Abstract
Background
Prolonged venous transit (PVT), defined as presence of time-to-maximum 10 s within the superior sagittal sinus (SSS) and/or torcula, is a novel, qualitatively assessed computed tomography perfusion surrogate parameter of venous outflow with potential utility in pretreatment acute ischemic stroke imaging for neuroprognostication. We aim to characterize the correlation between PVT and neurological functional outcomes in thrombectomy-treated patients.
Methods
A prospectively-collected database of large vessel occlusion acute ischemic stroke patients treated with thrombectomy was retrospectively analyzed. Spearman’s rank correlation coefficient and point-biserial correlations were performed between PVT status (i.e., no region, either SSS or torcula, or both), 90-day modified Rankin score (mRS), mortality (mRS 6), and poor functional outcome (mRS 4-6 vs 0-3).
Results
Of 128 patients, correlation between PVT and 90-day mRS ( = 0.35, p < 0.0001), mortality (r = 0.26, p = 0.002), and poor functional outcome (r = 0.27, p = 0.002) were significant.
Conclusion
There is a modest, significant correlation between PVT and severity of neurological functional outcome. Consequently, PVT is an easily-ascertained, qualitative metric that may be useful as an adjunct for anticipating a patient’s clinical course. Future analyses will determine the significance of incorporating PVT in clinical decision-making.
Keywords: Cerebrovascular circulation, computed tomography perfusion, neuroimaging, thrombectomy, treatment outcome, venous outflow
Introduction
Computed tomography perfusion (CTP) characterizes the physiological state of brain parenchyma. 1 In cerebral ischemia, compromised perfusion dynamics, which drives the extent of infarction, are captured by CTP. 1 Alongside altered arterial inflow and overall collateral status, venous outflow (VO) is thought to be strongly influential in stroke patient outcomes. 2
We propose a novel surrogate marker of delayed VO, reflecting the status of both superficial and deep VO drainage patterns. We define prolonged venous transit (PVT) as a multi-regional perfusion marker obtained from post-processed time-to-maximum (Tmax) maps derived from CTP. Prolonged transit delay, defined as Tmax 10s, 3 is thought to indicate compromised VO, and it can be qualitatively assessed from Tmax maps within specific venous structures. Superficial VO is approximated by assessing blood flow in the posterior superior sagittal sinus (SSS) 2 ; deep VO is approximated by assessing perfusion in the torcula. 4 Severity of PVT is characterized by whether it is absent in both the SSS and torcula, is present in one of the regions, or is present in both regions. Consequently, PVT is a novel, qualitative imaging paradigm collectively capturing the statuses of both superficial and VO tracts.
To assess the potential utility of this novel marker, we aim to characterize the association between PVT and neurological functional outcomes in endovascular thrombectomy (EVT)-treated stroke patients.
Methods
De-identified data will be made available upon reasonable request. This study was approved by the Institutional Review Board with waiver of informed consent. This study was performed in accordance with the ethical standards outlined in the 1964 Declaration of Helsinki. The results were reported in accordance to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.
A prospectively-recorded registry of acute ischemic stroke patients at three clinical sites was retrospectively reviewed for inclusion with the following criteria: (a) acute ischemic stroke by large vessel occlusion (distal intracranial internal carotid artery, M1 segment of the middle cerebral artery, and proximal M2 segment of the middle cerebral artery); (b) treated successfully with EVT; (c) available pretreatment CTP imaging; and (d) available 90-day modified Rankin score (mRS). Patients were excluded from analysis if significant premorbid disability (mRS >2) was present.
Additional demographic data collected included: age, sex, comorbidities associated with stroke risk (i.e., hypertension, diabetes mellitus, dyslipidemia, coronary artery disease, prior stroke/transient ischemic attack, atrial fibrillation, and tobacco use), admission National Institutes of Health Stroke Scale (NIHSS), premorbid mRS, occlusion location, occlusion laterality, treatment with IV thrombolysis (i.e., alteplase/tenecteplase), successful EVT (modified treatment in cerebral infarction [mTICI] 2 B/2 C/3), and discharge NIHSS.
Ascertaining PVT
Specifications for CTP acquisition are in accordance with the protocol described previously in Yedavalli et al. 5 Qualitative Tmax maps were generated using the post-processing function of commercially-available RAPID software (IschemaView, Menlo Park, CA, USA). These maps were retrospectively reviewed by an experienced neuroradiologist to assess whether SSS and/or torcula regions had prolonged venous transit delay of Tmax 10s.
Statistical analysis
Patients were grouped depending on whether PVT was evidenced in (1) neither the SSS nor torcula, (2) either the SSS or torcula, and (3) both the SSS and torcula. The primary outcome measure was the 90-day mRS score. Secondary sensitivity analyses were for the outcomes of 90-day mortality (mRS (6) and poor functional outcomes (90-day mRS 4-6 vs 0-3). Continuous variables were summarized as medians with interquartile range (IQR), and categorical variables were reported as frequencies and percentages. Spearman’s rank correlation coefficient was calculated between PVT and 90-day mRS. Point-biserial correlation coefficients were performed between PVT, mortality, and poor functional outcomes. Distribution of data was visualized with scatterplots with nonparametric densities. Missing data was minimal and not imputed. Significance was prespecified at 0.05. Statistical analyses were performed using JMP v17 (SAS Institute Inc, Carey, NC, USA).
Results
Of 128 patients included, the median age was 72 years (IQR 63-81), and 59.4% were female (Table 1). The most common comorbidity was hypertension (80.5%) (Table 1). Median NIHSS upon admission was 15 (IQR 11–20), and median premorbid mRS was 0 (0-1) (Table 1). The PVT was not present in 68.0% of patients, while 17.2% had PVT in one region only and 14.8% had PVT in both regions (Table 1). All patients achieved successful reperfusion (mTICI 2 B/2 C/3) (Table 1). Median NIHSS upon discharge was 4 (IQR 2-10) (Table 1). At 90-day follow-up, mortality (mRS 6) occurred in 18.8% of patients, and 37.5% of patients had poor functional outcome (mRS 4-6) (Table 1).
Table 1.
Baseline demographic and clinical characteristics.
| Variable | n = 128 |
|---|---|
| Demographics | |
| Age, median (IQR) | 72 (63-81) |
| Female, no. (%) | 76 (59.4%) |
| Past medical history, no. (%) | |
| Hypertension | 103 (80.5%) |
| Diabetes mellitus | 39 (30.5%) |
| Dyslipidemia | 68 (53.1%) |
| Coronary artery disease | 69 (53.9%) |
| Prior stroke | 26 (20.3%) |
| Atrial fibrillation | 56 (43.8%) |
| Tobacco use | 53 (41.4%) |
| Admission NIHSS, median (IQR) | 15 (11-20) |
| Premorbid mRS, median (IQR) | 0 (0-1) |
| Occlusion location, no. (%) | |
| Distal intracranial carotid artery | 4 (3.1%) |
| M1 middle cerebral artery | 95 (74.2%) |
| Proximal M2 middle cerebral artery | 29 (22.7%) |
| Left-sided occlusion, no. (%) | 69 (53.9%) |
| Prolonged venous transit, no. (%) | |
| Neither SSS or torcula | 87 (68.0%) |
| SSS Only or torcula only | 22 (17.2%) |
| Both SSS and torcula | 19 (14.8%) |
| Intervention and Recovery | |
| Thrombectomy alone, no. (%) | 87 (68.0%) |
| IV Thrombolysis + thrombectomy, no. (%) | 41 (32.0%) |
| mTICI, no. (%) | |
| 2B | 35 (27.3%) |
| 2C | 23 (18.0%) |
| 3 | 70 (54.7%) |
| Discharge NIHSS, median (IQR) | 4 (2-10) |
| 90-day mRS, no. (%) | |
| 0 | 20 (15.6%) |
| 1 | 22 (17.2%) |
| 2 | 25 (19.5%) |
| 3 | 13 (10.2%) |
| 4 | 19 (14.8%) |
| 5 | 5 (3.9%) |
| 6 | 24 (18.8%) |
Abbreviations: IQR = interquartile range, NIHSS = National Institutes of Health Stroke Scale, mRS = modified Rankin score, SSS = superior sagittal sinus, mTICI = modified treatment in cerebral infarction score.
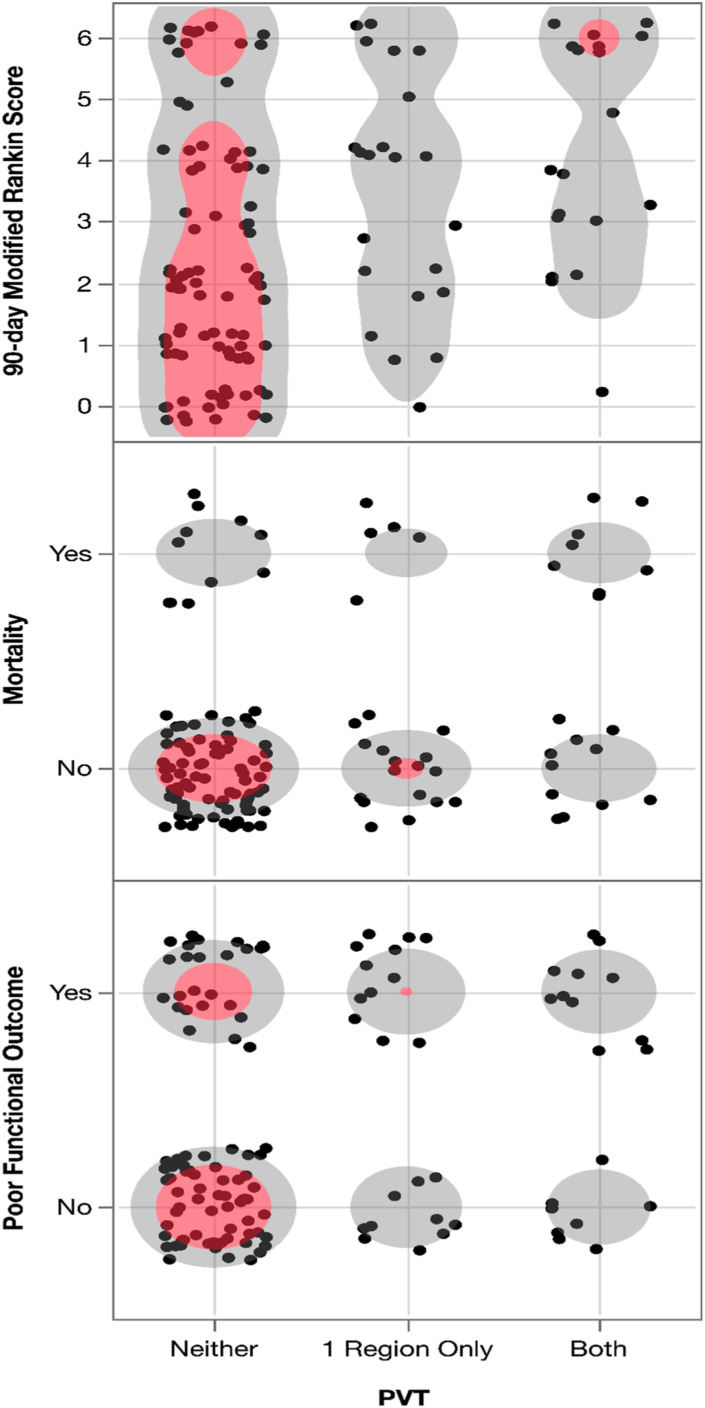
The correlation between PVT and 90-day mRS was significant ( = 0.35, p < 0.0001) (Figure 1). The significance of the correlation persisted when PVT was correlated with mortality (r = 0.26, p = 0.002) (Figure 1). The correlation between PVT and poor functional outcome was similarly significant (r = 0.27, p = 0.002) (Figure 1).
Figure 1.
Scatterplot showing prolonged venous transit (PVT) with (1) 90-day modified Rankin score (mRS), (2) 90-day mortality (mRS 6), and (3) poor functional outcome (mRS 4-6). The PVT is defined as time-to-maximum 10s volume in neither the superior sagittal sinus (SSS) nor torcula, in either the SSS or torcula, or in both regions.
Discussion
The PVT was statistically significantly correlated with 90-day neurological functional outcome. Hence, the PVT has potential utility in serving as a novel adjunct pretreatment imaging parameter for assessing functional recovery in stroke patients. Although the strength of the association was modest, it is important to note that several factors influence the post-treatment clinical course for stroke patients.6,7 Further analyses are required to ascertain the strength and independence of PVT as a pretreatment prognostic marker. Nonetheless, the relationship established between PVT and functional recovery serves as a foundation for future explorations utilizing this new perfusion parameter.
The correlation between PVT and poor functional recovery is consistent with prior studies that examined superficial and deep regional VO separately. For example, Sue et al. demonstrated delayed VO peak time in the SSS was independently associated with unfavorable recovery despite successful reperfusion therapy with EVT. 2 Similarly, Adusmulli et al. showed that VO peak time at the confluence of sinuses (i.e., torcula) was strongly correlated with cortical vein opacification scores (COVES), previously shown to correspond to post-EVT functional recovery.4,8 Separately, analyzing perfusion dynamics in each region has been strongly associated with post-EVT functional recovery.9–12
The PVT was derived from combining prior independent observations of the association between hemodynamics in the superficial and deep VO, respectively, and functional outcomes. Amongst the abundance of the ever-expanding list of clinical, imaging, and interventional parameters predicting clinical outcomes,2,4,13–24 the PVT offers a paradigm that consolidates and simplifies VO assessment into a single, easy-to-ascertain measure. In the era of simplification, future studies with similar objectives are warranted to consolidate valuable assessment components into user-friendly, cumulative indices for enhanced practical implementation into clinical workflows governing acute stroke care management.
Limitations
Given the retrospective nature of the analysis, this study is subject to limitations inherent to the study design. Several factors affect the degree of neurorehabilitation in the 90-day period, including comorbidities and discharge disposition 6 ; hence, the unadjusted effect of confounding likely explains the mild-to-moderate strength of findings demonstrated in this analysis. Moreover, PVT does not account for compensatory venous drainage from the nonaffected side, which may affect the degree of prolonged venous transit on the affected side. Future analyses in larger cohorts will be able to adequately control for these confounders. Given that this was a demonstration of a novel concept in a sample of moderate size, external validation in a larger cohort is needed to validate generalizability of findings.
Conclusion
The PVT is a novel, qualitative perfusion imaging marker reflecting severity of compromised cerebral VO in key regions of both superficial and deep VO tracts. There is a mild, significant correlation between PVT and neurological functional outcome. Consequently, PVT may be a useful adjunct measure in neuroprognostication efforts. Future analyses will characterize the performance of PVT within current clinical decision-making paradigms.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Drs. Vivek Yedavalli, Jeremy Heit, and Gregory Albers are consultants for iSchemaView (Menlo Park, CA), not related to submitted work.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical statement
Ethical approval
This study was approved by the institutional review board of the Johns Hopkins School of Medicine.
Informed consent
This retrospective study was approved by the IRB with waiver of informed consent.
ORCID iDs
Vivek S Yedavalli https://orcid.org/0000-0002-2450-4014
Dhairya A Lakhani https://orcid.org/0000-0001-7577-1887
Manisha Koneru https://orcid.org/0000-0001-5012-6793
Licia Luna https://orcid.org/0000-0003-3539-4831
Adam A Dmytriw https://orcid.org/0000-0003-0131-5699
Max Wintermark https://orcid.org/0000-0002-6726-3951
Data availability statement
De-identified data will be made available upon reasonable request to the corresponding author.*
References
- 1.Heit JJ, Wintermark M. Perfusion computed tomography for the evaluation of acute ischemic stroke: strengths and pitfalls. Stroke 2016; 47: 1153–1158. DOI: 10.1161/STROKEAHA.116.011873 [DOI] [PubMed] [Google Scholar]
- 2.Su M, Chen Z, Chen X, et al. Venous flow profiles on perfusion CT are associated with futile recanalization after thrombectomy. Neuropsychiatric Dis Treat 2022; 18: 933–942. DOI: 10.2147/NDT.S360626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mlynash M, Lansberg MG, De Silva DA, et al. Refining the definition of the malignant profile: insights from the DEFUSE-EPITHET pooled data set. Stroke 2011; 42: 1270–1275. DOI: 10.1161/STROKEAHA.110.601609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adusumilli G, Christensen S, Yuen N, et al. CT perfusion to measure venous outflow in acute ischemic stroke in patients with a large vessel occlusion. 2023. DOI: 10.1136/jnis-2023-020727 [DOI] [PubMed] [Google Scholar]
- 5.Yedavalli V, Hamam O, Mohseni A, et al. Pretreatment brain CT perfusion thresholds for predicting final infarct volume in distal medium vessel occlusions. 2023. DOI: 10.1111/jon.13142 [DOI] [PubMed] [Google Scholar]
- 6.Simić-Panić D, Bošković K, Milićević M, et al. The impact of comorbidity on rehabilitation outcome after ischemic stroke. Acta Clin Croat 2018; 57: 5–15. DOI: 10.20471/acc.2018.57.01.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vázquez-Guimaraens M, Caamaño-Ponte JL, Seoane-Pillado T, et al. Factors related to greater functional recovery after suffering a stroke. Brain Sci 2021; 11. DOI: 10.3390/brainsci11060802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffman H, Ziechmann R, Swarnkar A, et al. Cortical vein opacification for risk stratification in anterior circulation endovascular thrombectomy. J Stroke Cerebrovasc Dis 2019; 28: 1710–1717. DOI: 10.1016/j.jstrokecerebrovasdis.2019.02.016 [DOI] [PubMed] [Google Scholar]
- 9.Faizy TD, Kabiri R, Christensen S, et al. Favorable venous outflow profiles correlate with favorable tissue-level collaterals and clinical outcome. Stroke 2021; 52: 1761–1767. DOI: 10.1161/STROKEAHA.120.032242 [DOI] [PubMed] [Google Scholar]
- 10.Faizy TD, Kabiri R, Christensen S, et al. Association of venous outflow profiles and successful vessel reperfusion after thrombectomy. Neurology 2021; 96: e2903. DOI: 10.1212/WNL.0000000000012106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Horn N, Heit JJ, Kabiri R, et al. Cerebral venous outflow profiles are associated with the first pass effect in endovascular thrombectomy. J Neurointerventional Surg 2022; 14: 1056–1061. DOI: 10.1136/neurintsurg-2021-018078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heitkamp C, Winkelmeier L, Heit JJ, et al. Unfavorable cerebral venous outflow is associated with futile recanalization in acute ischemic stroke patients. Eur J Neurol 2023; 30: 2684–2692. DOI: 10.1111/ene.15898 [DOI] [PubMed] [Google Scholar]
- 13.Olivot JM, Mlynash M, Inoue M, et al. Hypoperfusion intensity ratio predicts infarct progression and functional outcome in the DEFUSE 2 Cohort. Stroke 2014; 45: 1018–1023. DOI: 10.1161/STROKEAHA.113.003857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rao VL, Mlynash M, Christensen S, et al. Collateral status contributes to differences between observed and predicted 24-h infarct volumes in DEFUSE 3. J Cerebr Blood Flow Metabol 2020; 40: 1966–1974. DOI: 10.1177/0271678X20918816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan Z, Parsons M, Bivard A, et al. Comparison of computed tomography perfusion and multiphase computed tomography angiogram in predicting clinical outcomes in endovascular thrombectomy. Stroke 2022; 53: 2926–2934. DOI: 10.1161/STROKEAHA.122.038576 [DOI] [PubMed] [Google Scholar]
- 16.Fainardi E, Busto G, Rosi A, et al. Tmax volumes predict final infarct size and functional outcome in ischemic stroke patients receiving endovascular treatment. Ann Neurol 2022; 91: 878–888. DOI: 10.1002/ana.26354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tonetti DA, Desai SM, Casillo S, et al. Successful reperfusion, rather than number of passes, predicts clinical outcome after mechanical thrombectomy. J Neurointerventional Surg 2020; 12: 548–551. DOI: 10.1136/neurintsurg-2019-015330 [DOI] [PubMed] [Google Scholar]
- 18.O’Connor KP, Hathidara MY, Danala G, et al. Predicting clinical outcome after mechanical thrombectomy: the GADIS (gender, age, diabetes mellitus history, infarct volume, and current smoker [corrected]) score. World Neurosurg 2020; 134: e1130–e1142. DOI: 10.1016/j.wneu.2019.11.127 [DOI] [PubMed] [Google Scholar]
- 19.Peisker T, Vaško P, Mikulenka P, et al. Clinical and radiological factors predicting stroke outcome after successful mechanical intervention in anterior circulation. Eur Heart J Suppl 2022; 24: B48–B52. DOI: 10.1093/eurheartjsupp/suac010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zaidat OO, Castonguay AC, Linfante I, et al. First pass effect: a new measure for stroke thrombectomy devices. Stroke 2018; 49: 660–666. DOI: 10.1161/STROKEAHA.117.020315 [DOI] [PubMed] [Google Scholar]
- 21.Baek J-H, Kim BM, Heo JH, et al. Number of stent retriever passes associated with futile recanalization in acute stroke. Stroke 2018; 49: 2088–2095. DOI: 10.1161/STROKEAHA.118.021320 [DOI] [PubMed] [Google Scholar]
- 22.Ozkara BB, Karabacak M, Hamam O, et al. Prediction of functional outcome in stroke patients with proximal middle cerebral artery occlusions using machine learning models. J Clin Med Res 2023; 12. DOI: 10.3390/jcm12030839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arthur KC, Huang S, Gudenkauf JC, et al. Assessing the relationship between LAMS and CT perfusion parameters in acute ischemic stroke secondary to large vessel occlusion. J Clin Med Res 2023; 12. DOI: 10.3390/jcm12103374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yedavalli V, Kihira S, Shahrouki P, et al. CTP-based estimated ischemic core: a comparative multicenter study between Olea and RAPID software. J Stroke Cerebrovasc Dis 2023; 32: 107297. DOI: 10.1016/j.jstrokecerebrovasdis.2023.107297 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
De-identified data will be made available upon reasonable request to the corresponding author.*