Abstract
Background:
Clinical trials have demonstrated the efficacy and safety of hybrid closed-loop (HCL) systems, yet few studies have compared outcomes in the real-world setting.
Method:
This retrospective study analyzed patients from an academic endocrinology practice between January 1, 2018, and November 18, 2022. The inclusion criteria were diagnosis code for type I diabetes (T1D), >18 years of age, new to any HCL system [Medtronic 670G/770G (MT), Tandem Control IQ (CIQ), or Omnipod 5 (OP5)], and availability of a pump download within three months. The outcomes included %time in range (TIR) of 70 to 180 mg/dL, %time below range (TBR) <70 mg/dL at 90 days, and HbA1c for 91 to 180 days.
Result:
Of the 176 participants, 47 were MT, 74 CIQ, and 55 OP5. Median (25%, 75%) change in HbA1c was −0.1 (−0.8, 0.3), −0.6 (−1.1, −0.15), and −0.55 (−0.98, 0)% for MT, CIQ, and OP5, respectively, (P = .04). TIR was 70 (57, 76), 67 (59, 75), and 68 (60, 76)% (P = .95) at 90 days while TBR was 2 (1, 3), 1 (0, 2), and 1 (0, 1)%, respectively, (P = .002). The %time in automated delivery was associated with TIR and change in HbA1c. After controlling other factors including %time in automated delivery, HCL type was not an independent predictor of change in HbA1c nor TIR but remained a significant predictor of TBR.
Conclusion:
There were significant reductions in HbA1c in CIQ and OP5. TIR was similar across pumps, but TBR was highest with MT. The %time in automated delivery likely explains differences in change in HbA1c but not TBR between HCL systems.
Keywords: automated insulin delivery, hybrid closed loop, insulin pump, real-world, type 1 diabetes
Introduction
An estimated 1.5 million individuals in the United States have type I diabetes (T1D), and the importance of maintaining glucose levels for preventing microvascular complications is well established. 1 However, less than half of individuals with T1D achieve an HbA1c <7%, and there are substantial disparities in glycemic control worldwide2,3 despite the availability of more physiologic insulins and improvements in insulin delivery. Earlier studies regarding insulin pump technology in patients with T1D have demonstrated glycemic benefit.4-6 Furthermore, insulin pump use is associated with reduced risk of severe hypoglycemia and diabetic ketoacidosis. 4 However, insulin pump use alone is limited in ability to get the majority of patients with T1D to target. 3 ,7-9
Automated insulin delivery (AID) systems provide additional benefit by altering insulin delivery in response to continuous glucose monitoring (CGM) data. 5 Hybrid closed-loop (HCL) systems are a type of AID which automatically adjust insulin delivery in response to glucose levels above or below target, although self-administered boluses at mealtime are still required for optimal outcomes. Multicenter trials evaluating HCL systems have demonstrated improved time in range (TIR, the percentage of time between 70 and 180 mg/dL) and time below range (TBR, the percentage of time below 70 mg/dL) before and after studies compared to standard therapy10,11 and in a randomized controlled trial against a sensor-augmented pump. 12
However, outcomes in real-world settings can differ from those observed in controlled environments of clinical trials. A one-year prospective study of 84 participants using Medtronic 670G showed that by three months, 28% of participants had stopped using automated delivery, and by nine months, the number increased to 35%. 13 While there are studies that have examined the performance of HCL pumps in real-world settings,13-15 few data compare multiple HCL systems. 16 This study compares the efficacy and safety of the three commercially available AID systems, Medtronic 670G/770G (MT), Tandem Control IQ (CIQ), and Omnipod 5 (OP5), in the real-world setting.
Methods and Materials
This was a retrospective cohort study of patients from an academic ambulatory endocrinology practice that started HCL therapy between January 1, 2018, and November 18, 2022. Patients were identified from insulin pump start visit records. Inclusion criteria were at least 18 years of age, a diagnosis code for T1D (ICD-10 E10*), and new to any HCL device. In cases where a patient used multiple HCL devices during the study period, the first pump that was accessed was the pump included in the analysis. Patients were excluded if there was no pump data within 90 days, if the patient did not start HCL delivery despite starting an HCL-enabled device, or if the patient was known to be pregnant. This study was approved by the Ohio State University’s Institutional Review Board (approval #00006378).
Demographics, duration of diabetes, mean household income by zip code (calculated with www.incomebyzipcode.com), microvascular and macrovascular complications, weight, body mass index (BMI), serum creatinine and estimated glomerular filtration rate (eGFR, determined using the Chronic Kidney Disease Epidemiology Collaboration [CKD-EPI] 2021 calculation) 17 within the past year, and hospitalization details were collected. Other outcomes included severe hypoglycemia requiring assistance, diabetic ketosis with or without acidosis, and infusion site failure within 90 days of pump start. Pump information and CGM data were collected from a 2 week period when available at three time intervals: baseline, between 0 and 30 days, and between 31 and 90 days while HbA1c was collected within three months prior to baseline, 30 to 90 days after baseline, and 91 to 180 days after baseline. Pump downloads were assessed using a minimum seven-day report. CGM data included % >250 mg/dL, % >180 mg/dL, % 70 to 180 mg/dL (TIR), %< 70 mg/dL (TBR), % <54 mg/dL, and coefficient of variation (CV). Finally, education visits were collected at 0 to 30 days and 31 to 90 days after pump start. An education visit was a telephone call or office visit with a Certified Diabetes Care and Education Specialist (CDCES). A standard education follow-up was utilized. CDCES reviewed pump data 24 to 48 hours after pump initiation through upload connections. CDCES continued to conduct weekly data reviews for up to six weeks. In-person education visits were scheduled for four to six weeks after starting the pump. If any changes to the pump were necessary, a phone call would be initiated until TIR reached over 70% and TBR was less than 4%. If TIR did not show improvement after four to six weeks, patients were instructed to consult with their endocrinologist.
Statistical Methods
Continuous variables with normal distribution were reported as mean (standard deviation), and differences between groups were determined using a one-way analysis of variance (ANOVA). Continuous variables with non-normal distribution were reported as median (interquartile range [IQR]) and analyzed using the Wilcoxon rank-sum test. Multiple comparisons were performed to determine the significance within pairs using Tukey–Kramer Honestly Significant Difference or Steel–Dwass method for normal and non-normal distributions, respectively. Matched pairs were analyzed using Wilcoxon signed rank test. Dichotomous/categorical variables were reported as number (percentage), and differences between groups were assessed using the Chi-square test. Statistical significance was considered when the P value was <.05.
Univariable associations between baseline characteristics and changes in HbA1c, TIR, and TBR were assessed. (Please see Supplemental Material for univariable tables.) Univariable predictors were included in multivariable models if the P value was <.2. An initial full model was created, followed by backward linear regression to a final reduced model. The models included log-transformed values for area-level income, diabetes duration, eGFR, and baseline HbA1c for better model fit. The analyses were performed using JMP Pro 17.0 software.
Results
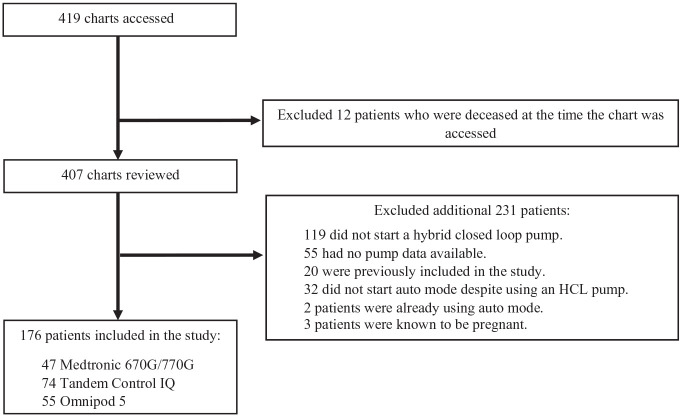
A total of 419 patient charts were accessed, resulting in an inclusion of 176 patients for the study (Figure 1). The final sample size consisted of 47 MT, 74 CIQ, and 55 OP5 patients. Only three individuals initiated the 770G system during the study period. Since the 770G algorithm is similar to the 670G (except greater time in automated delivery mode), 18 these patients were included in the 670G group. We excluded 119 patients who did not start an HCL pump and 55 patients with no post-HCL pump information available. Another 32 patients who did not start automated delivery despite starting an HCL pump were excluded. Three patients who were known to be pregnant were excluded. A total of 52 patients had pump and CGM information beyond the 90-day follow-up but within a 140-day timeframe and were included within the 90-day data.
Figure 1.
Patient flow diagram.
Baseline demographics and medical history across all devices were similar (Table 1), with a median age of 41 (IQR 30, 54) years, 87.5% white, 39.2% male, and median duration of diabetes of 22 (IQR 14-34) years. Significantly more patients in the MT group (87%) had previously used an insulin pump compared to 61% of CIQ and 67% of OP5 groups, respectively (P = .005). The use of a CGM at baseline also differed across groups, with 45% of MT users, compared to 89% of CIQ and 98% of OP5 users (P < .0001). Baseline AID type differed significantly among all three groups, with 27% of MT, 10% of CIQ, and 0% of OP5 groups using basal insulin suspension, and 4% of MT, 16% of CIQ, and 7% of OP5 using another HCL device previously (P < .0001) (Table 1).
Table 1.
Baseline Characteristics by HCL System.
| Total population | Medtronic 670G/770G | Control IQ | Omnipod 5 | ||
|---|---|---|---|---|---|
| (N = 176) | (N = 47) | (N = 74) | (N = 55) | P value | |
| Age (years) | 41 (30, 54) | 41 (34, 53) | 41 (30, 53) | 40 (27, 55) | .86 |
| Race, N (%) | |||||
| White | 154 (87.5) | 44 (93.6) | 64 (86.5) | 46 (83.6) | .26 a |
| Non-white | 22 (12.5) | 3 (6.4) | 10 (13.5) | 9 (16.4) | |
| Black | 16 (9.1) | 2 (4.3) | 7 (9.5) | 7 (12.7) | |
| Asian | 3 (1.7) | 0 (0) | 1 (1.4) | 2 (3.6) | |
| Other | 3 (1.7) | 1 (2.1) | 2 (2.7) | 0 (0) | |
| Male, N (%) | 69 (39.2) | 17 (36.2) | 27 (36.5) | 25 (45.5) | .52 |
| Duration of diabetes (years) | 22 (14, 33.8) | 24 (16, 27) | 23 (13.8, 34) | 21 (13, 34) | .85 |
| Area household income ($) | 96 314 (44 504) | 97 953 (37 794) | 92 204 (46 156) | 100 054 (50 192) | .61 |
| BMI, kg/m2 | 29.7 (7.0) | 29.6 (7.5) | 29.3 (7.2) | 30.1 (6.4) | .82 |
| Nephropathy, N (%) | 32 (18.2) | 6 (12.8) | 13 (17.6) | 13 (23.6) | .36 |
| Neuropathy, N (%) | 53 (30.1) | 15 (31.9) | 24 (32.4) | 14 (25.5) | .66 |
| Retinopathy, N (%) | 57 (32.4) | 19 (40.4) | 24 (32.4) | 14 (25.5) | .27 |
| CVD, N (%) | 22 (12.5) | 5 (10.6) | 7 (9.5) | 10 (18.2) | .32 |
| eGFR |
N = 173 101 (83, 114.4) |
N = 46 102 (85.0, 113.2) |
N = 72 101 (77.9, 113.6) |
N = 55 102 (84.6, 116.8) |
.92 |
| Baseline TDD (units) |
N = 121 47.7 (33.9, 72.2) |
N = 40 48.9 (34.4, 71.8) |
N = 45 46 (33.2, 72.2) |
N = 36 51.3 (34.9, 71.6) |
.96 |
| Baseline TDD (units/kg) |
N = 121 0.62 (0.20) |
N = 40 0.63 (0.19) |
N = 45 0.60 (0.17) |
N = 36 0.61 (0.25) |
.80 |
| Baseline basal dose (units) |
N = 175 25 (18.2, 36) |
N = 46 24.9 (18.5, 35.7) |
N = 74 26.4 (18, 36.4) |
N = 55 24.5 (18.5, 35) |
.98 |
| Baseline bolus count/day |
N = 120 5.18 (2.05) |
N = 40 5.72 (2.40) |
N = 45 4.69 (1.61) |
N = 35 5.19 (2.03) |
.07 |
| Baseline %bolus override |
N = 116 4.95 (0, 15.2) |
N = 38 10.3 (2.9, 15.6) |
N = 43 2.7 (0, 6) |
N = 35 8 (0, 23.8) |
.007 |
| Education 0- to 30-day N (%) | 102 (57.6) | 25 (53.2) | 43 (58.1) | 34 (61.8) | .68 |
| Education 31- to 90-day N (%) | 59 (33.5) | 18 (38.3) | 24 (32.4) | 17 (30.9) | .71 |
| Baseline pump type, N (%) | |||||
| None | 53 (30.1) | 6 (12.8) | 29 (39.2) | 18 (32.7) | .005 |
| Pump | 123 (69.9) | 41 (87.2) | 45 (60.8) | 37 (67.3) | |
| Medtronic 530/630G | 32 (18.2) | 22 (46.8) | 10 (13.5) | 0 (0) | |
| Medtronic 670/770G | 39 (22.2) | 4 (8.5) | 28 (37.8) | 7 (12.7) | |
| T-slim Basal IQ | 4 (2.3) | 0 (0) | 4 (5.4) | 0 (0) | |
| T-slim Control IQ | 2 (1.1) | 0 (0) | 0 (0) | 2 (3.6) | |
| T-slim other | 2 (1.1) | 1 (2.1) | 1 (1.4) | 0 (0) | |
| Omnipod 5 | 29 (16.5) | 0 (0) | 1 (1.4) | 28 (50.9) | |
| Others b | 15 (8.5) | 14 (29.8) | 1 (1.4) | 0 (0) | |
| Baseline AID type, N (%) | |||||
| None | 139 (79) | 33 (70.2) | 55 (74.3) | 51 (92.7) | <.0001 |
| Basal suspend | 19 (10.8) | 12 (25.5) | 7 (9.5) | 0 (0) | |
| Active HCL | 18 (10.2) | 2 (4.3) | 12 (16.2) | 4 (7.3) | |
| Baseline CGM use, N (%) | 141 (80.1) | 21 (44.7) | 66 (89.2) | 54 (98.2) | <.0001 |
Categorical variables were reported as number (percentage) and analyzed using the chi-square test. Continuous variables with normal distribution were reported as mean (standard deviation), and differences between groups were determined using a one-way ANOVA. Continuous variables with non-normal distribution were reported as median (lower quartile 25%, upper quartile 75%) and analyzed using the Wilcoxon rank-sum test.
AID, automated insulin delivery; BMI, body mass index; CGM, continuous glucose monitor; CVD, cardiovascular disease, eGFR, estimated glomerular filtration rate using CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration); HCL, hybrid closed loop; TDD, total daily insulin.
P-value represents comparison between white and non-white races; P = .29 for comparison against all races.
Others include Medtronic paradigm revel and Animas.
Insulin Use
Total daily insulin (TDI) use was similar across all three groups at baseline, 30 days, and 90 days (Table 2). However, there was a modest but significant difference in change in basal dose from baseline to 90 days (P = .04, Table 2). Bolus overrides were significantly different at 30 days (0 [IQR 0, 2.5]% for MT, 0 [IQR 0, 12]% for CIQ, and 3.7 [IQR 0, 19]% for OP5, P = .008) and 90 days (1.2 [IQR 0, 2.8]% for MT, 0 [IQR 0, 16]% for CIQ, and 3 [IQR 0, 39]% for OP5, P = .02) (Table 2). Pairwise comparison using Steel–Dwass method for multiple comparisons showed a significant difference in bolus overrides between MT and OP5 (P = .01) and CIQ and OP5 (P = .04) at 30 days and between MT and OP5 (P = .04) at 90 days. The %time in automated delivery was significantly different across groups with MT (87 [IQR 66, 95] %), compared to CIQ (93 [QR 89, 97] %), or OP5 (99 [IQR 89, 100] %), P < .001) at 30 days. Similarly, %time in automated delivery was 80 (IQR 54, 89), 95 (IQR 89, 98), and 95 (IQR 82, 100)%, at 90 days (P ≤ .0001, Table 2). The %time in automated delivery was similar at 30 days (87 [IQR 66, 95]% vs. 87 [IQR 64, 95]%) and 180 days (80 [IQR 54, 89]% vs 78 [IQR 47, 89]%) with or without inclusion of the three individuals initiating the 770G system respectively.
Table 2.
HbA1c and Pump Data by HCL System.
| Total population | Medtronic 670G/770G | Control IQ | Omnipod 5 | P value | |
|---|---|---|---|---|---|
| (N = 176) | (N = 47) | (N = 74) | (N = 55) | ||
| HbA1c baseline | N = 163 | N = 45 | N = 65 | N = 53 | .12 |
| % | 7.4 (6.8, 8.2) | 7.6 (6.7, 8.4) | 7.5 (6.85, 8.35) | 7.1 (6.7, 7.75) | |
| mmol/mol | 57 (51, 66) | 60 (50, 68) | 58 (51, 68) | 54 (50, 61) | |
| HbA1c 30 to 90 days | N = 97 | N = 36 | N = 36 | N = 25 | .51 |
| % | 7.1 (6.5, 7.6) | 7.1 (6.8, 7.78) | 7 (6.4, 7.58) | 7.1 (6.5, 7.45) | |
| mmol/mol | 54 (48, 60) | 54 (51, 62) | 53 (46, 59) | 54 (48, 58) | |
| HbA1c 91 to 180 days | N = 130 | N = 41 | N = 56 | N = 33 | .02 |
| % | 7.02 (0.82) | 7.30 (0.70) | 6.96 (0.90) | 6.78 (0.74) | |
| mmol/mol | 53 (9) | 56 (7.7) | 53 (9.8) | 51 (8.1) | |
| Change in HbA1c 91 to 180 day | N = 120 | N = 39 | N = 49 | N = 32 | .04 |
| % | −0.4 (−1, 0.1) | −0.1 (−0.8, 0.3) | −0.6 (−1.1, −0.15) | −0.55 (−0.98, 0) | |
| mmol/mol | −4.4 (−10.9, 1.1) | −1.1 (−8.7, 3.3) | −6.6 (−12, −1.6) | −6 (−10.7, 0) | |
| 30-day pump data | |||||
| TDD (units) |
N = 158 46.27 (33.6, 67.0) |
N = 46 45.2 (33.1, 68.1) |
N = 57 41.98 (33.4, 71.2) |
N = 55 48.7 (33.4, 61.5) |
.92 |
| TDD (units/kg) |
N = 158 0.60 (0.21) |
N = 46 0.62 (0.22) |
N = 57 0.60 (0.22) |
N = 55 0.58 (0.21) |
.69 |
| Bolus count (#/day) |
N = 158 5.22 (2.23) |
N = 46 5.66 (1.83) |
N = 57 4.99 (2.46) |
N = 55 5.10 (2.28) |
.28 |
| Bolus override (%) |
N = 158 0 (0, 9.05) |
N = 46 0 (0, 2.5) |
N = 57 0 (0, 12) |
N = 55 3.7 (0, 19.2) |
.008 |
| Automated delivery (%) |
N = 158 94 (81.75, 98) |
N = 46 86.5 (65.5, 95) |
N = 57 93 (89, 97) |
N = 55 99 (89, 100) |
<.0001 |
| Basal insulin (units/day) |
N = 158 25.35 (17.8, 34.3) |
N = 46 24.9 (18, 37.2) |
N = 57 24.9 (15.3, 37.36) |
N = 55 25.6 (21.4, 31.6) |
.77 |
| 90-day pump data | |||||
| TDD (units) |
N = 158 47.04 (36.5, 68) |
N = 46 47.05 (33.5, 66.8) |
N = 57 47.18 (37.4, 73.2) |
N = 55 46.9 (39.5, 63.2) |
.92 |
| TDD (units/kg) |
N = 158 0.62 (0.21) |
N = 46 0.63 (0.22) |
N = 57 0.63 (0.21) |
N = 55 0.60 (0.19) |
.75 |
| Bolus count (#/day) |
N = 158 4.8 (3.8, 6.59) |
N = 46 5.65 (4.48, 6.5) |
N = 57 4.9 (3.55, 7.5) |
N = 55 4.5 (3.7, 5.2) |
.08 |
| Bolus override (%) |
N = 158 1.05 (0, 8.85) |
N = 46 1.2 (0, 2.8) |
N = 57 0 (0, 15.5) |
N = 55 3.1 (0, 39.1) |
.02 |
| Auto mode (%) |
N = 158 92.5 (78, 97.3) |
N = 47 80 (54, 89) |
N = 56 95 (89.3, 97.8) |
N = 55 95 (82, 100) |
<.0001 |
| Basal insulin (units/day) |
N = 158 29.2 (12.7) |
N = 46 29.33 (13.7) |
N = 57 28.41 (12.7) |
N = 55 29.91 (12.0) |
.82 |
| Change in TDD (units/kg) |
N = 114 0.013 (−0.028, 0.063) |
N = 39 −0.003 (−0.063, 0.046) |
N = 39 0.028 (−0.0003, 0.062) |
N = 36 0.022 (−0.022, 0.072) |
.19 |
| Change in basal dose (units/day) |
N = 157 0.79 (−3.48, 4.5) |
N = 45 0.3 (−3, 4.45) |
N = 57 −0.49 (−5.69, 2.88) |
N = 55 2.1 (−1.2, 7.4) |
.04 |
| Severe Hypoglycemia, N (%) | 5 (2.8) | 1 (2.1) | 2 (2.7) | 2 (3.6) | .90 |
| DKA, N (%) | |||||
| Ketosis w/o acidosis | 6 (3.4) | 3 (6.4) | 3 (4.1) | 0 (0) | .17 |
| Ketoacidosis | 8 (4.6) | 1 (2.1) | 3 (4.1) | 4 (7.3) | |
| Infusion site failure, N (%) | 25 (14.2) | 7 (14.9) | 14 (18.9) | 4 (7.3) | |
Categorical variables were reported as number (percentage) and analyzed using the chi-square test. Continuous variables with non-normal distribution were reported as median (lower quartile 25%, upper quartile 75%) and analyzed using Wilcoxon/Kruskal–Wallis tests unless otherwise noted. Continuous variables with normal distribution were reported as mean (standard deviation) and differences between groups were determined using a one-way ANOVA.
DKA, diabetic ketoacidosis; HCL, hybrid closed loop; TDD, total daily insulin; w/o, without.
HbA1c
Of the total population, median HbA1c at baseline was 7.4 (IQR 6.8, 8.2)% (57 [IQR 51, 66] mmol/mol) while at 30 to 90 days, it was 7.1 (IQR 6.5, 7.6)% (54 [IQR 48, 60] mmol/mol). There was a significant decrease in HbA1c from baseline to 91 to 180 days in the CIQ (P < .0001) and OP5 (P = .0002) groups but not the MT group (P = .12, by Wilcoxon signed rank test). Change in HbA1c from baseline to 91 to 180 days was −0.1 (IQR −0.8, 0.3)% (−1.1 [IQR −8.7, 3.3] mmol/mol) for MT, −0.6 (IQR −1.1, −0.15) (−6.6 [IQR −12, −1.6] mmol/mol) for CIQ, and −0.55 (IQR −0.98, 0) (−6 [IQR −10.7, 0] mmol/mol) for OP5 (P = .04 across all three groups). Pairwise comparisons using Steel–Dwass method for multiple comparisons revealed a significant difference in change in HbA1c between the MT and CIQ groups (P = .04). (Figure 2).
Figure 2.
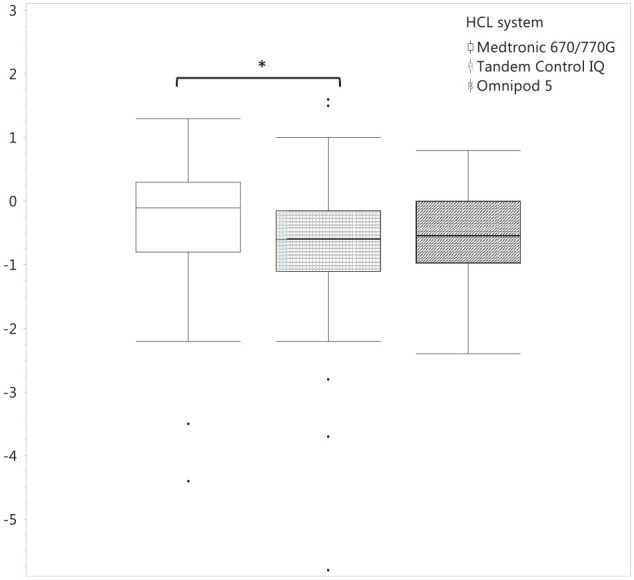
Change in HbA1c from 91 to 180 days by hybrid closed loop pump. A statistical difference was found between the Medtronic 670/770G and Tandem Control IQ groups (P = .04).
In the initial model for change in HbA1c, HCL device was not a significant predictor after adjusting for age and 30-day %time in automated delivery >90%. This did not change in the final model; however, 30-day %time in automated delivery >90% was a significant predictor (Table 3).
Table 3.
Multivariable Analysis of Change in HbA1c.
| Variable | Estimate | SE | P value |
|---|---|---|---|
| Initial model | |||
| HCL system | |||
| Medtronic | −0.04 | 0.14 | .80 |
| Control IQ | −0.03 | 0.14 | .81 |
| Omnipod 5 | 0.07 | 0.14 | .62 |
| Age | 0.005 | 0.007 | .43 |
| Baseline pump use | 0.23 | 0.11 | .03 |
| 30-day automated delivery >90% | −0.01 | 0.004 | .02 |
| Final model | |||
| HCL system | |||
| Medtronic | −0.03 | 0.14 | .80 |
| Control IQ | −0.04 | 0.13 | .79 |
| Omnipod 5 | 0.07 | 0.14 | .60 |
| Baseline pump use | 0.24 | 0.11 | .02 |
| 30-day automated delivery >90% | −0.009 | 0.004 | .03 |
HCL, hybrid closed loop; SE, standard error.
CGM Data
CGM data were available for most patients at 30 and 90 days (Table 4). Twelve patients had 7-day CGM reports instead of 14-day reports.
Table 4.
CGM Data by HCL System.
| Total population | Medtronic 670G/770G | Control IQ | Omnipod 5 | P value | |
|---|---|---|---|---|---|
| Baseline CGM | |||||
| %>180 mg/dL |
N = 124 42.8 (19.4) |
N = 5 37.26 (17.0) |
N = 67 43.5 (20.1) |
N = 52 42.34 (18.8) |
.77 |
| %70 to 180 mg/dL |
N = 124 54.76 (18.8) |
N = 5 58.46 (19.8) |
N = 67 53.66 (19.2) |
N = 52 55.81 (18.4) |
.75 |
| %<70 mg/dL |
N = 124 1.3 (0.53, 3.48) |
N = 5 2.8 (0.5, 8.85) |
N = 67 2 (0.8, 4.3) |
N = 52 1 (0.28, 2.35) |
.23 |
| %>250 mg/dL |
N = 119 13 (7, 27) |
N = 3 13 (2, 37) |
N = 65 14 (8, 26) |
N = 51 10.5 (4.3, 30) |
.47 |
| %<54 mg/dL |
N = 120 0 (0,0.5) |
N = 3 0 (0, 7) |
N = 66 0 (0, 0.5) |
N = 51 0 (0, 0.5) |
.60 |
| CV% |
N = 123 35.1 (31.4, 41.6) |
N = 5 36.9 (29.3, 45) |
N = 67 35.2 (32.5, 43.2) |
N = 51 34.7 (30, 39.2) |
.33 |
| 30-day CGM | |||||
| %>180 mg/dL |
N = 168 29 (21.1, 39) |
N = 45 28 (21.5, 35) |
N = 68 28.5 (22.3, 39.8) |
N = 55 32 (20, 42) |
.50 |
| %70 to 180 mg/dL |
N = 168 69 (60.25, 76) |
N = 45 70 (64.5, 76) |
N = 68 69.5 (59.1, 75) |
N = 55 67 (54, 78) |
.67 |
| %<70 mg/dL |
N = 168 1 (0, 2) |
N = 45 2 (1, 2) |
N = 68 1 (0, 1.98) |
N = 55 1 (0, 2) |
.06 |
| %>250 mg/dL |
N = 159 7 (3, 12) |
N = 43 5 (3,8) |
N = 61 7.7 (4.1, 14.5) |
N = 55 7 (3, 15) |
.07 |
| %<54 mg/dL |
N = 161 0 (0, 0) |
N = 45 0 (0, 0) |
N = 61 0 (0, 0) |
N = 55 0 (0, 0) |
.83 |
| CV% |
N = 168 33.7 (6.19) |
N = 45 33 (4.91) |
N = 68 34.88 (7.38) |
N = 55 32.8 (5.34) |
.12 |
| 90-day CGM | |||||
| %>180 mg/dL |
N = 170 30 (22, 40) |
N = 45 27 (22, 40.5) |
N = 70 32.4 (23, 39.6) |
N = 55 30 (22, 40) |
.96 |
| %70 to 180 mg/dL |
N = 170 67 (59, 76) |
N = 45 70 (57, 76) |
N = 70 67 (59, 75.2) |
N = 55 68 (60, 76) |
.95 |
| %<70 mg/dL |
N = 170 1 (0, 2) |
N = 45 2 (1, 3) |
N = 70 1 (0, 2) |
N = 55 1 (0, 1) |
.002 |
| %>250 mg/dL |
N = 166 7 (3, 13) |
N = 44 6.5 (2, 11) |
N = 67 8 (4, 14) |
N = 55 7 (3, 15) |
.50 |
| %<54 mg/dL |
N = 167 0 (0, 0) |
N = 45 0 (0, 1) |
N = 67 0 (0, 0) |
N = 55 0 (0, 0) |
.15 |
| CV% |
N = 170 33.7 (6.08) |
N = 45 33.2 (5.14) |
N = 70 34.0 (6.57) |
N = 55 33.7 (6.21) |
.82 |
Categorical variables were reported as number (percentage) and analyzed using the chi-square test. Continuous variables with non-normal distribution were reported as median (lower quartile 25%, upper quartile 75%) and analyzed using Wilcoxon/Kruskal–Wallis tests unless otherwise noted. Continuous variables with normal distribution were reported as mean (standard deviation) and differences between groups were determined using a one-way ANOVA.
CGM, continuous glucose monitor, CV, coefficient of variation; HCL, hybrid closed loop.
The three groups had similar 30-day CGM values for TIR, TBR, and time above range >180 mg/dL (TAR). As for 90-day data, TIR was also similar, 70 (IQR 57, 76)%, 67 (IQR 59, 75)%, and 68 (IQR 60, 76)%, for MT, CIQ, and OP5 devices respectively (P = .95). However, there was a significant difference in 90-day TBR %< 70 mg/dL: 2 (IQR 1, 3)%, 1 (IQR 0, 2)%, and 1 (IQR 0, 1)% for MT, CIQ, and OP5 devices respectively (P = .002), (Figure 3). Pairwise comparisons using Steel–Dwass method for multiple comparisons revealed a significant difference in TBR between the MT and CIQ (P = .009) as well as MT and OP5 groups (P = .002).
Figure 3.
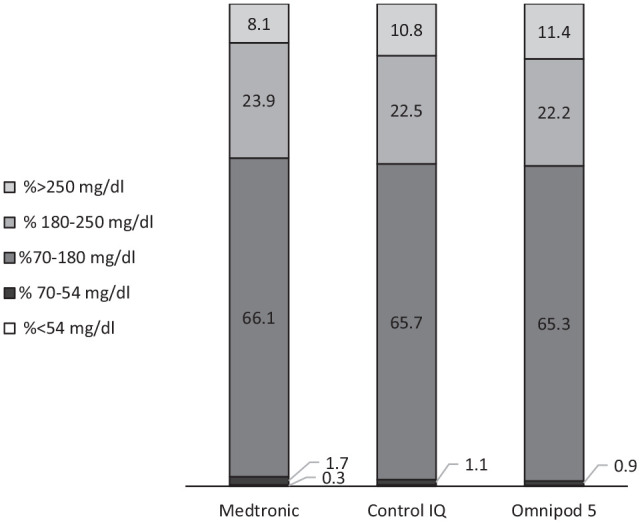
Average 90-day time in ranges by HCL pump type.
In the initial multivariate model adjusting for age, sex, income, diabetes duration, eGFR, baseline HbA1c, insulin use, and 90-day %time in automated delivery, HCL pump type was not an independent predictor of TIR, and this did not change in the final model or when the model was run without %time in automated delivery. However, older age, lower HbA1c at baseline, and %time in automated delivery >90% were independent predictors in the final models (Table 5).
Table 5.
Multivariable Analysis of 90-Day %TIR.
| With auto mode >90% | Without auto mode >90% | |||||
|---|---|---|---|---|---|---|
| Variable | Estimate | SE | P value | Estimate | SE | P value |
| Initial model | ||||||
| HCL system | ||||||
| Medtronic | 2.76 | 1.67 | .10 | −0.30 | 1.50 | .84 |
| Control IQ | −1.38 | 1.61 | .39 | 1.29 | 1.52 | .40 |
| Omnipod 5 | −1.39 | 1.59 | .38 | −1.00 | 1.61 | .54 |
| Age | 0.11 | 0.11 | .32 | 0.17 | 0.11 | .11 |
| Male | 1.22 | 1.11 | .28 | 1.55 | 1.14 | .18 |
| Area level income a | 4.58 | 2.95 | .12 | 4.56 | 3.03 | .14 |
| Diabetes duration a | 1.31 | 2.32 | .57 | −0.26 | 2.34 | .91 |
| eGFR a | 1.70 | 3.01 | .57 | 0.58 | 3.10 | .85 |
| Baseline AID | −0.25 | 1.25 | .84 | −0.41 | 1.26 | .74 |
| Baseline HbA1c a | −45.4 | 8.31 | <.0001 | −42.2 | 7.97 | <.0001 |
| 90-day automated delivery >90% | 3.69 | 1.24 | .004 | - | - | - |
| Baseline unit/kg | −2.51 | 5.56 | .65 | −6.60 | 5.67 | .25 |
| Final model | ||||||
| HCL system | ||||||
| Medtronic | 2.44 | 1.54 | .12 | 0.28 | 1.49 | .85 |
| Control IQ | −0.07 | 1.42 | .96 | 1.51 | 1.37 | .27 |
| Omnipod 5 | −2.37 | 1.36 | .08 | −1.79 | 1.43 | .21 |
| Age | 0.20 | 0.07 | .006 | 0.23 | 0.07 | .002 |
| Baseline HbA1c a | −34.6 | 6.56 | <.0001 | −43.5 | 6.40 | <.0001 |
| 90-day auto mode >90% | 3.92 | 1.11 | .0005 | - | - | - |
AID, automated insulin delivery; eGFR = estimated glomerular filtration rate; HCL, hybrid closed loop; SE, standard error; TIR, time in range.
Log-transformed values.
Since TBR did differ between groups at 90 days, an additional multivariable model was performed (Table 6). In this model, MT pump, younger age, lower baseline HbA1c, and lower pre-pump TDI were independently associated with higher 90-day TBR after adjusting for pump type, pre-pump TDI, 90-day %time in automated delivery, baseline HbA1c, and age. In contrast, OP5 pump use was associated with lower 90-day %TBR holding other variables constant. However, after the removal of >90% time in automated delivery in the final model, MT pump and OP5 were still independently associated with TBR.
Table 6.
Multivariable Analysis of 90-Day %TBR.
| Variable | Estimate | SE | P value |
|---|---|---|---|
| Initial model | |||
| HCL system | |||
| Medtronic | 0.47 | 0.23 | .05 |
| Control IQ | −0.07 | 0.21 | .73 |
| Omnipod 5 | −0.40 | 0.21 | .06 |
| Age | −0.03 | 0.01 | .01 |
| Baseline TDD | −0.01 | 0.006 | .02 |
| Baseline HbA1c a | −2.81 | 1.06 | .01 |
| 90-Day automated delivery >90% | 0.20 | 0.17 | .24 |
| Final model | |||
| HCL system | |||
| Medtronic | 0.64 | 0.20 | .002 |
| Control IQ | −0.24 | 0.19 | .22 |
| Omnipod 5 | −0.40 | 0.20 | .05 |
| Age | −0.03 | 0.01 | .007 |
| Baseline TDD | −0.01 | 0.006 | .04 |
| Baseline HbA1c a | −2.38 | 0.97 | .02 |
HCL, hybrid closed loop; SE, standard error; TBR, %time below range; TDD, total daily insulin.
Log-transformed values.
Safety Outcomes and Education Visits
There was no significant difference in the incidence of severe hypoglycemia, ketoacidosis, or infusion site failures among groups (Table 2). Education visits were similar across all pumps at 30 days (53% MT, 58% CIQ, 62% OP5; P = .70) and 90 days (38% MT, 32% CIQ, 31% OP5, P = .71) (Table 1). Furthermore, 30-day education visits were not associated with 91 to 180 HbA1c (P = .24), 90-day TIR (P = .46), nor 90-day %time in automated delivery (P = .53).
Discussion
This evaluated the efficacy and safety of three HCL devices in patients with T1D in the real-world setting and observed a significantly greater decrease in HbA1c in CIQ and OP5 compared to the MT group. All three pumps had similar TIR at 30 and 90 days, though 90-day TBR was highest in the MT group (Figure 3). However, HCL device was not an independent predictor for change in HbA1c nor 90-day TIR in multivariable models after adjusting for %time in automated delivery. To our knowledge, this is the first comparative analysis across all three HCL systems.
The type of HCL was not an independent predictor of change in HbA1c after adjustment for other factors, including age and %time in automated delivery. The %time in automated delivery differed between pumps at both 30 and 90 days with MT users having the lowest percentage of time. This finding is inherent to the system algorithm but may also be due to CGM performance and/or user satisfaction leading to exits from automated delivery.13,19 Newer systems have attempted to address these issues and may explain why there was higher %time in automated delivery among CIQ and OP5. Moreover, the recent FDA-approved MT 780G features multiple enhancements and a reported time in automated delivery of 92% to 94% in real-world studies.20,21 In the clinical trials, change in HbA1c for MT 670G was −0.5 (vs −0.1% in this study) but there were generally more favorable reductions with CIQ (−0.33% vs −0.6%) and OP5 (−0.38% vs −0.55%) with this study compared to that reported in clinical trials.10-12 This finding highlights the importance of comparative real-world evidence. While clinical trials are useful for understanding efficacy and safety under idealized conditions, often with healthier, highly motivated participants, frequent visits, and ample support, 12 they may not necessarily reflect outcomes in actual clinical practice. 22 In addition, comparisons made across individual trials published thus far are limited due to heterogeneity among study designs. 23
The type of HCL device was not an independent predictor of TIR; however, higher %time in automated delivery (>90%) was associated with greater TIR. Thus, when used as intended, AID systems are associated with clinically relevant improvements in glycemia in actual practice. 24
Finally, %TBR was highest in the MT group, even after adjusting for baseline characteristics. Consistent with the findings for change in HbA1c, this finding is likely explained by less %time in automated delivery as pump type was only significant after removing %time in automated delivery from the model. In contrast, OP5 use was associated with lower %TBR which could be due to differences in AID algorithms. It is unclear whether this difference is clinically relevant since there was no difference in %time below 54 mg/dL. Moreover, the differences in CGM performance may contribute to this finding.
Interestingly, there was a difference in bolus calculator overrides across groups, with patients who started MT having fewer and OP5 users having more overrides. We did not collect information related to reasons for overrides including whether the dose was increased or decreased; however, it is likely that differences between algorithms and/or pump mechanics might influence the tendency to override a bolus.25,26 For example, the override feature is not available in the MT system, though users may accomplish this by entering “fake” carbohydrates into the bolus calculator which are not generally identifiable in downloaded reports. On the other hand, the OP5 system allows more broadly adjustable targets and the bolus calculator incorporates a separate customizable target and correction factor, as well as CGM trends. Nevertheless, the frequency of bolus overrides was not associated with TIR or HbA1c. Thus, the difference between groups may have been offset by other features such as %time in automated delivery. Finally, education visits were similar across all three pumps at 30 and 90 days. While we did not observe a relationship between education visits and patient outcomes, the importance of proper education and support for managing diabetes is well established 27 and, follow-up visits with a trained diabetes educator may play a role in optimal outcomes.25,28,29
This study is the first comparative analysis of three separate HCL systems, featuring real-world patients from a heterogeneous population, including a wide range of socioeconomic status, duration of diabetes, as well as prior device use (new patients and experienced patients). Another strength is the addition of %time in automated delivery and frequency of bolus overrides to the analyses. However, it is important to consider several limitations. First, it was a single-center study, and consistent with previous reports, the sample had a low proportion of patients from non-white racial/ethnic backgrounds. 9 Thus, additional study, ideally from multicenter real-world populations, is needed. Second, there was a broad array of device use at baseline; however, this did not significantly influence glycemic outcomes. In addition, there was an insufficient number of 770G users compared to 670G. Baseline HCL use was considered a confounder as well; although, after running sensitivity analysis for the patients who had used an HCL device previously, the results were not statistically different. Moreover, data on timeframe of baseline CGM use were not collected for outcome comparison, which could have introduced potential confounding. However, it is worth noting that baseline CGM use was not a predictor of any of the outcomes. Inherent to a retrospective study, downloads were not available for a subset of patients, and the availability of downloads may be influenced by patient factors related to ongoing device use, especially %time in automated delivery. While HbA1c values were recorded up to six months, the timeframe for device downloads (CGM and pump information) was limited to 90 days, and thus longer-term follow-up studies are warranted. Other limitations include the inability to compare special features for various pumps such as sleep or exercise modes and target glucose to quantify entry of “fake carbs” 25 to manipulate the system to deliver more insulin. In addition, pumps used different CGMs, contributing to differences between systems. Finally, despite multivariable models, unmeasured factors may influence HbA1c and/or TIR. Thus, additional comparative effectiveness studies are needed to assess the effects of HCL technologies on glycemic outcomes as well as patient-reported outcomes and psychosocial factors that influence device use, particularly as additional new technologies emerge.
Conclusion
In conclusion, the use of HCL devices in clinical practice is associated with improved HbA1c, TIR, and TBR, but benefits are dependent upon %time in automated delivery. The study reiterates the importance of real-world studies as pivotal evidence for the efficacy and safety of current and future diabetes technology.
Supplemental Material
Supplemental material, sj-docx-1-dst-10.1177_19322968241234948 for Comparative Effectiveness of Hybrid Closed-Loop Automated Insulin Delivery Systems Among Patients with Type 1 Diabetes by Sara Folk, Janet Zappe, Kathleen Wyne and Kathleen M. Dungan in Journal of Diabetes Science and Technology
Supplemental material, sj-docx-2-dst-10.1177_19322968241234948 for Comparative Effectiveness of Hybrid Closed-Loop Automated Insulin Delivery Systems Among Patients with Type 1 Diabetes by Sara Folk, Janet Zappe, Kathleen Wyne and Kathleen M. Dungan in Journal of Diabetes Science and Technology
Supplemental material, sj-docx-3-dst-10.1177_19322968241234948 for Comparative Effectiveness of Hybrid Closed-Loop Automated Insulin Delivery Systems Among Patients with Type 1 Diabetes by Sara Folk, Janet Zappe, Kathleen Wyne and Kathleen M. Dungan in Journal of Diabetes Science and Technology
Footnotes
Abbreviations: AID, automated insulin delivery; ANOVA, analysis of variance; CDCES, Certified Diabetes Care and Education Specialist; CGM, continuous glucose monitor; CIQ, Tandem Control IQ; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; CV, coefficient of variation; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; HCL, hybrid closed loop; IQR, interquartile range; MT, Medtronic 670G/770G; OP5, Omnipod 5; T1D, type 1 diabetes; TAR, time above range >180 mg/dL; TBR, time below range <70 mg/dL; TDI, total daily insulin; TIR, time in range 70 to 180 mg/dL.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Kathleen Dungan reports research funding from Dexcom, Abbott, Insulet, Viacyte, and Sanofi; consulting from Dexcom, Eli Lilly, Insulet, Oppenheimer, and Elsevier; honoraria from Academy for Continued Healthcare Learning Med Learning Group and Medscape; and royalties from UpToDate. All other authors declare no conflicts of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by the Samuel J. Roessler Memorial Scholarship Medical Student Research Scholarship at the Ohio State University College of Medicine.
ORCID iDs: Sara Folk  https://orcid.org/0009-0009-3426-5289
https://orcid.org/0009-0009-3426-5289
Kathleen M. Dungan  https://orcid.org/0000-0003-1289-1595
https://orcid.org/0000-0003-1289-1595
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Diabetes Control and Complications Trial Research Group; Nathan DM, Genuth S, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977-986. [DOI] [PubMed] [Google Scholar]
- 2. Hermann JM, Miller KM, Hofer SE, et al. The transatlantic HbA1c gap: differences in glycaemic control across the lifespan between people included in the US T1D Exchange Registry and those included in the German/Austrian DPV registry. Diabet Med. 2020;37(5):848-855. [DOI] [PubMed] [Google Scholar]
- 3. Prigge R, McKnight JA, Wild SH, et al. International comparison of glycaemic control in people with type 1 diabetes: an update and extension. Diabet Med. 2022;39(5):e14766. [DOI] [PubMed] [Google Scholar]
- 4. Karges B, Schwandt A, Heidtmann B, et al. Association of insulin pump therapy vs insulin injection therapy with severe hypoglycemia, ketoacidosis, and glycemic control among children, adolescents, and young adults with type 1 diabetes. JAMA. 2017;318(14):1358-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weisman A, Bai JW, Cardinez M, Kramer CK, Perkins BA. Effect of artificial pancreas systems on glycaemic control in patients with type 1 diabetes: a systematic review and meta-analysis of outpatient randomised controlled trials. Lancet Diabetes Endocrinol. 2017;5(7):501-512. [DOI] [PubMed] [Google Scholar]
- 6. Bergenstal RM, Tamborlane WV, Ahmann A, et al. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med. 2010;363(4):311-320. [DOI] [PubMed] [Google Scholar]
- 7. Pickup JC. Is insulin pump therapy effective in Type 1 diabetes? Diabet Med. 2019;36(3):269-278. [DOI] [PubMed] [Google Scholar]
- 8. Faulds ER, Zappe J, Dungan KM. Real-world implications of hybrid close loop (HCL) insulin delivery system. Endocr Pract. 2019;25(5):477-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016–2018. Diabetes Technol Ther. 2019;21(2):66-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garg SK, Weinzimer SA, Tamborlane WV, et al. Glucose outcomes with the in-home use of a hybrid closed-loop insulin delivery system in adolescents and adults with type 1 diabetes. Diabetes Technol Ther. 2017;19(3):155-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brown SA, Forlenza GP, Bode BW, et al. Multicenter trial of a tubeless, on-body automated insulin delivery system with customizable glycemic targets in pediatric and adult participants with type 1 diabetes. Diabetes Care. 2021;44(7):1630-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brown SA, Kovatchev BP, Raghinaru D, et al. Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med. 2019;381(18):1707-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lal RA, Basina M, Maahs DM, Hood K, Buckingham B, Wilson DM. One year clinical experience of the first commercial hybrid closed-loop system. Diabetes Care. 2019;42(12):2190-2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Breton MD, Kovatchev BP. One year real-world use of the control-IQ advanced hybrid closed-loop technology. Diabetes Technol Ther. 2021;23(9):601-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Forlenza GP, Buckingham BA, Brown SA, et al. First outpatient evaluation of a tubeless automated insulin delivery system with customizable glucose targets in children and adults with type 1 diabetes. Diabetes Technol Ther. 2021;23(6):410-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Beato-Víbora PI, Chico A, Moreno-Fernandez J, et al. A multicenter prospective evaluation of the benefits of two advanced hybrid closed-loop systems in glucose control and patient-reported outcomes in a real-world setting. Diabetes Care. 2024;47:216-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Inker LA, Eneanya ND, Coresh J, et al. New creatinine- and cystatin C–based equations to estimate GFR without race. N Engl J Med. 2021;385(19):1737-1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nallicheri A, Mahoney KM, Gutow HA, Bellini N, Isaacs D. Review of automated insulin delivery systems for type 1 diabetes and associated time in range outcomes. Touchrev Endocrinol. 2022;18(1):27-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Messer LH, Berget C, Vigers T, et al. Real world hybrid closed-loop discontinuation: predictors and perceptions of youth discontinuing the 670G system in the first 6 months. Pediatr Diabetes. 2020;21(2):319-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Silva JD, Lepore G, Battelino T, et al. Real-world performance of the MiniMedTM 780G system: first report of outcomes from 4120 users. Diabetes Technol Ther. 2022;24(2):113-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Grassi B, Gómez AM, Calliari LE, et al. Real-world performance of the MiniMed 780G advanced hybrid closed loop system in Latin America: substantial improvement in glycaemic control with each technology iteration of the MiniMed automated insulin delivery system. Diabetes Obes Metab. 2023;25(6):1688-1697. [DOI] [PubMed] [Google Scholar]
- 22. Schneeweiss S, Patorno E. Conducting real-world evidence studies on the clinical outcomes of diabetes treatments. Endocr Rev. 2021;42(5):658-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Knoll C, Peacock S, Wäldchen M, et al. Real-world evidence on clinical outcomes of people with type 1 diabetes using open-source and commercial automated insulin dosing systems: a systematic review. Diabet Med. 2022;39(5):e14741. [DOI] [PubMed] [Google Scholar]
- 24. Duffus SH, Ta’ani ZA, Slaughter JC, Niswender KD, Gregory JM. Increased proportion of time in hybrid closed-loop “Auto Mode” is associated with improved glycaemic control for adolescent and young patients with adult type 1 diabetes using the MiniMed 670G insulin pump. Diabetes Obes Metab. 2020;22(4):688-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sherr JL, Heinemann L, Fleming GA, et al. Automated insulin delivery: benefits, challenges, and recommendations. A consensus report of the Joint Diabetes Technology Working Group of the European Association for the Study of Diabetes and the American Diabetes Association. Diabetes Care. 2022;45(12):3058-3074. [DOI] [PubMed] [Google Scholar]
- 26. Zhou K, Isaacs D. Closed-loop artificial pancreas therapy for type 1 diabetes. Curr Cardiol Rep. 2022;24(9):1159-1167. [DOI] [PubMed] [Google Scholar]
- 27. Mazzuca SA, Moorman NH, Wheeler ML, et al. The diabetes education study: a controlled trial of the effects of diabetes patient education. Diabetes Care. 1986;9(1):1-10. [DOI] [PubMed] [Google Scholar]
- 28. Usoh CO, Johnson CP, Speiser JL, Bundy R, Dharod A, Aloi JA. Real-world efficacy of the hybrid closed-loop system. J Diabetes Sci Technol. 2021;16(3):659-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nallicheri A, Mahoney KM, Gutow HA, Bellini N, Isaacs D. Review of automated insulin delivery systems for type 1 diabetes and associated time in range outcomes. Touchrev Endocrinol. 2022;18(1):27-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-dst-10.1177_19322968241234948 for Comparative Effectiveness of Hybrid Closed-Loop Automated Insulin Delivery Systems Among Patients with Type 1 Diabetes by Sara Folk, Janet Zappe, Kathleen Wyne and Kathleen M. Dungan in Journal of Diabetes Science and Technology
Supplemental material, sj-docx-2-dst-10.1177_19322968241234948 for Comparative Effectiveness of Hybrid Closed-Loop Automated Insulin Delivery Systems Among Patients with Type 1 Diabetes by Sara Folk, Janet Zappe, Kathleen Wyne and Kathleen M. Dungan in Journal of Diabetes Science and Technology
Supplemental material, sj-docx-3-dst-10.1177_19322968241234948 for Comparative Effectiveness of Hybrid Closed-Loop Automated Insulin Delivery Systems Among Patients with Type 1 Diabetes by Sara Folk, Janet Zappe, Kathleen Wyne and Kathleen M. Dungan in Journal of Diabetes Science and Technology