Abstract
Background:
Continuous glucose monitor (CGM) usage improves glycemia in people with type 1 diabetes (PWD) and is accepted as the standard of care. The CGM utilization is lower in patients with public insurance and minorized ethnicities. In 2022, California Medicaid reduced its barriers to obtaining CGM coverage for PWD. It is unknown whether this policy change is sufficient to increase CGM usage. We hypothesize that the change in Medicaid coverage improved CGM uptake in children and young adults with T1D.
Methods:
Data were extracted from electronic medical record of a large urban children’s hospital in 2021 and 2022. The CGM usage was determined based on clinician documentation or the presence of CGM downloads. Kruskal-Wallis tests, Wald tests, and χ2 tests were used to test hypothesis (P < .05). Mixed effects logistical regression analyses were performed.
Results:
We included 878 and 892 PWD (age ≤ 21 years) in 2021 and 2022, respectively. In 2022, Medicaid insured 59.3% of patients. Between 2021 and 2022, CGM usage did not change for privately insured patients (84%) but increased from 41% to 58% for patients receiving Medicaid. In our mixed effects logistic regression model, CGM usage was higher in 2022 and in English speakers. Public insurance, black race, and patients’ age were negatively associated with CGM usage.
Conclusion:
Our results suggest that Medicaid expansion of CGM coverage increases its utilization for pediatric PWD but did not eliminate the disparity. Future studies are needed to identify barriers that preclude equity in technology uptake.
Keywords: type 1 diabetes, pediatric, health equity, continuous glucose monitor
Introduction
More than 300 000 children and adolescents live with type 1 diabetes (T1D) in the United States. 1 Despite advancements in insulin analogues and diabetes technology, only 26% of children attain the American Diabetes Association hemoglobin A1c (HbA1c) target of 7% in 2021/2022. 2 Utilization of continuous glucose monitor (CGM) has been shown to improve glycemic outcome in people living with T1D (PWD).3-6 Laffel et al 7 demonstrated that adolescents and young adults randomized to CGM had a 0.4% reduction in HbA1c, compared with those randomized to usual care with glucometer (control). Alonso et al 8 showed that in a single-center retrospective study, mean HbA1c was 1.4% lower in CGM users for both patients on multiple daily insulin injections and insulin pump. Real-world data from 21 253 T1D Exchange (T1DX) Clinic Registry participants similarly demonstrated that CGM use was associated with lower HbA1c in all age groups. 3 In an observational crossover study of PWD, HbA1c improved when patients switched from self-monitoring of blood glucose to CGM. 9 The overwhelming evidence of CGM improving glycemic outcomes and reducing hypoglycemia has led to its acceptance as the standard of care in PWD.5,10
Despite the clear benefit of CGM use in PWD, only 45% of T1DX registry participants used CGM devices in 2021 to 2022. 2 Social determinants of health such as insurance coverage, race/ethnicity, health literacy, and familiarity with health technology may contribute to inequity in CGM usage.11,12 Clinician bias and personal preference are additional barriers to CGM utilization.11,13,14 Even when CGM is a covered insurance benefit, barriers include coverage as a durable medical equipment benefit instead of a pharmacy benefit, providing proof of minimum glucose checks per day, and prior authorizations.15,16 Public insurance tends to have more stringent requirements for CGM coverage, further amplifying health inequity already seen in PWD with lower income. 17
Whereas insurance barriers can reduce CGM access in PWD, it is unclear whether removal of such barriers is sufficient to eliminate inequity in CGM utilization. Recently, Ni et al 18 demonstrated that the expansion of Medicare coverage of CGM without minimum glucose requirement improved its utilization in PWD above the age of 18. It is unknown whether similar improvements in CGM usage occur in the pediatric cohort, who may have different perspectives on wearing a medical device than adults. Publicly insured, insulin-requiring children with T1D in California were able to access CGM if they met requirements including fear of hypoglycemia or permissive hyperglycemia due to fear of hypoglycemia, and demonstration of high motivation to use CGM (by submitting proof of blood glucose testing at least 3 times per day). 19 Starting January 1, 2022, CGM coverage no longer included these requirements for PWD. Concurrently, CGM prescription became available at retail pharmacies due to reclassification of CGM devices under pharmacy benefit. 20 We hypothesize that these policy changes will improve CGM usage for publicly insured children with T1D.
Methods
Data were extracted from the electronic medical record of a single-center, large urban children’s hospital in compliance with regulations set forth by the Children’s Hospital Los Angeles (CHLA) Institutional Review Board (Los Angeles, California). The study included patients with T1D (age ≤ 21 years) who had at least one ambulatory appointment in the diabetes center at CHLA between 2021 and 2022. Continuous glucose monitor and insulin pump usage was determined based on endocrinologist documentation and/or the presence of CGM or insulin pump report. We limited the data query to PWD who were seen at least once in the diabetes clinic each year. For patients with more than 1 visit during a year, clinical data from the most recent encounter were used. The language field refers to the preferred language of the caregivers. Patient/family self-identified race and ethnicity. Race and ethnicity were captured as 2 separate variables. We combined race and ethnicity into a single composite variable that better reflects Southern California’s large Latino population and to align with the recent updates to the Statistical Policy Directive No. 15: Standards for Maintaining, Collecting, and Presenting Federal Data on Race and Ethnicity.21,22 The original data set had 22 race values and 3 ethnicity values. These were recoded following the race and ethnicity data standards published by the Office of Minority Health. 23 After recoding, there were 7 unique race/ethnicity values (American Indian or Alaska Native [AIAN], Asian, Black or African American, Hispanic or Latino, Native Hawaiian or Other Pacific Islander [NHPI], Other, and white). Records with missing, unknown, or “declined to state” were collapsed into the category of “non-conforming data.” 24 It should be noted that the term “non-conforming data” here is descriptive and non-normative; it simply reflects that those variables do not conform to the Office of Management and Budget’s (OMB) race and ethnicity standards in either their original or expanded forms. Supplemental Table 1 shows the recoding and transformation logic that was applied to the original data set.
Summary statistics were used to describe variables in our data set. Count and percent were used to describe categorical variables, and continuous variables were plotted to determine whether to use mean and standard deviation or median and interquartile range (IQR). HbA1c data appeared to be positively skewed, so we decided that median and IQR would give a more accurate description of our HbA1c data. To test for differences in HbA1c between independent groups, we used independent-samples Mann-Whitney U tests. To explore the relationship between year and CGM use, we fit 2 separate mixed effects logistic regression models. One model contained year and insurance as predictors, and the second model contained year, insurance, race, age, and language. Wald tests were used for individual regression coefficients. In Figure 1, χ2 test was used to compare CGM usage between 2021 and 2022. Summary statistics and analysis were created using R statistical software.
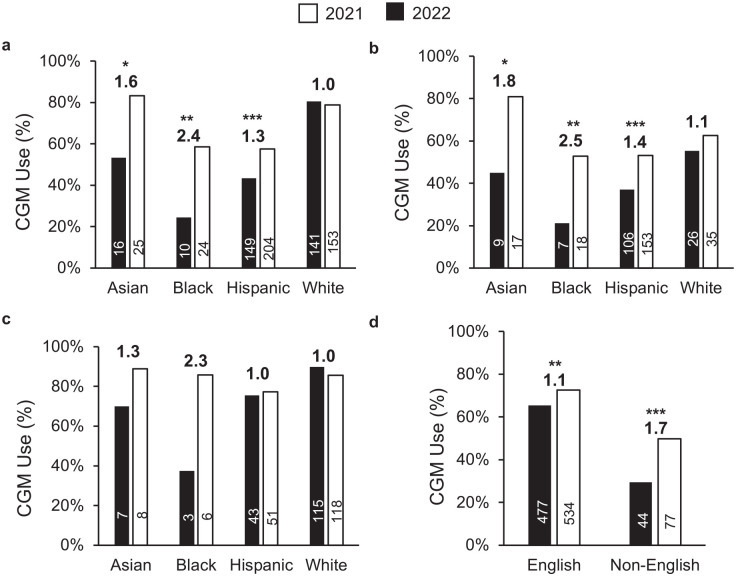
Figure 1.
Continuous glucose monitor use (%) by (a) all insurance types, (b) public insurance, (c) private insurance, and (d) language. Numbers above the bar graph reflects fold change between 2021 and 2022. Sample size shown within each bar.
*P < .05. **P < .01. ***P < .001.
Results
Continuous glucose monitor data for patients with T1D were available for 878 and 892 patients in 2021 and 2022, respectively, with 748 patients providing data in both years. As shown in Table 1, the mean age, sex, diabetes duration, race, ethnicity, and insurance status were comparable between the 2 years. The patient cohort in 2021 was 54.0% female with a mean (SD) age of 14.3 (4.5) years and diabetes duration of 6.1 (4.6) years. Our patient population is racially and ethnically diverse. In 2021, 19.9% identified as white and 39.1% identified as Hispanic. Similar racial and ethnic distributions were observed in 2022. We serve a primarily low-income population, with most patients receiving public insurance (57.9% in 2021, 59.3% in 2022). The median HbA1c [IQR] for all patients was 7.9% [7.0%-9.1%] in 2021 and 7.8% [6.9%-9.1%] in 2022. Patients with public insurance had higher HbA1c compared with those with private insurance (2021: 8.3% [7.3%-9.9%] vs 7.5% [6.8%-8.3%], P < .001; 2022: 8.2% [7.2%-9.7%] vs 7.2 [6.5%-8.1%], P < .001). Patients using CGM, irrespective of insulin regimen, had lower HbA1c levels compared with those not using CGM (Table 2). In 2022, the median HbA1c [IQR] for CGM users was 7.8% [6.8%-9.0%] for patients receiving insulin injections and 7.2% [6.5%-7.8%] for those using insulin pump therapy. The corresponding HbA1c [IQR] for non-CGM users was 9.2% [7.9%-10.9%] and 8.3% [7.6%-10.3%], respectively.
Table 1.
Patient Characteristics.
| 2021 | 2022 | |
|---|---|---|
| N | 878 | 892 |
| Age in years, mean (SD) | 14.3 (4.5) | 14.2 (4.5) |
| Gender = male (%) | 474 (54.0) | 491 (55.0) |
| Diabetes duration in years, mean (SD) | 6.1 (4.6) | 5.9 (4.5) |
| Insurance (%) | ||
| Private | 370 (42.1) | 361 (40.5) |
| Public | 508 (57.9) | 529 (59.3) |
| Self-pay | 0 (0.0) | 2 (0.2) |
| Race/Ethnicity (%) | ||
| American Indian or Alaska Native | 2 (0.2) | 2 (0.2) |
| Asian | 30 (3.4) | 30 (3.4) |
| Black or African American | 41 (4.7) | 41 (4.6) |
| Hispanic or Latino | 343 (39.1) | 354 (39.7) |
| Native Hawaiian and Other Pacific Islander | 1 (0.1) | 1 (0.1) |
| Non-conforming data | 53 (6.0) | 71 (8.0) |
| Other | 233 (26.5) | 199 (22.3) |
| White | 175 (19.9) | 194 (21.7) |
| Language | ||
| English | 729 (83.0) | 737 (82.6) |
| Non-English | 149 (17.0) | 155 (17.4) |
| HbA1c in % (median [IQR]) | 7.9 [7.0-9.1] | 7.8 [6.9-9.1] |
| Pump (%) | ||
| No | 454 (51.7) | 499 (55.9) |
| Yes | 341 (38.8) | 393 (44.1) |
| No data available | 83 (9.5) | 0 (0.0) |
Abbreviation: IQR, interquartile range.
Table 2.
Median [IQR] HbA1c (%) by CGM Usage Between 2021 and 2022.
| Insulin Regimen | +CGM | Count | −CGM | Count | P | |
|---|---|---|---|---|---|---|
| MDI | 2021 | 7.9 [7.0-8.8] | 124 | 8.8 [7.7-11.1] | 243 | <.001 |
| 2022 | 7.8 [6.8-9.0] | 221 | 9.2 [7.9-10.9] | 212 | <.001 | |
| CSII | 2021 | 7.4 [6.8-7.9] | 219 | 8.2 [7.5-9.1] | 37 | <.001 |
| 2022 | 7.2 [6.5-7.8] | 276 | 8.3 [7.6-10.3] | 34 | <.001 |
The 25 and 75 percentiles [IQR] enclosed in brackets. Kruskal-Wallis test is used to test differences between CGM users and non-CGM users.
Abbreviations: MDI, multiple daily insulin injections, CSII, continuous subcutaneous insulin infusion (also referred to as insulin pump).
We next examined the usage of CGM by insurance and year. Between 2021 and 2022, CGM usage increased by 1.2-fold for the entire cohort (from 59% to 68%, respectively, P < .0001). The CGM uptake did not change for privately insured patients (84% in 2021, 83% in 2022) but rose from 41% to 58% for publicly insured patients (P < .0001). Despite the increase in 2022, CGM usage rate remained lower in publicly insured patients compared with those with private insurance (58% vs 83%, P < .001).
Diabetes technology inequity is well-recognized in historically marginalized race and ethnicity groups. 2 Figure 1a shows that all non-white race/ethnicity groups experienced an increase in CGM usage between 2021 and 2022. Black youth had the lowest CGM use rate in 2021 (24%) but experienced the largest gain to 59% in 2022 (2.4-fold). When stratified by insurance types, however, the increase in CGM usage was only seen for Asian, black, and Hispanic, publicly insured patients (Figure 1b). There was no statistically significant difference in CGM usage in 2022 among privately insured patients (Figure 1c). The CGM usage increased among patients from English-speaking and non-English-speaking families, although the increase was more striking for the latter (Figure 1d).
Due to the intersectionality across race/ethnicity, insurance status, and language, we next performed logistic regression analysis to identify variables that contribute to CGM usage. However, a significant proportion of this study cohort had either missing race and ethnicity data (8.6%) or self-selected “other” race (23.7%) (Supplemental Table 1). To avoid discarding the data of patients with unknown race/ethnicity data, we decided to generate 2 mixed effects logistic regression models: one model using the full data set and a second model including only data from patients with race/ethnicity data. The first model used fixed effects for year (2021 or 2022) and insurance status (public or private). Table 3 shows that the adjusted odds of a patient being on CGM in 2022 were 2.86 times higher compared with 2021. Publicly insured patients were 0.13 times as likely to use CGM as privately insured patients. In the second regression model, the fixed effects included year, insurance status, language, age, and race/ethnicity. For race/ethnicity, we included Asian, black, Hispanic, and white race categories as these were the only ones with a significantly sized sample. In this model, we similarly observed that CGM use positively correlated with the year 2022 (compared with 2021; odds ratio = 3.16) and negatively correlated with public insurance (odds ratio = 0.23) (Table 4). In addition, age negatively correlated with CGM use (odds ratio = 0.88). Assuming all other variables remain the same, a patient from our sample who is 1 year older has 12% lower odds (or 88% the odds) of using CGM. This suggests that older patients are less likely to use CGM, compared with younger patients. The regression model also showed that patients with English-speaking families were 2.26 times more likely to use CGM. Finally, a statistically significant odds ratio was only seen for black (0.24), but not Asian or Hispanic race categories. This mixed effects logistic regression model not only highlights CGM use improvement in 2022, but also shows that usage was higher for patients with English-speaking families and lower for patients who were older, publicly insured, or black race.
Table 3.
Effect of Insurance Policy Change on CGM Use.
| Odds ratio | 95% CI for odds ratio | Log-odds | z value | P | |
|---|---|---|---|---|---|
| (Intercept) | 1.85 | 1.10-3.12 | 0.62 | 2.31 | .021 |
| Year [2022] | 2.86 | 1.88-4.35 | 1.05 | 4.89 | <.001 |
| Insurance [public] | 0.13 | 0.07-0.27 | −2.01 | −5.57 | <.001 |
Mixed effects logistic regression model with outcome variable of CGM use (coded as yes or no), fixed effects for year (2021 or 2022), and public insurance indicator and random intercepts for repeated measures. In total, 953 observations from 603 patients were included for analysis. Marginal R2/Conditional R2 = .131/.595.
Table 4.
Variables Associated With CGM Use.
| Odds ratio | 95% CI for odds ratio | Log-odds | z value | P | |
|---|---|---|---|---|---|
| (Intercept) | 6.05 | 1.67-21.98 | 1.8 | 2.74 | .006 |
| Year [2022] | 3.16 | 1.91-5.22 | 1.15 | 4.49 | <.001 |
| Insurance [public] | 0.23 | 0.11-0.52 | −1.45 | −3.59 | <.001 |
| Language [English] | 2.26 | 1.14-4.48 | 0.81 | 2.33 | .02 |
| Age in years | 0.88 | 0.82-0.94 | −0.13 | −3.65 | <.001 |
| Race/Ethnicity | |||||
| Asian | 1.81 | 0.42-7.80 | 0.59 | 0.79 | .427 |
| BAA | 0.24 | 0.07-0.80 | −1.43 | −2.32 | .02 |
| Hispanic | 0.57 | 0.26-1.24 | −0.56 | −1.42 | .155 |
Mixed effects logistic regression model with outcome variable of CGM use (coded as yes or no), fixed effects for year (2021 or 2022), public insurance indicator, English as preferred language indicator, age in years, and race and random intercepts for repeated measures. White is chosen as the baseline race and only patients categorized as white, Asian, black or African American (BAA) or Hispanic were included in this model because these were the only known race categories with a significantly sized sample. In total, 699 observations from 433 patients were included for analysis. Marginal R2/Conditional R2 = .216/.610.
Discussion
Health insurance coverage of CGM is essential in improving CGM access for PWD. We examined the impact of California Medicaid rule change on CGM utilization for PWD younger than the age of 22 in an urban pediatric diabetes center. Between 2021 and 2022, CGM uptake did not change among privately insured patients, but increased by 17% in publicly insured PWD. After controlling for age, race, language, and insurance, the mixed effects logistic regression model identified 3.16-fold higher odds of CGM usage between 2021 and 2022. This model showed no statistically significant difference in CGM usage in Hispanic or Asian patients, when adjusted for age, year, language, and insurance. However, a disparity in CGM usage persists for black PWD despite controlling for confounders in other pediatric and adult settings.25,26
Our findings suggest that disparity in CGM use in historically marginalized races is partly attributable to the health insurance status. Insurance policies that reduce barriers to CGM access have the potential to mitigate diabetes device disparity and health outcomes. We did not identify Hispanic race/ethnicity as a variable of CGM use in the regression model (odds ratio = 0.57, P = .155). One explanation may be the relatively small sample size of this single-center study. However, even with a small sample size, this study did show reduced CGM use in black patients. This finding aligns with prior reports demonstrating persistent 30% lower CGM use among black PWD. 2 One would need to consider the role of structural racism and mistrust of health care providers/systems in adaptation of CGM in non-white patients.
Clinicians may also contribute to CGM usage barriers through implicit bias. In a study of 109 diabetes providers in the United States, when presented with clinical vignettes, 61% exhibited insurance-mediated bias and 34% exhibited racial-ethnic bias (with 89% of the latter stating that they could recognize their own implicit bias). 27 Standardizing clinic practice by prescribing CGM to all PWD, adhering to the recommendation of the American Diabetes Association of initiating CGM at or shortly after diagnosis may reduce impact of clinician implicit bias. 28 Equity strategies focused on improved CGM access may include redesign of care delivery to remove structural barriers to CGM prescribing and implementation of communication tools with active PWD/caregiver engagement.29-31
Our findings provide the first evidence that the change in insurance coverage of CGM in California improved CGM access for publicly insured children and young adult. Despite the increased accessibility, CGM usage in publicly insured patients trails behind those with private insurance. Insurance prior authorizations are routinely needed for CGM dispensation. 22 Socioeconomically deprived families or PWD/caregivers with limited health literacy may not have the resources or recognize the need to follow up with pharmacies and prescribers, which further impedes CGM access. We advocate for eliminating prior authorization for CGM prescriptions for all PWD to eliminate burdensome paperwork for prescribers’ office and Medicaid staff, which would help to narrow the gap in diabetes care due to insurance coverage.
Many of the barriers described above are worsened in families with limited English proficiency. 32 Analysis from this study showed that patients with non-English-speaking family have lower CGM usage, corroborating findings from others. 33 Language barriers at clinical visits may limit CGM access if PWD and caregivers are not able to communicate with the health care team in their preferred language. They may also have less familiarity using and troubleshooting diabetes technology, especially if there is no one in the team or vendor representative that speaks their native language.
Additional barriers identified by PWD include site discomfort, past device inaccuracy, adhesive failure, and having a device on the body as additional barriers of CGM use.34,35 Families with lower health literacy may not understand expected differences between sensor and blood glucose levels and therefore question CGM accuracy. Similar reasons have also been endorsed by adolescents, an age group with lower CGM utilization rate but highest HbA1c level.25,36,37 Patient-centered approaches are needed to understand each PWD’s rationale for declining CGM use. Sharing strategies addressing those barriers may improve CGM uptake. Clinicians also need to consider the role of social stigma that a PWD may experience from wearing a CGM (or other devices) and drawing unwanted attention when it alarms. Supporting the PWD by developing a strategy with the PWD on how to comfortably wear the device may increase consistent CGM use. 38
The major strength of this work is the inclusion of a diverse cohort of pediatric PWD with different insurance coverage, to enable us to evaluate the role of public insurance policy change on CGM uptake. Inherent in a retrospective analysis is the limitation in determining causality. It is also unknown, the relative contribution of eliminating minimum blood glucose checks and the availability of CGM under pharmacy benefit to the increased CGM usage we observed in publicly insured patients in 2022. Furthermore, there was significant missing race/ethnicity data, which could influence the regression analysis if the missing was not random. Between 2020 and 2022, our institution expanded the number of options available to patients when self-reporting race and ethnicity. If a patient was not asked to update their race and ethnicity information during their visit, then both their race and ethnicity was recorded as “unknown.” This resulted in 32.3% (n = 330) of patients with essentially missing data in our analysis. Another limitation of this study is the small sample size for certain race categories (Asian, black, AIAN, and NHPI), precluding generalizability of our finding. Finally, because CGM use was dichotomized (yes or no), we are unable to discern the frequency and reason for suboptimal CGM wear (eg, unable to obtain sensor refill, sensor falling off early, or faulty sensor).
An important next step in advancing the care of PWD is advocacy that maximizes the therapeutic benefits of CGM. Clinical trials and real-world data have shown that automated insulin delivery (AID) systems lower HbA1c, improve time-in-range, and reduce diabetes burden in both children and adults, irrespective of insurance types. 28 Although Medicaid provides coverage of AID systems, current requirements (a low C-peptide level, HbA1c >7%, and diabetes diagnosis of at least 6 months) are additional barriers that limit PWD access to diabetes technology. 39 Such policies should be amended using existing evidence, to remove these requirements and reduce diabetes technology inequity. An additional opportunity to capitalize on the benefits of CGM is the development of a uniform integration of CGM device data into electronic monitoring system. This facilitates health care systems to evaluate the health benefit of specific diabetes treatments in their patient populations. It would also expand the feasibility of remote monitoring and the development of alert systems for case management when CGM-monitored glucometrics reach a high-risk threshold.
Conclusion
In summary, this analysis of CGM usage in children with T1D provides evidence that minimizing barriers to obtain CGM is accompanied by increased CGM usage. Similar policy change should be adopted by all insurances to improve the health of PWD.
Supplemental Material
Supplemental material, sj-docx-1-dst-10.1177_19322968241287217 for Expansion of Medicaid Coverage of Continuous Glucose Monitor Reduces Health Disparity in Children and Young Adults With Type 1 Diabetes by Brian Miyazaki, Troy Zeier, Rebecca Ortiz La Banca Barber, Juan Carlos Espinoza and Lily Chih-Chen Chao in Journal of Diabetes Science and Technology
Footnotes
Abbreviations: AIAN, American Indian or Alaska Native; CGM, continuous glucose monitor; CHLA, Children’s Hospital Los Angeles; CI, confidence interval; CSII, continuous subcutaneous insulin infusion; HbA1c, hemoglobin A1c; MDI, multiple daily insulin; NHPI, Native Hawaiian and Pacific Islander; OMB, Office of Management and Budget; PWD, people living with T1D; T1D, type 1 diabetes; T1DX, T1D Exchange.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JCE is a consultant for Sanofi. Sanofi played no role in the design, execution, analysis, writing, or in the decision to publish this manuscript and had no editorial input. The remaining authors declare that there is no conflict of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The data for this project were provided by the Real-World Evidence Demonstration Project supported by the Food and Drug Administration under award number P50FD006425 for The West Coast Consortium for Technology & Innovation in Pediatrics (PI: Espinoza). The funding sources were not involved in the development of this manuscript or in the decision to submit the paper for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the FDA.
ORCID iDs: Rebecca Ortiz La Banca Barber  https://orcid.org/0000-0001-6084-3657
https://orcid.org/0000-0001-6084-3657
Juan Carlos Espinoza  https://orcid.org/0000-0003-0513-588X
https://orcid.org/0000-0003-0513-588X
Lily Chih-Chen Chao  https://orcid.org/0000-0002-6294-0589
https://orcid.org/0000-0002-6294-0589
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Prevalence of diagnosed diabetes. Center for Disease Control and Prevention. Published May 15, 2024. https://www.cdc.gov/diabetes/php/data-research/index.html
- 2. Ebekozien O, Mungmode A, Sanchez J, et al. Longitudinal trends in glycemic outcomes and technology use for over 48,000 people with type 1 diabetes (2016-2022) from the t1d exchange quality improvement collaborative. Diabetes Technol Ther. 2023;25(11):765-773. doi: 10.1089/dia.2023.0320. [DOI] [PubMed] [Google Scholar]
- 3. Miller KM, Beck RW, Foster NC, Maahs DM. HbA1c levels in type 1 diabetes from early childhood to older adults: a deeper dive into the influence of technology and socioeconomic status on HbA1c in the T1D exchange clinic registry findings. Diabetes Technol Ther. 2020;22(9):645-650. doi: 10.1089/dia.2019.0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Champakanath A, Akturk HK, Alonso GT, Snell-Bergeon JK, Shah VN. Continuous glucose monitoring initiation within first year of type 1 diabetes diagnosis is associated with improved glycemic outcomes: 7-year follow-up study. Diabetes Care. 2022;45(3):750-753. doi: 10.2337/dc21-2004. [DOI] [PubMed] [Google Scholar]
- 5. ElSayed NA, Aleppo G, Aroda VR, et al. 7. Diabetes technology: standards of care in diabetes-2023. Diabetes Care. 2023;46(suppl 1):S111-S127. doi: 10.2337/dc23-S007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Demeterco-Berggren C, Ebekozien O, Noor N, et al. Factors associated with achieving target A1c in children and adolescents with type 1 diabetes: findings from the T1d exchange quality improvement collaborative. Clin Diabetes. 2022;41(1):68-75. doi: 10.2337/cd22-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Laffel LM, Kanapka LG, Beck RW, et al. Effect of continuous glucose monitoring on glycemic control in adolescents and young adults with type 1 diabetes: a randomized clinical trial. JAMA. 2020;323(23):2388-2396. doi: 10.1001/jama.2020.6940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alonso GT, Triolo TM, Akturk HK, et al. Increased technology use associated with lower A1c in a large pediatric clinical population. Diabetes Care. 2023;46(6):1218-1222. doi: 10.2337/dc22-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Noor N, Norman G, Sonabend R, et al. An observational crossover study of people using real-time continuous glucose monitors versus self-monitoring of blood glucose: real-world evidence using EMR data from more than 12,000 people with type 1 diabetes[published online ahead of print June 1, 2023]. J Diabetes Sci Technol. doi: 10.1177/19322968231178017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tauschmann M, Forlenza G, Hood K, et al. ISPAD clinical practice consensus guidelines 2022: diabetes technologies: glucose monitoring. Pediatr Diabetes. 2022;23(8):1390-1405. doi: 10.1111/pedi.13451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dos Santos TJ, Dave C, MacLeish S, Wood JR. Diabetes technologies for children and adolescents with type 1 diabetes are highly dependent on coverage and reimbursement: results from a worldwide survey. BMJ Open Diabetes Res Care. 2021;9(2):e002537. doi: 10.1136/bmjdrc-2021-002537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sinisterra M, Wang CH, Marks BE, et al. Patterns of continuous glucose monitor use in young children throughout the first 18 months following type 1 diabetes diagnosis. Diabetes Technol Ther. 2021;23(11):777-781. doi: 10.1089/dia.2021.0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Addala A, Hanes S, Naranjo D, Maahs DM, Hood KK. Provider implicit bias impacts pediatric type 1 diabetes technology recommendations in the United States: findings from the gatekeeper study. J Diabetes Sci Technol. 2021;15(5):1027-1033. doi: 10.1177/19322968211006476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marks BE, Wolfsdorf JI. Monitoring of pediatric type 1 diabetes. Front Endocrinol (Lausanne). 2020;11:128. doi: 10.3389/fendo.2020.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Miller EM. Using continuous glucose monitoring in clinical practice. Clin Diabetes. 2020;38(5):429-438. doi: 10.2337/cd20-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Center for Health Care Strategies. Continuous glucose monitor access for Medicaid beneficiaries living with diabetes: state-by-state coverage. https://www.chcs.org/media/CGM-Access-for-Medicaid-Beneficiaries-Living-with-Diabetes-State-By-State-Coverage.pdf
- 17. Anderson JE, Gavin JR, Kruger DF. Current eligibility requirements for cgm coverage are harmful, costly, and unjustified. Diabetes Technol Ther. 2020;22(3):169-173. doi: 10.1089/dia.2019.0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ni K, Tampe CA, Sol K, Richardson DB, Pereira RI. Effect of CGM access expansion on uptake among patients on Medicaid with diabetes. Diabetes Care. 2023;46(2):391-398. doi: 10.2337/dc22-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Continuous Glucose Monitoring (CGM) as a CCS/GHPP program benefit-revised with attachment. Department of Health Care Services, State of California-Health and Human Services Agency. Published August 29, 2018. https://www.dhcs.ca.gov/services/ccs/Documents/CCS.NL.14-0818.Continuous.Glucose.Monitoring.pdf
- 20. Medi-Cal Rx monthly bulletin. Department of Health Care Services, State of California-Health and Human Services Agency. Published January 1, 2022. Accessed February 15, 2024. https://medi-calrx.dhcs.ca.gov/cms/medicalrx/static-assets/documents/provider/bulletins/2022.01_B_Monthly_Bulletin_for_January.pdf
- 21. Federal Register. Revisions to OMB office’s statistical policy directive No.15: standards for maintaining, collecting, and presenting federal data on race and ethnicity. Published March 29, 2024. Accessed April 19, 2024. https://www.federalregister.gov/documents/2024/03/29/2024-06469/revisions-to-ombs-statistical-policy-directive-no-15-standards-for-maintaining-collecting-and
- 22. American Diabetes Association. Health equity and diabetes technology: a study of access to continuous glucose monitors by payer, geography and race. Accessed February 13, 2024. https://diabetes.org/sites/default/files/2023-09/ADA-CGM-Utilization-White-Paper-Oct-2022.pdf
- 23. Explanation of data standards for race, ethnicity, sex, primary language, and disability. US Department of Health & Human Services, Office of Minority Health. Accessed August 25, 2024. https://minorityhealth.hhs.gov/explanation-data-standards-race-ethnicity-sex-primary-language-and-disability
- 24. Cook L, Espinoza J, Weiskopf NG, et al. Issues with variability in electronic health record data about race and ethnicity: descriptive analysis of the national COVID cohort collaborative data enclave. JMIR Med Inform. 2022;10(9):e39235. doi: 10.2196/39235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1d exchange in 2016-2018. Diabetes Technol Ther. 2019;21(2):66-72. doi: 10.1089/dia.2018.0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Agarwal S, Crespo-Ramos G, Long JA, Miller VA. “I didn’t really have a choice”: qualitative analysis of racial-ethnic disparities in diabetes technology use among young adults with type 1 diabetes. Diabetes Technol Ther. 2021;23(9):616-622. doi: 10.1089/dia.2021.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Odugbesan O, Addala A, Nelson G, et al. Implicit racial-ethnic and insurance-mediated bias to recommending diabetes technology: insights from T1d exchange multicenter pediatric and adult diabetes provider cohort. Diabetes Technol Ther. 2022;24(9):619-627. doi: 10.1089/dia.2022.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. American Diabetes Association Professional Practice Committee. 7. Diabetes technology: standards of care in diabetes-2024. Diabetes Care. 2024;47(suppl 1):S126-S144. doi: 10.2337/dc24-S007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mathias P, Mahali LP, Agarwal S. Targeting technology in underserved adults with type 1 diabetes: effect of diabetes practice transformations on improving equity in cgm prescribing behaviors. Diabetes Care. 2022;45(10):2231-2237. doi: 10.2337/dc22-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Prahalad P, Ebekozien O, Alonso GT, et al. Multi-clinic quality improvement initiative increases continuous glucose monitoring use among adolescents and young adults with type 1 diabetes. Clin Diabetes. 2021;39(3):264-271. doi: 10.2337/cd21-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schmitt J, Fogle K, Scott ML, Iyer P. Improving equitable access to continuous glucose monitors for Alabama’s children with type 1 diabetes: a quality improvement project. Diabetes Technol Ther. 2022;24(7):481-491. doi: 10.1089/dia.2021.0511. [DOI] [PubMed] [Google Scholar]
- 32. Vrany EA, Hill-Briggs F, Ephraim PL, Myers AK, Garnica P, Fitzpatrick SL. Continuous glucose monitors and virtual care in high-risk, racial and ethnic minority populations: toward promoting health equity. Front Endocrinol (Lausanne). 2023;14:1083145. doi: 10.3389/fendo.2023.1083145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Loomba L, Bonanno S, Arellano D, Crossen S, Glaser N. Disparities in insulin pump use among Spanish-speaking children with type 1 diabetes compared to their non-Hispanic white peers: mixed methods study. JMIR Diabetes. 2023;8:e45890. doi: 10.2196/45890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Engler R, Routh TL, Lucisano JY. Adoption barriers for continuous glucose monitoring and their potential reduction with a fully implanted system: results from patient preference surveys. Clin Diabetes. 2018;36(1):50-58. doi: 10.2337/cd17-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Messer LH, Berget C, Beatson C, Polsky S, Forlenza GP. Preserving skin integrity with chronic device use in diabetes. Diabetes Technol Ther. 2018;20(S2):S254-S264. doi: 10.1089/dia.2018.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lacy ME, Lee KE, Atac O, et al. Patterns and trends in continuous glucose monitoring utilization among commercially insured individuals with type 1 diabetes: 2010-2013 to 2016-2019. Clin Diabetes. 2024;42(3):388-397. doi: 10.2337/cd23-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. van den Boom L, Karges B, Auzanneau M, et al. Temporal trends and contemporary use of insulin pump therapy and glucose monitoring among children, adolescents, and adults with type 1 diabetes between 1995 and 2017. Diabetes Care. 2019;42(11):2050-2056. doi: 10.2337/dc19-0345. [DOI] [PubMed] [Google Scholar]
- 38. Farrington C. Wearable technologies and stigma in diabetes: the role of medical aesthetics. Lancet Diabetes Endocrinol. 2016;4(7):566. doi: 10.1016/S2213-8587(16)00075-9. [DOI] [PubMed] [Google Scholar]
- 39. Insulin Infusion Pump. Cag-00041n. Centers for Medicare & Medicaid Services. Published August 26, 1999. Accessed August 25, 2024. https://www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&NCAId=40
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-dst-10.1177_19322968241287217 for Expansion of Medicaid Coverage of Continuous Glucose Monitor Reduces Health Disparity in Children and Young Adults With Type 1 Diabetes by Brian Miyazaki, Troy Zeier, Rebecca Ortiz La Banca Barber, Juan Carlos Espinoza and Lily Chih-Chen Chao in Journal of Diabetes Science and Technology