Abstract
Background:
We investigated the risk of incident diabetic retinopathy (DR) among high glycator compared to low glycator patients based on the hemoglobin glycation index (HGI). Visit-to-visit variations in HGI also were assessed.
Methods:
Glycated hemoglobin (HbA1c) and continuous glucose monitoring data were collected up to 7 years prior to the date of eye examination defining incident DR or no retinopathy (control). Hemoglobin glycation index was calculated as difference in measured HbA1c and an estimated A1c from sensor glucose (eA1c) to define high (HbA1c − eA1c >0%) or low (HbA1c − eA1c <0%) glycator. Stable glycators were defined as ≥75% of visits with same HGI category. Logistic regression was used to assess the association between glycation category and incident DR.
Results:
Of 119 adults with type 1 diabetes (T1D), 49 (41%) were stable low glycator (HbA1c − eA1c <0%), 36 (30%) were stable high glycator (HbA1c − eA1c >0%), and 34 (29%) were unstable glycator. Using alternate criteria to define high vs low glycator (consistent difference in HbA1c − eA1c of > 0.4% or <0.4%, respectively), 53% of the adults were characterized as unstable glycator. Compared to low glycators, high glycators did not have a significantly higher risk for incident DR over time when adjusted for age, T1D duration and continuous glucose monitoring (CGM) sensor type (odds ratio [OR] = 1.31, 95% confidence interval [CI] = 0.48-3.62, P = .15).
Conclusions:
The risk of diabetic retinopathy was not found to differ significantly comparing high glycators to low glycators in adults with T1D. Moreover, HbA1c − eA1c relationship was not stable in nearly 30% to 50% adults with T1D, suggesting that discordance in HbA1c and eA1c are mostly related either HbA1c measurements or estimation of A1c from sensor glucose rather than physiological reasons.
Keywords: diabetic retinopathy, glycator, HbA1c, type 1 diabetes
Introduction
The discordance between measured glycated hemoglobin (HbA1c) and A1c estimated (eA1c) from either sensor glucose or fructosamine level is well-known.1,2 As glycation of hemoglobin is non-enzymatic and considered to be regulated by genes,3,4 it has been recognized that some people may have higher or lower HbA1c than others at the same mean glucose levels. For example, African Americans have 0.4% to 0.5% higher HbA1c at similar mean glucose than non-Hispanic whites. 5 A meta-analysis of 12 studies involving 49 238 individuals without diabetes, HbA1c was significantly higher among blacks (by 0.26%), and Asians of Indian and Pakistan origin (by 0.24%) without much difference in Latino population (by 0.08%) compared to non-Hispanic whites at similar fasting plasma glucose levels. 6 Moreover, the discordance between mean glucose and HbA1c is also common among non-Hispanic whites. In the DCCT trial where most participants with type 1 diabetes (T1D) were non-Hispanic whites, 57% of participants had discordance between laboratory measured HbA1c and estimated A1c (eA1c) based on mean glucose. 7
Many methods have been described to study such discordance, eg, hemoglobin glycation index (HGI) (difference between measured HbA1c and eA1c), 7 a glycation gap (difference between the measured HbA1c and that predicted by the fructosamine concentration), 8 or a glycation index (ratio of HbA1c to 28-day rolling mean blood glucose). 9
Few studies have suggested a higher risk for diabetes complications among high glycators (measured HbA1c is higher than eA1c) compared to low glycators (measured HbA1c is lower than eA1c).7,8,10,11 However, this theory has been debated by others.12,13 The clinical implication of the glycation gap and its risk with diabetes complications is currently unknown.
In this article, we investigated the visit-to-visit variation in the relationship between measured HbA1c and eA1c in adults with T1D and the association between high vs low glycator and risk for incident diabetic retinopathy (DR).
Methods
Study Design and Participants
A previously published cohort was used for the current analysis. 14 In brief, adults with T1D and incident DR group (defined as presence of DR from at least one retinal examination during the study inclusion period with the two consecutive previous retinal examinations without DR) and without DR (control group) were identified from electronic medical records (EMRs) between June 2018 and March 2022. Their continuous glucose monitoring (CGM) data and HbA1c measurements were retrieved up to 7 years before the diagnosis of retinopathy. For each participant, raw CGM data were collected in CSV format for up to 90 days at each clinic visit performed after January 1, 2013, and prior to the date of diagnosis of retinopathy for the cases or the date of the last visit in the inclusion period for controls. From the visits over the same period, HbA1c measurements (point-of-care or venous) were collected from the EMR. The detailed description of inclusion and exclusion, definition of retinopathy, and CGM data collection methods have been described previously. 14
For this analysis, we used the individuals from the original cohort who had at least two clinic visits with non-missing HbA1c and sufficient CGM data to calculate mean glucose. Sufficient CGM data was defined as at least 70% of data available in the 28 days prior to the visit date.
Statistical Analysis
Continuous variables were shown as mean ± standard deviation (SD), and categorical variables were shown as absolute numbers and percentages. We fit a linear regression model with mean glucose as the predictor and HbA1c as the outcome. The model included a random intercept term to account for the correlation between measurements for the same participant. The intercept and slope from this model were used to calculate an estimated A1c (eA1c) for each participant and visit. Hemoglobin glycation index was calculated as the difference in measured HbA1c and an estimated A1c from sensor glucose (eA1c) to define high (HbA1c − eA1c >0%) or low (HbA1c − eA1c <0%) glycator.
A participant was considered a stable (consistent) high glycator if they were classified as a high glycator (HbA1c − eA1c >0%) for at least 75% of visits (ie, consistently higher measured HbA1c than expected from mean glucose). Similarly, a participant was considered a stable low glycator if they were classified as a low glycator (HbA1c − eA1c <0%) for at least 75% of visits. Otherwise, a participant was classified as “unstable” with respect to the HbA1c-mean glucose relationship.
As a second estimation of glycation and stability, we used a 0.4% (clinically meaningful HbA1c difference) cutoff for the difference between measured HbA1c and eA1c. HbA1c − eA1c >0.4% and <0.4% were considered high glycator and low glycator, respectively. Participants are considered stable if ≥75% of visits fall in the same category, otherwise, they are considered “unstable.”
We used a logistic regression model to assess the association between DR and glycation profile (high/low glycator/unstable), after adjusting for age, T1D duration, and CGM sensor type. SAS version 9.4 (SAS Institute, Inc, Cary, North Carolina) was used for statistical analysis.
Results
First, we used a consistent difference in HbA1c − eA1c of >0% or <0% to define high and low glycator, respectively. Using this definition, 49 adults with T1D (41%) were considered low glycator, and 36 (30%) were considered high glycator. The relationship between HbA1c and eA1c was not consistent in one direction for 34 adults (29%) who were characterized as unstable glycator. The baseline characteristics of adults with high glycation vs low glycation are provided in Table 1. There were no clinically meaningful differences in age, sex, race/ethnicity, and diabetes duration between high glycator and low glycator. Compared with the low glycator group (measured HbA1c − eA1c < 0%), there was no significant increase in risk for incident DR among the high glycator group, in both unadjusted (odds ratio [OR] = 1.19, 95% confidence interval [CI] =0.50-2.83, P = .32) and adjusted models for age, T1D duration, and CGM sensor type (OR = 1.31, 95% CI = 0.48-3.62, P = .15).
Table 1.
Patient Characteristics at Time of Study Inclusion.
| Overall (N = 119) |
Low glycator (N = 49) |
High glycator (N = 36) |
Unstable (N = 34) |
|
|---|---|---|---|---|
| Age (years) | ||||
| Mean ± SD | 34 ± 16 | 34 ± 15 | 36 ± 19 | 32 ± 15 |
| Range | 14 to 81 | 17 to 73 | 14 to 81 | 17 to 72 |
| Sex | ||||
| Female n (%) | 58 (49%) | 26 (53%) | 16 (44%) | 16 (47%) |
| Male n (%) | 61 (51%) | 23 (47%) | 20 (56%) | 18 (53%) |
| Race/ethnicity | ||||
| Non-Hispanic white n (%) | 97 (82%) | 40 (82%) | 28 (78%) | 29 (85%) |
| Other n (%) | 10 (8%) | 4 (8%) | 4 (11%) | 2 (6%) |
| Unknown n (%) | 12 (10%) | 5 (10%) | 4 (11%) | 3 (9%) |
| Health insurance | ||||
| Private n (%) | 101 (85%) | 43 (88%) | 27 (75%) | 31 (91%) |
| Medicaid n (%) | 15 (13%) | 4 (8%) | 8 (22%) | 3 (9%) |
| Military plan n (%) | 2 (2%) | 1 (2%) | 1 (3%) | 0 (0%) |
| Unknown n (%) | 1 (<1%) | 1 (2%) | 0 (0%) | 0 (0%) |
| BMI (kg/m 2 ) | ||||
| Mean ± SD | 26 ± 4 | 26 ± 4 | 25 ± 3 | 26 ± 5 |
| Underweight (<18.5 kg/m2) n (%) | 1 (<1%) | 0 (0%) | 0 (0%) | 1 (3%) |
| Normal weight (18.5 to <25.0 kg/m2) n (%) | 41 (34%) | 17 (35%) | 16 (44%) | 8 (24%) |
| Overweight (25.0 to <30.0 kg/m2) n (%) | 42 (35%) | 19 (39%) | 8 (22%) | 15 (44%) |
| Obese (≥30.0 kg/m2) n (%) | 15 (13%) | 7 (14%) | 3 (8%) | 5 (15%) |
| Missing n (%) | 20 (17%) | 6 (12%) | 9 (25%) | 5 (15%) |
| Duration of T1D (years) | ||||
| Mean ± SD | 18 ± 8 | 18 ± 8 | 19 ± 9 | 18 ± 7 |
| Range | 5 to 47 | 5 to 47 | 7 to 44 | 8 to 40 |
Consider the following categories: low glycator (measured HbA1c − estimated HbA1c < 0%), high glycator (measured HbA1c − estimated HbA1c > 0%). Participants are stable if ≥75% of visits fall in the same category, otherwise they are considered “unstable.”
BMI, body mass index; SD, standard deviation; T1D, type 1 diabetes.
Using alternate criteria to define high vs low glycator (consistent difference in HbA1c − eA1c of > or <0.4%, respectively), 53% of the adults were characterized as unstable glycator. Only 13 adults (11%) were stable low glycator (measured − estimated HbA1c <-0.4%) and eight (7%) were stable high glycator (measured − estimated HbA1c >0.4%) (Table 2). Using this criteria, there was no significant increase in risk for incident DR among high glycator group (measured HbA1c − eA1c >0.4%) compared to low glycators (measured HbA1c − eA1c <−0.4%), in both unadjusted (OR = 2.67, 95% CI = 0.43-16.39, P = .20) and adjusted models for age, T1D duration, and CGM sensor type (OR = 1.39, 95% CI = 0.18-10.75, P = .59). Visit-to-visit variation in measured HbA1c and eA1c for each participant is shown in Figure 1.
Table 2.
Frequency of Stable and Unstable Glycators Using Two Different Methods.
| Main definition a | Overall (N = 119) |
DR group (N = 49) |
Control group (N = 70) |
|---|---|---|---|
| Stable low glycator | 49 (41%) | 21 (43%) | 28 (40%) |
| Stable high glycator | 36 (30%) | 17 (35%) | 19 (27%) |
| Unstable | 34 (29%) | 11 (22%) | 23 (33%) |
| Alternate definition b | |||
| Stable low glycator | 13 (11%) | 5 (10%) | 8 (11%) |
| Stable between −0.4 to 0.4 | 35 (29%) | 12 (24%) | 23 (33%) |
| Stable high glycator | 8 (7%) | 5 (10%) | 3 (4%) |
| Unstable | 63 (53%) | 27 (55%) | 36 (51%) |
Consider the following categories: stable low (measured HbA1c − eA1c <0%), stable high (measured HbA1c − eA1c >0%). Participants are stable if ≥75% of visits fall in the same category, otherwise they are considered “unstable.”
Consider the following categories: stable low (measured HbA1c − eA1c < -0.4%), stable between −0.4% and 0.4%, and stable high (measured HbA1c − eA1c > 0.4%). Participants are stable if ≥75% of visits fall in the same category, otherwise they are considered “unstable.”
Figure 1.
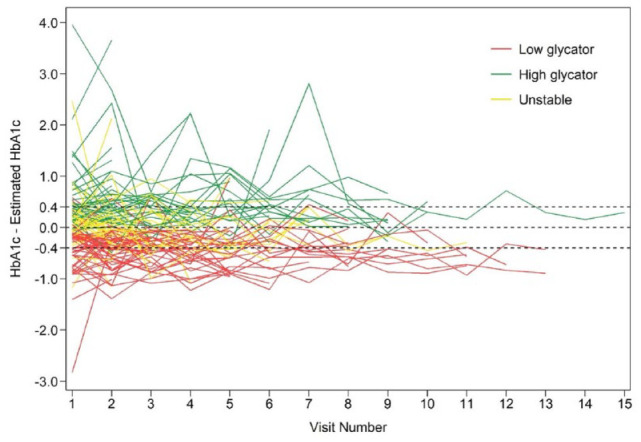
Spaghetti plot of difference between HbA1c and eA1c over time by participant.
Discussion
Using real-life, longitudinal data, we did not find a significant association between high glycators and incident DR in adults with T1D. This is in contrast to the analysis by McCarter and colleagues who analyzed DCCT data and reported a threefold increased risk for retinopathy among high glycators compared to low glycators. 7 However, that study had many major limitations. For example, they used only 1 day 7-point self-monitoring of blood glucose (SMBG) data to estimate A1c, which is insufficient compared with CGM data of our study. Lachin et al 12 used the same DCCT data and found a high correlation between HGI and HbA1c and when the analysis was adjusted for HbA1c, the association between HGI and retinopathy became insignificant suggesting that most of HGI risk was accounted for by HbA1c. Moreover, Lachin argued that HGI may not represent the biological variation in glycation as the relationship between mean glucose and HbA1c could be confounded by many factors such as red cell lifespan.
Our study found that nearly 30% of participants had an unstable relationship between measured HbA1c and eA1c; sometimes measured HbA1c was higher than eA1c, while other times, HbA1c was lower than eA1c. The percentage of participants with an unstable glycation gap increased when the glycation gap was defined as a consistent difference in HbA1c and eA1c of 0.4%. Thus, our data highlight that there are considerable visit-to-visit variations between measured HbA1c and eA1c. Previous literature is consistent with our results regarding the consistency and stability. Nayak et al 10 reported that only 549 of 1609 (34%) patients had consistently high or low glycation gaps in the repeated measures suggesting high discordance in the glycation directions between two visits. Thus, studies show 20% to 50% of people with diabetes do not have a consistent glycation profile. Therefore, HGI may not represent biological variation (genetic basis of non-enzymatic glycation) and the observed variation may represent the influence of various factors affecting either mean glucose (for eA1c) or HbA1c levels.
We believe that the variability/inconsistency of the calculation (measured HbA1c − eA1c) is mostly coming from the factors affecting HbA1c levels. Changes in diabetes therapy or management may influence HbA1c levels even in a short time frame. Glycation of hemoglobin reflects the weighted mean of preceding mean glucose over a considerably longer period of time. 15 For example, glucose levels during the most recent 4- to 6-week period will have a greater influence on the HbA1c result compared to levels from the prior 6 weeks. Hence, if a patient experiences a recent change in acute glucose levels (ie, use of automated insulin delivery systems or sickness or glucocorticoid treatment), the HbA1c will be disproportionately affected by the most recent glucose levels. Second, although point-of-care capillary HbA1c measurement devices are useful in clinical care, they are limited by the accuracy and higher variability between instruments, and therefore, the use of point-of-care HbA1c devices may be another factor in inducing HbA1c measurement errors. 16 Third, erythrocyte turnover issues and shorter erythrocyte lifespan would underrepresent earlier glucose management.1,2 Therefore, measured HbA1c and eA1c may not reflect the glycemic management of the same timeframe which may cause the discordance and unstable repeated measures.
The strength of this study is the longitudinal study design with data collection over 7 years with up to 15 clinic visits to evaluate the HbA1c-eA1c relationship. Moreover, we used 28 days of CGM data (compared to SMBG in previous studies) to estimate A1c from sensor mean glucose. The small sample size, non-Hispanic white predominant study population, retrospective medical record-based data, and non-standardization of HbA1c measurement with most measurements via point-of-care devices are limitations of this study.
Conclusions
In summary, we did not find an association between high HGI and incident DR over 7 years of follow-up. Most of the participants had alternating glycation status which highlights gaps in the glycation theory, and more research is needed to understand glycation of hemoglobin and its implication on diabetes complications.
Acknowledgments
The authors acknowledge the help of Sean Walker, Vamshi Krishna Karne, Vaishnavi Potluri, Madhavi Suyog Pagare, and Anagha Champakanath for collecting CGM data. The authors thank Bing Wang for doing the EMR search and EMR-based variable collection for this study. The authors also thank Lubna Qamar and Prakriti Joshee for their help with student supervision and data entry checking. The authors would like to thank all people with diabetes treated at the Barbara Davis Center for Diabetes without whom the data for this study would not have been available.
Footnotes
Abbreviations: CGM, continuous glucose monitoring; eA1c, A1c estimated; EMR, electronic medical record; HGI, hemoglobin glycation index; SD, standard deviation; SMBG, self-monitoring of blood glucose; T1D, type 1 diabetes.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: VNS has received research support from NovoNordisk, Alexion, Insulet, Tandem Diabetes Care, JDRF, and NIH and has received honoraria from Sanofi, NovoNordisk, Embecta, Insulet, Dexcom, Ascesia Diabetes Care, and Tandem Diabetes Care for speaking, consulting, or serving on an advisory board. RWB reports no personal financial disclosures but reports that his institution has received funding on his behalf as follows: grant funding, study supplies, and consulting fees from Insulet, Tandem Diabetes Care, and Beta Bionics; grant funding and study supplies from Dexcom; grant funding from Bigfoot Biomedical; study supplies from Medtronic, Ascencia, and Roche; consulting fees and study supplies from Eli Lilly and Novo Nordisk; and consulting fees from Embecta, Vertex, Hagar, Ypsomed, Sanofi, and Zucara. LGK, KEK, and CG do not report any conflict of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Juvenile Diabetes Research Foundation funded this study. The funder had no role in study design, data collection, or analyses.
ORCID iDs: Viral N. Shah  https://orcid.org/0000-0002-3827-7107
https://orcid.org/0000-0002-3827-7107
Lauren G. Kanapka  https://orcid.org/0000-0003-4440-5168
https://orcid.org/0000-0003-4440-5168
Kagan Ege Karakus  https://orcid.org/0000-0002-8552-5206
https://orcid.org/0000-0002-8552-5206
References
- 1. Nayak AU, Singh BM, Dunmore SJ. Potential clinical error arising from use of HbA1c in diabetes: effects of the glycation gap. Endocr Rev. 2019;40(4):988-999. [DOI] [PubMed] [Google Scholar]
- 2. Hempe JM, Hsia DS. Variation in the hemoglobin glycation index. J Diabetes Complications. 2022;36(7):108223. [DOI] [PubMed] [Google Scholar]
- 3. Cohen RM, Snieder H, Lindsell CJ, et al. Evidence for independent heritability of the glycation gap (glycosylation gap) fraction of HbA1c in nondiabetic twins. Diabetes Care. 2006;29:1739-1743. [DOI] [PubMed] [Google Scholar]
- 4. Snieder H, Sawtell PA, Ross L, Walker J, Spector TD, Leslie RD. HbA(1c) levels are genetically determined even in type 1 diabetes: evidence from healthy and diabetic twins. Diabetes. 2001;50(12):2858-2863. [DOI] [PubMed] [Google Scholar]
- 5. Bergenstal RM, Gal RL, Connor CG, et al. T1D exchange racial differences study group. racial differences in the relationship of glucose concentrations and hemoglobin A1c levels. Ann Intern Med. 2017;167(2):95-102. [DOI] [PubMed] [Google Scholar]
- 6. Cavagnolli G, Pimentel AL, Freitas PA, Gross JL, Camargo JL. Effect of ethnicity on HbA1c levels in individuals without diabetes: systematic review and meta-analysis. PLoS ONE. 2017;12(2):e0171315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McCarter RJ, Hempe JM, Gomez R, Chalew SA. Biological variation in HbA1c predicts risk of retinopathy and nephropathy in type 1 diabetes. Diabetes Care. 2004;27(6):1259-1264. [DOI] [PubMed] [Google Scholar]
- 8. Cohen RM, Holmes YR, Chenier TC, Joiner CH. Discordance between HbA1c and fructosamine: evidence for a glycosylation gap and its relation to diabetic nephropathy. Diabetes Care. 2003;26(1):163-167. [DOI] [PubMed] [Google Scholar]
- 9. Hudson PR, Child DF, Jones H, Williams CP. Differences in rates of glycation (glycation index) may significantly affect individual HbA1c results in type 1 diabetes. Ann Clin Biochem. 1999;36(pt 4):451-459. [DOI] [PubMed] [Google Scholar]
- 10. Nayak AU, Nevill AM, Bassett P, Singh BM. Association of glycation gap with mortality and vascular complications in diabetes. Diabetes Care. 2013;36(10):3247-3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hempe JM, Liu S, Myers L, et al. The hemoglobin glycation index identifies subpopulations with harms or benefits from intensive treatment in the ACCORD trial. Diabetes Care. 2015;38(6):1067-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lachin JM, Genuth S, Nathan DM, Rutledge BN. The hemoglobin glycation index is not an independent predictor of the risk of microvascular complications in the Diabetes Control and Complications Trial. Diabetes. 2007;56(7):1913-1921. [DOI] [PubMed] [Google Scholar]
- 13. van Steen SC, Woodward M, Chalmers J, et al. Haemoglobin glycation index and risk for diabetes-related complications in the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) trial. Diabetologia. 2018;61(4):780-789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shah VN, Kanapka LG, Akturk HK, et al. Time in range is associated with incident diabetic retinopathy in adults with type 1 diabetes: a longitudinal study. Diabetes Technol Ther. 2024;26:246-251. [DOI] [PubMed] [Google Scholar]
- 15. Tahara Y, Shima K. Kinetics of HbA1c, glycated albumin, and fructosamine and analysis of their weight functions against preceding plasma glucose level. Diabetes Care. 1995;18(4):440-447. [DOI] [PubMed] [Google Scholar]
- 16. Lenters-Westra E, Slingerland RJ. Six of eight hemoglobin A1c point-of-care instruments do not meet the general accepted analytical performance criteria. Clin Chem. 2010;56(1):44-52. [DOI] [PubMed] [Google Scholar]


