.78 | 1.57 ± 0.70 | 1.59 ± 0.57 | |||
| Sleep disturbance | 0.30 (0.740) | 59.52 (0.000) | 1.16 (0.324) | ||||
| tDCS + rTMS | 1.52 ± 0.60 | 1.00 ± 0.19 | 0.96 ± 0.27 | 1.00 ± 0.33 | |||
| sham tDCS + rTMS | 1.56 ± 0.76 | 1.08 ± 0.44 | 1.06 ± 0.37 | 1.00 ± 0.35 | |||
| tDCS + sham rTMS | 1.43 ± 0.50 | 1.00 ± 0.69 | 1.00 ± 0.63 | 1.12 ± 0.52 | |||
| Sleep medication | 1.45(0.239) | 91.28 (0.000) | 4.39 (0.002) | ||||
| tDCS + rTMS | 2.63 ± 0.95 | 2.73 ± 0.62 | 1.70 ± 0.99 | 1.48 ± 0.50 | |||
| sham tDCS + rTMS | 2.38 ± 1.16 | 2.78 ± 0.55 | 2.06 ± 0.79 | 1.72 ± 0.45 | |||
| tDCS + sham rTMS | 2.67 ± 0.55 | 2.55 ± 0.58 | 2.20 ± 0.78 | 1.75 ± 0.44 | |||
| Daytime dysfunction | 2.15 (0.120) | 208.43 (0.000) | 2.55(0.050) | ||||
| tDCS + rTMS | 2.55 ± 0.89 | 0.82 ± 0.43 | 0.84 ± 0.73 | 0.96 ± 0.33 | |||
| sham tDCS + rTMS | 2.26 ± 1.07 | 0.94 ± 0.79 | 1.00 ± 0.76 | 1.26 ± 0.44 | |||
| tDCS + sham rTMS | 2.18 ± 0.82 | 0.78 ± 0.64 | 0.75 ± 0.59 | 1.04 ± 0.34 |
PSQI Pittsburgh Sleep Quality Index. F and p for group effects. Significant results are highlighted (p < 0.05) in bold
The data are presented as mean ± standard deviation (SD)
Fig. 3.
A–H Changes in total PSQI and factor scores after three different treatment modalities in patients with chronic insomnia at 2 weeks, 4 weeks, and 3 months after treatment. * P < 0.05, ** P < 0.01, *** P < 0.001, tDCS + rTMS group vs sham tDCS + rTMS group; # P < 0.05, ## P < 0.01, ### P < 0.001, tDCS + rTMS group vs tDCS + sham rTMS group; & P < 0.05, && P < 0.01, &&& P < 0.001, sham tDCS + rTMS group vs tDCS + sham rTMS group; ns, no significant difference. PSQI, Pittsburgh Sleep Quality Index
Random-effects mixed-model analysis showed that the tDCS + rTMS group had a significantly reduced total PSQI score (tDCS + rTMS, 9.21 vs. sham tDCS + rTMS, 10.03; difference − 1.10; 95%CI − 1.82 to − 0.38; p = 0.003; tDCS + rTMS, 9.21 vs. tDCS + sham rTMS, 10.76; difference − 2.14; 95% CI − 2.90 to − 1.38; p < 0.001; sham tDCS + rTMS, 10.03 vs. tDCS + sham rTMS, 10.76; difference − 1.04; 95% CI − 1.82 to − 0.26; p = 0.010) (Table 3). The tDCS + rTMS group had significantly reduced sleep quality (tDCS + rTMS, 1.25 vs. sham tDCS + rTMS, 1.34; difference − 0.30; 95% CI − 0.48 to − 0.13; p = 0.001; tDCS + rTMS, 1.25 vs. tDCS + sham rTMS, 1.53; difference − 0.52; 95%CI − 0.71 to − 0.32; p < 0.001; sham tDCS + rTMS, 1.34 vs. tDCS + sham rTMS, 1.53; difference − 0.21; 95%CI − 0.42 to -0.01; p = 0.041). The tDCS + rTMS group had significantly reduced sleep duration (tDCS + rTMS, 0.94 vs. tDCS + sham rTMS, 1.33; difference − 0.33; 95%CI − 0.55 to − 0.12; p = 0.002; sham tDCS + rTMS, 1.14 vs. tDCS + sham rTMS, 1.33; difference − 0.36; 95%CI − 0.58 to − 0.14; p = 0.001). The tDCS + rTMS group had significantly reduced sleep efficiency (tDCS + rTMS, 1.09 vs. tDCS + sham rTMS, 1.78; difference − 0.71; 95%CI − 0.95 to − 0.48; p < 0.001; sham tDCS + rTMS, 1.28 vs. tDCS + sham rTMS, 1.78; difference − 0.55; 95%CI − 0.79 to − 0.31; p < 0.001). The tDCS + rTMS group had significantly reduced sleep medication usage (tDCS + rTMS, 2.13 vs. sham tDCS + rTMS, 2.24; difference − 0.24; 95%CI − 0.42 to − 0.05; p = 0.013; tDCS + rTMS, 2.13 vs. tDCS + sham rTMS, 2.29; difference − 0.26; 95%CI − 0.44 to − 0.08; p = 0.004). The tDCS + rTMS group had significantly reduced daytime dysfunction (tDCS + rTMS, 1.30 vs. tDCS + sham rTMS, 1.19; difference − 0.30; 95%CI − 0.44 to − 0.15; p < 0.001; sham tDCS + rTMS, 1.37 vs. tDCS + sham rTMS, 1.19; difference 0.22; 95%CI 0.07 to 0.37; p = 0.003).
Table 3.
Mixed-model results, including mean estimate under each treatment, the difference of treatments (tDCS + rTMS vs. sham tDCS + rTMS vs. tDCS + sham rTMS), effect size and significance level
| Primary outcomes | Adjusted mean (95% CI)a | Treatment effect estimate (95% CI) | Effect sizeb (p value) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment A | Treatment B | Treatment C | Treatment A vs C | Treatment B vs C | Treatment A vs B | Treatment A vs C | Treatment B vs C | Treatment A vs B | |
| PSQI total score | 9.21 (8.71 to 9.71) | 10.03 (9.51 to 10.56) | 10.76 (10.24 to 11.28) | − 2.14 (− 2.90 to − 1.38) | − 1.04 (− 1.82 to − 0.26) | − 1.10(− 1.82 to − 0.38) | − 0.82 (0.000) | − 0.39 (0.010) | − 0.44 (0.003) |
| Sleep quality | 1.25 (1.13 to 1.36) | 1.34 (1.22 to 1.46) | 1.53 (1.41 to 1.66) | − 0.52 (− 0.71 to − 0.32) | − 0.21 (− 0.42 to − 0.01) | − 0.30(− 0.48 to − 0.13) | − 0.66 (0.000) | − 0.44 (0.041) | − 0.21 (0.001) |
| Sleep latency | 1.38 (1.27 to 1.50) | 1.50 (1.38 to 1.62) | 1.50 (1.38 to 1.62) | − 0.121 (− 0.30 to − 0.06) | − 0.003 (− 0.19 to 0.19) | − 0.12(− 0.30 to − 0.05) | − 0.27 (0.194) | 0.00 (0.974) | − 0.27 (0.155) |
| Sleep duration | 0.94 (0.80 to 1.09) | 1.14 (0.98 to 1.29) | 1.33 (1.18 to 1.48) | − 0.33 (− 0.55 to − 0.12) | − 0.36 (− 0.58 to − 0.14) | 0.02(− 0.17 to 0.22) | − 0.72 (0.002) | − 0.36 (0.001) | − 0.36 (0.814) |
| Sleep efficiency | 1.09 (0.95 to 1.23) | 1.28 (1.13 to 1.43) | 1.78 (1.63 to 1.93) | − 0.71 (− 0.95 to − 0.48) | − 0.55 (− 0.79 to − 0.31) | − 0.17(− 0.41 to 0.08) | − 1.30 (0.000) | − 0.94 (0.000) | − 0.35 (0.185) |
| Sleep disturbance | 1.12 (1.02 to 1.22) | 1.18 (1.07 to 1.28) | 1.15 (1.04 to 1.24) | − 0.12 (− 0.27 to − 0.04) | − 0.12 (− 0.28 to − 0.04) | 0.00(− 0.20 to 0.20) | − 0.05 (0.136) | 0.10 (0.147) | − 0.15 (1.000) |
| Sleep medication | 2.13 (2.01 to 2.26) | 2.24 (2.10 to 2.37) | 2.29 (2.16 to 2.42) | − 0.26 (− 0.44 to − 0.08) | − 0.03 (− 0.21 to − 0.16) | − 0.24(− 0.42 to − 0.05) | − 0.32 (0.004) | − 0.11 (0.788) | − 0.21 (0.013) |
| Daytime dysfunction | 1.30 (1.18 to 1.41) | 1.37 (1.24 to 1.49) | 1.19 (1.07 to 1.31) | − 0.07 (− 0.22 to 0.07) | 0.22 (0.07 to 0.37) | − 0.30(− 0.44 to − 0.15) | 0.25 (0.301) | 0.41 (0.003) | − 0.16 (0.000) |
PSQI Pittsburgh Sleep Quality Index, CI confidence interval
Treatment A = tDCS + rTMS group, Treatment B = sham tDCS + rTMS group, Treatment C = tDCS + sham rTMS group
aMeans adjusted for sequence and period
bCohens d effect size
The remission and response rates of each of the three groups after 2 weeks, 4 weeks, and 3 months of treatment are shown in Table 4. Chi-squared analysis showed a significant difference in the response rates of the three groups at 2 weeks (χ2 = 22.507, p < 0.001). The tDCS + rTMS group had the highest response rate (53.57%, 30/56) compared to the sham tDCS + rTMS (34.00%, 17/50) and tDCS + sham rTMS groups (21.57%, 11/51). The tDCS + rTMS group had the highest remission rate (12.5%, 7/56), followed by the sham tDCS + rTMS (8.00%, 4/50) and tDCS + sham rTMS groups (3.92%, 2/51). However, there was no significant difference in remission rates among the three groups (χ2 = 5.378, p = 0.068). After 4 weeks of intervention, the highest remission rate (46.43%, 26/56) and response rate (64.29%, 36/56) were found in the tDCS + rTMS group, followed by the sham tDCS + rTMS (30.00%, 15/50; 44.00%, 22/50) and tDCS + sham rTMS groups (21.57%, 11/51; 27.45%, 14/51), with a significant difference among the three groups in remission rate (χ2 = 13.579, p = 0.001) and response rate (χ2 = 27.717, p < 0.001). Follow-up 3 months after the end of treatment showed the highest response rate in the tDCS + rTMS group (42.86%, 24/56), followed by the sham tDCS + rTMS (36.00%, 18/50) and tDCS + sham rTMS groups (23.53%, 12/51), with a significant difference between groups (χ2 = 8.191, p = 0.017). The tDCS + rTMS group had the highest remission rate (19.64%, 11/56), followed by the sham tDCS + rTMS (14.00%, 7/50) and tDCS + sham rTMS groups (9.80%, 5/51). However, there was no significant difference in remission rates among groups (χ2 = 4.048, p = 0.132).
Table 4.
Remission and response rates at 2 weeks, 4 weeks and 4 months in each group
| tDCS + rTMS Group (n = 56) | sham tDCS + rTMS Group (n = 50) | tDCS + sham rTMS Group (n = 51) | χ2 | p value | |
|---|---|---|---|---|---|
| Remission %(n) | |||||
| 2 weeks | 12.50% (7) | 8.00% (4) | 3.92% (2) | 5.378 | 0.068 |
| 4 weeks | 46.43% (26) | 30.00% (15) | 21.57% (11) | 13.579 | 0.001 |
| 4 months | 19.64% (11) | 14.00% (7) | 9.80% (5) | 4.048 | 0.132 |
| Response %(n) | |||||
| 2 weeks | 53.57% (30) | 34.00% (17) | 21.57% (11) | 22.507 | < 0.001 |
| 4 weeks | 64.29% (36) | 44.00% (22) | 27.45% (14) | 27.717 | < 0.001 |
| 4 months | 42.86% (24) | 36.00% (18) | 23.53% (12) | 8.191 | 0.017 |
Response was defined as the percentage of those with at least a 50% reduction in the PSQI total score from baseline. Remission was defined as a PSQI total score < 5. PSQI Pittsburgh Sleep Quality Index, χ2 and p for group effects. Significant results are highlighted (p < 0.05) in bold
Secondary outcomes
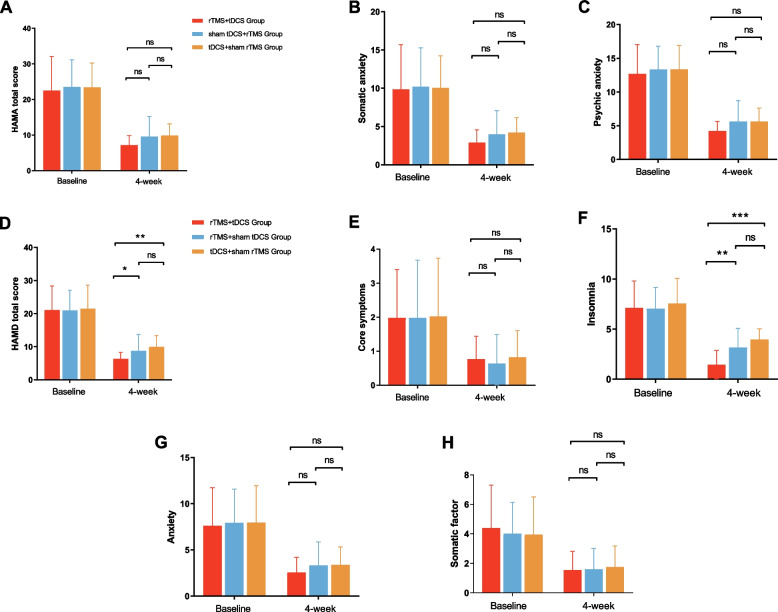
Longitudinal changes in anxiety and depression severity over 4 weeks in the three groups were revealed by the total score and scores for each factor of the HAMA and HAMD, as shown in Fig. 4. One-way ANOVA was performed on the HAMA total score and factor delta scores before and after treatment among the three treatment groups, yielding results indicating no significant differences among the three groups (p > 0.05). Subsequently, a one-way ANOVA was conducted on the total score and factor scores of the HAMD to determine the differences among the three treatment groups. The results showed a significant difference in the total HAMD score (F = 4.490, p = 0.013) and insomnia (F = 10.068, p < 0.001) among the three groups, whereas there was no significant difference in the other three items of HAMD. The results of a further two-by-two comparison are shown that the total HAMD and insomnia factor scores of the tDCS + rTMS treatment group were significantly lower than those of the other two groups (p < 0.05).
Fig. 4.
A–H Changes in total and factor scores on the HAMA scale as well as the HAMD scale at baseline and after 20 treatments in patients with chronic insomnia after three different treatment modalities in three groups, as well as two-by-two comparisons after 20 treatments. * P < 0.05, ** P < 0.01, *** P < 0.001; ns, no significant difference. HAMA, Pittsburgh Sleep Quality Index. HAMD, Hamilton Depression Scale. HAMA, Hamilton Anxiety Scale
Adverse events and safety
After 20 interventions, all patients reported that the sessions were well-tolerated. The most significant adverse symptoms were mild skin redness in two patients, mild pruritus in one patient, and transient headache in three patients in the tDCS + rTMS group. The most significant adverse symptoms were transient headache in three patients in the sham tDCS + rTMS group. The most significant adverse symptoms were mild skin redness in two patients and mild pruritus in two patients in the tDCS + sham rTMS group (Additional file 1: Table S1). However, the above symptoms were quickly relieved after a single treatment; the pain disappeared after 20 min and did not persist during the subsequent treatment period. Importantly, none of the patients reported adverse events such as seizures, mania, dizziness, or nausea.
Integrity of blinding
Patients were asked if they knew which type of stimuli they received during the treatment. In total, 89.29% (50/56) of patients in the tDCS + rTMS group, 86.00% (43/50) in the sham tDCS + rTMS group, and 86.27% (44/51) in the tDCS + sham rTMS group believed that they had received the actual stimulus and felt improved. The chi-squared test showed there was no significant difference between the three groups in the proportion of patients who correctly guessed which stimulus they received (χ2 = 0.323, p = 0.851).
Discussion
This study examined the safety, feasibility, and efficacy of combining tDCS with rTMS in comparison with monotherapy for treating chronic insomnia in adult patients. The study involved 20 sessions of different neurotherapy protocols delivered over 4 consecutive weeks. Participants in the tDCS + rTMS treatment group showed significantly improved sleep status after 2 weeks of intervention compared to baseline. This improvement was sustained, with a further significant decrease in sleep scores observed after 4 weeks of intervention. At the 3-month follow-up after the end of treatment, it was found that the combination therapy group performed significantly better than the other two groups in terms of treatment continuity. These findings indicate that tDCS + rTMS may offer a rapid and effective approach to improving insomnia. Simultaneously, analysis of the response rate, remission rate, and changes in the scores of PSQI sub-items revealed a more significant therapeutic effect for the rTMS + tDCS treatment compared to the other two treatments. All indices consistently showed better effects in the group which underwent the combined treatment. Meanwhile, the combined tDCS + rTMS regimen group did not report more side effects, compared with the sham tDCS + rTMS and tDCS + sham rTMS groups. This indicates that the combined treatment regimen was well-tolerated and safe.
The results of this study showed that among the three groups, the tDCS + rTMS group had the highest response rate at the end of the 2-week intervention, which was significantly higher than that of the other two groups. This indicates that tDCS + rTMS effectively improved patients’ sleep. However, there was no difference in the remission rate among the three groups at the end of the 2-week period. This indicates that compared to the baseline, patients’ sleep scores had significantly decreased but had not yet reached the level of the normal population. Subsequently, at the end of the 4-week intervention, the tDCS + rTMS group continued to demonstrate the highest response and remission rates, both of which were significantly higher than those of the other two groups. This indicates the significantly better treatment effect of the combined intervention, which embodied the rapid and effective enhancement in patients’ sleep with chronic insomnia. The follow-up after 3 months of intervention showed that the persistence of treatment effects was more pronounced in the combination therapy group, which may be attributed to the comprehensive effect of the treatment plan, emphasising the potential advantages of the combination therapy strategy in improving treatment stability and long-term benefits. Further analysis revealed significant improvements in the PSQI total and sub-item scores, as well as in the total HAMD and sleep factor scores. Comparison among groups revealed that the combined treatment regimen of tDCS + rTMS showed more pronounced efficacy. It outperformed the other two treatment regimens in two aspects: PSQI total score and sleep duration. Additionally, it outperformed the tDCS + sham rTMS regimen in terms of sleep quality and sleep efficiency. Simultaneously, the combined tDCS + rTMS regimen was also significantly better than the other two regimens in two aspects: HAMD total score and sleep factor score. This suggests that tDCS combined with rTMS treatment could achieve a more obvious improvement in sleep quality during the same period, which could help accelerate the treatment of chronic insomnia.
We excluded participants with psychiatric disorders by strictly following the diagnostic criteria of the M.I.N.I. Although the HAM-D and HAM-A scores are relatively high, these scales do not have diagnostic functions and are only used to assess the severity of emotional and anxiety symptoms. All participants were not diagnosed with psychiatric disorders, despite the possibility of emotional and anxiety symptoms. Studies have shown that depressive or anxiety symptoms are common in patients with insomnia, especially in patients with long-term chronic insomnia [33]. It is possible that high HAM-D and HAM-A scores may occur owing to obvious depression or anxiety symptoms caused by long-term insomnia before seeking medical attention. However, it does not mean that these symptoms are independent variables affecting the effectiveness of treatment. During data analysis, we controlled for these symptoms to ensure the independence and reliability of the effects of the three neurotherapy regimens. The results showed that neurotherapy with different regimens significantly improved chronic insomnia symptoms, whereas the presence of emotional and anxiety symptoms did not significantly affect the statistical and clinical significance of the intervention effect, indicating that despite the high scores of HAM-D and HAM-A, the main conclusions of the study are still valid.
Further subgroup analyses of gender and age revealed that sleep quality was more likely to be affected by neurotherapy in men than in women. At the 2-week, 4-week, and 3-month follow-up after treatment, the total PSQI score was significantly improved in the male tDCS + rTMS group compared to the female group. A previous study showed that male participants responded to rTMS treatment for insomnia more than female subjects as measured by the PSQI [34]. Another study evaluating sleep depth after tDCS treatment in student athletes with subclinical sleep disorders showed the effectiveness of tDCS in increasing total sleep time after treatment in males but not in females, as measured by polysomnography [35]. The similar phenomenon was also demonstrated in our study, which may be due to differences in cortical excitability, neurotransmitter systems, and brain connectivity patterns between males and females, resulting in different responses to neuromodulation techniques. In addition, fluctuating hormone levels in females, especially hormonal changes associated with the menstrual cycle, pregnancy, and menopause, may further affect the response of females to tDCS and rTMS [36, 37]. Another important finding of our study is that tDCS + rTMS treatment significantly improved sleep quality in patients with insomnia under 50 years old. Although tDCS + rTMS significantly improved sleep quality in patients over 50 years old, this effect was only significant during the treatment period and did not persist for 3 months after the end of treatment. This is consistent with other findings that young people respond more significantly to tDCS and rTMS than the elderly, possibly because nervous systems of young people are easily modulated by external stimuli. On the contrary, old adults have low neural plasticity and reduced cortical thickness, which may decrease the treatment effectiveness [38, 39]. The brain undergoes significant structural and functional changes with age, which may affect the therapeutic efficacy of neuromodulation techniques.
In this study, tDCS combined with rTMS was applied for the first time for the treatment of chronic insomnia, effectively improving the quality of sleep and achieving significant relief from insomnia after 4 weeks of treatment. Some studies have demonstrated the effectiveness of combination therapies in healthy participants and patients with other diseases. For example, in healthy participants, high-frequency rTMS stimulation was applied to the M1 region of the left primary motor cortex, while cathodal tDCS stimulation was applied to the right M1. This approach aimed to elicit inter-hemispheric inter-regulatory interactions, inducing neuromodulatory effects and thus modulating cortical excitability and motor function [40]. Subsequent studies have shown that the combination of cathodal tDCS stimulation and low-frequency rTMS administered simultaneously to the left cortex of the right dorsal interosseous muscle of the first interosseous muscle resulted in greater suppression of motor-evoked potential amplitude than tDCS stimulation or rTMS stimulation alone, with a stronger inhibitory effect [41]. This further demonstrates that the simultaneous application of tDCS and rTMS is more powerful in modulating cortical excitability than either tDCS or rTMS alone. In another study, the bilateral angular gyrus in the combined stimulation group was found to be effective in improving neuropsychiatric symptoms, cognitive function, and sleep quality in patients with Alzheimer’s disease, whereas there were no significant changes in the tDCS and rTMS groups [42].
tDCS and rTMS have been shown to have individual therapeutic effects on insomnia. From a neurophysiological perspective, both tDCS and rTMS can affect the electrophysiological activity of neurones and directly modulate brain hyperexcitability [10, 11, 16]. From a neurochemical perspective, both tDCS and rTMS improve sleep by modulating the concentration or activity of 5-hydroxytryptamine (5-HT) and gamma-aminobutyric acid [43, 44]. Research has shown that, compared with healthy participants, patients with insomnia exhibit increased cortical excitability, decreased intracortical facilitation, and cortical overexcitement in both awake and sleeping states [45–47]. Based on these previous findings, we further validated that the modulation of cortical excitability may be an interesting approach to treat insomnia and improve sleep quality in patients with other types of clinical disorders with worsening sleep. Compared with a single intervention, the combination of tDCS and rTMS stimulation resulted in better efficacy after the 2-week intervention, significantly improved insomnia by week 4, and led to sustained treatment effects for 3 months after the end of the intervention. This indicates that combination therapy can not only accelerate the relief of insomnia symptoms and achieve faster improvement in the short term, but also have long-term sustained effects. Patients receiving combination therapy received two stimulation types, potentially increasing the effective treatment duration through longer stimulation times, more types, and targeting more stimulation sites in a single session.
Another possible reason for the improvement in insomnia may be the pretreatment effect of tDCS. In this study, our treatment plan involved patients receiving tDCS before rTMS treatment. This approach considered the distinct working mechanisms and principles of each technique. tDCS not only affects the electrophysiological activity state of neurones to change the excitability of the cerebral cortex but also has an effect on the concentration of neurochemical transmitters. It can effectively promote the release of 5-HT and β-endorphin in the body, alleviating stress and promoting relaxation, which can improve the symptoms of insomnia [48]. rTMS can directly hyperpolarise neurones in the DLPFC through pulsed magnetic fields. This can induce neurones to produce an LTP/LTD effect, altering brain plasticity and further inhibiting the over-excitation (hyperarousal) state of the cerebral cortex [49]. Therefore, the treatment sequence involved cathodal tDCS stimulation of the right DLPFC first, inducing hyperpolarisation of right DLPFC neurones and reducing cortical excitability. Subsequently, patients received low-frequency rTMS stimulation of the same area, leading to more lasting inhibition of hyperexcitability and neuroplasticity changes in the cerebral cortex. One study found that the interaction between tDCS and rTMS follows an internal steady-state mechanism [50], indicating that the pretreatment effect of tDCS is not simply a cumulative effect. In this study, the pretreatment of the tDCS cathode to the right DLPFC resulted in a better effect of rTMS, which led to a more pronounced improvement of insomnia symptoms. However, it is unclear whether the pretreatment effect of tDCS on the DLPFC follows the same homeostatic mechanism, and further studies are needed.
Participants were treated with zopiclone throughout the trial. The goal of our trial was to investigate the changes in the efficacy of different neurotherapy regimens for patients with chronic insomnia under medication conditions. We chose participants who had long-term chronic insomnia, these patients who were already taking sedative hypnotics for a long period of time at the time of their visit, and different hypnotic medications have different therapeutic effects. In order to avoid differences due to medication, as well as to maintain the compliance of patient, we allowed patients to take the same hypnotic medication (zopiclone) throughout the study. Despite zopiclone may have the potential impact on the outcomes, the medication use was balanced and uniformed among different groups, with no statistical differences. Therefore, the overall trend and main conclusions of the study remain statistically significant and valuable for exploration.
We have chosen tDCS and TMS schemes based on many previous literatures. tDCS and rTMS stand for electrical and magnetic stimulation, respectively. In addition, tDCS and rTMS not only have high safety and good clinical popularity, but also improve insomnia symptoms and sleep disturbances across different types of neurological and neuropsychiatric diseases [51]. Previous studies have confirmed that the selected tDCS protocol could effectively improve the sleep status of patients with depression and insomnia [52]. Moreover, it was demonstrated that the applying of TMS protocol could produce significant therapeutic effects on patients with chronic insomnia [44, 53, 54]. Our previous study showed that the combination of these two specific tDCS and TMS protocols had significant therapeutic effects, thus providing a theoretical and empirical basis for our selection [55]. Furthermore, these protocols have been proven to be safe in many clinical trials with fewer side effects and better tolerability among participants, which ensures successful of trials and safety of participants. Meanwhile, these two specific tDCS and TMS protocols are available and easy to implement in our research facility, ensuring the operability of the study and the reliability of the data.
Although two neurotherapy methods were used in this study, the results did not show any serious adverse events. The main adverse symptoms after the intervention were mild skin irritation, itching, redness, swelling, and transient headache, which may be related to the stimulation site and the somatosensory system. Importantly, none of the patients experienced seizures or manic symptoms. Overall, 95.7% of the participants completed the experiment, indicating that tDCS combined with rTMS had good tolerance and safety.
This study has some limitations. First, objective measurements of sleep parameters, such as polysomnography and other techniques, were not performed. Second, all participants were treated with zopiclone during the trial. Although zopiclone is safe and commonly used in clinical practice, its sedative and hypnotic effects may affect the cognitive function and behavioural performance of participants, which might have an impact on our findings. In the further study, it is suggested to use a control group design, and to compare the differences of outcomes between participants with and without zopiclone treatment to improve the reliability and effectiveness of the results. Third, future research can be further refined by grouping and analysing symptoms of depression and anxiety to different degrees, to verify the specific impact of these symptoms on intervention effectiveness. Fourth, it might be subjective and unreliable to record side effects by using open-ended questions at the end of the trial. Therefore, in future research, we will introduce standardised side effects scales and perform regular evaluation and objective measurement. Such improvements will help to evaluate side effects systematically and objectively, ensuring the reliability and integrity of data.
Conclusions
In summary, this study provides evidence that tDCS combined with rTMS effectively relieves chronic insomnia symptoms in adults. Notably, the intervention led to a significant therapeutic effect after just 2 weeks, demonstrating the rapid and effective augmentation of this combination therapy. In the later follow-up, the durability of the treatment effect was also demonstrated, emphasising the advantages of combination therapy in improving treatment stability and long-term benefits. These findings suggest that the tDCS and rTMS regimen may be a safe and effective method for treating adult patients with chronic insomnia. Further research is encouraged to explore how tDCS and rTMS improve sleep quality using objective tools to definitely evaluate their efficacy. Additionally, investigating the underlying mechanism through neuroelectrophysiological methods could provide valuable insights into how this treatment works for insomnia.
Supplementary Information
Additional file 1: Table S1. Adverse events among patients with chronic insomnia receiving treatment.
Acknowledgements
The authors are grateful to all participants for their excellent cooperation.
Abbreviations
- 5-HT
5-Hydroxytryptamine
- ANOVA
Analysis of variance
- DLPFC
Dorsolateral prefrontal cortex
- HAMA
Hamilton Anxiety Scale
- HAMD
Hamilton Depression Scale
- ICD-11
International Classification of Diseases-11
- LTD
Long-term depression
- LTP
Long-term potentiation
- M.I.N.I
Mini-International Neuropsychiatric Interview
- PSQI
Pittsburgh Sleep Quality Index
- rTMS
Repetitive transcranial magnetic stimulation
- tDCS
Transcranial direct current stimulation
Authors’ contributions
HHY and DSZ: conception and design. YC, QW, and YFT: conduction. WHZ: randomization and blinding. QZ and TMZ: statistical analysis. QZ: drafting of the manuscript. ZWL, YC, QW and TMZ: administrative, technical, and material support. QZ, HHY and DSZ: critical revision of the manuscript for important intellectual content. All authors have read and approved the final version of the manuscript.
Funding
This research was supported by the Natural Science Foundation of Zhejiang (No.LTGD24H090001), the Ningbo Public Welfare Project (No. 2022S022), the Ningbo Natural Science Foundation Project (No. 2021J275 and 2022J269), the Traditional Chinese Medicine Science and Technology Plan Project of Zhejiang Province (No. 2021ZB266), the Ningbo Clinical Medical Research Centre for Mental Health (No.2022L002), the Ningbo medical and health brand discipline (No.PPXK 2024–07), and the Ningbo Top Medical and Health Research Program (No.2022030410). The funding organisations had no role in the design and conduct of the study, the collection, management, analysis, and interpretation of the data, the preparation, review, and approval of the manuscript, or the decision to submit the manuscript for publication.
Data availability
The data are available by making a request through Dr. Zhou per the new Data Management and Sharing Agreement plan.
Declarations
Ethics approval and consent to participate
All procedures were approved by the Clinical Research Ethics Committee of Ningbo Kangning Hospital (No. NBKNYY-2019-LC-20), ensuring compliance with the ethical standards and regulations of the Declaration of Helsinki on Human Research. This study was registered as a clinical trial with the China Clinical Trial Registration Center (ChiCTR2100052681). All participants were fully informed of the study procedure and voluntarily signed a written informed consent form.
Consent for publication
All authors have seen and approved the final version of the manuscript being submitted. We warrant that the article is the authors’ original work, has not received prior publication, and is not under consideration for publication elsewhere.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Qi Zhou and Zhiwang Liu contributed equally to this work.
References
- 1.Riemann D, Nissen C, Palagini L, Otte A, Perlis M, Spiegelhalder K. The neurobiology, investigation, and treatment of chronic insomnia. Lancet Neurol. 2015;14(5):547–58. [DOI] [PubMed] [Google Scholar]
- 2.Buysse D. Insomnia. JAMA. 2013;309(7):706–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo Y, Zhao X, Liu X, Liu J, Li Y, Yue L, Yuan F, Zhu Y, Sheng X, Yu D, et al. Electroencephalography microstates as novel functional biomarkers for insomnia disorder. Gen Psychiatry. 2023;36(6):e101171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sophie W-P, Danica CS, Daniel JT. Insomnia and cognitive performance: A systematic review and meta-analysis. Sleep Med Rev. 2019;48:101205. [DOI] [PubMed] [Google Scholar]
- 5.Nicholson K, Rodrigues R, Anderson K, Wilk P, Guaiana G, Stranges S. Sleep behaviours and multimorbidity occurrence in middle-aged and older adults: findings from the Canadian Longitudinal Study on Aging (CLSA). Sleep Med. 2020;75:156–62. [DOI] [PubMed] [Google Scholar]
- 6.Sutton E. Insomnia. Ann Int Med. 2021;174(3):ITC33–48. [DOI] [PubMed] [Google Scholar]
- 7.Morin C, Inoue Y, Kushida C, Poyares D, Winkelman J. Endorsement of European guideline for the diagnosis and treatment of insomnia by the World Sleep Society. Sleep Med. 2021;81:124–6. [DOI] [PubMed] [Google Scholar]
- 8.Everitt H, Baldwin D, Stuart B, Lipinska G, Mayers A, Malizia A, Manson C, Wilson S. Antidepressants for insomnia in adults. Cochrane Database Syst Rev. 2018;5(5):CD010753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klomjai W, Katz R, Lackmy-Vallée A. Basic principles of transcranial magnetic stimulation (TMS) and repetitive TMS (rTMS). Ann Phys Rehabil Med. 2015;58(4):208–13. [DOI] [PubMed] [Google Scholar]
- 10.Qian F, He Y, Du X, Lu H, He R, Fan J. Repetitive transcranial magnetic stimulation promotes neurological functional recovery in rats with traumatic brain injury by upregulating synaptic plasticity-related proteins. Neural Regen Res. 2023;18(2):368–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iglesias A. Transcranial magnetic stimulation as treatment in multiple neurologic conditions. Curr Neurol Neurosci Rep. 2020;20(1):1. [DOI] [PubMed] [Google Scholar]
- 12.Sun N, He Y, Wang Z, Zou W, Liu X. The effect of repetitive transcranial magnetic stimulation for insomnia: a systematic review and meta-analysis. Sleep Med. 2021;77:226–37. [DOI] [PubMed] [Google Scholar]
- 13.Shi X, Shi X, Shi X, Guo Y, Guo Y, Guo Y, Zhu L, Zhu L, Zhu L, Wu W. Electroencephalographic connectivity predicts clinical response to repetitive transcranial magnetic stimulation in patients with insomnia disorder. Sleep Med. 2021;88:171–9. [DOI] [PubMed] [Google Scholar]
- 14.Herrero Babiloni A, Bellemare A, Beetz G, Vinet S, Martel M, Lavigne G, De Beaumont L. The effects of non-invasive brain stimulation on sleep disturbances among different neurological and neuropsychiatric conditions: a systematic review. Sleep Med Rev. 2021;55:101381. [DOI] [PubMed] [Google Scholar]
- 15.Nitsche M, Fricke K, Henschke U, Schlitterlau A, Liebetanz D, Lang N, Henning S, Tergau F, Paulus W. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J Physiol. 2003;553:293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santarnecchi E, Feurra M, Barneschi F, Acampa M, Bianco G, Cioncoloni D, Rossi A, Rossi S. Time course of corticospinal excitability and autonomic function interplay during and following monopolar tDCS. Front Psych. 2014;5:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nitsche M, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527(Pt 3):633–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lefaucheur J, Antal A, Ayache S, Benninger D, Brunelin J, Cogiamanian F, Cotelli M, De Ridder D, Ferrucci R, Langguth B, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol. 2017;128(1):56–92. [DOI] [PubMed] [Google Scholar]
- 19.Cirillo G, Di Pino G, Capone F, Ranieri F, Florio L, Todisco V, Tedeschi G, Funke K, Di Lazzaro V. Neurobiological after-effects of non-invasive brain stimulation. Brain Stimul. 2017;10(1):1–18. [DOI] [PubMed] [Google Scholar]
- 20.Hadoush H, Al-Sharman A, Khalil H, Banihani SA, Al-Jarrah M. Sleep quality, depression, and quality of life after bilateral anodal transcranial direct current stimulation in patients with Parkinson’s disease. Med Sci Monit Basic Res. 2018;24:198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saebipour MR, Joghataei MT, Yoonessi A, Sadeghniiat-Haghighi K, Khalighinejad N, Khademi S. Slow oscillating transcranial direct current stimulation during sleep has a sleep-stabilizing effect in chronic insomnia: a pilot study. J Sleep Res. 2015;24(5):518–25. [DOI] [PubMed] [Google Scholar]
- 22.Kuan-Wei H, Pao-Yen L, Yu L, Yu-Chi H, Chi-Fa H, Sheng-Yu L, Chih-Ken C, Liang-Jen W. Validation of the Chinese version of the schizophrenia cognition rating scale. Psychiatry Investig. 2022;19(7):511–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lancet T. ICD-11. Lancet. 2019;393(10188):2275. [DOI] [PubMed] [Google Scholar]
- 24.Magno M, Utheim T, Snieder H, Hammond C, Vehof J. The relationship between dry eye and sleep quality. Ocul Surf. 2021;20:13–9. [DOI] [PubMed] [Google Scholar]
- 25.Klem G, Lüders H, Jasper H, Elger C. The ten-twenty electrode system of the International Federation. The International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol. 1999;52:3–6. [PubMed] [Google Scholar]
- 26.Lee H, Schreiner L, Jo S, Sieghartsleitner S, Jordan M, Pretl H, Guger C, Park H. Individual finger movement decoding using a novel ultra-high-density electroencephalography-based brain-computer interface system. Front Neurosci. 2022;16:1009878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riemann D, Baglioni C, Bassetti C, Bjorvatn B, Dolenc Groselj L, Ellis J, Espie C, Garcia-Borreguero D, Gjerstad M, Gonçalves M, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26(6):675–700. [DOI] [PubMed] [Google Scholar]
- 28.Natalie AG, Shantha MWR, Darren M, Tracey LS, Gershon S, Jennie LP. Efficacy of melatonin for sleep disturbance following traumatic brain injury: a randomised controlled trial. BMC Med. 2018;16(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Antczak J, Poleszczyk A, Wichniak A, Rakowicz M, Parnowski T. The influence of the repetitive transcranial magnetic stimulation on sleep quality in depression. Psychiatr Pol. 2017;51(5):845–57. [DOI] [PubMed] [Google Scholar]
- 30.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldberg J, Siu C, Tocco M, Pikalov A, Loebel A. The effect of lurasidone on anxiety symptoms in patients with bipolar depression: a post hoc analysis. J Clin Psychiatry. 2023;84(4):22m14732. [DOI] [PubMed] [Google Scholar]
- 32.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. New Jersey: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 33.Niloofar S, Abolfazl A, Arman S, Mohammad Javad A, Mohammad Mobin TA, Omid S, Parsa F, Alireza E, Omid R, Amir Hossein S, et al. The global prevalence of depression, anxiety, and sleep disorder among patients coping with Post COVID-19 syndrome (long COVID): a systematic review and meta-analysis. BMC Psychiatry. 2024;24(1):105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haixia M, Jingxia L, Jiali H, Lo DHT, Hector WHT. Effectiveness of TES and rTMS for the treatment of insomnia: meta-analysis and meta-regression of randomized sham-controlled trials. Front Psychiatry. 2021;12:744475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siqi W, Jinjin W, Wenmin G, Hang Y, Xinbo L, Jun L, Haoli Z. Gender difference in gender bias: transcranial direct current stimulation reduces male’s gender stereotypes. Front Hum Neurosci. 2019;13:403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mark JS, Linda FA, Peter JS, David RR, Eric MW. Effects of ovarian hormones on human cortical excitability. Ann Neurol. 2002;51(5):599–603. [DOI] [PubMed] [Google Scholar]
- 37.Josette JW, Sangeetha H, Claire ELP. Why sex matters. Elife. 2021;10:e74935.
- 38.Hakuei F, Mark RH, Matthew WS, Michael IG, Jeffery JS. Age-related differences in corticospinal excitability and inhibition during coordination of upper and lower limbs. Neurobiol Aging. 2012;33(7):1484.e1–14. [DOI] [PubMed] [Google Scholar]
- 39.Sandra D, Carolyn W, Hubert Z, Jutta K. Plasticity in brain activity dynamics after task-shifting training in older adults. Neuropsychologia. 2019;136:107285. [DOI] [PubMed] [Google Scholar]
- 40.Wang H, Wang X, Jin J, Zhang W, Li Y, Liu Z, Yin T. Simultaneous stimulation using rTMS and tDCS produces the most effective modulation of motor cortical excitability in healthy subjects: a pilot study. Neurosci Lett. 2019;694:46–50. [DOI] [PubMed] [Google Scholar]
- 41.Han T, Xu Z, Liu C, Li S, Song P, Huang Q, Zhou Q, Lin Y, Wang Y. Simultaneously applying cathodal tDCS with low frequency rTMS at the motor cortex boosts inhibitory aftereffects. J Neurosci Methods. 2019;324:108308. [DOI] [PubMed] [Google Scholar]
- 42.Hu Y, Jia Y, Sun Y, Ding Y, Huang Z, Liu C, Wang Y. Efficacy and safety of simultaneous rTMS-tDCS over bilateral angular gyrus on neuropsychiatric symptoms in patients with moderate Alzheimer’s disease: a prospective, randomized, sham-controlled pilot study. Brain Stimul. 2022;15(6):1530–7. [DOI] [PubMed] [Google Scholar]
- 43.Poh EZ, Hahne D, Moretti J, Harvey AR, Clarke MW, Rodger J. Simultaneous quantification of dopamine, serotonin, their metabolites and amino acids by LC-MS/MS in mouse brain following repetitive transcranial magnetic stimulation. Neurochem Int. 2019;131:104546. [DOI] [PubMed]
- 44.Jie F, Qing Z, Chengliang Z, Zhongmin W, Xianju Z. The Effect of sequential bilateral low-frequency rTMS over dorsolateral prefrontal cortex on serum level of BDNF and GABA in patients with primary insomnia. Brain Behav. 2019;9(2):e01206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fernandez-Mendoza J, Li Y, Vgontzas AN, Fang J, Gaines J, Calhoun SL, et al. Insomnia is associated with cortical hyperarousal as early as adolescence. Sleep. 2016;39(5):1029–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bonnet M, Arand D. Hyperarousal and insomnia: state of the science. Sleep Med Rev. 2010;14(1):9–15. [DOI] [PubMed] [Google Scholar]
- 47.van der Werf Y, Altena E, van Dijk K, Strijers R, De Rijke W, Stam C, van Someren E. Is disturbed intracortical excitability a stable trait of chronic insomnia? A study using transcranial magnetic stimulation before and after multimodal sleep therapy. Biol Psychiat. 2010;68(10):950–5. [DOI] [PubMed] [Google Scholar]
- 48.Kirsch D, Nichols F. Cranial electrotherapy stimulation for treatment of anxiety, depression, and insomnia. Psychiatr Clin North Am. 2013;36(1):169–76. [DOI] [PubMed] [Google Scholar]
- 49.Namgung E, Kim M, Yoon S. Repetitive transcranial magnetic stimulation in trauma-related conditions. Neuropsychiatr Dis Treat. 2019;15:701–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Siebner HR. Preconditioning of low-frequency repetitive transcranial magnetic stimulation with transcranial direct current stimulation: evidence for homeostatic plasticity in the human motor cortex. J Neurosci. 2004;24(13):3379–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alberto HB, Audrey B, Gabrielle B, Sophie-A V, Marc OM, Gilles JL, Louis DB. The effects of non-invasive brain stimulation on sleep disturbances among different neurological and neuropsychiatric conditions: a systematic review. Sleep Med Rev. 2020;55:101381. [DOI] [PubMed] [Google Scholar]
- 52.Qi Z, Chang Y, Haihang Y, Yuanyuan Z, Zhiwang L, Zhenyu H, Ti-Fei Y, Dongsheng Z. The effects of repeated transcranial direct current stimulation on sleep quality and depression symptoms in patients with major depression and insomnia. Sleep Med. 2020;70:17–26. [DOI] [PubMed] [Google Scholar]
- 53.Penghui S, Hua L, Siran L, Li W, Jianghong L, Ning L, Yuping W. Repetitive transcranial magnetic stimulation (rTMS) modulates time-varying electroencephalography (EEG) network in primary insomnia patients: a TMS-EEG study. Sleep Med. 2019;56:157–63. [DOI] [PubMed] [Google Scholar]
- 54.Yang-Pu Z, Wei-Jing L, Wen-Guang X. Effect of acupuncture cooperated with low-frequency repetitive transcranial magnetic stimulation on chronic insomnia: a randomized clinical trial. Curr Med Sci. 2018;38(3):491–8. [DOI] [PubMed] [Google Scholar]
- 55.Qi Z, Zhiwang L, Shengnan Z, Jia Y, Dongsheng Z, Weiqian X, Yuanyuan Z. Transcranial magnetic stimulation combined with transcranial direct current stimulation in patients with chronic insomnia: a case report. J Clin Sleep Med. 2022;18(12):2871–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Adverse events among patients with chronic insomnia receiving treatment.
Data Availability Statement
The data are available by making a request through Dr. Zhou per the new Data Management and Sharing Agreement plan.