Abstract
Background
This study aimed to understand the vision health status of adolescents in the Western Pacific Region (WPR) using Global Burden of Disease (GBD) data from 1990 to 2019.
Methods
We conducted a comprehensive analysis of blindness and vision loss using GBD data from 1990 to 2019, analyzed trends in the prevalence and burden of blindness and vision loss over time using joinpoint regression, and analyzed their independent effects on blindness and vision loss in three dimensions using age-period-cohort (APC) modeling.
Results
The prevalence of blindness and vision loss among adolescents in the WPR showed an increasing trend between 1990 and 2019 (AAPC: 0.56%) and a slight increase in YLD (AAPC: 0.11%). The joinpoint regression showed a decreasing trend after 2017 (AAPC: -2.45%). The prevalence and burden trended downward in most Western Pacific countries, with only China rising significantly (AAPC prevalence: 0.57%). Refractive disorders had the highest prevalence among the subgroups. The risk of blindness and vision loss in adolescents reduced with age but grew later in time and at later birth. In addition, prevalence and burden were generally higher in females than males.
Conclusions
In the context of a declining global trend in the prevalence and burden of blindness and vision loss among adolescents, there is an increasing trend among adolescents in WPR, with the most pronounced rise in China. This finding has important implications for WPR, suggesting that more attention should be given to adolescent eye health in the region.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12889-024-20607-5.
Keywords: Vision loss, Disease burden, Joinpoint regression, Age-period-cohort, Western pacific
Introduction
Blindness and vision loss are important global health problems and are the third most important disorders after anemia and hearing loss [1]. Eye diseases that can lead to vision loss or even blindness include age-related macular degeneration, cataracts, corneal opacity, diabetic retinopathy, glaucoma, refractive errors, and trachoma [2]. According to the World Health Organization, at least 2.2 billion people worldwide suffer from visual impairment, of which at least 1 billion suffer from preventable or unresolved visual impairment [3]. These striking figures indicate that visual problems are widespread.
Blindness and visual impairment significantly affects an individual’s quality of life [4]. Individuals with blindness and vision loss are unable to participate in common daily activities, such as exercise, learning, work, and social interactions, which can lead to limited mobility or reduced cognitive ability [5]. Additionally, blindness and visual impairment impose a significant socioeconomic burden. Particularly noteworthy is the reported economic burden caused by uncorrected myopia in East Asia, South Asia, and Southeast Asia, which is more than twice that of other regions, equivalent to over 1% of the gross domestic product (GDP) [6]. The global burden of vision problems is evident, and although it affects people of all ages, the visual health of adolescents is of particular concern.
Adolescence is an important stage in the development of the visual system, and any damage to vision during this stage may have long-term effects on an individual’s future [7, 8]. Unfortunately, we found that blindness and visual impairment can cause significant harm to the adolescent population. Certain eye diseases commonly occur during childhood, including myopia [9], retinopathy of prematurity [10], and amblyopia [11]. These visual impairments may not only lead to limitations in physical activity, such as restricted mobility due to environmental barriers (e.g., transportation barriers) [12], but may also affect academic performance and limit developmental potential [13]. Moreover, visual impairment can trigger psychological and emotional stress, influencing social interactions and mental health [14]. For example, research has demonstrated a correlation between visual impairment and lower self-esteem [15].
With the development of global medical resources, some early vision problems in children and adolescents have improved significantly. However, the prevalence of certain visual conditions increases during adolescence. A trend analysis study using GBD data showed that the global burden of visual impairment in children under 5 years of age has decreased markedly, whereas the burden of myopia in adolescents aged 15–19 years has increased dramatically [16]. This trend is particularly evident in some regions, with studies finding that eye health problems among adolescents in East and Southeast Asia are more severe than those in other regions [17–19]. In China alone, 6.4 million children and adolescents are visually impaired, and this number is expected to increase to 180 million by 2030 according to a projection study on the burden of vision [20]. Other studies have found that the prevalence of myopia among adolescents is 70% in Singapore [21], 94.9% in Japan [22], and 96.5% in South Korea [23]. Although some of the data relied on self-reports or school physical examination records, which may be subject to error, they were derived from government-supported national surveys and research projects by academic institutions using large sample sizes and standardized methodologies, lending them high credibility. However, it is worth noting that these alarming data are concentrated in the Western Pacific Region (WPR), raising concerns regarding adolescent visual health.
The WPR encompasses 31 countries, including parts of East and Southeast Asia and many island nations, and spans multiple cultural and economic contexts, making it one of the most complex regions in the world [24]. As a result, the challenges facing adolescents’ vision of health vary from country to country. For instance, high-income countries in the region, such as Singapore and Japan, may have relatively high levels of visual impairment owing to educational pressures [21]. However, developing countries in the region are also exposed to stress on visual health due to rapid urbanization [25] as well as economic factors that limit people’s ability to access appropriate healthcare resources and eye health services [26]. Furthermore, complex geographical factors also play an important role in cross-national inequalities in eye health [27]. A recent review of eye health in Pacific Island countries found that refractive errors and cataracts are of little concern in these islands [28]; however, they are the main causes of visual impairment globally. It is clear that Western Pacific countries face complex challenges to adolescent visual health because of multiple social, economic, and geographic conditions. A 2015 study also confirmed the high burden of visual impairment in this region [29]. Therefore, eye health research targeting this specific region is essential for addressing these issues.
However, we found that although there are studies analyzing adolescent vision health issues, they either focus on the global situation [30], lack in-depth analysis of the WPR, or are outdated and unable to reflect on recent trends [31]. As a result, there have been no specialized studies in recent years focusing on adolescent blindness and vision loss in WPR. Therefore, the core objective of this study was to systematically analyze trends in the prevalence of blindness and vision loss among adolescents in the WPR using data from the Global Burden of Disease (GBD) study from 1990 to 2019. Additionally, the secondary objective of the study was to analyze the prevalence of blindness and vision loss over time using joinpoint regression and to assess the impact of age, period, and cohort on these trends using the age-period-cohort (APC) model. The study results showed that despite the global decline in the prevalence and burden of adolescent blindness and vision loss, the WPR, particularly in China, showed an opposite upward trend. This finding highlights the unique challenges within the region and suggests potential shortcomings in current preventive measures and policies addressing adolescent vision problems. Our research provides strong evidence for the future development of targeted policies and plans to tackle adolescent vision health issues in WPR more effectively.
Materials and methods
Data sources
The Global Burden of Disease Study 2019 (GBD 2019) data we used are freely available from the Global Health Data Exchange (GHDx) (https://vizhub.healthdata.org/gbd-results/). GBD 2019 provides data on disease incidence, prevalence, burden, and other indicators from 1990 to 2019, covering 369 diseases and injuries across 204 countries and territories. A detailed description of the original data and general methodology for blindness and vision loss in GBD have been presented in previous studies [32]. In short, the GBD team collected data on the prevalence and burden of visual impairment by reviewing relevant published research and other available epidemiological surveys [32]. MR-BRT (Meta-Regression, Bayesian, Regularized, Trimmed) models were used to adjust for uncertainty and trim anomalous input data, and the Bayesian mixed-effects meta-regression tool, DisMod-MR 2.1 was used to ensure consistency between parameters [33].
The International Classification of Diseases (ICD)-10 codes for blindness and vision loss are H25-H28.8, H31-H36.8, H40-H40.9, H42-H42.8, and H46-H54.9, which include conditions such as glaucoma, cataracts, age-related macular degeneration, refractive disorders, near vision loss, and other vision loss [27]. According to the Snellen chart, it can be categorized into the following severity levels [34]: (1) Moderate: 6/18 > visual acuity ≥ 6/60 (2), Severe: 6/60 > visual acuity ≥ 3/60, and (3) Blindness: Visual acuity of < 3/60 or < 10% visual field around central fixation. Near vision loss is defined as a progressive inability to focus on near objects with age (presbyopia) and near visual acuity of < 6/12 distance equivalent. Refractive error is blurred vision caused by the inability of the lens to focus (including myopia, astigmatism, etc.). Glaucoma is characterized by elevated intraocular pressure that can lead to optic nerve damage. A cataract is clouding of the lens of the eye due to protein buildup that impairs vision. Age-related macular degeneration is a progressive retinal disease that develops with age, primarily affecting the macular area and can lead to loss of central vision.
We explored blindness and vision loss with data from 31 countries in the WPR: American Samoa, Australia, Brunei Darussalam, Cambodia, China, Cook Islands, Fiji, Guam, Japan, Kiribati, Lao People’s Democratic Republic, Malaysia, Marshall Islands, Micronesia (Federated States of), Mongolia, Nauru, New Zealand, Niue, Northern Mariana Islands, Palau, Papua New Guinea, Philippines, Republic of Korea, Samoa, Singapore, Solomon Islands, Tokelau, Tonga, Tuvalu, Vanuatu and Viet Nam [24, 35].
Our study focuses on adolescents aged 10–24, divided into three age groups: 10–14, 15–19, and 20–24 years. 10–24 years of age is a period of critical physiological change and social transition, but the physiological conditions as well as the academic, life, and social pressures they face differ at each stage [36, 37]. Therefore, this division allows for a more accurate capture of changes in visual health. In addition, since glaucoma and age-related macular degeneration develop after the age of 45 years and cataracts after 20 years of age [34], only cataracts (20–24 years age group), refractive disorders, near vision loss, and other vision loss were selected in the subgroups.
The data used in this study come from public databases and consist of aggregated data that do not involve direct human experiments or personal privacy-related information. Therefore, ethical approval and informed consent were not required. Additionally, the Global Burden of Disease (GBD) study strictly adheres to the Guidelines for Accurate and Transparent Health Estimates Reporting (GATHER), ensuring the transparency, completeness, and scientific integrity of the data.
Statistical analysis
The number of cases and rates (per 100,000 population) of prevalence and years lived with disability (YLD) were obtained directly from the GBD together with 95% uncertainty intervals (UIs) for each estimate (based on the ranked 1,000 estimates consisting of the 2.5th and 97.5th percentiles). As GBD offers only all-age standardized rates, we also calculated age-standardized rates for ages 10–24, based on the standard population to facilitate comparisons. Current data characterize the prevalence trends and disease burden of blindness and vision loss by sex, age, year, country, and disease subgroup strata.
Kim [38] showed that joinpoint regression analysis can divide longitudinal changes into different zones through segmental regression and identify statistically significant trends in these zones. Briefly, the joinpoint regression model first determines the interval segmentation function connection points using log-linear modeling and the grid search method (GSM). Then, the Monte Carlo permutation test was applied to establish the optimal model for joinpoint regression, and the number of potential joints was set between 0 and 5. Finally, the optimal model was used to calculate the annual percent change (APC) between turning points and the average annual percent change (AAPC). This method is simple and flexible, allowing for clear identification of each key trend turning point, which is particularly critical when dealing with long-term data. Therefore, we used APCs, AAPCs, and the corresponding 95% confidence intervals (CIs) obtained from the joinpoint regression analyses to describe the trends in the prevalence and burden of blindness and vision loss in adolescents from 1990 to 2019. Z-test and significance level of 0.05 were used to determine the difference between APC or AAPC and 0.
Age-period-cohort analysis based on Poisson distribution can eliminate the linear relationship between age, cohort, and other factors, and accurately reflect the effects of age, period, and cohort on disease [39]. Compared with other statistical models that can only analyze a single effect, it is more comprehensive in revealing the independent roles of multidimensional factors in disease prevalence trends [40]. Therefore, the APC model was used to simultaneously assess the age, period and cohort effects of the trend in the prevalence of blindness and vision loss to disaggregate and explore the underlying physiological, social, historical and environmental factors [41]. In the APC model of this study, age, period, and cohort were categorized into three age groups with 5-year intervals (10–14 years, 15–19 years, 20–24 years), six periods with 5-year intervals (1990–1994, 1995–1999, ., 2015–2019) and eight birth cohorts (1965–1974, ., 2000–2009), with partial overlap of neighboring birth cohorts. The model provides various estimates such as (1) net drift representing the overall time trend in prevalence, which is the overall annual percentage change over the entire period; (2) local drift the time trend in prevalence within each age group, which is expressed as the annual percentage change in prevalence for a specific age group; (3) longitudinal age effect which refers to changes caused by physical, psychological and social status shifts associated with changes in biological age; (4) period effect the relative risk of the period compared to the reference period adjusted for age and nonlinear effects; and (5) cohort effect the relative risk of the birth cohort compared to the reference cohort adjusted for age and nonlinear effects. These parameters were tested for statistical significance using the Wald χ2 test with a significance level of 0.05.
All data processing, analysis, and visualization were performed using Excel 2016 and version R 4.2.1.
Results
Prevalence and YLDs of blindness and vision loss in adolescents
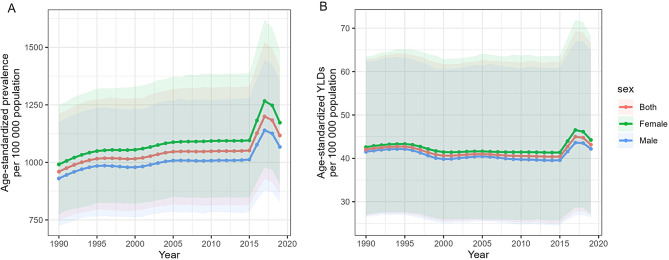
Table 1 presents the prevalence (per 100,000 population) and YLDs (per 100,000 population) of blindness and vision loss. Global blindness and vision loss showed a decreasing trend, with the prevalence declining from 1333.89 (95% UI: 1041.18 to 1682.05) in 1990 to 1290.80 (95% UI: 997.71 to 1637.20) in 2019, AAPC of -0.11% (95% CI: − 0.13% to -0.08%), and YLD also decreased from 56.82 (95% UI: 36.20 to 84.62) in 1990 to 50.99 (95% UI: 31.98 to 77.03) in 2019, and the AAPC was − 0.36% (95% CI: -0.41% to -0.31%). The prevalence and YLDs were also reduced to varying degrees in the other regions. However, the opposite was true for the WPR. Its prevalence grew from 959.96 (95% UI: 749.47 to 1210.71) in 1990 to 1117.08 (95% UI: 866.50 to 1414.64) in 2019, AAPC of 0.56% (95% CI: 0.35–0.76%), and the YLD also increased from 42.03 (95% UI: 26.74 to 62.90) in 1990 to 43.16 (95% UI: 26.84 to 66.51) in 2019, AAPC of 0.11% (95% CI: -0.04–0.26%) (Fig. 1, Figure S1).
Table 1.
Prevalence and YLDs of blindness and vision loss and their AAPCs among adolescents, global and various regions, 1990 to 2019
| location | Prevalence | YLDs | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case(n), 1990 | Age-standardized prevalence (per 100,000 population), 1990 | Case(n), 2019 | Age-standardized prevalence (per 100,000 population), 2019 | AAPC (%), 1990–2019 | P Value | Case(n), 1990 | Age-standardized YLDs (per 100,000 population), 1990 | Case(n), 2019 | Age-standardized YLDs (per 100,000 population), 2019 | AAPC (%), 1990–2019 | P Value | |
| Global |
20,673,337 (16720186 to 25239548) |
1333.89 (1041.18 to 1682.05) |
24,071,797 (19400459 to 29441243) |
1290.80 (997.71 to 1637.20) |
-0.11 (-0.13 to -0.08) |
< 0.001 |
881,087 (565901 to 1301049) |
56.82 (36.20 to 84.62) |
950,963 (600554 to 1426687) |
50.99 (31.98 to 77.03) |
-0.36 (-0.41 to -0.31) |
< 0.001 |
| African Region |
1,479,777 (1207073 to 1792287) |
921.67 (722.33 to 1157.90) |
2,989,614 (2425469 to 3666032) |
845.31 (653.98 to 1070.82) |
-0.30 (-0.31 to -0.28) |
< 0.001 |
72,704 (47838 to 103695) |
45.30 (29.51 to 65.37) |
135,592 (86985 to 198173) |
38.33 (24.46 to 56.33) |
-0.58 (-0.59 to -0.56) |
< 0.001 |
| Eastern Mediterranean Region |
2,341,737 (1876234 to 2877599) |
1957.54 (1523.51 to 2478.15) |
3,686,263 (2980518 to 4505995) |
1747.25 (1350.32 to 2219.87) |
-0.39 (-0.41 to -0.38) |
< 0.001 |
104,694 (68359 to 152264) |
87.64 (56.41 to 129.18) |
150,797 (95224 to 226553) |
71.50 (45.10 to 107.29) |
-0.70 (-0.72 to -0.69) |
< 0.001 |
| European Region |
2,728,977 (2182751 to 3358370) |
1386.37 (1062.26 to 1784.10) |
2,221,409 (1773163 to 2756857) |
1360.91 (1037.38 to 1757.97) |
-0.06 (-0.07 to -0.06) |
< 0.001 |
104,526 (64477 to 158240) |
53.11 (32.67 to 80.72) |
82,415 (50636 to 125460) |
50.51 (30.58 to 78.02) |
-0.17 (-0.18 to -0.16) |
< 0.001 |
| Region of the Americas |
3,536,000 (2826865 to 4374241) |
1787.11 (1369.13 to 2305.56) |
3,975,134 (3177663 to 4929079) |
1704.86 (1295.90 to 2196.72) |
-0.15 (-0.16 to -0.14) |
< 0.001 |
142,177 (90473 to 211620) |
71.86 (44.82 to 108.33) |
151,363 (94061 to 229390) |
64.96 (39.50 to 99.24) |
-0.34 (-0.35 to -0.33) |
< 0.001 |
| South-East Asia Region |
6,070,870 (4940595 to 7358610) |
1518.57 (1184.72 to 1905.45) |
7,410,828 (5979830 to 8992428) |
1323.23 (1023.50 to 1684.56) |
-0.49 (-0.54 to -0.44) |
< 0.001 |
258,356 (166991 to 377985) |
64.72 (41.22 to 95.93) |
284,296 (178506 to 424882) |
50.73 (31.75 to 77.02) |
-0.84 (-0.92 to -0.76) |
< 0.001 |
| Western Pacific Region |
4,455,977 (3607607 to 5380890) |
959.96 (749.47 to 1210.71) |
3,715,042 (2960207 to 4550058) |
1117.08 (866.50 to 1414.64) |
0.56 (0.35 to 0.76) |
< 0.001 |
196,342 (126723 to 289213) |
42.03 (26.74 to 62.90) |
143,809 (90875 to 218372) |
43.16 (26.84 to 66.51) |
0.11 (-0.04 to 0.26) |
0.153 |
Notes: YLD, years lived with disability; AAPC, average annual percent change
Fig. 1.
The age-standardized (A) prevalence and (B) YLDs of blindness and vision loss among adolescents in the Western Pacific Region, 2019. Notes: YLD, years lived with disability
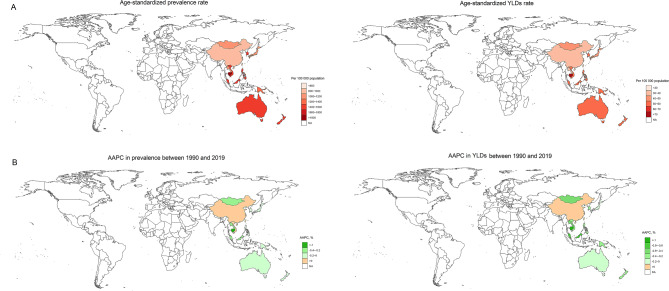
For countries in the WPR, the prevalence and burden of blindness and vision loss among adolescents were notably high in Cambodia, Singapore, New Zealand, Vietnam, and Australia. For example, although the overall trend of visual impairment in Cambodia declined significantly, the prevalence in 2019 remained high, reaching 1888.71 (95% UI: 1438.30 to 2432.63), with YLD being the highest among the WPR.(Table 2; Fig. 2A and Figure S2). However, particularly noteworthy was China, whose prevalence rose from 826.56 (95% UI: 643.01 to 1039.02) in 1990 to 987.95 (95% UI: 762.94 to 1249.61) in 2019, AAPC was 0.57% (95% CI: 0.12–1.01%), and YLD rose from 37.96 (95% UI: 24.41 to 56.50) to 39.42 (95% UI: 24.70 to 60.58), AAPC was 0.16% (95% CI: -0.02–0.34%) (Table 2; Fig. 2B). This trend is in sharp contrast to the overall downward trend observed globally and in other Western Pacific countries.
Table 2.
Prevalence and YLDs of blindness and vision loss and their AAPCs among adolescents in the Western Pacific Region, 1990 to 2019
| location | Prevalence | YLDs | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case(n), 1990 | Age-standardized prevalence (per 100,000 population), 1990 | Case(n), 2019 | Age-standardized prevalence (per 100,000 population), 2019 | AAPC (%), 1990–2019 | P Value | Case(n), 1990 | Age-standardized YLDs (per 100,000 population), 1990 | Case(n), 2019 | Age-standardized YLDs (per 100,000 population), 2019 | AAPC (%), 1990–2019 | P Value | |
| American Samoa |
134 (108 to 166) |
889.27 (669.97 to 1158.29) |
146 (114 to 182) |
852.49 (636.51 to 1113.61) |
-0.14 (-0.20 to -0.09) |
< 0.001 |
5 (3 to 8) |
35.17 (20.97 to 54.31) |
6 (3 to 8) |
32.32 (19.24 to 50.42) |
-0.29 (-0.33 to -0.25) |
< 0.001 |
| Australia |
61,458 (49322 to 76857) |
1548.11 (1161.92 to 2031.57) |
69,482 (55626 to 87050) |
1531.05 (1155.26 to 2006.69) |
-0.04 (-0.05 to -0.02) |
< 0.001 |
2190 (1321 to 3376) |
55.20 (32.92 to 87.52) |
2441 (1494 to 3762) |
53.81 (32.09 to 84.87) |
-0.08 (-0.11 to -0.04) |
< 0.001 |
| Brunei Darussalam |
1115 (874 to 1391) |
1460.48 (1093.58 to 1901.58) |
1533 (1207 to 1909) |
1418.58 (1062.99 to 1856.41) |
-0.10 (-0.12 to -0.07) |
< 0.001 |
46 (28 to 68) |
59.65 (36.66 to 90.50) |
60 (37 to 91) |
55.43 (33.53 to 85.99) |
-0.25 (-0.28 to -0.22) |
< 0.001 |
| Cambodia |
82,225 (65490 to 98570) |
2546.67 (1941.74 to 3145.23) |
86,108 (67721 to 107504) |
1888.71 (1438.30 to 2432.63) |
-1.04 (-1.16 to -0.93) |
< 0.001 |
3462 (2194 to 5181) |
108.10 (66.94 to 161.84) |
3240 (1997 to 4940) |
70.93 (42.85 to 110.22) |
-1.46 (-1.52 to -1.39) |
< 0.001 |
| China |
2,967,932 (2395798 to 3582731) |
826.56 (643.01 to 1039.02) |
2,228,552 (1785508 to 2719154) |
987.95 (762.94 to 1249.61) |
0.57 (0.12 to 1.01) |
0.012 |
137,348 (90001 to 200852) |
37.96 (24.41 to 56.50) |
89,064 (56454 to 135135) |
39.42 (24.70 to 60.58) |
0.16 (-0.02 to 0.34) |
0.077 |
| Cook Islands |
53 (42 to 65) |
907.05 (682.26 to 1165.64) |
35 (27 to 43) |
836.52 (623.38 to 1094.43) |
-0.28 (-0.32 to -0.25) |
< 0.001 |
2 (1 to 4) |
40.31 (24.44 to 61.86) |
1 (1 to 2) |
33.44 (19.82 to 52.7) |
-0.64 (-0.68 to -0.59) |
< 0.001 |
| Fiji |
2348 (1857 to 2913) |
986.84 (738.34 to 1280.13) |
2206 (1732 to 2720) |
934.48 (694.19 to 1209.86) |
-0.20 (-0.23 to -0.17) |
< 0.001 |
98 (61 to 148) |
41.23 (25.02 to 63.69) |
87 (53 to 132) |
36.83 (21.77 to 56.92) |
-0.40 (-0.44 to -0.37) |
< 0.001 |
| Guam |
340 (272 to 423) |
865.96 (646.42 to 1129.88) |
347 (270 to 438) |
831.95 (615.85 to 1099.32) |
-0.14 (-0.16 to -0.12) |
< 0.001 |
13 (8 to 20) |
32.81 (19.67 to 51.67) |
13 (8 to 20) |
30.35 (18.09 to 48.36) |
-0.27 (-0.31 to -0.23) |
< 0.001 |
| Japan |
332,688 (265080 to 413383) |
1181.60 (904.25 to 1519.46) |
204,496 (161565 to 253790) |
1152.13 (871.25 to 1485.89) |
-0.08 (-0.10 to -0.07) |
< 0.001 |
13,391 (8382 to 20165) |
47.57 (29.45 to 72.31) |
8002 (4936 to 12193) |
45.12 (27.60 to 69.38) |
-0.18 (-0.20 to -0.17) |
< 0.001 |
| Kiribati |
208 (166 to 256) |
928.56 (692.95 to 1198.34) |
310 (245 to 385) |
895.25 (664.71 to 1162.28) |
-0.12 (-0.17 to -0.08) |
< 0.001 |
9 (6 to 14) |
40.32 (24.32 to 62.08) |
13 (8 to 19) |
36.55 (21.94 to 56.51) |
-0.34 (-0.39 to -0.30) |
< 0.001 |
| Lao People’s Democratic Republic |
16,130 (12869 to 20147) |
1236.40 (939.26 to 1578.10) |
23,461 (18513 to 29246) |
1116.74 (841.06 to 1449.41) |
-0.36 (-0.39 to -0.33) |
< 0.001 |
594 (361 to 921) |
45.77 (27.40 to 72.21) |
809 (488 to 1281) |
38.46 (22.51 to 61.36) |
-0.60 (-0.64 to -0.57) |
< 0.001 |
| Malaysia |
84,508 (68454 to 103824) |
1566.94 (1201.16 to 2003.48) |
117,314 (92264 to 145599) |
1450.39 (1096.31 to 1876.06) |
-0.27 (-0.31 to -0.22) |
< 0.001 |
3447 (2144 to 5209) |
64.07 (40.02 to 98.20) |
4268 (2549 to 6602) |
52.53 (31.13 to 83.08) |
-0.69 (-0.73 to -0.64) |
< 0.001 |
| Marshall Islands |
140 (110 to 173) |
920.33 (689.78 to 1189.86) |
144 (114 to 181) |
876.39 (652.36 to 1142.05) |
-0.17 (-0.21 to -0.14) |
< 0.001 |
6 (4 to 9) |
38.61 (23.34 to 59.23) |
6 (3 to 9) |
34.47 (20.70 to 53.61) |
-0.40 (-0.43 to -0.36) |
< 0.001 |
| Micronesia (Federated States of) |
310 (244 to 386) |
908.16 (681.16 to 1177.76) |
274 (214 to 343) |
858.28 (627.90 to 1118.10) |
-0.20 (-0.24 to -0.15) |
< 0.001 |
13 (8 to 20) |
37.84 (23.06 to 58.38) |
11 (6 to 16) |
32.96 (19.19 to 51.52) |
-0.47 (-0.50 to -0.44) |
< 0.001 |
| Mongolia |
8224 (6626 to 10129) |
1165.59 (886.51 to 1494.47) |
8003 (6368 to 9910) |
1095.94 (818.62 to 1410.97) |
-0.21 (-0.23 to -0.19) |
< 0.001 |
370 (233 to 538) |
52.57 (32.28 to 78.74) |
328 (204 to 496) |
44.93 (27.30 to 69.11) |
-0.55 (-0.59 to -0.50) |
< 0.001 |
| Nauru |
27 (21 to 33) |
883.85 (651.11 to 1150.00) |
28 (22 to 35) |
849.92 (628.16 to 1115.74) |
-0.14 (-0.16 to -0.11) |
< 0.001 |
1 (1 to 2) |
35.06 (20.85 to 55.08) |
1 (1 to 2) |
31.70 (18.65 to 50.4) |
-0.34 (-0.37 to -0.32) |
< 0.001 |
| New Zealand |
13,674 (10848 to 16955) |
1625.78 (1234.8 to 2108.92) |
13,908 (10879 to 17224) |
1571.77 (1187.79 to 2043.91) |
-0.12 (-0.13 to -0.10) |
< 0.001 |
503 (313 to 767) |
59.82 (36.54 to 92.58) |
496 (298 to 767) |
56.11 (33.56 to 87.68) |
-0.22 (-0.23 to -0.2) |
< 0.001 |
| Niue |
6 (4 to 7) |
865.21 (650.09 to 1131.24) |
3 (3 to 4) |
820.18 (604.56 to 1070.56) |
-0.18 (-0.22 to -0.15) |
< 0.001 |
0 (0 to 0) |
33.22 (19.77 to 51.88) |
0 (0 to 0) |
29.43 (17.20 to 46.78) |
-0.43 (-0.44 to -0.41) |
< 0.001 |
| Northern Mariana Islands |
110 (87 to 137) |
866.54 (644.25 to 1128.62) |
89 (69 to 111) |
839.76 (616.22 to 1102.57) |
-0.11 (-0.15 to -0.07) |
< 0.001 |
4 (3 to 6) |
32.98 (19.45 to 51.21) |
3 (2 to 5) |
30.66 (18.05 to 48.78) |
-0.25 (-0.28 to -0.22) |
< 0.001 |
| Palau |
40 (32 to 51) |
858.53 (641.24 to 1107.86) |
29 (23 to 37) |
821.61 (612.92 to 1076.28) |
-0.16 (-0.18 to -0.14) |
< 0.001 |
2 (1 to 2) |
32.57 (19.27 to 51.05) |
1 (1 to 2) |
29.52 (17.48 to 47.46) |
-0.34 (-0.37 to -0.31) |
< 0.001 |
| Papua New Guinea |
17,402 (13757 to 21466) |
1330.50 (994.64 to 1725.89) |
37,321 (29241 to 46057) |
1278.89 (953.13 to 1656.82) |
-0.13 (-0.16 to -0.11) |
< 0.001 |
719 (442 to 1082) |
55.04 (33.33 to 84.45) |
1459 (900 to 2185) |
49.99 (30.41 to 77.18) |
-0.33 (-0.35 to -0.31) |
< 0.001 |
| Philippines |
307,298 (247180 to 377973) |
1493.07 (1149.18 to 1889.66) |
456,617 (364109 to 563859) |
1426.32 (1091.97 to 1818.61) |
-0.16 (-0.17 to -0.15) |
< 0.001 |
11,596 (7244 to 17670) |
56.50 (34.72 to 86.32) |
16,407 (9932 to 25288) |
51.21 (30.87 to 79.51) |
-0.34 (-0.35 to -0.33) |
< 0.001 |
| Republic of Korea |
193,522 (152900 to 239946) |
1464.82 (1105.80 to 1912.67) |
118,501 (94975 to 146629) |
1401.69 (1049.42 to 1825.49) |
-0.14 (-0.17 to -0.12) |
< 0.001 |
8006 (4984 to 11996) |
60.60 (37.29 to 91.86) |
4579 (2842 to 6964) |
54.21 (33.19 to 83.98) |
-0.38 (-0.43 to -0.33) |
< 0.001 |
| Samoa |
513 (403 to 630) |
874.75 (641.49 to 1128.24) |
582 (459 to 727) |
849.83 (630.74 to 1110.10) |
-0.10 (-0.12 to -0.07) |
< 0.001 |
20 (13 to 31) |
34.62 (20.55 to 53.32) |
22 (13 to 34) |
32.01 (18.40 to 50.51) |
-0.26 (-0.31 to -0.22) |
< 0.001 |
| Singapore |
15,082 (11786 to 18990) |
1817.44 (1330.86 to 2414.46) |
13,341 (10320 to 16980) |
1769.35 (1301.87 to 2352.06) |
-0.09 (-0.11 to -0.07) |
< 0.001 |
570 (348 to 865) |
68.70 (41.45 to 106.62) |
488 (298 to 753) |
64.77 (38.72 to 102.51) |
-0.20 (-0.23 to -0.17) |
< 0.001 |
| Solomon Islands |
1088 (862 to 1348) |
944.97 (710.43 to 1217.70) |
1815 (1417 to 2243) |
896.71 (666.10 to 1163.37) |
-0.18 (-0.21 to -0.15) |
< 0.001 |
47 (29 to 69) |
40.72 (24.39 to 62.05) |
72 (44 to 111) |
35.78 (21.23 to 55.88) |
-0.44 (-0.49 to -0.39) |
< 0.001 |
| Tokelau |
4 (3 to 5) |
894.37 (668.68 to 1162.28) |
3 (2 to 4) |
837.18 (616.50 to 1094.51) |
-0.23 (-0.25 to -0.20) |
< 0.001 |
0 (0 to 0) |
36.01 (21.77 to 55.89) |
0 (0 to 0) |
30.92 (18.29 to 49.06) |
-0.53 (-0.56 to -0.49) |
< 0.001 |
| Tonga |
224 (180 to 280) |
673.05 (502.54 to 872.49) |
193 (152 to 242) |
628.22 (458.54 to 825.97) |
-0.23 (-0.27 to -0.20) |
< 0.001 |
9 (5 to 13) |
25.89 (15.49 to 40.30) |
7 (4 to 11) |
23.00 (13.49 to 36.34) |
-0.40 (-0.45 to -0.34) |
< 0.001 |
| Tuvalu |
23 (18 to 28) |
916.72 (682.72 to 1184.18) |
29 (23 to 36) |
853.72 (638.62 to 1116.02) |
-0.25 (-0.27 to -0.22) |
< 0.001 |
1 (1 to 1) |
38.37 (23.07 to 58.41) |
1 (1 to 2) |
32.53 (18.97 to 51.12) |
-0.56 (-0.58 to -0.54) |
< 0.001 |
| Vanuatu |
375 (293 to 464) |
795.60 (590.16 to 1028.38) |
682 (534 to 841) |
761.56 (559.88 to 996.21) |
-0.15 (-0.18 to -0.12) |
< 0.001 |
14 (9 to 22) |
30.10 (17.82 to 47.12) |
25 (15 to 39) |
27.43 (16.13 to 43.97) |
-0.33 (-0.38 to -0.27) |
< 0.001 |
| Viet Nam |
335,886 (267917 to 407730) |
1554.21 (1194.73 to 1977.13) |
327,329 (260326 to 404387) |
1563.68 (1203.30 to 2007.02) |
0.03 (-0.04 to 0.09) |
0.420 |
13,505 (8499 to 20384) |
62.67 (38.28 to 95.47) |
11,863 (7186 to 18537) |
56.53 (33.69 to 89.24) |
-0.33 (-0.40 to -0.27) |
< 0.001 |
Notes: YLD, years lived with disability; AAPC, average annual percent change
Fig. 2.
Distribution of blindness and vision loss among adolescents in the Western Pacific Region in 2019. (A) age-standardized rates; (B) AAPCs. Notes: YLD, years lived with disability; AAPC, average annual percent change
Subgroups of blindness and vision loss
We examined the contribution of several subgroups to blindness and vision loss. Between 1990 and 2019, refractive disorders were the leading cause of vision impairment across all age groups regardless of sex. Although its prevalence decreased with age, it remained dominant throughout adolescence, having the greatest impact on overall trends in blindness and vision impairment. As age increased, the impact of near vision loss gradually became more apparent, particularly in the 20–24 age group, where the burden increased significantly. The proportion of other causes of vision loss remained relatively stable across the different age groups, whereas the effect of cataracts was only observed in the 20–24 age group (Tables S1-S4, Figures S3-S5). Overall, refractive errors were the primary driver of vision impairment among adolescents, while near vision loss and cataracts had a greater impact in the older age groups.
The distribution of vision loss varies by country. Cataracts had the highest prevalence and burden in Papua New Guinea, Kiribati, and Cambodia. Near vision loss was found to have the highest prevalence and YLDs in China, Mongolia, and Papua New Guinea. Refractive disorders also show a higher prevalence and burden of disease in Cambodia, Singapore, and New Zealand. Other vision loss observed in Singapore, Brunei Darussalam, the Republic of Korea, and Mongolia was the most widely distributed (Tables S1-S4, Figures S6-S7, Figures S8-S11 A).
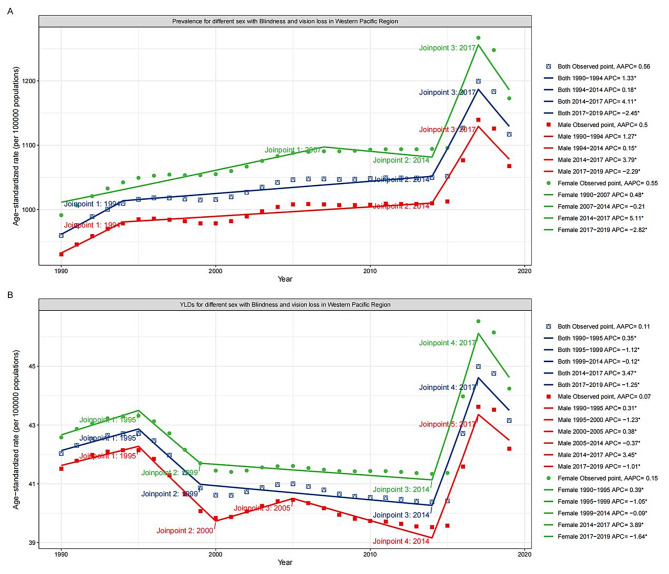
Joinpoint regression analysis
Across WPR, the prevalence of blindness and vision loss generally showed an upward trend. Specifically, the prevalence rose more rapidly between 1990 and 1994, with an APC of 1.33%, and increased slowly and steadily from 1994 to 2014, with an APC of 0.18%. The most dramatic increase was from 2014 to 2017, with an APC of 4.11%. However, after 2017, the prevalence exhibited a sudden downward trend, with an APC of -2.45%. The prevalence trends for both males and females were broadly similar to the overall trend, although there was a slow reduction in female prevalence between 2007 and 2014 (Fig. 3A).
Fig. 3.
Joinpoint regression analysis of age-standardized (A) prevalence and (B) YLDs of blindness and vision loss among adolescents in the Western Pacific Region, 1990–2019. Notes: YLD, years lived with disability; AAPC, average annual percent change; APC, annual percent change
Similar to the prevalence, YLD for blindness and vision loss in WPR showed an overall upward trend. 1990 to 1995 trended upward, with an APC of 0.35%. Between 1995 and 1999, there was a rapid drop, with an APC of -1.12%. Between 1999 and 2014, the fall rate was slower (APC = -0.12%). Between 2014 and 2017, there was a similar rapid increase (APC = 3.47%), followed by a sudden decline after 2017 (APC = -1.25%)(Fig. 3B). Approximately parallel YLDs and overall trends were observed for both males and females. However, both prevalence and YLD were markedly higher in females than in males.
Among the different causes of vision loss, cataract prevalence and YLD showed an overall decreasing trend but increased after 2015 (Figure S12). Near vision loss increased from 1990 to 1999 and then showed a downward trend (Figure S13). Refractive disorders were closest to the trends for blindness and vision loss, with an upward trend from 1990 to 2017 followed by a swift fall after 2017 (Figure S14). Other vision loss decreased overall, but the YLD increased after 2016 (Figure S15). The prevalence and YLD trends for all causes were broadly similar for males, females, and both, and were higher for females than for males, except for other vision loss.
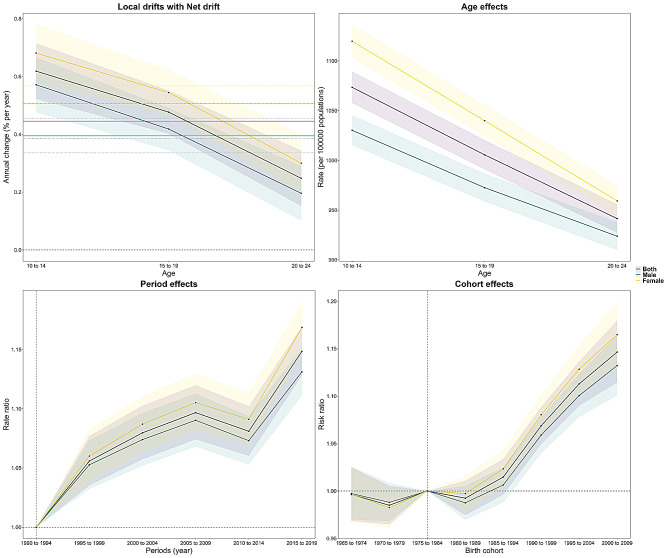
Age-period-cohort analyses
Figure 4 illustrates the age-period cohort effect in the WPR. A net drift of 0.45% between blindness and vision loss was observed over the entire study period (Table S6), which suggests increasingly severe vision loss. Local drift was also greater than 0% in every age group, with a maximum in the 10–14 years age group (0.62%) and a minimum in the 20–24 years age group (0.25%) (Table S7). The age effect showed that the risk was highest in the adolescent stage of 10 to 14 years but decreased with increasing age (Table S8). Compared to 1990, the period effect showed a gradual increase in risk from 1995 to 2009 but a slight decrease from 2010 to 2014, followed by a very large increase in relative risk (Table S9), with an overall increase in the risk of disease as the period moved backward. Concerning birth cohort effects, those born before 1985 had a relatively low risk of disease with weak upward and downward fluctuation, characterized by a decline followed by an increase and then a decline, whereas those born after 1985 had an increased risk of disease (Table S10). Moreover, regardless of the effect, girls were at higher risk.
Fig. 4.
Age-period-cohort analysis of the prevalence of blindness and vision loss among adolescents in the Western Pacific Region
There was considerable variation across countries, with only China and Vietnam showing a significant increase in the risk of disease (net drift of 0.52% and 0.16%, respectively) (Table S5, Table S6). Localized drift increased with age in Australia, China, Malaysia, and Vietnam; decreased with age in Cambodia; and decreased or increased with age in the rest of the countries with non-significant homogeneous decreases (Table S7, Figure S16). In terms of the age effect, the risk of the disease improved with age in Cambodia, China, Laos, Malaysia, the Philippines, and Vietnam, but adolescents in Japan and Korea were significantly more at risk (Table S8, Figure S17). The time effect showed that the overall prevalence risk of blindness and vision loss declined in almost all countries and was most pronounced in Cambodia. In China, however, there was only an improvement from 2000 to 2009, with adolescents in later periods more likely to be at risk (Table S9, Figure S18). Similarly, birth cohorts also showed a higher risk of disease the later the cohort was born in China, the more marked the improvement in Cambodia (Table S10, Figure S19). In addition, there was a very high overlap between the prevalence patterns in China and WPR. The prevalence varied between men and women in each country; however, in general, girls were worse off in most cases.
Discussion
To our knowledge, this is the first study to explore blindness and vision loss in adolescents aged 10–24 years in WPR. Our results showed an overall increasing trend in both the prevalence and YLD of blindness and vision loss among adolescents in the WPR, with an opposite pattern observed globally and in other regions. Moreover, the prevalence and burden of blindness and vision loss among adolescents in China are also rising, with a dramatic impact on WPR. We also found that refractive disorders dominate the visual impairment in adolescents in this region. Thus, the overall eyesight of Western Pacific adolescents was not encouraging.
Trends in the prevalence and burden of blindness and vision loss among adolescents
The complex composition of countries in the WPR, which is home to a large number of low-income countries as well as high-income countries with favorable economic status [42], all of which face different forms of vision loss pressures (such as barriers to access to primary health care services [43] or intensified education [44]), have contributed to the overall worsening of visual impairment in adolescents. However, amid an overall increase in prevalence and burden, vision deterioration eased after 2017, a trend attributed to multiple factors. First, this improvement is closely related to socioeconomic development and enhancement of healthcare policies. As economies gradually improved, countries in the WPR increased their investments in public health, particularly ophthalmic care. Several nations have launched large-scale intervention programs that promote early screening and treatment, which significantly reduce the burden of eye diseases [45–48]. Technological advances have also played a key role in reversing this trend. For example, the widespread adoption of refractive correction techniques and cataract surgery has enabled many patients to receive timely treatment, reducing the long-term accumulation of vision impairments [49, 50]. Additionally, the implementation of the WHO Global Action Plan for Eye Health (2014–2019) in the WPR had a positive impact [51]. The plan aimed to reduce avoidable vision impairment by increasing access to and coverage of eye care services in low- and middle-income countries through international aid and government funding, further improving the region’s vision health.
Interestingly, we observed that this particular trend in adolescent vision problems in the WPR may be largely attributable to China’s influence. This is because China’s trend is consistent with the overall trend in the Western Pacific, whereas most other countries show a downward trend, consistent with previous reports [16]. This may be due to excessive Internet use among Chinese adolescents, which significantly increases screen time [52, 53]. Additionally, the “Chinese-style education” system requires children to spend most of their time studying, leaving little time for outdoor activities [54, 55]. Therefore, it is unsurprising that most teenagers in China have poor eyesight. A previous study reported that China’s teens and young adults went from less than 20% nearsighted in approximately 60 years to 90% in 2015 [19], which is a frightening figure. However, this situation has improved since 2017 and this turning point may be closely related to a series of proactive policies and measures. For example, the Chinese government issued an Implementation Plan for Comprehensive Prevention and Control of Myopia among Children and Adolescents [56], which effectively alleviated vision impairment by promoting nationwide vision screening and preventive measures. Additionally, the deepening of healthcare reform has significantly enhanced the capacity of primary healthcare services, and ophthalmic care resources in remote areas have also been strengthened [57]. Furthermore, technological advancements have played an important role in reducing the burden of vision impairments in China.
Among the countries where the burden of vision impairment has eased, Cambodia’s improvement has been particularly remarkable (despite having the highest prevalence). Historically, because of the Khmer Rouge regime, many educated professionals were killed or displaced. Coupled with economic difficulties, the country faced a severe shortage of ophthalmic resources, leading to serious vision impairment issues. However, Cambodia improved this situation through a series of measures, becoming the second country after China signed in support of the “Vision 2020” initiative [58]. With the gradual economic recovery and support of international aid, Cambodia strengthened community education and early screening, established multiple specialized ophthalmology hospitals, and to some extent improved the accessibility and quality of eye care by training a large number of ophthalmologists and nurses. After years of effort, the cataract surgery rate increased from 1,182 per million in 2009 to 2,791 per million in 2019. In 2015, the country successfully eliminated trachoma [59]. Cambodia’s success demonstrates that the effective integration of policies and resources can significantly improve vision and health, even in low- and middle-income countries. Therefore, developed countries such as South Korea, Japan, and Singapore are even better positioned to reduce the burden of adolescent vision impairment through comprehensive multi-faceted measures.
Blindness and visual loss subgroups
In our study, refractive disorders predominated in adolescents with visual impairment, which has also been reported in other studies [16]. More than one study has shown that refractive disorders in adolescents are still mainly the result of close work and intensive education [60–62]. What caught our attention was that the trend in the prevalence of overall vision loss in Western Pacific adolescents was extremely similar to that in the prevalence of refractive disorders. It is not difficult to understand that refractive disorders are the leading cause of blindness and vision loss in the entire population [34], as well as in adolescents [16], and when it produces a change it can greatly implicate the overall change in vision loss. The prevalence of near vision loss increased with age, similar to previous studies [17, 63]. Although the definition of near vision loss in GBD is relatively limited (it encompasses only correctable near vision loss), its burden can be mitigated through prevention and intervention. Thus, it an equally high public health significance. In contrast to refractive disorders and near vision loss, there was a notable reduction in the prevalence and burden of cataracts and other vision losses. This may be attributed to the global initiative to eliminate avoidable blindness, “Vision 2020: The Right to Sight“ [64], launched by the WHO in 1999. The initiative focuses on causes such as cataracts because it has cost-effective interventions that are already in place, and after many years of effort, its achievements are evident.
Refractive disorders pose a significant threat to adolescents’ optical well-being. Although some policies and measures currently implemented by various countries have yielded certain results, there is still a need to strengthen public health education, raise awareness among parents and adolescents regarding prevention and control, conduct regular vision screenings, and promote intervention measures such as increasing outdoor activities and limiting screen time [65, 66]. Simultaneously, governments should continue to enhance policy support, promote international cooperation, and share preventive and control experiences. Furthermore, future interventions should be personalized and offer tailored prevention and correction plans for high-risk adolescents. In summary, we call for more comprehensive and improved preventive and interventional efforts.
Age-period-cohort effects of blindness and vision loss in adolescents
We deconstructed the relative contributions of age, period, and cohort to vision impairment using the age-period-cohort (APC) model, revealing the roles of the underlying physiological, social, historical, and environmental factors. The results of the age effect showed that the risk of vision impairment among adolescents in the Western Pacific was highest at ages 10–14 and improved with increasing age. This finding may be related to the developmental characteristics of the human eye. One study on adolescent eye development used 13 years of age as the terminal stage of normal eye growth [8]. Another study on visual stability showed that refractive error in juveniles stabilizes in late adolescence (90% stabilization by age 21) [67]. In addition, myopia in adolescents usually progresses rapidly in the early years and then slows down [68]. These physiological factors are consistent with the age effect we observed.
Regarding the period effect, this study presents us with the fact that the later the period, the stronger the overall risk of the disease. Rapid economic development has introduced several risks. For example, technological advancements have transformed electronic devices from traditional large computers to portable smartphones, which have become an indispensable part of daily life. For instance, since the launch of smartphones, more than half of the children have exceeded the recommended guidelines for daily screen time use [69]; however, prolonged near-eye use increases the risk of eye disease. Studies have shown that as the use of digital media by adolescents increases, the incidence of vision problems is rising rapidly worldwide, especially in Asia, with the age of onset becoming younger [70–73]. Although exposure to outdoor natural light can help prevent vision decline, the development of the economy and technology has changed the way adolescents work and entertain themselves, with more choosing indoor electronic entertainment or studying, leading to reduced time spent in natural light, thereby worsening vision problems [74]. Additionally, air pollution (such as PM2.5, PM10) is also associated with vision impairment, and the decline in air quality due to industrialization has exposed adolescents living in cities to greater risks to vision health [75].
For the birth cohort, we determined that the risk of disease increased only for those born after 1985. This increasing trend may be attributed to specific sociocultural factors at that time. Sociocultural factors are closely related to economic development. Once people meet their basic living needs, higher pursuits such as education, entertainment, and diet follow. Those born during these periods of sociocultural transformation are inevitably influenced by these changes. Today higher education is increasingly promoted [76], and in many countries, children are constantly devoting more time to their studies to achieve their further educational goals. Several studies have confirmed that higher education is a risk factor for vision problems [77–79]. For example, it was reported that children who read more than two books per week had substantially longer axial lengths than those children who read less than two books per week [80]. In addition, the dietary quality of many adolescents has declined in today’s fast-paced society. Poor eating habits, such as consumption of fast food, may negatively impact overall health, including vision [81, 82]. Of course, the increasing use of electronic devices and gradual reduction in outdoor activities are also part of this societal shift. Consequently, countries should strengthen regular vision screening activities for children and adolescents as early detection and intervention can be effective in reducing the risk of vision loss.
Gender inequality in adolescent blindness and vision loss
We found, unsurprisingly, that blindness and vision loss were worse for girls than for boys, which is consistent with many prior studies [34, 83–85]. This is often related to the lower economic level and less access to education for females, resulting in fewer opportunities to obtain health information and eye care services [86, 87]. It may also be correlated with factors such as women spending more time at home and being exposed to higher levels of household air pollution [84]. To address this issue of gender inequality, countries in the WPR should take the following measures: enhance eye health education and awareness campaigns targeted at girls to improve their knowledge of eye care; improve the accessibility of ophthalmic services by establishing mobile eye clinics in low-income areas; formulate policies that encourage families to support girls’ education, particularly in health education; and strengthen cooperation between governments, non-governmental organizations, and communities to promote the status of women in health and education. Through these measures, we aimed to alleviate gender inequality in adolescent vision impairment and improve overall eye health.
Limitations
Subject to the GBD data, this study also has some limitations. First, different measures were used to estimate visual acuity, which may have led to an increase in the measurement error. Such errors may affect the estimation of the actual prevalence of blindness and visual loss, leading to an impact on the comparability of the data, which may lead to misinterpretation of the overall trend. Second, GBD estimates of disease burden were calculated in all regions based on available epidemiologic data; however, sparse data in some countries resulted in estimates that did not reflect true variation or had too large an uncertainty interval and overestimated disease prevalence and burden. Such inaccurate data will hinder our understanding of the actual challenges faced by these countries and further limit our comprehensive understanding of the overall trends. In addition, the GBD 2019 does not provide data on factors associated with visual impairment in adolescents. Consequently, it is not possible to fully identify the factors that contribute to marked changes in blindness and vision loss. Finally, in the GBD data, blindness and vision loss were only categorized into three subtypes; refractive disorders, near vision loss, and other vision loss for 10- to 19-year-olds, with an additional cataract subtype for 20- to 24-year-olds. However, GBD 2019 did not have data on specific eye diseases associated with vision loss in adolescents, such as amblyopia and ocular trauma. These ocular diseases have different developmental processes and poor adult outcomes, which cause differences in individual disease burden. Therefore, future studies on GBD should consider more specific eye diseases.
Conclusions
In conclusion, the burden of vision loss among adolescents has decreased significantly globally and in other regions; however, high burdens persist in the WPR, with a high burden of refractive disorders being of particular concern. In countries where the prevalence and burden continue to rise, such as China, a robust response is necessary. In the future, addressing the issue of declining vision among adolescents will hinge on developing and implementing more targeted preventive and intervention measures. First, public health policies should focus on strengthening early screening and prevention of common eye diseases among adolescents, particularly for high-prevalence conditions, such as refractive errors. Countries should promote eye health education, encourage outdoor activities, and advocate the use of electronic devices to reduce myopia. Additionally, policymakers should tailor healthcare resource allocation to the socioeconomic context of each country, ensuring that low-income populations have access to basic ophthalmic services. Furthermore, cross-sector collaboration should be promoted, encompassing fields such as education, health, and social security, to establish a systematic approach to eye health intervention. These measures aim to effectively reduce the burden of vision impairment among adolescents and improve the overall eye health in the region.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank all GBD collaborators for preparing these publicly available data.
Author contributions
Wei Wang and Xiaojuan Wang conceived the study; Yunjiao Luo and Qingzhi Wang participated in data curation and writing—original draft preparation; Yingxue Wang, Louisa Esi Mackay, Na Yan, Yuhao Wang, Blen Dereje Shiferaw, Yihan Wang, Jingjing Wang, Jie Tang, and Ya Liao supervised and validated the study; Wei Wang participated in writing—reviewing and editing.
Funding
This work was supported by the National Natural Science Foundation of China [82003484], Education Science ‘14th Five-Year Plan’ General Project in Jiangsu Province [D/2021/01/163], Jiangsu Province Colleges ‘Qinglan’ Project, and Higher Education Scientific Research Planning Project of the China Association of Higher Education [24BJ0415].
Data availability
Data is publically available at Global Health Data Exchange (GHDx) online website (https://vizhub.healthdata.org/gbd-results/).
Declarations
Ethics approval and consent to participate
Because these data were extracted from a publicly accessible repository rather than a primary study, no patients were involved in data collection or the identification of research questions, outcome measures, or study design, and no ethical approval or informed consent was required.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Yunjiao Luo and Qingzhi Wang contributed equally as co-first authors.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xiaojuan Wang, Email: 153803468@qq.com.
Wei Wang, Email: weiwang90@163.com.
References
- 1.Disease GBD, Injury I, Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of Disease Study 2017. Lancet. 2018;392(10159):1789–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Blindness and vision impairment. 2023 [ https://www.who.int/news-room/fact-sheets/detail/blindness-and-visual-impairment
- 3.World Health Organization. World report on vision. 2019 [ https://www.who.int/publications/i/item/9789241516570
- 4.Cahill MT, Banks AD, Stinnett SS, Toth CA. Vision-related quality of life in patients with bilateral severe age-related macular degeneration. Ophthalmology. 2005;112(1):152–8. [DOI] [PubMed] [Google Scholar]
- 5.Guthrie DM, Davidson JGS, Williams N, Campos J, Hunter K, Mick P, et al. Combined impairments in vision, hearing and cognition are associated with greater levels of functional and communication difficulties than cognitive impairment alone: analysis of interRAI data for home care and long-term care recipients in Ontario. PLoS ONE. 2018;13(2):e0192971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naidoo KS, Fricke TR, Frick KD, Jong M, Naduvilath TJ, Resnikoff S, et al. Potential lost Productivity resulting from the global burden of myopia: systematic review, Meta-analysis, and modeling. Ophthalmology. 2019;126(3):338–46. [DOI] [PubMed] [Google Scholar]
- 7.Voss P. Sensitive and critical periods in visual sensory deprivation. Front Psychol. 2013;4:664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fledelius HC, Christensen AS, Fledelius C. Juvenile eye growth, when completed? An evaluation based on IOL-Master axial length data, cross-sectional and longitudinal. Acta Ophthalmol. 2014;92(3):259–64. [DOI] [PubMed] [Google Scholar]
- 9.Pan CW, Dirani M, Cheng CY, Wong TY, Saw SM. The age-specific prevalence of myopia in Asia: a meta-analysis. Optom Vis Sci. 2015;92(3):258–66. [DOI] [PubMed] [Google Scholar]
- 10.Blencowe H, Lawn JE, Vazquez T, Fielder A, Gilbert C. Preterm-associated visual impairment and estimates of retinopathy of prematurity at regional and global levels for 2010. Pediatr Res. 2013;74(Suppl 1):35–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rajavi Z, Sabbaghi H, Baghini AS, Yaseri M, Moein H, Akbarian S, et al. Prevalence of Amblyopia and refractive errors among Primary School Children. J Ophthalmic Vis Res. 2015;10(4):408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith L, Pardhan S, Gorely T, Barnett Y, Jacob L, López-Sánchez GF, et al. Physical activity and visual difficulties in 36 low- and middle-income countries. Eye (Lond). 2022;36(3):585–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toledo CC, Paiva AP, Camilo GB, Maior MR, Leite IC, Guerra MR. Early detection of visual impairment and its relation to academic performance. Rev Assoc Med Bras (1992). 2010;56(4):415–9. [DOI] [PubMed]
- 14.Nollett C, Ryan B, Bray N, Bunce C, Casten R, Edwards RT, et al. Depressive symptoms in people with vision impairment: a cross-sectional study to identify who is most at risk. BMJ Open. 2019;9(1):e026163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Augestad LB, Elmer S. Self-concept and self-esteem among children and young adults with visual impairment: a systematic review. Cogent Psychol. 2017;4(1).
- 16.Liu L, Jiao J, Yang X, Zhang J, Yu H, Li C, et al. Global, Regional, and National Burdens of Blindness and Vision Loss in children and adolescents from 1990 to 2019: a Trend Analysis. Ophthalmology. 2023;130(6):575–87. [DOI] [PubMed] [Google Scholar]
- 17.Abdolalizadeh P, Chaibakhsh S, Falavarjani KG. Global burden of paediatric vision impairment: a trend analysis from 1990 to 2017. Eye (Lond). 2021;35(8):2136–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morgan IG, French AN, Ashby RS, Guo X, Ding X, He M, et al. The epidemics of myopia: Aetiology and prevention. Prog Retin Eye Res. 2018;62:134–49. [DOI] [PubMed] [Google Scholar]
- 19.Dolgin E. The myopia boom. Nature. 2015;519(7543):276–8. [DOI] [PubMed] [Google Scholar]
- 20.Sun HP, Li A, Xu Y, Pan CW. Secular trends of reduced visual acuity from 1985 to 2010 and disease burden projection for 2020 and 2030 among primary and secondary school students in China. JAMA Ophthalmol. 2015;133(3):262–8. [DOI] [PubMed] [Google Scholar]
- 21.Wu HM, Seet B, Yap EP, Saw SM, Lim TH, Chia KS. Does education explain ethnic differences in myopia prevalence? A population-based study of young adult males in Singapore. Optom Vis Sci. 2001;78(4):234–9. [DOI] [PubMed] [Google Scholar]
- 22.Matsumura H, Hirai H. Prevalence of myopia and refractive changes in students from 3 to 17 years of age. Surv Ophthalmol. 1999;44(Suppl 1):S109–15. [DOI] [PubMed] [Google Scholar]
- 23.Jung SK, Lee JH, Kakizaki H, Jee D. Prevalence of myopia and its association with body stature and educational level in 19-year-old male conscripts in Seoul, South Korea. Invest Ophthalmol Vis Sci. 2012;53(9):5579–83. [DOI] [PubMed] [Google Scholar]
- 24.Kang S, Eum S, Chang Y, Koyanagi A, Jacob L, Smith L, et al. Burden of neurological diseases in Asia from 1990 to 2019: a systematic analysis using the global burden of Disease Study data. BMJ Open. 2022;12(9):e059548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morgan IG, Ohno-Matsui K, Saw SM. Myopia Lancet. 2012;379(9827):1739–48. [DOI] [PubMed] [Google Scholar]
- 26.Low JR, Gan ATL, Fenwick EK, Gupta P, Wong TY, Teo ZL, et al. Role of socio-economic factors in visual impairment and progression of diabetic retinopathy. Br J Ophthalmol. 2021;105(3):420–5. [DOI] [PubMed] [Google Scholar]
- 27.Li Y, Wang H, Guan Z, Guo C, Guo P, Du Y et al. Persistence of severe global inequalities in the burden of blindness and vision loss from 1990 to 2019: findings from the global burden of Disease Study 2019. Br J Ophthalmol. 2023;108(2):301–9. [DOI] [PubMed]
- 28.Hamm LM, Wainiqolo I, Pant N, Bhatta S, Petrie-Deely D, Silwal P, et al. Research about eye health and eye health services in Pacific Island Countries and territories: a scoping review. Lancet Reg Health West Pac. 2024;50:101152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keeffe JE, Casson RJ, Pesudovs K, Taylor HR, Cicinelli MV, Das A, et al. Prevalence and causes of vision loss in South-East Asia and Oceania in 2015: magnitude, temporal trends and projections. Br J Ophthalmol. 2019;103(7):878–84. [DOI] [PubMed] [Google Scholar]
- 30.Liu L, Jiao J, Yang X, Zhang J, Yu H, Li C, et al. Global, Regional, and National Burdens of Blindness and Vision Loss in children and adolescents from 1990 to 2019. Ophthalmology. 2023;130(6):575–87. [DOI] [PubMed] [Google Scholar]
- 31.Keeffe JE, Konyama K, Taylor HR. Vision impairment in the Pacific region. Br J Ophthalmol. 2002;86(6):605–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Global burden. Of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of Disease Study 2019. Lancet. 2020;396(10258):1204–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bourne R, Steinmetz JD, Flaxman S, Briant PS, Taylor HR, Resnikoff S, et al. Trends in prevalence of blindness and distance and near vision impairment over 30 years: an analysis for the global burden of Disease Study. Lancet Global Health. 2021;9(2):e130–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Causes of blindness and vision impairment. In 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the right to Sight: an analysis for the global burden of Disease Study. Lancet Glob Health. 2021;9(2):e144–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Organization. Report of the Regional Director: the work of WHO in the Western Pacific Region, 1 July 2021–30 June 2022. 2022 [ https://www.who.int/china/publications-detail/9789290619963
- 36.Best O, Ban S. Adolescence: physical changes and neurological development. Br J Nurs. 2021;30(5):272–5. [DOI] [PubMed] [Google Scholar]
- 37.U.S. Department of Health & Human Services. Adolescent Development Explained. 2009 [ https://opa.hhs.gov/adolescent-health/adolescent-development-explained
- 38.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335–51. [DOI] [PubMed] [Google Scholar]
- 39.Holford TR. Understanding the effects of age, period, and cohort on incidence and mortality rates. Annu Rev Public Health. 1991;12:425–57. [DOI] [PubMed] [Google Scholar]
- 40.Yang Y, Land K. Age-Period-Cohort Analysis: New Models, Methods, and Empirical Applications2013. 355 p.
- 41.Dong Z, Wang QQ, Yu SC, Huang F, Liu JJ, Yao HY, et al. Age-period-cohort analysis of pulmonary tuberculosis reported incidence, China, 2006–2020. Infect Dis Poverty. 2022;11(1):85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ravuvu A, Waqa G. Childhood obesity in the Pacific: challenges and opportunities. Curr Obes Rep. 2020;9(4):462–9. [DOI] [PubMed] [Google Scholar]
- 43.World Health Organization. Strengthening primary health care for the future. 2022 [ https://www.who.int/westernpacific/publications/m/item/strengthening-primary-health-care-for-the-future-rc73
- 44.Grzybowski A, Kanclerz P, Tsubota K, Lanca C, Saw SM. A review on the epidemiology of myopia in school children worldwide. BMC Ophthalmol. 2020;20(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.World Health Organization. Towards universal eye health: a regional action plan for the Western Pacific (2014–2019). 2014 [ https://www.who.int/publications/i/item/9789290616702
- 46.The International Agencyfor the Prevention of Blindness. Regional Plan for Universal Eye Health: Western Pacific. 2015 [ https://www.iapb.org/news/regional-plan-for-universal-eye-health-western-pacific/
- 47.The International Agencyfor the Prevention of Blindness. China five year National Plan for Eye Health 2016–2020. 2016 [ https://www.iapb.org/learn/resources/china-five-year-national-plan-for-eye-health-2016-2020/
- 48.The International Agencyfor the Prevention of Blindness. Long-term Strategy for Blindness Prevention endorsed by, Vietnam PM. 2017 [ https://www.iapb.org/news/long-term-strategy-for-blindness-prevention-endorsed-by-vietnam-pm/
- 49.Han X, Zhang J, Liu Z, Tan X, Jin G, He M, et al. Real-world visual outcomes of cataract surgery based on population-based studies: a systematic review. Br J Ophthalmol. 2023;107(8):1056–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sato M, Kamiya K, Hayashi K, Tabuchi H, Kojima T, Goto N, et al. Changes in cataract and refractive surgery practice patterns among JSCRS members over the past 20 years. Jpn J Ophthalmol. 2024;68(5):443–62. [DOI] [PubMed] [Google Scholar]
- 51.World Health Organization. Universal eye health: a global action plan 2014–2019. 2013 [ https://www.who.int/publications/i/item/universal-eye-health-a-global-action-plan-2014-2019
- 52.Ma A, Yang Y, Guo S, Li X, Zhang S, Chang H. Adolescent resilience and mobile phone addiction in Henan Province of China: impacts of chain mediating, coping style. PLoS ONE. 2022;17(12):e0278182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu S, Ye S, Xi W, Zhang X. Electronic devices and myopic refraction among children aged 6–14 years in urban areas of Tianjin, China. Ophthalmic Physiol Opt. 2019;39(4):282–93. [DOI] [PubMed] [Google Scholar]
- 54.Jan C, Li SM, Kang MT, Liu L, Li H, Jin L, et al. Association of visual acuity with educational outcomes: a prospective cohort study. Br J Ophthalmol. 2019;103(11):1666–71. [DOI] [PubMed] [Google Scholar]
- 55.Morgan IG, Rose KA. Myopia and international educational performance. Ophthalmic Physiol Opt. 2013;33(3):329–38. [DOI] [PubMed] [Google Scholar]
- 56.Ministry of Education of the People’s Republic of China. Circular of the Ministry of Education and Eight Other Departments on the Issuance of the Implementation Plan for Comprehensive Prevention and Control of Myopia among Children and Adolescents. 2018 [ http://www.moe.gov.cn/srcsite/A17/moe_943/s3285/201808/t20180830_346672.html
- 57.Jakovljevic M, Chang H, Pan J, Guo C, Hui J, Hu H, et al. Successes and challenges of China’s health care reform: a four-decade perspective spanning 1985–2023. Cost Eff Resour Alloc. 2023;21(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sogbesan E, Yutho U. Cambodia’s National Eye Care Programme and VISION 2020: the right to Sight. Community Eye Health. 2000;13(36):57–9. [PMC free article] [PubMed] [Google Scholar]
- 59.The Phnom Penh Post. Cambodia a step closer to ending avoidable blindness by 2030. 2021 [ https://phnompenhpost.com/opinion/cambodia-step-closer-ending-avoidable-blindness-2030
- 60.Jonas JB, Ang M, Cho P, Guggenheim JA, He MG, Jong M, et al. IMI Prevention of Myopia and its progression. Invest Ophthalmol Vis Sci. 2021;62(5):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ip JM, Huynh SC, Robaei D, Kifley A, Rose KA, Morgan IG, et al. Ethnic differences in refraction and ocular biometry in a population-based sample of 11-15-year-old Australian children. Eye (Lond). 2008;22(5):649–56. [DOI] [PubMed] [Google Scholar]
- 62.French AN, Morgan IG, Mitchell P, Rose KA. Patterns of myopigenic activities with age, gender and ethnicity in Sydney schoolchildren. Ophthalmic Physiol Opt. 2013;33(3):318–28. [DOI] [PubMed] [Google Scholar]
- 63.Ma Y, Qu X, Zhu X, Xu X, Zhu J, Sankaridurg P, et al. Age-specific prevalence of visual impairment and refractive error in children aged 3–10 years in Shanghai, China. Invest Ophthalmol Vis Sci. 2016;57(14):6188–96. [DOI] [PubMed] [Google Scholar]
- 64.World Health Organization. Vision 2020: The Right to Sight. 2009 [ https://iris.who.int/bitstream/handle/10665/206523/B4317.pdf?sequence=1&isAllowed=y
- 65.Ang M, Flanagan JL, Wong CW, Müller A, Davis A, Keys D, et al. Review: myopia control strategies recommendations from the 2018 WHO/IAPB/BHVI meeting on myopia. Br J Ophthalmol. 2020;104(11):1482–7. [DOI] [PubMed] [Google Scholar]
- 66.World Health Organization. Eye care, vision impairment and blindness: Refractive errors. 2024 [ https://www.who.int/news-room/questions-and-answers/item/blindness-and-vision-impairment-refractive-errors
- 67.Myopia stabilization and. Associated factors among participants in the correction of myopia evaluation trial (COMET). Invest Ophthalmol Vis Sci. 2013;54(13):7871–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang Z, Jin G, Li Z, Liao Y, Gao X, Zhang Y, et al. Global disease burden of uncorrected refractive error among adolescents from 1990 to 2019. BMC Public Health. 2021;21(1):1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.LeBlanc AG, Katzmarzyk PT, Barreira TV, Broyles ST, Chaput JP, Church TS, et al. Correlates of total sedentary time and screen time in 9–11 year-old children around the World: the International Study of Childhood obesity, Lifestyle and the Environment. PLoS ONE. 2015;10(6):e0129622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang SK, Guo Y, Liao C, Chen Y, Su G, Zhang G, et al. Incidence of and factors Associated with myopia and high myopia in Chinese children, based on refraction without Cycloplegia. JAMA Ophthalmol. 2018;136(9):1017–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li Y, Liu J, Qi P. The increasing prevalence of myopia in junior high school students in the Haidian District of Beijing, China: a 10-year population-based survey. BMC Ophthalmol. 2017;17(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li L, Zhong H, Li J, Li CR, Pan CW. Incidence of myopia and biometric characteristics of premyopic eyes among Chinese children and adolescents. BMC Ophthalmol. 2018;18(1):178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang J, Li M, Zhu D, Cao Y. Smartphone overuse and visual impairment in children and young adults: systematic review and Meta-analysis. J Med Internet Res. 2020;22(12):e21923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li M, Lanca C, Tan CS, Foo LL, Sun CH, Yap F, et al. Association of time outdoors and patterns of light exposure with myopia in children. Br J Ophthalmol. 2023;107(1):133–9. [DOI] [PubMed] [Google Scholar]
- 75.Yang BY, Guo Y, Zou Z, Gui Z, Bao WW, Hu LW, et al. Exposure to ambient air pollution and visual impairment in children: a nationwide cross-sectional study in China. J Hazard Mater. 2021;407:124750. [DOI] [PubMed] [Google Scholar]
- 76.Lee SS, Mackey DA. Prevalence and risk factors of myopia in young adults: review of findings from the Raine Study. Front Public Health. 2022;10:861044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pan CW, Klein BE, Cotch MF, Shrager S, Klein R, Folsom A, et al. Racial variations in the prevalence of refractive errors in the United States: the multi-ethnic study of atherosclerosis. Am J Ophthalmol. 2013;155(6):1129–e381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rahi JS, Cumberland PM, Peckham CS. Myopia over the lifecourse: prevalence and early life influences in the 1958 British birth cohort. Ophthalmology. 2011;118(5):797–804. [DOI] [PubMed] [Google Scholar]
- 79.Han SB, Jang J, Yang HK, Hwang JM, Park SK. Prevalence and risk factors of myopia in adult Korean population: Korea national health and nutrition examination survey 2013–2014 (KNHANES VI). PLoS ONE. 2019;14(1):e0211204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Saw SM, Carkeet A, Chia KS, Stone RA, Tan DT. Component dependent risk factors for ocular parameters in Singapore Chinese children. Ophthalmology. 2002;109(11):2065–71. [DOI] [PubMed] [Google Scholar]
- 81.Broadhead GK, Hong T, Bahrami B, Flood V, Liew G, Chang AA. Diet and risk of visual impairment: a review of dietary factors and risk of common causes of visual impairment. Nutr Rev. 2021;79(6):636–50. [DOI] [PubMed] [Google Scholar]
- 82.Li L, Sun N, Zhang L, Xu G, Liu J, Hu J, et al. Fast food consumption among young adolescents aged 12–15 years in 54 low- and middle-income countries. Global Health Action. 2020;13(1):1795438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bourne RRA, Flaxman SR, Braithwaite T, Cicinelli MV, Das A, Jonas JB, et al. Magnitude, temporal trends, and projections of the global prevalence of blindness and distance and near vision impairment: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(9):e888–97. [DOI] [PubMed] [Google Scholar]
- 84.Li S, Ye E, Huang J, Wang J, Zhao Y, Niu D, et al. Global, regional, and national years lived with disability due to blindness and vision loss from 1990 to 2019: findings from the global burden of Disease Study 2019. Front Public Health. 2022;10:1033495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xu T, Wang B, Liu H, Wang H, Yin P, Dong W, et al. Prevalence and causes of vision loss in China from 1990 to 2019: findings from the global burden of Disease Study 2019. Lancet Public Health. 2020;5(12):e682–91. [DOI] [PubMed] [Google Scholar]
- 86.Bahremani E, Alizadeh M, Nejadghaderi SA, Noori M, Sullman MJM, Kolahi AA, et al. The burden of vision loss in the Middle East and North Africa region, 1990–2019. Arch Public Health. 2023;81(1):172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ulldemolins AR, Lansingh VC, Valencia LG, Carter MJ, Eckert KA. Social inequalities in blindness and visual impairment: a review of social determinants. Indian J Ophthalmol. 2012;60(5):368–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is publically available at Global Health Data Exchange (GHDx) online website (https://vizhub.healthdata.org/gbd-results/).