Abstract
Background
Knee osteoarthritis (KOA) is characterized by mitochondrial damage and increased inflammation. Circulating cell-free mitochondrial DNA (ccf-mtDNA), which originates from damaged mitochondria, is an endogenous damage-associated molecular pattern (DAMPs) molecule that may trigger inflammation and is recognized as a potential biomarker for various diseases. In this study, we investigated the potential association between plasma ccf-mtDNA content and its use as a diagnostic biomarker in patients with KOA.
Methods
We collected plasma samples from patients with KOA and healthy controls (HC). Subsequently, quantitative real-time polymerase chain reaction (qRT-PCR) was used to detect ccf-mtDNA content in the plasma samples. We used the Kellgren–Lawrence (K-L) classification criteria to classify patients with KOA into four grades: I-IV. Disease severity in patients with KOA was assessed using the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC). Next, Spearman analysis was performed to observe the correlation between ccf-mtDNA content and the K-L classification and WOMAC score. Logistic regression analysis was used to evaluate the relationship between ccf-mtDNA and KOA risk.
Results
In total, we enrolled 60 patients with KOA and HC who were matched for age, sex, and body mass index (BMI). We found that plasma ccf-mtDNA contents were significantly higher in patients with KOA (median, 2.44; quartile range, 1.10–3.79) than in HC (median, 1.08; quartile range, 0.52–2.12) (P < 0.0001). Plasma ccf-mtDNA content sequentially increased following the KOA class I-IV group (P = 0.040) and positively correlated with the K-L classification (r = 0.369, P = 0.004) and WOMAC scores (r = 0.343, P = 0.007). The ccf-mtDNA content did not significantly differ between patients with bilateral and those with single KOA (P = 0.083). Patients with high levels of ccf-mtDNA had a significantly increased risk of KOA compared with those with low levels of ccf-mtDNA (odds ratio [OR], 4.15, 95% confidence interval [CI], 1.71–10.07; P = 0.002). Quartile analysis revealed a significant dose-dependent association (P trend < 0.001).
Conclusion
Our study’s findings showed that plasma ccf-mtDNA was highly expressed in patients with KOA compared with HC. Furthermore, ccf-mtDNA content is significantly associated with the severity and risk of KOA. Therefore, its detection may provide insight into the prevention and treatment of KOA.
Keywords: Knee osteoarthritis, Circulating cell-free mitochondrial DNA, Liquid biopsy biomarker, Case–control study
Introduction
Knee osteoarthritis (KOA) is a chronic degenerative musculoskeletal disease characterized by synovial inflammation, subchondral osteosclerosis, and osteoid formation [1]. A population-based research study showed that KOA affects 16.0% of individuals over 15 years of age, with prevalence increasing with age up to 22.9% among those aged 40 years. Furthermore, the prevalence of KOA has increased due to a developing aging population and an increase in obesity. Notably, KOA is one of the leading causes of functional impairment and chronic disability worldwide [2]. Current research suggests that the development of KOA is a complex process involving inflammatory and metabolic factors, in which chronic overload and impaired joint biomechanics lead to the destruction of articular cartilage, leading to inflammation. Subsequently, the inflammation leads to joint stiffness, swelling, and loss of mobility [3]. Early symptoms of KOA are subtle, with hidden or intermittent pain as the main symptom, whereas late KOA shows signs of deformed and enlarged joints, deformity, joint effusion, and persistent pain accompanied by joint dysfunction, such as difficulty in walking up and down the stairs and squatting. This dysfunction leads to physical disability, thus affecting the patient's quality of life [4]. KOA develops gradually; however, when obvious symptoms are seen, the disease is already in the advanced stage, with no effective treatment available to reverse the pathological changes. Therefore, early diagnosis is important for timely intervention, slowing disease progression, and improving a patient’s quality of life. Currently, the diagnosis of KOA is mainly based on clinical symptoms combined with imaging. However, when imaging shows arthropathy, the disease is already in its advanced stage, causing severe and irreparable damage [5]. Consequently, there is growing interest among researchers worldwide for sensitive biomarkers that can identify disease activity and progression.
Notably, mitochondria are double-membrane organelles in the cytoplasm of eukaryotic cells that contain their DNA [6]. In addition to their primary role in energy production, they are a major source of oxidative stress from byproducts, such as reactive oxygen species (ROS) [7]. Sustained uncontrolled oxidative stress leads to increased mitochondrial membrane permeability and leakage of mitochondrial DNA (mtDNA) into the cytoplasm and extracellular space. The mtDNA is referred to as circulating cell-free mitochondrial DNA (ccf-mtDNA) and can be detected in different biological fluids (plasma or serum) [8]. Damage-associated molecular patterns (DAMPs) are molecules released in response to cellular stress or tissue injury. They are endogenous signals of danger because they induce an effective inflammatory response during non-infectious inflammation by activating the innate immune system [9, 10]. ccf-mtDNA, a DAMP originating from the mitochondria, stimulates a systemic pro-inflammatory response by activating the toll-like receptor system [11]. Therefore, ccf-mtDNA can be considered a potential liquid biopsy biomarker for mitochondrial dysfunction and oxidative stress. Moreover, liquid biopsy biomarkers based on circulating DNA samples have unique advantages over other samples because of their non-invasive nature and the ability to be repeatedly sampled [12]. Increasing evidence suggests that mitochondria play several regulatory roles in the pathogenesis of KOA, including bioenergetic metabolism, inflammatory responses, apoptosis, senescence-related responses, ROS production, and calcium metabolism [13]. Additionally, mitochondrial dysfunction and mtDNA variants contribute to cartilage degeneration and are implicated in the pathogenesis of KOA [14]. However, the role of ccf-mtDNA in KOA remains unclear.
Therefore, in the current study, we aim to investigate whether the plasma ccf-mtDNA content is potentially associated with the severity of KOA and whether it can serve as a liquid biomarker in patients with KOA.
Methods
Participants
This study was approved by the Ethics Committee of the First Affiliated Hospital of Gannan Medical University (approval number: LLSC2023-156), and informed consent was obtained from each participant. We collected samples from patients with osteoarthritis of one or both knees aged ≥ 40 years who attended the First Affiliated Hospital of Gannan Medical University between June 2023 and January 2024. Sample collection was performed following the diagnostic criteria for osteoarthritis of the knee established by the American College of Rheumatology. Participants were excluded if they had a history of knee trauma, infection, surgery, other inflammatory arthritis, autoimmune diseases, or blood-related diseases. All healthy controls (HC) were matched to the trial group for age, sex, and body mass index (BMI) and showed no evidence of clinical symptomatic changes in KOA.
The imaging severity of KOA was scored using the Kellgren (K-L) classification [15], and the severity of KOA symptoms was scored using the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) [16]. The symptoms of KOA included knee pain, joint stiffness, and joint dysfunction. Greater pain intensity correlates with increased stiffness, and more severe joint dysfunction, resulting in higher assessment scores. For patients with bilateral KOA, only the more severely affected knee was evaluated.
Plasma sample collection
Approximately 5 mL of blood was drawn into an EDTA anticoagulant blood tube in the morning. After collection, the tube was gently inverted and mixed after collection ensuring that the anticoagulant or protective agent made full contact with the blood. Subsequently, plasma was separated from the cellular components within 2 h. Using a centrifuged pre-cooled to 4℃, the sample was first centrifuged at 1,900 g for 10 min. After centrifugation, if severe hemolysis and lipemia were observed, the sample tube was gently excluded from further analysis. The plasma supernatant was transferred to a prepared 1.5 mL enzyme-free sterilization centrifugal tube, avoiding suction to the white membrane layer during the suction process. The 1.5 mL centrifuge tube containing plasma was subjected to a second centrifugation at 16,000 g, 4 °C, for 10 min to remove cells and cell debris. Finally, the centrifuged supernatant was transferred to a new 1.5 mL enzyme-free sterilized centrifuge tube, taking care not to aspirate the precipitate, and the separated plasma was stored in a -80 °C refrigerator.
Extraction of DNA sample and quantification of ccf-mtDNA in plasma
Plasma was thawed at room temperature. Subsequently, the circulating cell-free DNA (ccf-DNA) was extracted from 1 mL of plasma using the QIAamp MinElute ccfDNA kit (Qiagen, Hilden, Germany), following the manufacturer’s protocol for blood and body fluids. ccf-DNA was eluted with 55 μL of ultra-clean water. In addition, ccf-mtDNA content in the plasma was measured using quantitative real-time polymerase chain reaction (qRT-PCR) employing a modified protocol described in a previous study [17]. In this protocol, the ratio of the copy number of the mitochondrial ND1 gene to that of the human single copy of the gene 36B4 was used to determine the relative ccf-mtDNA content. In summary, the 20 μL qRT-PCR reaction for the ND1 and 36B4 genes comprised 10 μL 2 × ChamQ Universal SYBR qPCR Master Mix (Vazyme, Jiangsu, CHN), 2 μL of each primer (2 μM) (Sangong, Shanghai, CHN) and purified plasma DNA sample, and 4 μL of nuclease-free water (Solarbio, Beijing, CHN). The primer sequences are as follows: Forward primer of ND1: 5'-CCCCTAAAACCCGCCACATCT-3'; Reverse primer of ND1: 5'-GAGCGATGGTGAGAGCTAAGGT-3'; Forward primer of 36B4: 5'-CAGCAAGTGGGAAGGTGTAATCC-3; Reverse primer of 36B4: 5'-CCCATTCTATCATCAACGGGGTACAA-3'. Additionally, the thermal cycling conditions are as follows: Pre-denaturation at 95 °C for 2 min, followed by 40 cycles, each including a denaturation step at 95 °C for 15 s, an annealing step at 60 °C for 15 s, and an extension step at 72 °C for 30 s. All samples were analyzed repeatedly using a StepOne™ qRT-PCR System (Applied Biosystems) in a 48-well plate. We used the 2ΔΔCT method to analyze relative gene expression differences. Specifically, the relative content of ccf-mtDNA in each plasma sample was calculated using the formula 2ΔΔCT = 2(CT(KOAND1−KOA36B4)) − (CT(HCND1−HC36B4)), where CT represents the threshold cycle.
Statistical analysis
We used the Statistical Package for Social Sciences (SPSS) 26.0 was used to analyze the relevant data. The normality of the data was assessed using the Kolmogorov–Smirnov test and the normally distributed data was expressed as mean ± standard deviation (SD). Furthermore, comparisons between two groups were made using the student t-test, with the Student’s t-test, used for unequal variance, whereas for multiple groups the one-way analysis of variance was used, with the Krukal–Wallis H-test used for unequal variance. Non-normally distributed data were expressed as medians with quartile ranges, and comparisons between the two groups were analyzed using the Mann–Whitney test. Additionally, comparisons between multiple groups were performed using the Kruskal–Wallis H test. Chi-square was used to compare qualitative data between the two groups. In addition, Spearman’s correlation was used to analyze the correlation between plasma ccf-mtDNA levels and the degree of joint degeneration in patients with KOA. Notably, we used ccf-mtDNA as a categorical variable and performed the analysis using the cut-off value at the median or quartile value in the control group. Furthermore, unadjusted and adjusted odds ratios (OR) and 95% confidence intervals (CI) were determined by univariate and multivariate logistic regression analyses, respectively, to assess the correlation between plasma ccf-mtDNA levels and KOA risk. All statistical tests were conducted using a two-sided test using the significance α = 0.05 as the significance level. P < 0.05 was considered statistically significant.
Results
General population characteristics
In total, we included 72 patients with KOA in this study. Of these, some patients were excluded due to missing imaging data or insufficient plasma volumes (< 1 mL). Finally, 60 patients were included, comprising 48 females and 12 males, with female composition representing 80% of the sample and an age range of 43–88 years old. Sixty HC were matched to the patients with KOA by age, sex, and BMI. Among the patients with KOA, 17 had osteoporosis, with a composition ratio of 28.33%, and 15 had hypertension, with a composition ratio of 25%. Eighteen patients had osteoarthritis of the left knee, 23 had at the right knee, and 19 had it in both knees. Furthermore, patients with KOA were categorized following the X-ray K-L classification criteria: 9 patients had grade I, 22 had grade II, 18 had grade III, and 11 had grade IV. The mean and SD of the WOMAC total score for joint symptoms were 48.92 ± 25.62, with mean and SD of 13.78 ± 6.11, 6.43 ± 2.83, and 28.52 ± 17.21 for joint pain, joint stiffness, and functional impairment, respectively. (Table 1).
Table 1.
Characteristics of the study population
| Variables | KOA (n = 60) | HC (n = 60) | P value |
|---|---|---|---|
| Age (mean ± SD) | 60.73 ± 10.01 | 59.53 ± 9.54 | 0.503 |
| Gender (M/F) | 12/48 | 12/48 | 1 |
| Height (mean ± SD) | 1.59 ± 0.06 | 1.60 ± 0.06 | 0.284 |
| Weight (mean ± SD) | 60.30 ± 8.15 | 59.82 ± 7.54 | 0.737 |
| BMI (mean ± SD) | 23.97 ± 3.03 | 23.40 ± 2.31 | 0.241 |
| < 18.5 (%) | 1 (1.67) | 2 (3.33) | > 0.9999 |
| 18.5–23.9 (%) | 32 (53.33) | 34 (56.67) | 0.714 |
| 24–27.9 (%) | 18 (30.00) | 20 (33.33) | 0.695 |
| ≥ 28kg (%) | 9 (15.00) | 4 (6.67) | 0.142 |
| Comorbid condition (%) | |||
| Osteoporosis | 17 (28.33) | ||
| Hypertension | 15 (25.00) | ||
| Sides of KOA (%) | |||
| Left side | 18 (30.00) | ||
| Right side | 23 (38.33) | ||
| Bilateral | 19 (31.67) | ||
| K-L grading scale (%) | |||
| K-L = 1 | 9 (15.00) | ||
| K-L = 2 | 22 (36.67) | ||
| K-L = 3 | 18 (30.00) | ||
| K-L = 4 | 11 (18.33) | ||
| WOMAC score (mean ± SD) | |||
| Total score | 48.92 ± 25.62 | ||
| Pain score | 13.78 ± 6.11 | ||
| Stiffness score | 6.43 ± 2.83 | ||
| Physical function score | 28.52 ± 17.21 | ||
Distribution of ccf-mtDNA content in cases and controls by patient characteristics
Overall, ccf-mtDNA contents were significantly higher in patients with KOA than in HC (median, 2.44; quartile range, 1.10–3.79) vs. (median, 1.08; quartile range, 0.52–2.12) (P < 0.0001). In the stratified analyses, patients with KOA had significantly higher ccf-mtDNA in all strata except those with BMI > 23.69 than HC. In addition, we compared the differences in ccf-mtDNA levels between strata for each variable between patients with KOA and HC. In HC, ccf-mtDNA levels were higher in shorter subjects than in taller subjects (P = 0.043). The strata of other variables in the HC group did not significantly differ from all variables in the KOA group (Table 2).
Table 2.
Distributions of plasma ccf-mtDNA content by host characteristics in cases and controls
| ccf-mtDNA: Median (Quartile range) | |||
|---|---|---|---|
| Variables | KOA (n = 60) | HC (n = 60) | Pa value |
| Overall | 2.44 (1.10–3.79) | 1.08 (0.52–2.12) | < 0.0001 |
| Age (years) | |||
| ≤ 60.1 | 2.86 (1.26–3.72) | 1.21 (0.59–2.16) | 0.000 |
| > 60.1 | 1.88 (0.90–4.66) | 0.87 (0.43–2.00) | 0.008 |
| Pb value | 0.318 | 0.346 | |
| Gender | |||
| Female | 2.63 (1.04–3.92) | 1.21 (0.52–2.32) | 0.000 |
| Male | 1.98 (1.17–2.96) | 0.70 (0.46–1.45) | 0.002 |
| Pb value | 0.526 | 0.135 | |
| Height (m) | |||
| ≤ 1.59 | 2.86 (1.11–4.42) | 1.47 (0.49–2.59) | 0.001 |
| > 1.59 | 1.95 (1.03–3.10) | 0.80 (0.58–1.12) | 0.001 |
| Pb value | 0.100 | 0.043 | |
| Weight (kg) | |||
| ≤ 60 | 2.69 (1.31–4.18) | 1.21 (0.48–2.29) | 0.000 |
| > 60 | 1.36 (0.91–3.64) | 0.89 (0.59–1.81) | 0.013 |
| Pb value | 0.138 | 0.527 | |
| BMI (kg/m2) | |||
| ≤ 23.69 | 2.58 (1.37–4.28) | 0.73 (0.45–2.08) | < 0.0001 |
| > 23.69 | 2.09 (0.94–3.54) | 1.32 (0.86–2.38) | 0.114 |
| Pb value | 0.231 | 0.170 | |
Pa: Differences between KOA cases and HC
Pb: Differences between the two strata of each host variables
Comparison of the general conditions and ccf-mtDNA content among patients with different K-L grades of KOA
Patients with KOA were divided into four groups following the X-ray K-L classification. The WOMAC scores (including pain, stiffness, dysfunction, and total score) significantly differed between the groups under the different K-L classifications (P < 0.0001). Furthermore, ccf-mtDNA expression increased continuously with increasing K-L grade, and the difference was statistically significant (P = 0.040). No statistically significant differences were observed in age, sex, height, weight, or BMI among the different K-L classifications (P = 0.136, 0.409, 0.261, 0.563, and 0.975, respectively) (Table 3).
Table 3.
Comparison of the general conditions and ccf-mtDNA content among patients with different K-L grades of KOA
| K-L n | I 9 | II 22 | III 18 | IV 11 | P value |
|---|---|---|---|---|---|
| Age (mean ± SD) | 54.44 (9.52) | 60.59 (11.00) | 61.56 (9.65) | 64.82 (7.14) | 0.136 |
| Female (%) | 66.67 | 86.36 | 72.22 | 90.91 | 0.409 |
| Height (mean ± SD) | 1.62 (0.06) | 1.58 (0.05) | 1.59 (0.07) | 1.57 (0.04) | 0.261 |
| Weight (mean ± SD) | 64.44 (13.58) | 59.77 (13.58) | 59.67 (5.54) | 59.00 (7.73) | 0.563 |
| BMI (mean ± SD) | 24.39 (4.01) | 23.95 (2.34) | 23.81 (3.27) | 23.94 (3.37) | 0.975 |
| WOMAC (mean ± SD) | |||||
| Total score | 18.89 (3.72) | 34.18 (9.06) | 56.72 (12.18) | 90.18 (12.80) | < 0.0001 |
| Pain score | 7.89 (2.15) | 10.14 (2.66) | 15.28 (3.21) | 23.45 (4.13) | < 0.0001 |
| Stiffness score | 3.56 (1.59) | 4.91 (1.66) | 7.28 (1.74) | 10.45 (1.64) | < 0.0001 |
| Physical function score | 7.44 (1.94) | 19.14 (6.66) | 33.61 (7.46) | 56.18 (8.60) | < 0.0001 |
| ccf-mtDNA content (median, quartile range) | 1.22 (0.84–2.65) | 2.26 (0.98–3.12) | 3.45 (1.50–4.66) | 4.52 (1.09–5.84) | 0.040 |
Correlation analysis of plasma ccf-mtDNA contents with K-L classifications and WOMAC in patients with KOA
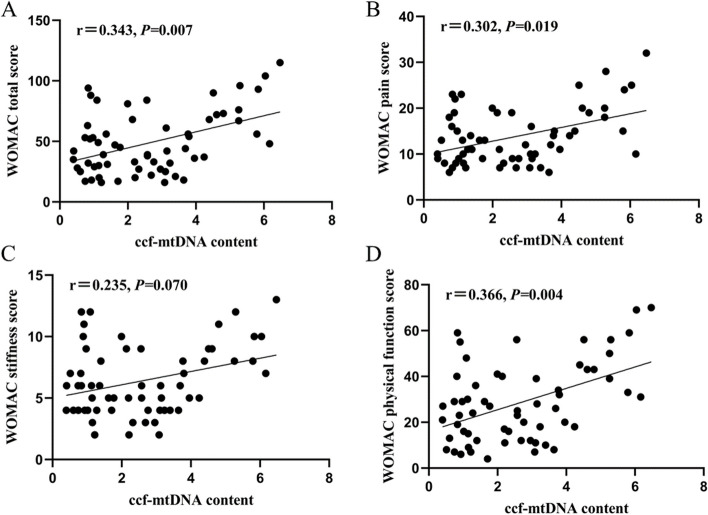
Results from Spearman's analysis showed that plasma ccf-mtDNA content in patients with KOA positively correlated with K-L grading (r = 0.369, P = 0.004) (Fig. 1). Furthermore, Spearman correlation analysis showed that plasma ccf-mtDNA content was positively correlated with the total WOMAC score, pain score, and joint function score (r = 0.343, 0.302, and 0.366; P = 0.007, 0.019, and 0.004, respectively); however, no correlation was observed the stiffness score (r = 0.235; P = 0.070) (Fig. 2).
Fig. 1.
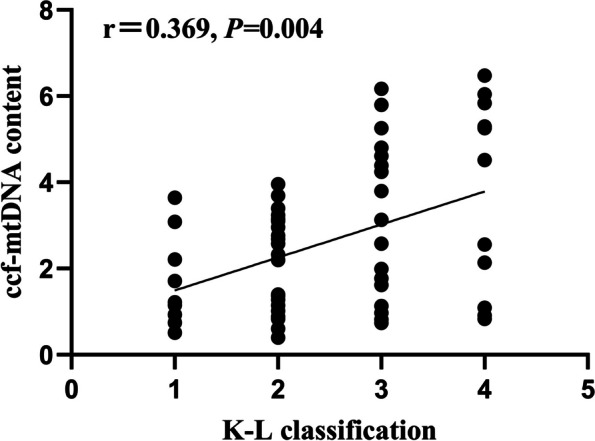
Correlation of plasma ccf-mtDNA with K-L classification
Fig. 2.
Correlation of plasma ccf-mtDNA with WOMAC score. A Correlation of plasma ccf-mtDNA with WOMAC total score. B Correlation of plasma ccf-mtDNA with WOMAC pain score. C Correlation of plasma ccf-mtDNA with WOMAC stiffness score. D Correlation of plasma ccf-mtDNA with WOMAC physical function score
Comparison of general conditions and ccf-mtDNA content between patients with bilateral KOA and single KOA
Patients with KOA were divided into two groups based on whether the patient had bilateral knee osteoarthritis. The characteristics and expression of ccf-mtDNA were analyzed between the two groups. The results showed that there were no significant differences in age, sex, height, weight, body mass index, or ccf-mtDNA content between the two groups (P = 0.270, 0.835, 0.220, 0.755, 0.289, and 0.083, respectively (Table 4).
Table 4.
Comparison of general conditions and ccf-mtDNA content between patients with bilateral KOA and single KOA
| Variables | Bilateral KOA (n = 19) | Single KOA (n = 41) | P value |
|---|---|---|---|
| Age (mean ± SD) | 62.84 (11.50) | 59.76 (9.23) | 0.270 |
| Female (%) | 78.95 | 80.49 | 0.835 |
| Height (mean ± SD) | 1.57 (0.06) | 1.59 (0.06) | 0.220 |
| Weight (mean ± SD) | 60.79 (8.18) | 60.07 (8.23) | 0.755 |
| BMI (mean ± SD) | 24.59 (3.10) | 23.69 (2.99) | 0.289 |
|
ccf-mtDNA content (median, quartile range) |
3.69 (0.91–5.26) | 2.14 (1.11–3.18) | 0.083 |
Association between ccf-mtDNA content and KOA risk
The association between ccf-mtDNA content and KOA risk was modeled using unconditional logistic regression by analyzing ccf-mtDNA content as a categorical variable based on the cut-off value of the median or interquartile distribution of ccf-mtDNA content in the control group. The results showed that patients with KOA who had higher ccf-mtDNA contents (> 1.08) had a significantly increased risk of KOA in both univariate (unadjusted OR, 3.29; 95% CI, 1.50–7.20; P = 0.003) and multivariate analyses (adjusted OR, 4.15; 95% CI, 1.71–10.07; P = 0.002) compared with those with lower ccf-mtDNA contents (≤ 1.08). In quartile analyses, using patients with the lowest ccf-mtDNA levels as a reference, those with higher ccf-mtDNA levels had a significantly increased risk of KOA in both univariate and multifactorial analyses, with a clear dose-dependent relationship (P for trend = 0.001 and < 0.001, respectively) (Table 5).
Table 5.
Association of plasma ccf-mtDNA content with the risk of KOA
| Unadjusted | Adjusted | |||||
|---|---|---|---|---|---|---|
| ccf-mtDNA | KOA | HC | OR (95% CI) | P value | OR (95% CI) | P value |
| By median | ||||||
| Lowe | 14 | 30 | ||||
| Higher | 46 | 30 | 3.29 (1.50-7.20) | 0.003 | 4.15 (1.71-10.07) | 0.002 |
| By quartile | ||||||
| 1st quartile | 3 | 15 | 1 | - | 1 | - |
| 2nd quartile | 11 | 15 | 3.67 (0.85-15.85) | 0.082 | 3.35 (0.76-14.84) | 0.112 |
| 3rd quartile | 12 | 15 | 4.00 (0.93-17.12) | 0.062 | 4.49 (0.99-20.47) | 0.052 |
| 4th quartile | 34 | 15 | 11.33 (2.85-45.05) | 0.001 | 13.36 (3.18-56.19) | 0.000 |
| P for trend | 0.001 | < 0.001 | ||||
Adjusted for age, gender, height, weight, and BMI
Association between ccf-mtDNA content and KOA risk stratified by patients’ characteristics
We further assessed the association between ccf-mtDNA content and the risk of KOA, stratified by patient characteristics, using multivariate analyses corrected for age, sex, height, weight, and BMI, where appropriate. In the stratified analyses, increased ccf-mtDNA levels significantly increased the risk of developing KOA in all strata, except for patients > 60.1 years (P = 0.203), weighing > 60 kg (P = 0.211), and those with BMI > 23.69 (P = 0.355) (Table 6).
Table 6.
Association of plasma ccf-mtDNA content with KOA risk stratified by host characteristics
| Variables | ccf-mtDNA | KOA | HC | OR (95% CI) | P value |
|---|---|---|---|---|---|
| Age | |||||
| ≤ 60.1 | Lower | 5 | 15 | 1 | 0.005 |
| Higher | 29 | 21 | 7.49 (1.83–30.70) | ||
| > 60.1 | Lower | 9 | 15 | 1 | 0.203 |
| Higher | 17 | 9 | 2.31 (0.64–8.42) | ||
| Gender | |||||
| Female | Lower | 12 | 22 | 1 | 0.012 |
| Higher | 36 | 26 | 3.63 (1.33–9.92) | ||
| Male | Lower | 2 | 8 | 1 | 0.030 |
| Higher | 10 | 4 | 9.76 (1.25–76.10) | ||
| Height | |||||
| ≤ 1.59 | Lower | 9 | 14 | 1 | 0.035 |
| Higher | 29 | 24 | 3.94 (1.10–14.10) | ||
| > 1.59 | Lower | 5 | 16 | 1 | 0.004 |
| Higher | 17 | 6 | 8.84 (2.04–38.36) | ||
| Weight | |||||
| ≤ 60 | Lower | 7 | 15 | 1 | 0.008 |
| Higher | 30 | 21 | 6.29 (1.62–24.47) | ||
| > 60 | Lower | 7 | 15 | 1 | 0.211 |
| Higher | 16 | 9 | 2.39 (0.61–9.31) | ||
| BMI | |||||
| ≤ 23.69 | Lower | 6 | 20 | 1 | 0.001 |
| Higher | 26 | 15 | 8.34 (2.27–30.70) | ||
| > 23.69 | Lower | 8 | 10 | 1 | 0.355 |
| Higher | 20 | 15 | 1.88 (0.49–7.18) | ||
Adjusted for age, gender, height, weight, and BMI where appropriate
Discussion
In this study, we found that the plasma ccf-mtDNA content was higher in patients with KOA than in HC. In addition, ccf-mtDNA significantly correlated with KOA severity based on the Spearman analysis of the correlation of ccf-mtDNA levels with the X-ray KL classification and WOMAC scores. Logistic regression analysis revealed that ccf-mtDNA may be a risk factor for developing KOA. Therefore, ccf-mtDNA is a marker of mitochondrial dysfunction and cellular stress, and detecting its levels may help prevent and treat KOA.
Consistent with the epidemiological results, we included a high proportion of female participants (80%) in this study, indicating a greater prevalence of KOA among females than males. Additionally, 45% of patients in the KOA group were obese and the age of the patients increased as the K-L grade increased, suggesting that sex, obesity, and age may be risk factors in the pathogenesis of KOA, similar to the findings of a previous study [18].
Notably, the mitochondria do not have the same complex DNA repair mechanisms as the nucleus, and the absence of protective histones in mtDNA is responsible for the damage. Subsequently, the damaged mtDNA fragments are released into the cytoplasm or extracellular space [19, 20]. Following the release into the extracellular space, mtDNA fragments such as ccf-mtDNA can be detected in the blood or other body fluid samples [21, 22]. ccf-mtDNA behaves similarly to DAMPs by activating toll-like receptor 9 (TLR9), inflammatory vesicles, the interferon gene stimulator pathway, and the stimulator pathway to induce inflammation [23]. Increasing evidence suggests that innate response pathways may significantly influence the onset and progression of KOA, particularly TLR [24–26]. Reports revealed that TLR-9 activates nuclear transcription factor-κB (NF-κB) transcription factors, leading to the production of pro-inflammatory cytokines, thereby promoting the development of KOA [27]. Mitochondrial dysfunction maintains an active regenerative cycle through oxidative stress, increased ROS, and mtDNA damage, which are regarded as hallmarks of chronic degenerative diseases such as KOA. The accumulation of ROS and mtDNA damage can activate the NF-κB pathway, which majorly regulates inflammation [28]. Significant increases in ccf-mtDNA levels have been observed in diseases with chronic inflammatory states, such as trauma, sepsis, aging, cancer, and immune-mediated diseases [29–33]. In this study, we found that plasma ccf-mtDNA levels were significantly higher in patients with KOA than in HC. However, our findings are not consistent with those of Panagopoulou et al. Panagopoulou et al. did not find a significant difference in plasma ccf-mtDNA levels between patients with KOA and those of HC (P = 0.852) [34]. This inconsistency could be attributed to several factors. In their study, the comparison of ccf-mtDNA between patients with KOA and HC was a secondary analysis that did not strictly control for major confounders. Additionally, the study by Panagopoulou et al. had a small sample size, which may have led to unstable estimates. Finally, this inconsistency could also be due to other factors, such as differences in assay methods (TaqMan method vs. SYBR Green method) or patient ethnicity (Chinese vs. Greek). Hence, further large, well-controlled studies are required to validate these results.
Furthermore, we categorized patients with KOA into four groups following the X-ray KL classification criteria based on the severity of KOA imaging. Additionally, we used WOMAC to assess the severity of symptoms in these patients. Märtens et al. performed a study on the correlation of radiographic changes and pain sensation in shoulder osteoarthritis with patient age. The results show that age was positively correlated with the X-ray K-L classification; the higher the K-L classification, the older the age [35]. Similarly, in this study, we found that as the radiographic K-L grade increased, the age of patients also tended to increase. Furthermore, the WOMAC pain, stiffness, joint function, and total scores were significantly higher as the K-L grade increased, which suggests that the severity of KOA imaging findings is associated with the severity of its symptoms. However, further studies revealed that plasma ccf-mtDNA levels increased sequentially from KOA I–IV groups, suggesting that ccf-mtDNA may be involved in the development of KOA. Notably, joint pain, stiffness, and dysfunction are common symptoms of KOA that affect and quality of life of patients with KOA [36]. Spearman correlation analysis showed that plasma ccf-mtDNA positively correlated with the K-L classification, WOMAC total score, pain score, and joint function score; however, no correlation was found with the stiffness score, suggesting that plasma ccf-mtDNA levels could reflect the severity of KOA. Meanwhile, we analyzed the number of affected joints, and our results showed that ccf-mtDNA content did not significantly differ between patients with bilateral KOA and those with a single KOA. However, ccf-mtDNA content in patients with bilateral KOA tends to be higher than that in patients with single KOA, which may be attributed to the small sample size; hence partially masking the correlation between ccf-mtDNA and the K-L grade. Therefore, future studies with larger sample sizes are needed to investigate the relationship between ccf-mtDNA and the number of affected joints in patients with KOA.
In the early stage of KOA, before irreversible structural alterations occur in the joints, several biomarkers in blood already show alterations. These include inflammatory indicators such as C-reactive protein and interleukin-6 (IL-6), cartilage metabolism indicators, including type II collagen carboxy-terminal telopeptide and cartilage oligomeric matrix protein, lipid metabolism indicators such as cholesterol and fatty acids, and circulating nucleic acids such as microRNAs and cfDNA [37–39]. Detection biomarkers in plasma reflecting the severity of the disease can predict the destruction of bone and cartilage at an earlier stage, help to further understand the pathogenesis of KOA, and provide a new research direction for the treatment of the disease. The higher number of mtDNA copies makes it easier to detect mtDNA content than nuclear DNAs in body fluid samples, such as plasma and serum, where the total DNA concentration is deficient [40]. In addition, ccf-mtDNA as a liquid biopsy biomarker has the advantages of noninvasiveness and repeated sampling [41]. Therefore, detection of abnormal changes in ccf-mtDNA has become an increasingly important tool for early disease diagnosis. However, plasma ccf-mtDNA is a non-specific marker, as it is significantly elevated in other inflammatory immune diseases, mitochondrial diseases, and tumors [42–44]. Because most KOA patients are older, they are likely to have some associated comorbidities, which may lead to increased ccf-mtDNA levels. Therefore, future studies need larger sample sizes to control for confounding effects of other diseases through multivariate analysis. The irreversible nature of cartilage damage in the knee joints of patients with KOA makes it difficult to treat, and the etiology and pathogenesis of the disease have not yet been clarified. Therefore, it is clinically important to control the risk factors to reduce morbidity. Studies have shown that age, sex, and obesity are risk factors for KOA [45]. In this study, plasma ccf-mtDNA was found to be a pathogenic factor for KOA. In addition to the risk factors reported in previous studies, attention should be paid to patients with elevated plasma ccf-mtDNA levels so that timely measures can be taken to treat KOA.
In stratified analyses, we found that plasma ccf-mtDNA levels were significantly higher in patients with KOA; however, plasma ccf-mtDNA levels greatly differed between patients with KOA and HC, patients with age > 60.1, weight > 60, or BMI > 23.69 compared with patients with age ≤ 60.1, weight ≤ 60, or BMI ≤ 23.69. The close association between age, obesity, and KOA may have masked the relatively modest association conferred by a greater amount of ccf-mtDNA. Furthermore, because of the lack of statistical validity, we cannot exclude the possibility that a smaller number of participants may have contributed to these observations. Therefore, larger independent studies are needed to further explore the interaction between ccf-mtDNA and key demographic variables associated with KOA risk.
Previous findings suggest that ccf-mtDNA may contribute to inflammation and, therefore, may be directly involved in the pathogenesis of KOA. mtDNA activates TLR9 in the endolysosomal membrane when its fragments are released extracellularly and persist in the extracellular fluid, such as ccf-mtDNA. Therefore, activating NF-κB and the transcription of pro-inflammatory genes [46]. Notably, hydroxychloroquine has potential as a disease-modifying osteoarthritis drug (DMOAD) for treating KOA owing to its inhibitory effect on TLR signaling and ability to promote its degradation via a pro-inflammatory pathway [47]. Recent studies have shown that the cGAS-STING pathway may be activated by cytoplasmic mtDNA, and its activation by mtDNA leads to the expression of tumor necrosis factor-alpha (TNF-α) and IL-6, cytokines that are therapeutic targets for KOA [42, 47].
The present study has some limitations. First, only the plasma levels of ccf-mtDNA were measured in this study, whereas the levels of ccf-mtDNA in the joint fluid were not; therefore, the correlation between ccf-mtDNA and KOA in the joint fluid remains unclear. Second, this study was affected by many potential confounding factors. Future studies with large sample sizes, varying patient-level factors, and controlling for confounding factors are needed. Additionally, patients with KOA undergo surgical or exercise therapy shortly after admission, so we cannot determine the progression of their disease and, therefore, cannot detect the progression of these patients through analysis of baseline ccf-mtDNA. Hence, future community studies are needed to investigate the association between plasma ccf-mtDNA and the progression of KOA in patients. Finally, the study’s design was cross-sectional, which did not allow us to conclude a causal relationship between ccf-mtDNA and KOA development. More prospective cohort studies are needed to further confirm this finding.
Conclusion
In summary, ccf-mtDNA was highly expressed in the plasma of patients with KOA, and the plasma ccf-mtDNA level significantly correlated with the severity of KOA, which is a risk factor for the development of KOA. The detection of ccf-mtDNA may provide clues for the prevention and treatment of KOA, and its pro-inflammatory pathway may become a pharmacological target; however, its specific mechanism requires further study.
Acknowledgements
Not applicable.
Abbreviations
- KOA
Knee osteoarthritis
- ROS
Reactive oxygen species
- mtDNA
Mitochondrial DNA
- ccf-mtDNA
Circulating cell-free mitochondrial DNA
- DAMPs
Damage-associated molecular patterns
- HC
Healthy controls
- BMI
Body mass index
- K-L
Kellgren-Lawrence
- WOMAC
Western Ontario and McMaster Universities Osteoarthritis Index
- ccf-DNA
Circulating cell-free DNA
- qRT-PCR
Quantitative real-time polymerase chain reaction
- SD
Standard deviation
- OR
Odds ratio
- 95% CI
95% Confidence intervals
- TLR
Toll-like receptor
- NF-κB
Nuclear transcription factor-κB
- DMOAD
Disease-modifying osteoarthritis drug
- TNF-α
Tumor necrosis factor-alpha
- IL-6
Interleukin-6
Authors’ contributions
Yan-lin Wu collected and analyzed the data and wrote the manuscript. Shaogui Wan supervised the study and revised the manuscript. Long and Ye used the K-L and WOMAC scores to assess knee injury severity in patients with KOA. Yang and Luo collected blood samples from patients with KOA and HC and isolated plasma samples. Zhong and Xiao extracted ccf-DNA. Chen et al. used qRT-PCR to detect ccf-mtDNA content. Mao-Yuan Wang conceived and designed the study.
Funding
Mao-Yuan Wang was supported by the National Natural Science Foundation of China (82060420), a major project of the Ganzhou Science and Technology Bureau (2023LNS37155) and the Science and the Technology Program Project of Jiangxi Provincial Health and Wellness Commission (202410058).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request at: wmy.gmu.kf@gmail.com.
Declarations
Ethics approval and consent to participate
The study was conducted following the principles of the Declaration of Helsinki. This study was approved by the Ethics Committee of the First Affiliated Hospital of Gannan Medical University (approval number: LLSC2023-156) and informed consent was obtained from each participant.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vincent KR, Conrad BP, Fregly BJ, Vincent HK. The pathophysiology of osteoarthritis: a mechanical perspective on the knee joint. Pm r. 2012;4(5 Suppl):S3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cui A, Li H, Wang D, Zhong J, Chen Y, Lu H. Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. EClinicalMedicine. 2020;29–30:100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jang S, Lee K, Ju JH. Recent Updates of Diagnosis, Pathophysiology, and Treatment on Osteoarthritis of the Knee. Int J Mol Sci. 2021;22(5):2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol. 2007;213(3):626–34. [DOI] [PubMed] [Google Scholar]
- 5.Mahmoudian A, Lohmander LS, Mobasheri A, Englund M, Luyten FP. Early-stage symptomatic osteoarthritis of the knee - time for action. Nat Rev Rheumatol. 2021;17(10):621–32. [DOI] [PubMed] [Google Scholar]
- 6.Malik AN, Czajka A. Is mitochondrial DNA content a potential biomarker of mitochondrial dysfunction? Mitochondrion. 2013;13(5):481–92. [DOI] [PubMed] [Google Scholar]
- 7.Nilsson MI, Tarnopolsky MA. Mitochondria and aging—The role of exercise as a countermeasure. Biology. 2019;8(2):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gambardella S, Limanaqi F, Ferese R, Biagioni F, Campopiano R, Centonze D, Fornai F. ccf-mtDNA as a Potential Link Between the Brain and Immune System in Neuro-Immunological Disorders. Front Immunol. 2019;10:1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen GY, Nuñez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10(12):826–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vénéreau E, Ceriotti C, Bianchi ME. DAMPs from Cell Death to New Life. Front Immunol. 2015;6:422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCarthy CG, Wenceslau CF, Goulopoulou S, Ogbi S, Baban B, Sullivan JC, Matsumoto T, Webb RC. Circulating mitochondrial DNA and Toll-like receptor 9 are associated with vascular dysfunction in spontaneously hypertensive rats. Cardiovasc Res. 2015;107(1):119–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.González-Masiá JA, García-Olmo D, García-Olmo DC. Circulating nucleic acids in plasma and serum (CNAPS): applications in oncology. Onco Targets Ther. 2013;6:819–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Picard M, McManus MJ, Gray JD, Nasca C, Moffat C, Kopinski PK, Seifert EL, McEwen BS, Wallace DC. Mitochondrial functions modulate neuroendocrine, metabolic, inflammatory, and transcriptional responses to acute psychological stress. Proc Natl Acad Sci U S A. 2015;112(48):E6614–6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blanco FJ, Valdes AM, Rego-Pérez I. Mitochondrial DNA variation and the pathogenesis of osteoarthritis phenotypes. Nat Rev Rheumatol. 2018;14(6):327–40. [DOI] [PubMed] [Google Scholar]
- 15.Kohn MD, Sassoon AA, Fernando ND. Classifications in brief: Kellgren-Lawrence classification of osteoarthritis. Clin Orthop Relat Res. 2016;474(8):1886–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833–40. [PubMed] [Google Scholar]
- 17.Zhou G, Li Y, Li S, Liu H, Xu F, Lai X, Zhang Q, Xu J, Wan S. Circulating cell-free mtDNA content as a non-invasive prognostic biomarker in HCC patients receiving TACE and traditional chinese medicine. Front Genet. 2021;12:719451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong Y, Yan Y, Zhou J, Zhou Q, Wei H. Evidence on risk factors for knee osteoarthritis in middle-older aged: a systematic review and meta analysis. J Orthop Surg Res. 2023;18(1):634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krysko DV, Agostinis P, Krysko O, Garg AD, Bachert C, Lambrecht BN, Vandenabeele P. Emerging role of damage-associated molecular patterns derived from mitochondria in inflammation. Trends Immunol. 2011;32(4):157–64. [DOI] [PubMed] [Google Scholar]
- 20.Wang L, Xie L, Zhang Q, Cai X, Tang Y, Wang L, Hang T, Liu J, Gong J. Plasma nuclear and mitochondrial DNA levels in acute myocardial infarction patients. Coron Artery Dis. 2015;26(4):296–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan E, Liu D, Perry L, Zhu J, Cid-Serra X, Deane A, Yeo C, Ajani A. Cell-free DNA as a potential biomarker for acute myocardial infarction: A systematic review and meta-analysis. Int J Cardiol Heart Vasc. 2023;47:101246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oka T, Hikoso S, Yamaguchi O, Taneike M, Takeda T, Tamai T, Oyabu J, Murakawa T, Nakayama H, Nishida K, et al. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature. 2012;485(7397):251–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.West AP, Shadel GS. Mitochondrial DNA in innate immune responses and inflammatory pathology. Nat Rev Immunol. 2017;17(6):363–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barreto G, Manninen M, Eklund K K. Osteoarthritis and Toll-Like Receptors: When Innate Immunity Meets Chondrocyte Apoptosis. Biology (Basel). 2020;9(4):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13(5):816–25. [DOI] [PubMed] [Google Scholar]
- 26.Chow YY, Chin KY. The role of inflammation in the pathogenesis of osteoarthritis. Mediators Inflamm. 2020;2020:8293921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng M, Shi S, Zheng Q, Wang Y, Ying X, Jin Y. Association between TLR-9 gene rs187084 polymorphism and knee osteoarthritis in a Chinese population. Biosci Rep. 2017;37(5):BSR20170844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Minguzzi M, Cetrullo S, D’Adamo S, Silvestri Y, Flamigni F, Borzì RM. Emerging players at the intersection of chondrocyte loss of maturational arrest, oxidative stress, senescence and low-grade inflammation in osteoarthritis. Oxid Med Cell Longev. 2018;2018:3075293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simmons JD, Lee YL, Mulekar S, Kuck JL, Brevard SB, Gonzalez RP, Gillespie MN, Richards WO. Elevated levels of plasma mitochondrial DNA DAMPs are linked to clinical outcome in severely injured human subjects. Ann Surg. 2013;258(4):591–6 (discussion 596-598). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gu X, Yao Y, Wu G, Lv T, Luo L, Song Y. The plasma mitochondrial DNA is an independent predictor for post-traumatic systemic inflammatory response syndrome. PLoS ONE. 2013;8(8):e72834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hajizadeh S, DeGroot J, TeKoppele JM, Tarkowski A, Collins LV. Extracellular mitochondrial DNA and oxidatively damaged DNA in synovial fluid of patients with rheumatoid arthritis. Arthritis Res Ther. 2003;5(5):R234–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pinti M, Cevenini E, Nasi M, De Biasi S, Salvioli S, Monti D, Benatti S, Gibellini L, Cotichini R, Stazi MA, et al. Circulating mitochondrial DNA increases with age and is a familiar trait: Implications for “inflamm-aging.” Eur J Immunol. 2014;44(5):1552–62. [DOI] [PubMed] [Google Scholar]
- 33.Liu S, Feng M, Guan W. Mitochondrial DNA sensing by STING signaling participates in inflammation, cancer and beyond. Int J Cancer. 2016;139(4):736–41. [DOI] [PubMed] [Google Scholar]
- 34.Panagopoulou M, Karaglani M, Tzitzikou K, Kessari N, Arvanitidis K, Amarantidis K, Drosos GI, Gerou S, Papanas N, Papazoglou D, et al. Mitochondrial Fraction of Circulating Cell-Free DNA as an Indicator of Human Pathology. Int J Mol Sci. 2024;25(8):4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Märtens N, März V, Bertrand J, Lohmann CH, Berth A. Radiological changes in shoulder osteoarthritis and pain sensation correlate with patients’ age. J Orthop Surg Res. 2022;17(1):277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu Q, Chen B, Wang Y, Wang X, Han D, Ding D, Zheng Y, Cao Y, Zhan H, Zhou Y. The effectiveness of manual therapy for relieving pain, stiffness, and dysfunction in knee osteoarthritis: a systematic review and meta-analysis. Pain Physician. 2017;20(4):229–43. [PubMed] [Google Scholar]
- 37.Ostojic M, Oliveira JP, Kordic D, Mouton C, Prill R, Becker R. Blood and urine biomarkers for the diagnosis of early stages of knee osteoarthritis: A systematic review. J Exp Orthop. 2024;11(3):e12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mobasheri A, Thudium CS, Bay-Jensen AC, Maleitzke T, Geissler S, Duda GN, Winkler T. Biomarkers for osteoarthritis: Current status and future prospects. Best Pract Res Clin Rheumatol. 2023;37(2):101852. [DOI] [PubMed] [Google Scholar]
- 39.Budd E, Nalesso G, Mobasheri A. Extracellular genomic biomarkers of osteoarthritis. Expert Rev Mol Diagn. 2018;18(1):55–74. [DOI] [PubMed] [Google Scholar]
- 40.Kohler C, Radpour R, Barekati Z, Asadollahi R, Bitzer J, Wight E, Bürki N, Diesch C, Holzgreve W, Zhong XY. Levels of plasma circulating cell free nuclear and mitochondrial DNA as potential biomarkers for breast tumors. Mol Cancer. 2009;8:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nikanjam M, Kato S, Kurzrock R. Liquid biopsy: current technology and clinical applications. J Hematol Oncol. 2022;15(1):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao X, Yu M, Zhao Y, Zheng Y, Meng L, Du K, Xie Z, Lv H, Zhang W, Liu J, et al. Circulating cell-free mtDNA release is associated with the activation of cGAS-STING pathway and inflammation in mitochondrial diseases. J Neurol. 2022;269(9):4985–96. [DOI] [PubMed] [Google Scholar]
- 43.Yu M. Circulating cell-free mitochondrial DNA as a novel cancer biomarker: opportunities and challenges. Mitochondrial DNA. 2012;23(5):329–32. [DOI] [PubMed] [Google Scholar]
- 44.Zhong XY, Guo Y, Fan Z. Increased level of free-circulating MtDNA in maintenance hemodialysis patients: Possible role in systemic inflammation. J Clin Lab Anal. 2022;36(7):e24558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yao Q, Wu X, Tao C, Gong W, Chen M, Qu M, Zhong Y, He T, Chen S, Xiao G. Osteoarthritis: pathogenic signaling pathways and therapeutic targets. Signal Transduct Target Ther. 2023;8(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee YH. Efficacy of hydroxychloroquine for knee osteoarthritis. Korean J Intern Med. 2022;37(1):51–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen Q, Sun L, Chen ZJ. Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat Immunol. 2016;17(10):1142–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request at: wmy.gmu.kf@gmail.com.