Abstract
Dilated perivascular spaces (PVSs) are common and easily recognized on imaging. However, rarer giant tumefactive PVSs (GTPVSs) can have unusual multilocular cystic configurations, and are often confused for other pathologic entities, including neoplasms, cystic infarctions, and neuroepithelial cysts. Because GTPVSs are scarcely encountered and even more infrequently operated upon, many radiologists are unaware of the imaging and pathologic features of these lesions. Here, a case of a resected GTPVS is presented, highlighting both its radiologic and histologic characteristics, and discussing how such lesions can be differentiated from their closest mimickers on imaging.
Keywords: Tumor, pathology, tumefactive
Brief history
The patient is a 27-year-old right-hand dominated female without notable past medical history who presented to an outside clinic with a 1-month history of dizziness and headaches. She described her headaches as occurring daily. Her headaches were holo-cephalic, involving her entire head. She reported variable headache severity, waxing and waning each day. In terms of her dizziness, the patient reported general unsteadiness that came on during times of strenuous activity. However, her symptoms tended to be transient, and responded well to rest. Her review of symptoms was otherwise negative; she denied focal neurologic deficits, seizures, or difficulties with mentation. She was sent for MRI imaging for further evaluation.
Imaging
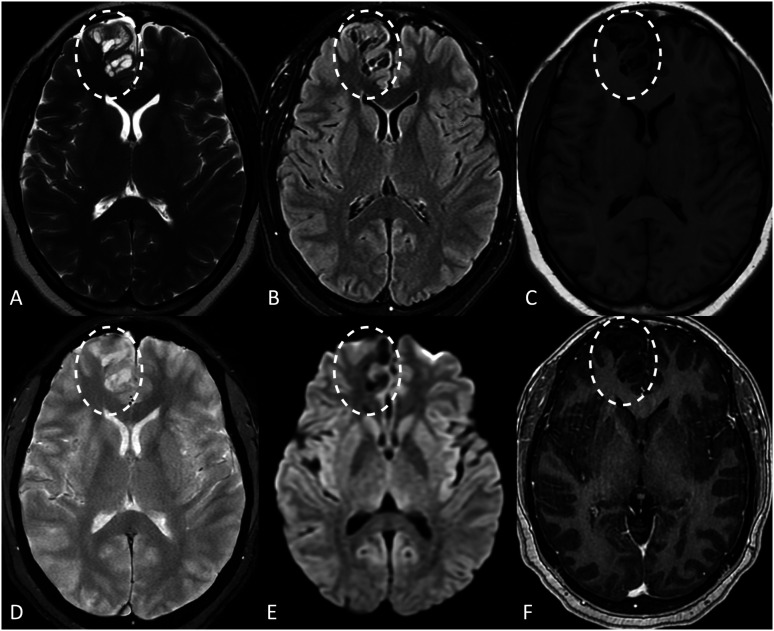
Imaging demonstrated a T2 hyperintense abnormality in the subcortical region of the medial right frontal lobe. The abnormality appeared multi-cystic and “bubbly” on T2-weighted images, with intra-lesional signal dropout on FLAIR sequences. There was no intra-lesional restricted diffusion or enhancement. The overlying cortex was preserved. Notably, there was no observable locoregional mass effect, and the signal in the adjacent parenchyma was normal (Figure 1). No other intracranial abnormalities were present.
Figure 1.
Imaging characteristics of the patient’s giant tumefactive perivascular space on MRI (dashed ovals on all images). On axial T2-weighted (a), FLAIR (b), and T1-weighted (c) images, there is a multi-cystic well-defined lesion in the medial right frontal lobe. Intra-cystic signal follows that of CSF on each sequence. There was no abnormal signal in the adjacent parenchyma. No intra-lesional calcifications or hemosiderin were noted on GRE (d), and no restricted diffusion was noted on DWI (e). The lesion also lacked pathologic enhancement (f).
At the outside clinic, the MRI was initiated interpreted as being compatible with a dysembryoplastic neuroepithelial tumor (DNET). On further review, the differential was broadened to encompass other low-grade neoplasms, including a gangliocytoma or a ganglioglioma; it was also mentioned that this could represent a cluster of perivascular cysts. Given the uncertainty of her diagnosis and her persistent headache, the decision was made to perform an excisional biopsy/resection of the abnormality.
Operative report
The patient was placed in a supine position in a Mayfield head rest, and a pre-procedure brain MRI was used for neuro-navigation. A craniotomy was completed directly over the right frontal lobe lesion. A neuro-navigational probe was used to guide the corticectomy and resection. Intra-operatively, abnormal tissue with intra-lesional cysts was encountered, corresponding with the pre-procedural MRI findings. At the completion of the procedure, it was felt that a subtotal resection had been performed. The operative cavity was then layered with fibular hemostatic agent, and the craniotomy bone flap was reattached to the skull.
Pathologic analysis
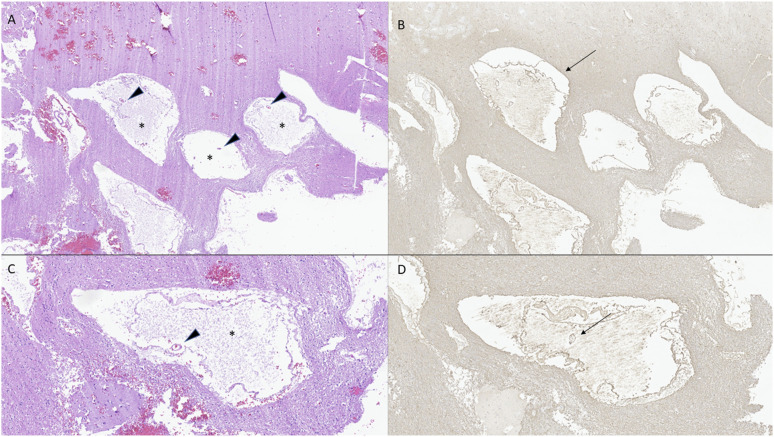
Histologic examination of the excision material revealed fragments of cerebral cortex and subcortical white matter, in which markedly dilated cystic spaces were noted to be scattered throughout the cortex (Figure 2). The surrounding cortex showed a mild degree of reactive gliosis, but no evidence of glial atypia, dysmorphic neurons, or acute inflammation was identified. Within dilated cystic spaces, small cortical capillaries were noted.
Figure 2.
H&E-stained sections (a,c) demonstrate markedly dilated perivascular spaces (asterisks) in cerebral cortex and white matter, harboring small cerebrovascular structures (arrowheads). GFAP immunohistochemistry (b, d) corroborates the presence of glia limitans delineating perivascular spaces (arrows).
Multiple features distinguished this process from other etiologies presenting as cystic lesions within the brain. In the differential spectrum of neoplasms that may radiographically mimic this process, DNET is notable for distinctive cytoarchitecture (the so-called “specific glioneuronal element”), in which columns of neoplastic glial cells arranged perpendicularly to the cortical surface are separated by pyramidal neurons suspended in a myxoid/mucoid matrix. Other low-grade neoplasms associated with cystic appearances, including multinodular and vacuolating neuronal tumor (MVNT), ganglioglioma, and pilocytic astrocytoma, are also defined by specific architectures. MVNT represents a neoplasm of neuronal elements, which are characteristically arranged in coalescent nodules within the deep cortex and superficial subcortical white matter, with characteristic vacuolization present within the background matrix of these coalescent nodules as well as within the cytoplasm of individual cells. Gangliogliomas are characterized by dual-population proliferation of morphologically atypical (“dysmorphic”) ganglion and glial cells. The neoplastic cell population in pilocytic astrocytomas is solely glial, but the neoplastic cells in this tumor type have a characteristic “biphasic” architecture, in which areas of tumor cells with compact elongated (“piloid”) processes alternate with areas of myxoid to microcystic tumor background. The present lesion, importantly, is devoid of any evidence of a neoplastic glial or ganglion/neuronal cell population, and shows no evidence of an organized architecture associated with cystic changes, rendering it consistent with a non-neoplastic process. 1
An important cause of cystic lesions in the brain includes those borne of infectious processes. In this differential spectrum, neurocysticercosis is histologically characterized by the creation of thin-walled cysts by the larva of Taenia solium. In this context, the cystic structure represents the infectious organism itself, in which anatomic components (including the scolex) are histologically identifiable. If the organism dies, the cyst may degenerate, with fibrotic thickening of the wall. In the present lesion, no evidence of an organism was identified to support this infectious process.
Developmental cysts of the central nervous system represent an important differential consideration in this imaging context, and can be broadly divided into three major groups (ectodermal, neuroectodermal, and endodermal) based upon embryologic origin, anatomic localization, and type of cyst lining. Neuroectodermal cysts demonstrate a lining derived from the respective CNS structure from which they derive: ependymal, arachnoid, or choroid plexus. Endodermal cysts are characterized by ciliated, simple to pseudostratified epithelium with goblet cells. Ectodermal cysts, namely, epidermoid and dermoid cysts, are histologically defined by squamous epithelial linings, the latter also including the presence of dermal skin appendage structures. In the present lesion, no epithelial or neuroectodermal lining was present to suggest a cyst within this spectrum of entities. 2
Remote ischemic events and infarct may produce cystic cavities within the cerebral cortex, following resorption and clearance of infarcted and necrotic parenchyma. Evidence of extensive parenchymal disruption and destruction will delineate these cavities, including rarefaction of the neuropil with associated axonal loss and spheroid formation. While a mild degree of reactive gliosis was noted of the present lesion, no concomitant surrounding parenchymal destruction was present to suggest a remote destructive event, as these cystic spaces in fact demonstrated a preserved glia limitans (as elucidated by GFAP immunohistochemistry).
Ultimately, the histopathologic features of the cystic structures seen in the present lesion, harboring small cortical capillaries, delineated by a preserved glia limitans and intact adjacent cortical parenchyma, and lacking a discernible neoplastic cell or parasitic organism component, were assessed as most consistent with giant tumefactive perivascular spaces.
Discussion
Perivascular spaces (PVSs), also known as Virchow–Robin spaces, are small cavities located along penetrating vessels. These spaces form a network throughout the brain that is part of the glymphatic system. 3 Although fluid-filled, they are nearly always microscopic and thus invisible on imaging. When greater than 2 mm in size, the spaces are considered “enlarged” or “dilated.” 4 Typically, dilated PVSs are located in the basal ganglia along lenticulostriate arteries (Type I), in the subcortical white matter along perforating medullary arteries (Type II), and in the midbrain (Type III).5,6 They are common; the estimated prevalence in a healthy population is 1.6%–4.8%. 7 Both their size and incidence increase with age. 8 Although dilated PVSs are considered benign, they are associated with numerous pathologies including dementia, hypertension multiple sclerosis, traumatic brain injury, sleeping difficulties, and neuropsychiatric disorders.5,9,10
The common forms of dilated PVS are easily recognized, incidentally discovered, and clinical unimportant, despite their aforementioned possible associations. 11 However, atypically large Virchow–Robin spaces are distinctly different in their appearance. These unusual PVSs can have bizarre cystic configurations that mimic pathologic processes such as tumors, cystic infarctions, or parasitic infections. When PVSs expand to the point of causing mass effect—or, by other definitions, to the point of exceeding 15 mm in diameter—they are referred to as giant tumefactive perivascular spaces (GTPVSs). 6
On CT, GTPVSs appear as cystic non-enhancing hypodense lesions, sometimes with an internal vessel noted on contrast-enhanced exams. 3 On MRI, the lesions are unilocular or multilocular cystic regions that match CSF intensity on all sequences.12,13 They are most commonly located in the midbrain and thalamus region. When hemispheric, cysts within a GTPVS tend to stretch and displace the overlying cortex. On sagittal and/or coronal images, the lesions may demonstrate a radiating linear morphology along perivascular spaces. In some cases, mild peri-lesional signal abnormalities on FLAIR images can be observed, possibly related to chronic ischemic changes related to mass effect.14,15 The lesions classically lack pathologic enhancement, restricted diffusion, or perfusional abnormalities. A recent study found that a perforating vessel was seen in over half of lesions. 16
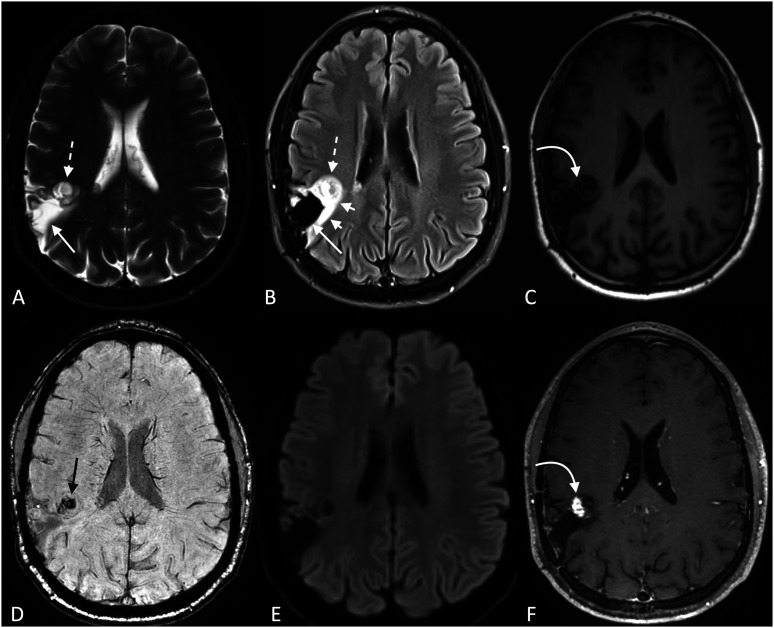
GTPVSs can often be confused for other pathologic processes, particularly cystic neoplasms such as DNETs or multinodular and vacuolating neuronal tumors (MVNTs), neuroepithelial cysts, parasitic cysts, and cystic infarctions. 17 However, multiple imaging features can help distinguish a GTPVS from its closest mimickers (Table 1). DNETs, for example, often involve the cortex, may have solid components and/or enhance, and demonstrate variable intra-cystic signal on FLAIR images. DNETs may also have peri-lesional hyperintense signal on FLAIR images—the so-called “Ring Sign”—while peri-lesional signal around GTPVSs tends to be absent or minimal (Figure 3). 18 MVNTs can also appear “bubbly,” though the intra-cystic signal does not suppress on FLAIR images. 19 Chronic cystic infarctions are associated with volume loss, are surrounded by gliosis, and involve the cortex if hemispheric. 20 Neuroepithelial cysts are mostly common unilocular. Neurocysticercosis tends to be multifocal, with a variable imaging appearance that evolves over the lifecycle of the infection.
Table 1.
Comparison of imaging features between giant tumefactive perivascular spaces (GTPVSs), dysembryoplastic neuroepithelial tumors (DNETs), and neuroepithelial cysts.
| GTPVS | DNET | Neuroepithelial cyst | |
|---|---|---|---|
| Typically multi-cystic or “bubbly” | + | + | - |
| Contents match CSF on all sequences | + | +/− | + |
| Intra-lesional enhancement | - | +/− | - |
| Solid components | - | +/− | - |
| Normal adjacent parenchyma | +/− | +/− | + |
| Typically involves cortex | - | + | - |
Figure 3.
Example of a dysembryoplastic neuroepithelial tumor (DNET), shown to highlight differences in imaging features between DNETs and giant tumefactive perivascular spaces (GTPVSs). In contrast to GTPVSs, some of the intratumoral cysts match CSF on T2 (a) and FLAIR (b) (long solid straight white arrows), while others do not (dashed white arrows). Peri-tumoral hyperintense signal is also notably present on FLAIR images (short solid white arrows). Some solid components appear to be present on pre-contrast T1 (c), which enhance on post-contrast images (f) (curved white arrows). In this solid region, blooming foci (either calcification or hemorrhage) is seen on SWI (d) (straight black arrow). No restricted diffusion is present on DWI (e).
Because GTPVSs are so infrequently resected, pathologic descriptions of these lesions are sparse. Histologic descriptions that available indicate pial-lined cysts delineated by reactive astrocytosis. 9 These features must be, by definition, devoid of evidence of a neoplastic cell population, infectious organism component, or parenchymal destruction suggestive of remote infarct or alternate causes of cystic encephalomalacia. Correlated with the characteristic imaging appearance, the histologic features of GTPVSs can aid in confirming the clinical and radiologic impression.
Patients commonly present with findings that are not attributable to GTPVSs. 12 When symptomatic, the clinical presentation of GTPVS varies on the size and location of the lesions. The most common symptom, present in nearly half of patients, is headache, though it has not been proven that these are secondary to GTPVSs. Dizziness, visual changes, cranial neuropathy, and imbalance have also been reported, though it is doubtful that such symptoms can be convincingly attributed to the lesions in most cases. 12 In rare cases, mass effect can lead to obstructive hydrocephalus. 9 In asymptomatic patients, most clinicians elect to monitor the lesions with imaging.
In the case presented here, non-invasive monitoring with imaging certainly could have been an option; not all patients with suspected GTPVs need biopsy and/or resection. Nevertheless, the imaging in this case was unusual, and a low-grade glioma was a distinct possibility based on the MRI features. This was further complicated by the lack of prior imaging that could be used to establish stability over time. Given this uncertainty and the possibility of an underlying neoplasm, it was considered reasonable to proceed with surgical resection of the abnormality. However, given the patient’s relatively vague symptoms on presentation, is also would have been reasonable to conclude that this was an incidental finding, and that it could have been monitored with serial imaging.
Most GTPVSs are stable on long-term follow-up, and in exceedingly rare cases can even spontaneously regress.21,22 In rare cases, GTPVSs can also increase in size, either spontaneously or following surgery, even necessitating a repeat operation. 23 According to Kwee et al., however, type 2 GTPVs (as seen in this case) do not require further follow-up. Thus, no imaging surveillance of the post-operative site is planned. 23 To date, the patient presented in this case has reported no recurrence of her symptoms, and no new neurologic deficits. She is expected to make a full recovery.
Case summary
• GTPVSs are benign lesions that may mimic the imaging appearance of other pathologic entities, including DNETs, chronic infarctions, and neuroepithelial cysts.
• The most common symptom of GTPVSs is headache. Typically, however, the lesions are thought to be incidental, with clinical presentations that are not attributable to the findings.
• On imaging, GTPVSs typically appear as non-enhancing, multi-cystic lesions without involvement of the overlying cortex. Peri-lesional signal abnormalities, if present, tend to be minimal.
• GTPVSs are histologically characterized by enlarged cystic spaces exhibiting an external glia limitans (corresponding to the pial surface of the perivascular space), and, by definition, lack evidence of a neoplastic cell population, infectious organism component, or parenchymal destruction.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs
John C Benson https://orcid.org/0000-0002-4038-5422
Ian T Mark https://orcid.org/0000-0002-4036-2992
Ajay A Madhavan https://orcid.org/0000-0003-1794-4502
Derek R Johnson https://orcid.org/0000-0002-4217-5517
References
- 1.Board WC of TE . Central nervous system Tumours, Lyon: International Agency for Research; on Cancer. https://publications.iarc.fr/Book-And-Report-Series/Who-Classification-Of-Tumours/Central-Nervous-System-Tumours-2021 Accessed 18 August 2023. [Google Scholar]
- 2.Hirano A, Hirano M. Benign cysts in the central nervous system: neuropathological observations of the cyst walls. Neuropathology 2004; 24(1): 1–7. DOI: 10.1111/j.1440-1789.2003.00526.x. [DOI] [PubMed] [Google Scholar]
- 3.Conforti R, Capasso R, Franco D, et al. Giant tumefactive perivascular space: advanced fusion MR imaging and tractography study-a case report and a systematic review. Diagn Basel Switz 2023; 13(9): 1602. DOI: 10.3390/diagnostics13091602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi Y, Nam Y, Choi Y, et al. MRI-visible dilated perivascular spaces in healthy young adults: a twin heritability study. Hum Brain Mapp 2020; 41(18): 5313–5324. DOI: 10.1002/hbm.25194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwee RM, Kwee TC. Virchow-Robin spaces at MR imaging. Radiographics 2007; 27(4): 1071–1086. DOI: 10.1148/rg.274065722. [DOI] [PubMed] [Google Scholar]
- 6.Woo PYM, Cheung E, Zhuang JTF, et al. A giant tumefactive perivascular space: a rare cause of obstructive hydrocephalus and Monoparesis. Asian J Neurosurg 2018; 13(4): 1295–1300. DOI: 10.4103/ajns.AJNS_108_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bilginer B, Narin F, Hanalioglu S, et al. Virchow-Robin spaces cyst. Childs Nerv Syst 2013; 29(12): 2157–2162. DOI: 10.1007/s00381-013-2240-3. [DOI] [PubMed] [Google Scholar]
- 8.Heier LA, Bauer CJ, Schwartz L, et al. Large Virchow-Robin spaces: MR-clinical correlation. AJNR Am J Neuroradiol 1989; 10(5): 929–936. [PMC free article] [PubMed] [Google Scholar]
- 9.Al Abdulsalam H, Alatar AA, Elwatidy S. Giant tumefactive perivascular spaces: a case report and Literature review. World Neurosurg 2018; 112: 201–204. DOI: 10.1016/j.wneu.2018.01.144. [DOI] [PubMed] [Google Scholar]
- 10.Baril AA, Pinheiro AA, Himali JJ, et al. Lighter sleep is associated with higher enlarged perivascular spaces burden in middle-aged and elderly individuals. Sleep Med 2022; 100: 558–564. DOI: 10.1016/j.sleep.2022.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Idiculla PS, Gurala D, Siddiqui JH. Giant tumefactive perivascular spaces: an incidental finding. Acta Neurol Belg 2020; 120(6): 1443–1444. DOI: 10.1007/s13760-020-01481-5. [DOI] [PubMed] [Google Scholar]
- 12.Salzman KL, Osborn AG, House P, et al. Giant tumefactive perivascular spaces. AJNR Am J Neuroradiol 2005; 26(2): 298–305. [PMC free article] [PubMed] [Google Scholar]
- 13.Lahiri AK, Slaney PL. Tumefactive perivascular spaces: a rare incidental finding. Pract Neurol 2017; 17(3): 229–230. DOI: 10.1136/practneurol-2017-001596. [DOI] [PubMed] [Google Scholar]
- 14.Lakhani DA, Joseph J. Giant tumefactive perivascular spaces. Radiology 2023; 307(4): e222559. DOI: 10.1148/radiol.222559. [DOI] [PubMed] [Google Scholar]
- 15.Shiratori K, Mrowka M, Toussaint A, et al. Extreme, unilateral widening of Virchow-Robin spaces: case report. Neuroradiology 2002; 44(12): 990–992. DOI: 10.1007/s00234-002-0840-9. [DOI] [PubMed] [Google Scholar]
- 16.Ayyildiz V, Koksal A, Taydas O, et al. Contribution of advanced MRI to the diagnosis of giant tumefactive perivascular spaces. Acta Radiol 2022; 63(11): 1554–1562. DOI: 10.1177/02841851211047240. [DOI] [PubMed] [Google Scholar]
- 17.Sankararaman S, Velayuthan S, Ambekar S, et al. Giant tumefactive perivascular spaces: a further case. J Pediatr Neurosci 2013; 8(2): 108–110. DOI: 10.4103/1817-1745.117837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parmar HA, Hawkins C, Ozelame R, et al. Fluid-attenuated inversion recovery ring sign as a marker of dysembryoplastic neuroepithelial tumors. J Comput Assist Tomogr 2007; 31(3): 348–353. DOI: 10.1097/01.rct.0000243453.33610.9d. [DOI] [PubMed] [Google Scholar]
- 19.Nunes RH, Hsu CC, da Rocha AJ, et al. Multinodular and vacuolating neuronal tumor of the cerebrum: a new “leave me alone” lesion with a characteristic imaging pattern. AJNR Am J Neuroradiol 2017; 38(10): 1899–1904. DOI: 10.3174/ajnr.A5281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nour M, Liebeskind DS. Imaging of cerebral ischemia: from acute stroke to chronic disorders. Neurol Clin 2014; 32(1): 193–209. DOI: 10.1016/j.ncl.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stephens T, Parmar H, Cornblath W. Giant tumefactive perivascular spaces. J Neurol Sci 2008; 266(1-2): 171–173. DOI: 10.1016/j.jns.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 22.Eluvathingal Muttikkal TJ, Raghavan P. Spontaneous regression and recurrence of a tumefactive perivascular space. NeuroRadiol J 2014; 27(2): 195–202. DOI: 10.15274/NRJ-2014-10034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwee RM, Kwee TC. Tumefactive Virchow-Robin spaces. Eur J Radiol 2019; 111: 21–33. DOI: 10.1016/j.ejrad.2018.12.011. [DOI] [PubMed] [Google Scholar]