Abstract
Background:
The Glycemia Risk Index (GRI) describes the quality of glycemic control, emphasizing extreme hypoglycemia and hyperglycemia more than less extreme values. However, a pregnancy-specific GRI (pGRI), tailored to the tighter target glucose range required during pregnancy, has not been established.
Methods:
We retrospectively evaluated clinical, metabolic, and Continuous Glucose Monitoring (CGM) data across pregnancy in women with insulin-treated diabetes, managed between September 2021 and March 2024 at the University Hospital of Pisa. First and second levels of hyperglycemia (TAR1: 140-180 mg/dL, TAR2: >180 mg/dL) and hypoglycemia (TBR1: 63-54 mg/dL, TBR2: <54 mg/dL) were used to calculate the pGRI at each trimester. Logistic regression analysis investigated the association between pGRI and risk of at least one adverse neonatal outcome (among preterm delivery, macrosomia, large for gestational age, small for gestational age, neonatal hypoglycemia, neonatal jaundice, and neonatal intensive care unit admission).
Results:
Of 45 pregnant women, 25 (56%) experienced at least one adverse neonatal outcome. In the third trimester, women with adverse outcomes had significantly higher total TAR (26 [12-32]% vs 10 [4-23]%, P = .018) and lower TIR (71 [64-83]% vs 88 [75-92]%, P = .007). Specifically, the difference was notable in TAR2 (6 [2-15]% vs 1 [0-4]%, P = .004), whereas TAR1 was comparable between the 2 groups. Accordingly, third trimester pGRI was higher in women with adverse neonatal outcomes (38 [18-49]% vs 18 [10-31]%, P = .013) and, at logistic regression, slightly but significantly increased the risk of adverse neonatal outcomes (1.044 [1.004-1.086], P = .024).
Conclusions:
Pregnant women with insulin-treated diabetes reporting adverse neonatal outcomes spent more time in hyperglycemia, particularly in extreme hyperglycemia. Therefore, the level of hyperglycemia should always be assessed during pregnancy. The pGRI, emphasizing extreme hyperglycemia, may be a novel comprehensive tool for assessing the risk of adverse neonatal outcomes.
Keywords: adverse neonatal outcomes, diabetes, Glycemic Risk Index, GRI, hyperglycemia level, pregnancy
Introduction
Pregnant women with diabetes have an increased risk of adverse maternal and neonatal outcomes, largely related to the degree of maternal hyperglycemia. 1 Therefore, in these women, achieving and maintaining euglycemia as safely as possible, prior to conception and throughout gestation, is necessary to improve pregnancy outcomes. 2
The use of Continuous Glucose Monitoring (CGM) systems in pregnant women with type 1 diabetes (T1D) may optimize maternal glycemic control, decreasing the risk of adverse neonatal outcomes as large for gestational age (LGA) infants, neonatal hypoglycemia, and neonatal intensive care unit (NICU) admission. 3
Benefits of CGM in pregnant women with type 2 diabetes (T2D) or gestational diabetes (GDM) treated with intensive insulin therapy are less evident. 4
The International Consensus of Time In Range (TIR) 5 established a tight desirable glucose range, between 63 and 140 mg/dL, for pregnant women with diabetes. Pregnant women with T1D should spend at least 70% of their time in this range, with less than 25% of glucose values above 140 mg/dL (time above range [TAR]), and less than 4% below 63 mg/dL (time below range [TBR]), including less than 1% of severe hypoglycemia (<54 mg/dL). Due to limited evidence for pregnant women with T2D and GDM, the appropriate percentage of TIR, TAR, and TBR in these women has not been determined yet. Moreover, despite the linear association between maternal glucose values and adverse neonatal outcomes, 1 current recommendations do not identify different levels of hyperglycemia in pregnant women with diabetes. However, in several recent studies on pregnant women with T1D, the percentage of severe hyperglycemia (TAR > 180 mg/dL) is assessed together with the total TAR (>140 mg/dL).6-8
Indeed, even outside of pregnancy, extreme hypoglycemia and hyperglycemia have greater impact on average glycemic control of people with diabetes, compared with less extreme values. Recently, a composite CGM metric, the Glycemic Risk Index (GRI), has been identified. The GRI describes the quality of glycemic control in adults with diabetes, weighting very low (TBR < 54 mg/dL) and very high (TAR >250 mg/dL) glucose values more than less extreme values (TBR 70-54 mg/dL and TAR 180-250 mg/dL)1. Based on the opinion of expert clinicians, the GRI gives more weight to the hypoglycemic than the hyperglycemic component, as indicated by the formula used to its calculation—GRI = (3.0 × Very Low) + (2.4 × Low) + (1.6 × Very High) + (0.8 × High). 9
Previously, other metrics derived from CGM have been described to assess the quality of glycemic control in individuals with diabetes.10,11 It has been shown that the GRI is strongly correlated to other CGM-derived metrics and to glycemic variability.12,13 Growing evidence supports the association between GRI and diabetic complications as retinopathy 14 and nephropathy 15 in people with T2D. Recent findings support the use of GRI to assess glycemic control also in pediatric patients with T1D. 16
Currently, no study has assessed the use of GRI in pregnant women with diabetes. Due to the tighter target glucose range required during pregnancy, a pregnancy-specific GRI (pGRI) should be established, by using pregnancy-specific thresholds for level 1 and level 2 of hypoglycemia and hyperglycemia. Despite specific recommendations on the 2 levels of hyperglycemia in pregnancy are lacking, a threshold of 180 mg/dL for the higher level of hyperglycemia seems reasonable. Therefore, the aim of this study was to assess the relationship between different levels of hyperglycemia (level 1: 140-180 mg/dL and level 2: >180 mg/dL) and adverse neonatal outcomes. The efficacy of pGRI in predicting the risk of adverse neonatal outcomes was then assessed.
Materials and Methods
Study Design and Subjects
An observational, retrospective, single-center study was conducted on pregnant women with insulin-treated diabetes (T1D, T2D, or GDM), managed during gestation and who delivered at the University Hospital of Pisa a live singleton infant between September 2021 and March 2024. All women used a CGM system (Abbott—FreeStyle Libre 2 or Dexcom G6) for glucose monitoring during pregnancy. In 3 T1D women, the CGM (Dexcom G6) was integrated with an insulin pump (Tandem Control IQ); all the other women were on multiple daily insulin injections.
Women with pregestational diabetes used CGM from the first trimester of pregnancy, or from the preconception period in case of pregnancy planning, whereas those with GDM from the second or third trimester, when intensive insulin treatment was required. In Italy, the National Health System provides free access to new technologies for people with diabetes. Specifically, CGM is reimbursed for women with pregestational diabetes starting from the phase of pregnancy planning. Moreover, in Tuscany region, women with GDM may also have access to it if intensive insulin treatment is required.
During pregnancy, women were managed by a multidisciplinary team (including expert diabetologist, dietitian, nurse, and obstetrician) as for standard of care. In particular, CGM-data, insulin therapy, blood pressure, and weight were assessed every 2 to 3 weeks across gestation on outpatient visits. At the third trimester of pregnancy, hemoglobin A1c (HbA1c) and lipid profile were also assessed. The HbA1c assay was performed by high-efficiency/resolution liquid chromatography.
The study complies with the Declaration of Helsinki and women provided informed consent for personal data treatment.
Methods
The main clinical and metabolic features of women during pregnancy were collected by medical records.
The CGM data at 12 ± 2 (for women with pregestational diabetes), 24 ± 2, and 36 ± 2 gestational weeks were downloaded by specific digital platforms (Libreview, Clarity, or Glooko). The following CGM-derived metrics were recorded: TIR (63-140 mg/dL), TAR (>140 mg/dL), TBR (<63 mg/dL), mean glucose, glycemic management indicator (GMI), and coefficient of variation (CV). Moreover, in the hyperglycemic range, level 1 (TAR1: 140-180 mg/dL) and level 2 (TAR2: >180 mg/dL) of TAR were distinguished. Likewise, level 1 (TBR1: 63-54 mg/dL) and level 2 (TBR2: <54 mg/dL) of TBR were assessed.
The GRI at each trimester of pregnancy was obtained through the specific calculator available online. 17 For the 2 levels of hypoglycemia, we used those identified in pregnancy by the International Consensus, 5 while the threshold to distinguish the 2 levels of hyperglycemia in pregnancy was arbitrarily chosen at 180 mg/dL, as several recent studies have considered this cutoff to assess severe hyperglycemia in pregnancy among exploratory secondary outcomes.6-8 The GRI obtained using the pregnancy-specific thresholds of hypoglycemia and hyperglycemia was defined as pGRI.
Neonatal data (sex, gestational age, length, weight, head circumference, Apgar at 5 minutes, delivery method, perinatal complications) were collected by hospital discharges. Babies were classified according to international anthropometric standards as small for gestational age (SGA) when weight was under the 10th percentile, and LGA when weight was over the 90th percentile. 18 Macrosomia was defined as birth weight >4000 g. 19 Delivery that occurred before 37 gestational weeks was considered preterm. 20 Neonatal hypoglycemia requiring intravenous dextrose, jaundice treated with phototherapy, and NICU admission were considered perinatal complications.
Statistical Analysis
Data analysis was performed using the IBM SPSS Statistics V.20 statistical analysis program. Continuous variables were expressed as mean ± standard deviation (SD), if normally distributed, and as median (interquartile range) if not; categorical variables were expressed as percentages. Normality was checked using the Shapiro-Wilk test.
Analysis of variance with Bonferroni test for post hoc comparisons, Kruskal-Wallis test, and chi-square test were used to compare clinical and metabolic data during gestation and neonatal outcomes among different types of diabetes. Independent samples t-test, Mann-Whitney test, and chi-square test were used to compare metabolic and CGM data between women with and without adverse neonatal outcomes. Logistic regression analysis was used to assess the main factors associated with the risk of at least one adverse neonatal outcome, among preterm delivery, macrosomia, LGA, SGA, neonatal hypoglycemia, neonatal jaundice, NICU admission. Cesarean delivery was not included, due to the high rate in women with T1D, as per internal regulation of our hospital. A P value of <.05 was considered statistically significant.
Results
Forty-five women with insulin-treated diabetes (20 with T1D, 17 with T2D, and 8 with GDM) were evaluated (Table 1). All women used CGM for glucose monitoring (37 [82%] Freestyle Libre 2 and 8 (18%) Dexcom G6). Except 3 (15%) women with T1D, treated with insulin pump, all women were on basal-bolus insulin therapy. Moreover, 6 (35%) T2D women were treated with metformin in addition to basal-bolus insulin therapy.
Table 1.
Main Clinical and Metabolic Features During Pregnancy of Women Included in the Study.
| All women (n = 45) | Type 1 diabetes (n = 20) | Type 2 diabetes (n = 17) | Gestational diabetes (n = 8) | P value | |
|---|---|---|---|---|---|
| Age (years) | 34±5 | 34±5 | 34±4 | 32±3 | .540 |
| Pregestational BMI (kg/m2) | 26 [22-30] | 22 [21-25] | 30 [27-33] | 28 [25-33] | <.001 c |
| Weight gain (kg) | 11±5 | 13±3 | 9±5 | 12±4 | .022 d |
| Pregnancy planning (%) a | 15 (41%) | 11(55%) | 4 (24%) | / | .043 |
| Preconception HbA1c (%) a | 6.3 [5.9-7.4] | 6.3 [5.9-7.4] | 7.0 [5.9-8.1] | / | .240 |
| Diabetes duration(years) a | 11 [4-21] | 20 [11-27] | 4 [1-9] | / | <.001 |
| First trimester TDD/kg (UI/kg) a | 0.4 [0.1-0.6] | 0.6 [0.4-0.7] | 0.1 [0-0.4] | / | .001 |
| Second trimester TDD/kg (UI/kg) | 0.6 [0.3-0.9] | 0.7 [0.6-1.0] | 0.4 [0.2-1.0] | 0.1 [0-0.4] | .006 e |
| Third trimester TDD/kg (UI/kg) | 0.8 [0.4-1.0] | 0.8 [0.7-1.1] | 0.5 [0.3-1.2] | 0.3 [0-0.8] | .056 |
| Third trimester HbA1c b (mmol/mol) | 5.8 [5.5-6.4] | 5.8 [5.5-6.4] | 5.7 [5.4-6.5] | 5.5 [5.3-5.7] | .909 |
Data are shown for all women and based on type of diabetes. ANOVA with post hoc Bonferroni test, Kruskal-Wallis, and chi-square test were used as appropriate to compare data among different types of diabetes. Data are expressed as mean ± standard deviation, if normally distributed, and as median [interquartile range] if not; categorical variables are expressed as percentages. The 3-way P values are shown in bold in the last column.
Abbreviations: BMI, body mass index; TDD, total daily insulin dose.
Data available for women with pregestational diabetes.
Data available for 30 women.
The post-hoc pairwise comparison showed a significant difference between T1D and T2D (P <.001) and between T1D and GDM (P .003).
The post-hoc pairwise comparison showed a significant difference between T1D and T2D (P .019).
The post-hoc pairwise comparison showed a significant difference between between T1D and GDM (P .009).
Overall, 25 (56%) women experienced at least one adverse outcome (including neonatal hypoglycemia, jaundice, LGA, SGA, macrosomia, preterm delivery, and NICU admission) (Table 2).
Table 2.
Neonatal Outcomes.
| All women (n = 45) | Type 1 diabetes (n = 20) | Type 2 diabetes (n = 17) | Gestational diabetes (n = 8) | P value | |
|---|---|---|---|---|---|
| Gestational age (weeks) | 37 [37-38] | 37 [36-38] | 38 [36-38] | 38 [37-39] | .037 a |
| Birthweight (g) | 3139±527 | 3077±444 | 3084±668 | 3431±271 | .280 |
| Birthweight percentile | 63±30 | 64±26 | 57±36 | 71±27 | .577 |
| Apgar score 5’ | 9 [9-9] | 9 [9-9] | 9 [9-9] | 9 [9-9] | .912 |
| Cesarean delivery | 24 (53%) | 14 (70%) | 7 (41%) | 3 (38%) | .144 |
| Preterm delivery | 10 (22%) | 6 (30%) | 4 (24%) | 0 | .243 |
| Macrosomia | 2 (4%) | 1 (5%) | 1 (6%) | 0 | .803 |
| LGA | 11 (24%) | 5 (25%) | 4 (24%) | 1 (13%) | .984 |
| SGA | 2 (4%) | 0 | 2 (12%) | 0 | .182 |
| Neonatal hypoglycemia | 10 (22%) | 4 (20%) | 6 (35%) | 0 | .150 |
| Neonatal jaundice | 9 (20%) | 5 (25%) | 4 (24%) | 0 | .299 |
| NICU admission | 2 (4%) | 1 (5%) | 1 (6%) | 0 | .791 |
| At least one adverse outcome b | 25 (56%) | 15 (75%) | 9 (53%) | 1 (13%) | .025 c |
Data are shown for all women and based on type of diabetes. ANOVA with post hoc Bonferroni test, Kruskal-Wallis, and chi-square test were used as appropriate to compare data among different types of diabetes. Data are expressed as mean ± standard deviation, if normally distributed, and as median [interquartile range] if not; categorical variables are expressed as percentages. The 3-way P values are shown in the last column.
Abbreviations: LGA, large for gestational age; SGA, small for gestational age; NICU, neonatal intensive care unit.
The post hoc pairwise comparison showed a significant difference between T1D and GDM women (P = .037).
Among preterm delivery, macrosomia, LGA, SGA, neonatal hypoglycemia, neonatal jaundice, NICU admission.
The post hoc pairwise comparison showed a significant difference between T1D and GDM women (P = .007).
The CGM-derived metrics at first and second trimester of pregnancy were comparable between women with and without adverse neonatal outcomes (data not shown), whereas at third trimester of pregnancy, despite similar HbA1c, women with adverse outcomes had significantly higher mean glucose, GMI, CV, total TAR, and lower TIR than women without adverse outcomes (Table 3). Notably, within the hyperglycemic range, the difference was apparent in TAR2, whereas TAR1 was comparable between the 2 groups. Higher levels of TAR2 resulted in the higher pGRI in women with adverse neonatal outcomes.
Table 3.
CGM-Derived Metrics and Metabolic Data at Third Trimester of Pregnancy in Women Without or With At Least One Adverse Neonatal Outcome.*
| Without adverse neonatal outcomes (n = 20) | With adverse neonatal outcomes (n = 25) | P value | |
|---|---|---|---|
| Mean glucose (mg/dL) | 107 [92-120] | 118 [106-129] | .049 |
| Total TAR (>140) (%) | 10 [4-23] | 26 [12-32] | .018 |
| TAR1 (140-180) (%) | 9 [4-20] | 16 [9-22] | .090 |
| TAR2 (>180) (%) | 1 [0-4] | 6 [2-15] | .004 |
| TIR (63-140) (%) | 88 [75-92] | 71 [64-83] | .007 |
| Total TBR (<63) (%) | 3 [0.3-6] | 1 [0-5] | .921 |
| TBR1 (63-54) (%) | 2 [0-4] | 1 [0-5] | .969 |
| TBR2 (<54) (%) | 0 [0-1] | 0 [0-1] | .740 |
| pGRI (%) | 18 [10-31] | 38 [18-49] | .013 |
| GMI (mmol/mol) | 40 [37-43] | 43 [40-46] | .046 |
| CV (%) | 24 [20-28] | 30 [25-35] | .009 |
| TDD/kg (UI/lg) | 0.5 [0.2-0.9] | 0.8 [0.7-1.2] | .070 |
| Weight gain (kg) | 10 [8-14] | 13 [9-15] | .609 |
| HbA1c (%) | 5.7 [5.5-6.7] | 5.8 [5.5-6.4] | .536 |
Among preterm delivery, macrosomia, large for gestational age, small for gestational age, neonatal hypoglycemia, neonatal jaundice, neonatal intensive care unit admission.
Data are expressed as median [interquartile range].
Abbreviations: TAR, time above range; TIR, time in range; TBR, time below range; pGRI, pregnancy-specific Glycemic Risk Index; GMI: glucose management indicator; CV, coefficient of variation; TDD, total daily insulin dose.
The third trimester pGRI was significantly higher in T1D women compared with T2D (44 [26-54] vs 17 [11-37]%, P = .006) and GDM (44 [26-54] vs 12 [7-23]%, P = .001) women.
At univariate logistic regression analysis, third trimester pGRI, CV, and T1D increased the risk of adverse neonatal outcomes. The significant association was not maintained at the multivariate analysis (Table 4).
Table 4.
Univariate and Multivariate Logistic Analysis of Factors Associated With Increased Risk of Adverse Neonatal Outcomes.*
| Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Third trimester mean glucose | 1.037 [0.997-1.078] | .073 | / | / |
| Third trimester GMI | 1.031 [0.943-1.127] | .508 | / | / |
| Third trimester CV | 1.153 [1.019-1.305] | .024 | 0.690 [0.284-1.676] | .413 |
| Third trimester pGRI | 1.044 [1.004-1.086] | .030 | 1.477 [0.712-3.060] | .295 |
| Type 1 diabetes | 3.429 [1.037-11.332] | .043 | 0.014 [0.000-528.9] | .425 |
At least one among preterm delivery, macrosomia, large for gestational age, small for gestational age, neonatal hypoglycemia, neonatal jaundice, neonatal intensive care unit admission.
Data are presented as odds ratio [95% confidence interval].
Abbreviations: OR, odds ratio; GMI, Glycemic Management Indicator; CV, coefficient of variation.
At receiver operating characteristic (ROC) curve analysis, pGRI ≥25% significantly increased the risk of adverse neonatal outcomes, with sensitivity 70% and specificity 74% (Figure 1).
Figure 1.
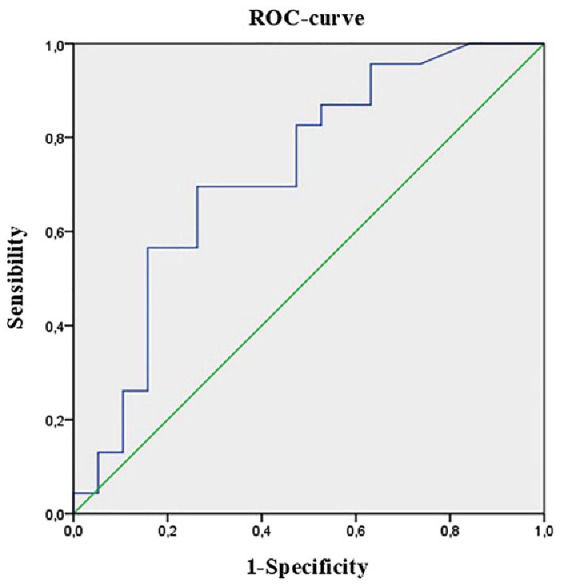
ROC curve of pregnancy-specific GRI and the risk of at least one adverse neonatal outcome, among preterm delivery, macrosomia, large for gestational age, small for gestational age, neonatal hypoglycemia, neonatal jaundice, neonatal intensive care unit admission. AUC: 0.725 [0.566-0.885], P = .013.
Discussion
Our findings suggest that pGRI, placing greater emphasis on severe hyperglycemia compared with less extreme values, may be a novel comprehensive tool for assessing the risk of adverse neonatal outcomes in insulin-treated pregnant women. In fact, our results show that pregnant women with insulin-treated diabetes experiencing adverse neonatal outcomes spend more time in hyperglycemia during the third trimester of pregnancy, compared with those without adverse outcomes. The association between hyperglycemia and adverse pregnancy outcomes has already been reported by other authors, both in women with GDM 21 and pregestational diabetes.22,23 In addition, in our study, we found that the difference was notable particularly for extreme values of hyperglycemia (>180 mg/dL), suggesting that the level of hyperglycemia should always be assessed during pregnancy. This result was reflected by the higher pGRI in the third trimester of pregnancy found in women with adverse neonatal outcomes.
At univariate logistic analysis, third trimester pGRI increased the risk of adverse neonatal outcomes in pregnant women with insulin-treated diabetes. The risk significantly increased for pGRI ≥25%, similar to the cutoff of 20% that identifies the lower glycemic risk in the GRI used outside of pregnancy. 9 However, this association was not maintained in the multivariate analysis, probably due to the interdependency among pGRI, other CGM-derived metrics, and type of diabetes. The strong correlation between GRI and other CGM parameters was previously shown outside of pregnancy.12,13 Moreover, in our study, we found higher level of pGRI in T1D women compared with T2D and GDM women. The greater instability of glucose control in pregnant women with T1D women is well known: Murphy et al 24 showed that pregnant women with T2D spent less time in hyperglycemia and hypoglycemia, compared with T1D women, achieving a TIR (70-140 mg/dL) approximately of 90%. To date, due to insufficient evidence, specific recommendations about the desirable percentage of pregnancy-specific TIR in women with T2D and GDM are lacking. However, more stringent targets should be achieved in these women, as suggested in the International Consensus of Time In Range, in which a wider green area corresponding to TIR is graphically reported in these women, compared with those with T1D. 5 Likewise, the pGRI cutoff associated with the risk of adverse pregnancy outcomes could be higher in women with T1D compared with those with T2D and GDM. In our study, probably due to the small sample size, the separate ROC curve analysis was not significant, but further studies with larger sample size may address this point.
Notably, in our cohort, the median TIR at third trimester of pregnancy was >70% even in women reporting adverse neonatal outcomes, suggesting that the exclusive evaluation of TIR may not be sufficient to assess this risk. Indeed, due to the wide range of TIR, different thresholds of TIR in the fasting and postmeal periods were proposed. 25 On the contrary, the median TAR in women experiencing adverse outcomes was >25%. These findings confirm the predominant impact of hyperglycemia in determining the risk of adverse outcomes, and may therefore suggest the potential role of pGRI in predicting this risk.
The relatively small sample size, the retrospective nature of the study, and the inclusion of women with different types of diabetes represent limitations of our study. Moreover, in our study, the correlation between the pGRI in early pregnancy and the risk of developing GDM was not assessed, as CGM was applied only after GDM diagnosis and when intensive insulin therapy was required. Furthermore, due to the retrospective nature of the study, we could not assess the correlation between the pGRI at each week of pregnancy and the risk of adverse outcomes. Another limitation of our study lies in the formula we used to obtain the pGRI. In fact, we used the formula available online for the calculation of GRI outside of pregnancy, arbitrarily choosing the threshold to distinguish the 2 levels of hyperglycemia at 180 mg/dL, that is, the cutoff considered to assess severe hyperglycemia in pregnancy in several recent studies.6-8 Furthermore, in the development of GRI outside of pregnancy, more weight was posed on hypoglycemic than the hyperglycemic component. 14 Instead, greater value should be given to the hyperglycemic component in pregnancy, that is, the most associated with the risk of adverse neonatal outcomes. 1 Therefore, further studies are needed to develop and validate a proper pGRI, using not only pregnancy-specific thresholds of hypoglycemia and hyperglycemia, but also specific weights for the hypoglycemic and hyperglycemic components. In this way, the potential role of pGRI in predicting the risk of adverse neonatal outcomes may be even higher.
Conclusion
Pregnant women with insulin-treated diabetes experiencing adverse neonatal outcomes spent more time in hyperglycemia, particularly in severe hyperglycemia (>180 mg/dL), in the third trimester of pregnancy, than women without adverse outcomes. Therefore, together with the total time spent in hyperglycemia, the level of hyperglycemia should always be assessed during gestation. These findings suggest the opportunity to implement the current recommendations on glucose targets in pregnancy, identifying different levels of hyperglycemia, as already occurs outside of pregnancy.
The pGRI, placing greater emphasis on extreme hyperglycemia and hypoglycemia, may be a novel comprehensive tool for assessing the risk of adverse neonatal outcomes in these women. The pGRI used in our study was obtained using pregnancy-specific thresholds of hyperglycemia and hypoglycemia, but the same weights for the hypoglycemic and hyperglycemic components used outside of pregnancy. This pGRI was associated with greater risk of adverse neonatal outcomes in our cohort of women. The potential role of pGRI in predicting adverse neonatal outcomes may be probably increased, giving more weight to the hyperglycemic than the hypoglycemic component. Our findings foster the need of further studies to identify and validate an appropriate formula for the calculation of pGRI, including pregnancy-specific levels of hypoglycemia and hyperglycemia and giving more weight to the hyperglycemic than the hypoglycemic component.
Acknowledgments
FC and PM have been supported by the European Union—Next Generation EU, through the Italian Ministry of University and Research under PNRR—M4C2-1.3, Project PE_00000019 HEAL ITALIA.
Footnotes
Abbreviations: GRI, Glycemic Risk Index; CGM, continuous glucose monitoring; TIR, time in range; TAR, time above range; TBR, time below range; LGA, large for gestational age; SGA, small for gestational age; NICU, neonatal intensive care unit.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: FC, CB, TB, FG, CV, LB, PM, AB, and MA declare no conflict of interest.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Fabrizia Citro  https://orcid.org/0009-0000-1673-2327
https://orcid.org/0009-0000-1673-2327
References
- 1. Metzger BE, Lowe LP, Dyer AR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358(19):1991-2002. [DOI] [PubMed] [Google Scholar]
- 2. 15. Management of diabetes in pregnancy: standards of care in diabetes-2024. Diabetes Care. 2024;47(suppl 1):S282-S294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Feig DS, Donovan LE, Corcoy R, et al. Continuous glucose monitoring in pregnant women with type 1 diabetes (CONCEPTT): a multicentre international randomised controlled trial. Lancet. 2017;390(10110):2347-2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Byford AR, Forbes K, Scott EM. Glucose treatment targets in pregnancy: a review of evidence and guidelines. Curr Diabetes Rev. 2023;19(2):e220422203917. [DOI] [PubMed] [Google Scholar]
- 5. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42(8):1593-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee TTM, Collett C, Bergford S, et al. Automated insulin delivery in women with pregnancy complicated by type 1 diabetes. N Engl J Med. 2023;389(17):1566-1578. [DOI] [PubMed] [Google Scholar]
- 7. Levy CJ, Kudva YC, Ozaslan B, et al. At-home use of a pregnancy-specific zone-MPC closed-loop system for pregnancies complicated by type 1 diabetes: a single-arm, observational multicenter study. Diabetes Care. 2023;46(7):1425-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Benhalima K, Beunen K, Van Wilder N, et al. Comparing advanced hybrid closed loop therapy and standard insulin therapy in pregnant women with type 1 diabetes (CRISTAL): a parallel-group, open-label, randomised controlled trial. Lancet Diabetes Endocrinol. 2024;12:390-403. [DOI] [PubMed] [Google Scholar]
- 9. Klonoff DC, Wang J, Rodbard D, et al. A glycemia risk index (GRI) of hypoglycemia and hyperglycemia for continuous glucose monitoring validated by clinician ratings. J Diabetes Sci Technol. 2023;17(5):1226-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rodbard D. Metrics to evaluate quality of glycemic control: comparison of time in target, hypoglycemic, and hyperglycemic ranges with “risk indices..” Diabetes Technol Ther. 2018;20(5):325-334. [DOI] [PubMed] [Google Scholar]
- 11. Monnier L, Bonnet F, Colette C, Renard E, Owens D. Key indices of glycaemic variability for application in diabetes clinical practice. Diabetes Metab. 2023;49(6):101488. [DOI] [PubMed] [Google Scholar]
- 12. Kim JY, Yoo JH, Kim JH. Comparison of glycemia risk index with time in range for assessing glycemic quality. Diabetes Technol Ther. 2023;25(12):883-892. [DOI] [PubMed] [Google Scholar]
- 13. Pérez-López P, Férnandez-Velasco P, Bahillo-Curieses P, de Luis D, Díaz-Soto G. Impact of glucose variability on the assessment of the glycemia risk index (GRI) and classic glycemic metrics. Endocrine. 2023;82(3):560-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang Y, Lu J, Ni J, et al. Association between glycaemia risk index (GRI) and diabetic retinopathy in type 2 diabetes: a cohort study. Diabetes Obes Metab. 2023;25(9):2457-2463. [DOI] [PubMed] [Google Scholar]
- 15. Yoo JH, Kim JY, Kim JH. Association between continuous glucose monitoring-derived glycemia risk index and albuminuria in type 2 diabetes. Diabetes Technol Ther. 2023;25(10):726-735. [DOI] [PubMed] [Google Scholar]
- 16. Piona C, Marigliano M, Roncarà C, et al. Glycemia risk index as a novel metric to evaluate the safety of glycemic control in children and adolescents with type 1 diabetes: an observational, multicenter, real-life cohort study. Diabetes Technol Ther. 2023;25(7):507-512. [DOI] [PubMed] [Google Scholar]
- 17. Glycemia risk index calculator. https://www.diabetestechnology.org/gri/. Accessed October 12, 2024.
- 18. Villar J, Cheikh Ismail L, Victora CG, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet. 2014;384(9946):857-868. [DOI] [PubMed] [Google Scholar]
- 19. Araujo Júnior E, Peixoto AB, Zamarian AC, Elito Júnior J, Tonni G. Macrosomia. Best Pract Res Clin Obstet Gynaecol. 2017;38:83-96. [DOI] [PubMed] [Google Scholar]
- 20. Slattery MM, Morrison JJ. Preterm delivery. Lancet. 2002;360(9344):1489-1497. [DOI] [PubMed] [Google Scholar]
- 21. Liang X, Fu Y, Lu S, et al. Continuous glucose monitoring-derived glycemic metrics and adverse pregnancy outcomes among women with gestational diabetes: a prospective cohort study. Lancet Reg Health West Pac. 2023;39:100823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sibiak R, Gutaj P, Mrzewka-Rogacz B, Mantaj U, Wender-Ozegowska E. Novel continuous glucose monitoring metrics and large-for-gestational-age risk: an exploratory retrospective cohort study in pregnancies with type 1 diabetes. Diabetes Technol Ther. 2022;24(1):42-53. [DOI] [PubMed] [Google Scholar]
- 23. McLean A, Barr E, Tabuai G, Murphy HR, Maple-Brown L. Continuous glucose monitoring metrics in high-risk pregnant women with type 2 diabetes. Diabetes Technol Ther. 2023;25(12):836-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Murphy HR, Rayman G, Duffield K, et al. Changes in the glycemic profiles of women with type 1 and type 2 diabetes during pregnancy. Diabetes Care. 2007;30(11):2785-2791. [DOI] [PubMed] [Google Scholar]
- 25. Citro F, Bertolotto A, Aragona M, Nicolì F, Bianchi C. Do we risk getting lost in such a wide range? Thoughts on interpreting the continuous glucose monitoring-derived metrics. Diabetes Technol Ther. 2023;25(8):574-575. [DOI] [PubMed] [Google Scholar]


