Abstract
Study Design
Prospective, single-center study.
Objective
To evaluate the clinical relevance of the validated intraoperative bleeding severity scale (VIBe) in thoracolumbar spine surgery.
Methods
Adult patients aged 18 through 88 undergoing elective decompression, instrumentation, and fusion of the thoracolumbar spine were prospectively enrolled after informed consent was provided and written consent was obtained. Validated intraoperative bleeding severity scores were recorded intraoperatively. Univariate analysis consisted of Student T-tests, Pearson’s χ2 Tests, Fisher’s Exact Tests, linear regression, and binary logistic regression. Multivariable regression was conducted to adjust for baseline characteristics and potential confounding variables.
Results
A total of N = 121 patients were enrolled and included in the analysis. After adjusting for confounders, VIBe scores were correlated with an increased likelihood of intraoperative blood transfusion (β = 2.46, P = .012), postoperative blood transfusion (β = 2.36, P = .015), any transfusion (β = 2.49, P < .001), total transfusion volume (β = 180.8, P = .020), and estimated blood loss (EBL) (β = 409, P < .001). Validated intraoperative bleeding severity scores had no significant association with length of hospital stay, 30-day readmission, 30-day reoperation, 30-day emergency department visit, change in pre- to post-op hemoglobin and hematocrit, total drain output, or length of surgery.
Conclusion
The VIBe scale is associated with perioperative transfusion rates and EBL in patients undergoing thoracolumbar spine surgery. Overall, the VIBe scale has clinically relevant meaning in spine surgery, and shows potential utility in clinical research.
Level of evidence
Level II.
Keywords: thoracolumbar, blood transfusion, bleeding, clinician reported scale, validated intraoperative bleeding scale, spinal instrumentation, spinal fusion, hemostasis
Introduction
Intraoperative blood loss is common in spine surgery due to large open incisions, tissue dissection, and bleeding from bone surfaces. Maintaining hemostasis is critical, as other works have shown that perioperative blood transfusion is associated with increased morbidity, mortality, health care costs, and resource utilization.1-3 Further, the risk of medical complications, length of stay, and mortality may increase in a dose-dependent manner with transfusion. 4 Blood loss is minimized through the use of electrocautery devices, as well as intravenous (IV) and topical hemostatic agents. Commonly used hemostatic agents include tranexamic acid (TXA), oxidized regenerated cellulose (Surgicel®), and gelatin matrices (Surgifoam®, FloSeal®, Surgiflo®) (Ethicon, Cincinnati, Ohio, and Baxter International, Deerfield, Illinois). However, the lack of a real-time standardized bleeding metric previously hindered comparison of these agents in clinical studies.
In 2016, the United States Food and Drug Administration (FDA) requested the development of a clinician-reported scale (CRS) for categorizing intraoperative bleeding severity. To this end, Lewis et al were the first to describe the Validated Intraoperative Bleeding Severity Scale (VIBe). 5 The VIBe scale is a Likert-type scale in which the surgeon assigns a grade of 0 to 4 based on visual presentation, anatomic appearance, qualitative severity, and visually estimated volumetric rate of blood loss. Qualitatively, the scale ranges from no bleeding to life threatening hemorrhage that obscures the visual field. In their initial study, Lewis et al demonstrated high intra- and inter-observer reliability between surgeon raters. Further, the VIBe scale met all of the FDA’s essential criteria for a CRS, including clarity, validity, relevance, repeatability, reproducibility, response distribution, usability, and the ability to detect change. 5
The aim of the VIBe scale was to allow for better comparison of hemostatic agents in clinical studies. The scale has been met with strong approval by surgeons as a useful tool for clinical research. 5 The study by Lewis et al was initially most applicable to general surgery, as it was conducted with porcine models in the thoracic, abdominal, and pelvic anatomy. Subsequently, the VIBe scale was successfully applied to compare hemostatic agents in pre-clinical bleeding models and in hepatic surgery.5,6 Finally, in 2022, Sciubba et al validated the reliability of the VIBe scale in spine surgery. 7 However, there is currently no existing literature which investigates the utility of the VIBe scale in assessing surgical bleeding or transfusion risk in spine surgery.
Surgical decompression, instrumentation, and fusion is a routine procedure for symptomatic degenerative disease of the thoracolumbar spine.8,9 Traditionally, an open posterior approach with a midline incision is used, and blood loss is a well-known complication. 10 Transfusion rates have been reported from 4 to 17% in lumbar fusion, and up to 31% in thoracic fusion.11,12 The purpose of this study was to evaluate the clinical relevance of the VIBe scale in patients undergoing elective thoracolumbar spine surgery using an open posterior approach. We hypothesized that greater intraoperative VIBe scores would be independently associated with greater rates of transfusion and bleeding complications. This study may provide a basis for future research in spine surgery hemostasis by confirming the clinical significance of the VIBe scale.
Methods
Patient Selection
Following Institutional Review Board approval at our institution, we prospectively enrolled N = 121 patients undergoing elective decompression, instrumentation, and fusion of the thoracolumbar spine from March 2022 to March 2023. All patients enrolled voluntarily with informed and written consent. All surgeries were indicated for degenerative pathology. All surgeries were performed using a traditional open midline posterior approach. Only adult patients >18 years old were included. Patients were excluded if their indication for surgery was due to trauma, tumor, or infection, or if their procedure occurred through any approach other than the traditional posterior approach.
Demographic and Surgical Characteristics
Demographic data for each patient was collected from the electronic medical record, including age, sex, race, body mass index, smoking history, history of anti-coagulant use, and primary surgical diagnosis. Surgical characteristics were collected from the operative report, including primary vs revision surgery, length of surgery, estimated blood loss, implant type, number of levels instrumented, use of intraoperative topical hemostatic agents, and use of cell saver. This was a multiple surgeon study, and use of intraoperative hemostatic agents were determined according to the clinical judgment of the attending surgeon during the course of surgery.
VIBe Score Measurements
We used the VIBe scale as described by Lewis et al in 2016. 5 Grade 0 referred to no bleeding present. Grade 1 represented capillary bleeding that oozed or bled intermittently. Grade 2 was continuous bleeding from a venule or arteriolar source. Grade 3 was non-central venous or arterial bleeding that spurted with overwhelming flow. Grade 4 was bleeding from a central arterial or venous source that obscured the bleeding source and surgical field (Table 1). Educational materials, training videos, and practice simulations were provided from Baxter International on how to appropriately assign score using the VIBe scale. All authors reviewed these materials and passed a competency test on scoring accuracy prior to data collection.
Table 1.
The Validated Intraoperative Bleeding Severity (VIBe) Scale.
| VIBe Score | Qualitative Description | Anatomic Significance | Volumetric Rate (milliliters/minute) |
|---|---|---|---|
| 0 | No bleeding or not clinically relevant | None | <1.0 |
| 1 | Mild, oozing/intermittent flow | Capillary-like | >1.0-5.0 |
| 2 | Moderate, continuous flow | Arteriolar or venule | >5.0-10.0 |
| 3 | Severe, controllable, spurting/overwhelming flow | Non-central arterial or venous | >10.0-50.0 |
| 4 | Life threatening, view-obstructing spurting or gush | Major central artery or vein | >50.0 |
Table adapted from Lewis et al. 5
Data Collection
During surgery, maximum VIBe measurements (the highest score observed) were recorded during each of 5 phases of surgery as determined by the senior attending surgeon. We collected VIBe measurements during (1) exposure, (2) decompression, (3) instrumentation, (4) fusion, and (5) closure. These scores were recorded on a data collection sheet. Patients were followed through the electronic medical record throughout their hospitalization and up to 60 days after discharge.
Primary and Secondary Outcomes
The primary outcomes of interest were the intraoperative, postoperative, and overall perioperative blood transfusion rates. Secondary outcomes included total transfusion volume, EBL, postoperative drain output, length of stay, change in pre- to post-operative hemoglobin and hematocrit, 30-day readmission, 30-day reoperation, and 30-day emergency department visit. For preoperative hemoglobin values, the last documented lab hemoglobin and hematocrit recorded in the patient chart prior to the OR were used. If the last lab values were collected following any procedure that resulted in blood loss, the lab results preceding the procedure were used.
Statistical Analysis
We utilized R software for data analysis (R Core Team 2023. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria). Significance was set at an a priori alpha of .05. Descriptive statistics were calculated for all continuous variables, and percentages were calculated for categorical variables. For univariate outcomes analysis, continuous variables were analyzed with two-sample Student T-tests, and categorical variables were analyzed with Pearson’s χ2 Test and Fisher’s Exact Test. Univariate regression analysis was conducted. For multivariable analysis, binary outcomes were analyzed using multivariable logistic regression and continuous variables were analyzed with multiple linear regression. Adjustments in the multivariable analyses included age, biological sex, race, body mass index (BMI), smoking status, anticoagulation history, and number of levels instrumented.
Results
Cohort Characteristics
A total of N = 121 patients were included in the analysis. The mean age at presentation was 63.9 ± 10.8 years old and 69 (57.0%) patients were female. The mean BMI at presentation was 30.8 ± 2.8. A total of 17 (14%) patients had a history of coagulopathy or anticoagulation prior to surgery. Regarding preoperative diagnosis, 92 (76%) patients had stenosis, 25 (20.7%) had scoliosis, 15 (12.4%) had pseudoarthrosis, and 40 (33.1%) had spondylolisthesis (Table 2).
Table 2.
Population Characteristics.
| Demographic Characteristics | n (%) or Mean ± SD | Surgical Characteristics | n (%) or Mean ± SD |
|---|---|---|---|
| Total | 121 | Primary surgery | 49 (40.5%) |
| Age | 63.9 ± 10.8 | Revision surgery | 72 (59.5%) |
| Sex | Length of surgery (min) | 228.3 ± 99.2 | |
| Female | 69 (57.0%) | Estimated blood loss (mL) | 490.1 ± 395.6 |
| Male | 52 (43.0%) | Use of interbody cage | 50 (41.3%) |
| Race | Number of Levels Instrumented | 3.4 ± 2.9 | |
| White | 93 (76.9%) | 1-2 levels | 67 |
| Black | 25 (20.7%) | 3-6 levels | 39 |
| Hispanic | 3 (2.5%) | 7+ levels | 15 |
| BMI | 30.8 ± 2.8 | Use of Intraoperative: | |
| BMI Class | Topical thrombin | 101 (83.5%) | |
| <35 | 87 (71.9%) | Tranexamic acid | 40 (33.1%) |
| 35-40 | 24 (19.8%) | Porcine gelatin matrix | 73 (60.3%) |
| 40+ | 10 (8.3%) | Bovine gelatin matrix | 8 (6.6%) |
| Smoking Status | Porcine gelatin sponge | 4 (3.3%) | |
| Current | 12 (9.9%) | Cell saver | 13 (10.7%) |
| Former | 46 (38.0%) | VIBe Score | |
| Never | 63 (52.1%) | Mean VIBe score | 1.1 ± 0.3 |
| History of coagulopathy or Anticoagulation | 17 (14.0%) | Max VIBe score | 1.7 ± 0.5 |
| Preoperative Diagnosis | Exposure | 1.4 ± 0.6 | |
| Stenosis | 92 (76.0%) | Decompression | 1.3 ± 0.5 |
| Spondylolisthesis | 40 (33.1%) | Instrumentation | 1.2 ± 0.6 |
| Scoliosis | 25 (20.7%) | Fusion | 1.1 ± 0.4 |
| Pseudarthrosis | 15 (12.4%) | Closure | .6 ± 0.5 |
Abbreviations: VIBe, validated intraoperative bleeding scale; BMI, body mass index; SD, standard deviation; min, minutes; mL, milliliters.
Data presented as n (%) for categorical data, and mean ± standard deviation for continuous values.
Surgical Features
Of the 121 patients, 72 (59.5%) were revision cases. There were 3 (2.5%) patients who had posterior lumbar interbody fusion, 5 (4.1%) patients who had transforaminal lumbar interbody fusion, and 33 (27.3%) patients who had posterior column osteotomy. The mean surgical time was 228.3 ± 99.2 minutes and the mean EBL was 490.1 ± 395.6 mL. The mean number of instrumented levels was 3.4 ± 2.9 levels and 50 (41.3%) patients had an interbody cage implanted. Intravenous TXA was used intra-operatively for 40 (33.1%) patients, topical thrombin was used for 101 (83.5%) patients, Surgicel® was used for 73 (60.3%) patients, FloSeal® was used for 8 (6.6%) patients, and Surgifoam® was used for 4 (3.3%) patients. Cell saver was used in 13 (10.7%) patients (Table 2).
Surgical Phase VIBe Scores
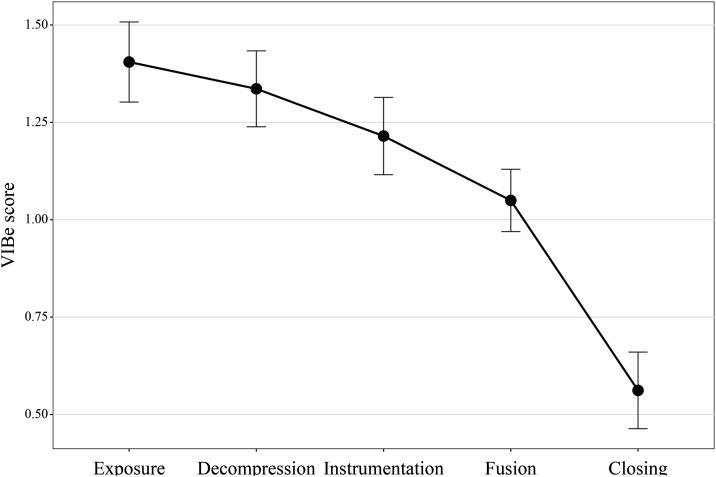
Across all patients, the mean VIBe score was 1.4 ± .6 during exposure, 1.3 ± .5 during decompression, 1.2 ± .6 during instrumentation, 1.1 ± .4 during fusion, and .6 ± .5 during closure (Figure 1). The maximum VIBe score during any phase of surgery per patient had a mean of 1.7 ± .5. The overall mean of all patient’s mean VIBe scores (calculated per patient as the mean of 5 VIBe scores for each phase of surgery) was 1.1 ± .3 (Table 2).
Figure 1.
Mean validated intraoperative bleeding severity (VIBe) score1 across all patients during exposure, decompression, instrumentation, fusion, and closure phases of thoracolumbar surgery.
Outcomes of Interest
A total of 34 patients (28.1%) received a transfusion. Of these patients, 18 (14.9%) received an intraoperative transfusion and 22 (18.0%) received a post-operative transfusion. The mean EBL was 490.1 ± 395.6 mL, the mean preoperative to postoperative change in hemoglobin was −1.6 ± 3.6 grams/deciliter. The mean post-op day 1 drain output was 310.1 ± 209.4 mL, the mean total drain output was 839.7 ± 452.5 mL, and the mean length of stay was 4.2 ± 5.5 days. Within 30-days of discharge, there were 8 (6.6%) patients who were readmitted, 18 (14.9%) who presented to the ER, and 4 (3.3%) who had a reoperation. (Table 3). Of the 4 reoperations, 1 was for suspected infection, 1 was for implant loosening, 1 was for retained drain fragment, and 1 was for postoperative sacral fracture.
Table 3.
Outcomes of Interest, Univariate Analysis Stratified by Mean Validated Intraoperative Bleeding Severity Score Range.
| Outcome | Total | Mean VIBe Score Range: | |||
|---|---|---|---|---|---|
| 0 to .49 | .50 to .99 | 1.00 to 1.49 | 1.50 to 1.99 | ||
| Total | 121 | 4 | 25 | 73 | 19 |
| Transfusion (Any) | 34 (28.1%) | 1 (25%) | 4 (16.0%) | 16 (21.9%) | 13 (68.4%) |
| RR: 1.14, P a = 1 | RR: .73, P = .526 | RR: 1.00, (Ref) | RR: 3.12, P < .001 | ||
| Intraoperative transfusion | 18 (14.9%) | 0 (.0%) | 1 (4.0%) | 10 (13.7%) | 7 (36.8%) |
| RR: 0, P = 1 | RR: .29, P = .280 | RR: 1.00, (Ref) | RR: 2.69, P = .021 | ||
| Postoperative transfusion | 22 (18.2%) | 1 (25%) | 3 (12%) | 9 (12.3%) | 9 (47.4%) |
| RR: 2.08, P = .434 | RR: .98, P = 1 | RR: 1.00, (Ref) | RR: 3.85, P < .001 | ||
| Estimated blood loss (mL) | 490.1 ± 395.6 b | 437.5 ± 154.8 | 397.3 ± 282.1 | 445.3 ± 290.2 | 873.7 ± 645.6 |
| RD: .98, P = .931 | RD: .89, P = .470 | RD: 1.00, (Ref) | RD: 1.96, P = .011 | ||
| Drain output, POD1 | 310.1 ± 209.4 | 162.5 ± 93 | 311 ± 209.9 | 281.2 ± 192.2 | 402.5 ± 237.2 |
| RD: .58, P = .075 | RD: 1.11, P = .535 | RD: 1.00, (Ref) | RD: 1.43, P = .050 | ||
| Drain output, total | 839.7 ± 452.5 | 707.2 ± 236.5 | 823.8 ± 441.8 | 800.1 ± 425.3 | 1002 ± 557.8 |
| RD: .88, P = .508 | RD: 1.03, P = .816 | RD: 1.00, (Ref) | RD: 1.25, P = .155 | ||
| Length of stay | 4.2 ± 5.5 | 4.8 ± 1 | 3.8 ± 7.6 | 4.2 ± 2.4 | 5.3 ± 3.2 |
| RD: 1.14, P = .342 | RD: .90, P = .798 | RD: 1.00, (Ref) | RD: 1.26, P = .175 | ||
| Change in hemoglobin | −1.7 ± 3.5 | −1.5 ± 1.5 | −1.5 ± 4.4 | −1.8 ± 2.9 | −2.1 ± 1.4 |
| RD: .83, P = .733 | RD: .83, P = .753 | RD: 1.00, (Ref) | RD: 1.17, P = .523 | ||
| Change in hematocrit | −5.5 ± 11.1 | −7.4 ± 8.3 | −4.8 ± 14.2 | −5.7 ± 8.8 | −6.7 ± 4.1 |
| RD: 1.30, P = .715 | RD: .84, P = .769 | RD: 1.00, (Ref) | RD: 1.18, P = .476 | ||
| 30-Day readmission | 8 (6.6%) | 0 (0%) | 1 (4.0%) | 5 (6.8%) | 2 (10.5%) |
| RR: 0, P = 1 | RR: .38, P = 1 | RR: 1.00, (Ref) | RR: 1.54, P = .631 | ||
| 30-Day reoperation | 4 (3.3%) | 0 (0%) | 1 (4.0%) | 3 (4.1%) | 0 (0%) |
| RR: 0, P = 1 | RR: 1.00, P = 1 | RR: 1.00, (Ref) | RR: 0, P = 1 | ||
| 30-Day ED visit | 18 (14.9%) | 0 (0%) | 1 (4.0%) | 13 (17.8%) | 4 (21.1%) |
| RR: 0, P = 1 | RR: .22, P = .108 | RR: 1.00, (Ref) | RR: 1.19, P = .746 | ||
Abbreviations: VIBe, validated intraoperative bleeding severity scale; Ref, reference; RR; risk ratio; RD, relative difference (ratio compared to reference); POD1, postoperative day 1; ED, emergency department.
Bold values represent significant values with P<0.05. Data presented as n (%) for categorical data, and mean ± standard deviation for continuous values.
aP-values derived from Pearson’s χ2 Test, Fisher’s Exact Test, or Welch’s two-sample t-test.
bMean ± standard deviation.
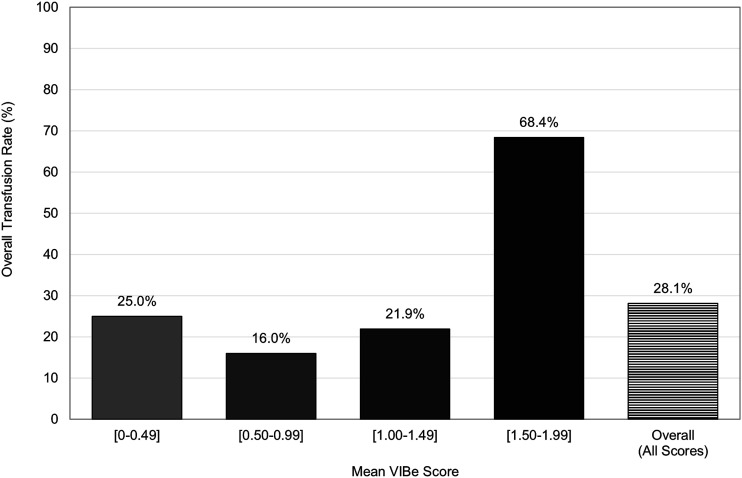
Patients with mean VIBe scores in the range of 1.50 to 1.99 had greater rates of overall transfusion (68.4%, RR: 3.12, P < .001), intraoperative transfusion (36.8%, RR: 2.69, P = .021), postoperative transfusion (47.4%, RR: 3.85, P < .001), and had greater EBL (873.7 ± 645.6 mL, RD: 1.96, P = .011) when compared to the overall cohort. (Table 3, Figure 2).
Figure 2.
90-day perioperative transfusion rates, by mean validated intraoperative bleeding severity (VIBe) score thresholds.
Univariate regression analysis showed that patients with a greater mean VIBe score across the 5 surgical phases were more likely to require an intraoperative blood transfusion (β = .30, P = .002), postoperative blood transfusion (β = .29, P = .007), any transfusion (β = 2.49, P < .001), and total transfusion volume (β = 180.8, P = .020) (Figure 2). They also had a greater EBL (β = 415.8, P < .001) and postoperative day 1 drain output (β = 118.6, P = .038).
Multivariable regression analysis showed that, after adjusting for confounders, patients with a greater mean VIBe were more likely to require a blood transfusion (β = 2.89, P = .015), whether the transfusion was intraoperative (β = 2.46, P = .012) or postoperative (β = 2.36, P = .015). In addition, they had a greater EBL (β = 409.2, P = .015) and total transfusion volume (β = 169.33, P = .024) (Table 4).
Table 4.
Regression Analysis of Outcomes of Interest by Mean Validated Intraoperative Bleeding Severity Score, Adjusted for Confounders. a
| Outcome | Univariate Regression Coefficient ( ) | P-Value b | Multivariable Regression Coefficient ( ) | P-Value |
|---|---|---|---|---|
| Transfusion (Any) | 2.49 | <.001 | 2.89 | .015 |
| Intraoperative transfusion | .30 | .002 | 2.46 | .012 |
| Postoperative transfusion | .29 | .007 | 2.36 | .015 |
| Transfusion volume (mL) | 180.8 | .020 | 169.33 | .024 |
| Estimated blood loss (mL) | 415.8 | <.001 | 409.24 | <.001 |
| Change in pre- to post-op hemoglobin | −.99 | .318 | −1.19 | .266 |
| Change in pre- to post-op hematocrit | −2.99 | .343 | −3.71 | .272 |
| Drain output, postoperative day 1 (mL) | 118.6 | .038 | 106.43 | .063 |
| Drain output, total (mL) | 173.5 | .162 | 143.81 | .246 |
| Length of surgery (minutes) | 4.37 | .876 | 11.67 | .993 |
| Length of stay (days) | 2.18 | .148 | 2.06 | .190 |
| 30-day readmission | .05 | .451 | .40 | .805 |
| 30-day reoperation | −.03 | .491 | −3.01 | .313 |
| 30-day emergency department visit | 1.43 | .075 | 1.38 | .124 |
Abbreviations: VIBe, validated intraoperative bleeding severity scale; mL; milliliters.
Bolded values indicated as being under a significance threshold of P < .05.
aAdjusted for age, biological sex, race, body mass index, smoking status, coagulopathy, number of levels fused, use of intraoperative tranexamic acid (TXA).
bMultivariable logistic regression for categorical outcomes, multiple linear regression for continuous outcomes.
Discussion
In this study, we observed that VIBe scores were highest during exposure (mean of 1.4 ± .6) and lowest during closure (mean of .6 ± .5). Using regression analysis, we showed that the mean of 5 surgical-phase-specific VIBe scores per procedure was significantly associated with intra- and post-operative blood transfusions, total transfusion volume, EBL, and the amount of drain output through post operative day 1. Length of hospital stay, 30-day readmission, 30-day reoperation, 30-day emergency department visit, change in pre- to post-op hemoglobin and hematocrit, total drain output, and length of surgery were not significantly correlated with the mean VIBe score.
Blood transfusion was the primary outcome of interest in this study, and was found to be associated with intraoperative VIBe scores. In addition, a mean VIBe score threshold of 1.50 or greater was seen to be associated with greater transfusion rates. Transfusion presents new risks to the patient, in addition to signifying substantial blood loss. First, blood products may rarely predispose the patient to transfusion reactions. Second, blood products may impair the immune system and increase the incidence of surgical wound infections.15-17 Lastly, the setting of pronounced blood loss and transfusion can result in physiologic fluid shifts and impair the function of the cardiovascular, pulmonary, and renal systems. 18 There have been numerous published research articles aiming to predict transfusion risk in spinal fusion. In general, these studies have identified risk factors, proposed predictive scoring models, or employed advanced computer algorithms.19-21 However, all these factors are preoperative in nature and do not provide the surgeon with real time data as a case evolves. An advantage of the VIBe scale is that it is quick and easy to assign. Clinically, the results of this study show that the VIBe scale may provide a real-time benchmark for surgeons regarding transfusion risk and need for hemostasis.
At our institution, VIBe scores decreased as a case progressed from beginning to end, implying that the greatest rate of blood loss occurred during early portions of the procedure. This is the first data which looks at blood loss by surgical phase in adult posterior spinal fusion for degenerative conditions. The only comparison available is the literature on pediatric scoliosis, which reports the highest rate of blood loss during deformity correction.13,14 Overall, our findings are plausible given the degree of tissue dissection during exposure and the implementation of hemostatic methods to reduce bleeding rate throughout the operation. Decompression and instrumentation are also plausible phases of high blood loss, as this is during the initial violation of bone. By nature, the VIBe score is more indicative of the rate of blood loss at a given point in time, rather than the total volume of blood loss. Therefore, each surgical phase’s duration should be considered when conceptualizing the total blood loss. Anticipating the surgical phase of the most rapid blood loss may be helpful to spine surgeons.
Finally, VIBe scores may offer research utility. A major limitation of hemostasis research in the past was the lack of a standardized definition or classification for intraoperative bleeding severity, making it difficult for surgeons to discern meaningful clinical differences between hemostatic agents. 5 This study followed up on the validation of the VIBe scale in spine surgery by confirming that it is clinically meaningful regarding transfusion risk. The findings of this study may provide a basis for future clinical research aimed at improving hemostasis in spine surgery. For example, one application of the VIBe scale is the evaluation and comparison of hemostatic agents. 5 In future studies, VIBe scores could serve as an outcome that can be extrapolated to clinical outcomes such as transfusion. Alternatively, hemostatic agents could be evaluated on their effectiveness in the setting of different grades of bleeding severity. In addition, future studies are needed to confirm the utility of the VIBe scale in different spine procedures or regions, such as the cervical spine. Lastly, VIBe scores could be used to draw comparisons between surgical techniques. Overall, these findings confirm the VIBe scale as a clinically relevant CRS for research purposes.
Limitations
This research has limitations. First, while the VIBe scale has been validated and determined to have high intra- and inter-reporter reliability, the scale relies on subjective measurements that can be influenced by user bias and experience. For some collected variables, there was unavoidable variability. For example, the exact timing of preoperative and postoperative hemoglobin lab values varied from patient to patient. In this instance, it was less consequential to our findings because the change in hemoglobin did not significantly differ between groups in our analysis. While this study is appropriately powered when analyzing the patient cohort as a whole, some sub-stratifications may lack sufficient power to detect an effect, such as when comparing discrete VIBe score ranges. In addition, mean VIBe scores were determined to be the most useful variable for analysis, although these scores are assigned as whole numbers during surgery. Finally, this was a single-center study conducted at an urban academic institution, which may not be generalizable to all other patient populations and practice settings. Further research may look at repeating these findings in different practice settings, at multiple institutions, or in niche patient populations.
Conclusion
This study evaluated the clinical significance of the VIBe scale in elective thoracolumbar decompression and fusion using an open posterior approach. The VIBe scale is an independent predictor of intraoperative, postoperative, and overall blood transfusion, EBL, and postoperative day 1 drain output. The surgical phases with the highest VIBe scores were exposure and decompression. Overall, the VIBe scale is clinically relevant and has potential utility in both the clinical and research settings for improving hemostasis.
Acknowledgments
Chixiang Chen, PhD, Assistant Professor, Department of Epidemiology & Public Health, University of Maryland School of Medicine.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: In addition, the following conflicts of interest and funding sources have been declared by the authors below. For the remaining authors none were declared. BS: Maryland Orthopaedic Association: Board or committee member, JJ: Children: Editorial or governing board, KC: Cerapedics: Paid presenter or speaker, Stryker: Paid consultant, CS: Executive Committee of the American Association of Neurosurgery Spine Section: Board or committee member Journal of Neurosurgery: Spine: Editorial or governing board Nuvasive: IP royalties; Paid consultant Stryker: IP royalties, DC: Alphatec Spine: Paid consultant; Stock or stock Options, EK: Alphatec Spine: Stock or stock Options, SL: AAOS: Board or committee member Alphatec Spine: IP royalties; Stock or stock Options American Board of Orthopaedic Surgery, Inc.: Board or committee member American Orthopaedic Association: Board or committee member AO Spine North America Spine Fellowship Support: Research support ASIP, ISD: Stock or stock Options Atlas Spine: IP royalties Baxter: Research support Cervical Spine Research Society: Board or committee member Contemporary Spine Surgery: Editorial or governing board DePuy, A Johnson & Johnson Company: IP royalties LSRS: Board or committee member MDC: Stock or stock Options Nuvasive: IP royalties; Paid consultant; Paid presenter or speaker; Stock or stock Options OMEGA: Research support Smiss: Board or committee member Stryker: IP royalties The Spine Journal: Editorial or governing board.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Our division is currently receiving a grant (#52-6002033) from Baxter International Inc in support of the current study.
IRB Approval: This research has received approval by our institutional review board (HP-00097392).
ORCID iDs
Alexandra E. Thomson https://orcid.org/0000-0001-5378-4417
Steven C. Ludwig https://orcid.org/0000-0002-3962-5724
References
- 1.Janssen SJ, Braun Y, Wood KB, et al. Allogeneic blood transfusions and postoperative infections after lumbar spine surgery. Spine J. 2015;15:901-909. [DOI] [PubMed] [Google Scholar]
- 2.Purvis TE, Goodwin CR, De la Garza-Ramos R, et al. Effect of liberal blood transfusion on clinical outcomes and cost in spine surgery patients. Spine J. 2017;17:1255-1263. [DOI] [PubMed] [Google Scholar]
- 3.Mikhail C, Pennington Z, Arnold PM, et al. Minimizing blood loss in spine surgery. Global Spine J. 2020;10:71S-83S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferraris VA, Davenport DL, Saha SP, et al. Surgical outcomes and transfusion of minimal amounts of blood in the operating room. Arch Surg. 2012;147:49-55. [DOI] [PubMed] [Google Scholar]
- 5.Lewis KM, Li Q, Jones DS, et al. Development and validation of an intraoperative bleeding severity scale for use in clinical studies of hemostatic agents. Surgery. 2017;161:771-781. [DOI] [PubMed] [Google Scholar]
- 6.Uranues S, Fingerhut A, Levin E, et al. Effectiveness of Hemopatch® versus surgicel® original to control mild and moderate liver bleeding. BMC Surg. 2022;22:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sciubba DM, Khanna N, Pennington Z, et al. VIBe Scale: validation of the intraoperative bleeding severity scale by spine surgeons. Int J Spine Surg. 2022;16:740-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choma TJ, Mroz TE, Goldstein CL, et al. Emerging techniques in degenerative thoracolumbar surgery. Neurosurgery. 2017;80:S55. [DOI] [PubMed] [Google Scholar]
- 9.Reid PC, Morr S, Kaiser MG. State of the union: a review of lumbar fusion indications and techniques for degenerative spine disease: JNSPG 75th anniversary invited review article. J Neurosurg Spine. 2019;31:1-14. [DOI] [PubMed] [Google Scholar]
- 10.Cho K-J, Suk S-I, Park S-R, et al. Complications in posterior fusion and instrumentation for degenerative lumbar scoliosis. Spine (Phila Pa 1976). 2007;32:2232-2237. [DOI] [PubMed] [Google Scholar]
- 11.Yoshihara H, Yoneoka D. Trends in the utilization of blood transfusions in spinal fusion in the United States from 2000 to 2009. Spine (Phila Pa 1976). 2014;39:297-303. [DOI] [PubMed] [Google Scholar]
- 12.Aoude A, Nooh A, Fortin M, et al. Incidence, predictors, and postoperative complications of blood transfusion in thoracic and lumbar fusion surgery: an analysis of 13,695 patients from the American college of surgeons national surgical quality improvement program database. Global Spine J. 2016;6:756-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wahlquist S, Wongworawat M, Nelson S. When does intraoperative blood loss occur during pediatric scoliosis correction? Spine Deform. 2017;5:387-391. [DOI] [PubMed] [Google Scholar]
- 14.Shirasawa E, Saito W, Miyagi M, et al. Intraoperative blood loss at different surgical-procedure stages during posterior spinal fusion for idiopathic scoliosis. Medicina. 2023;59:387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carson JL, Altman DG, Duff A, et al. Risk of bacterial infection associated with allogeneic blood transfusion among patients undergoing hip fracture repair. Transfusion. 1999;39:694-700. [DOI] [PubMed] [Google Scholar]
- 16.Murphy P, Heal JM, Blumberg N. Infection or suspected infection after hip replacement surgery with autologous or homologous blood transfusions. Transfusion. 1991;31:212-217. [DOI] [PubMed] [Google Scholar]
- 17.Triulzi DJ, Vanek K, Ryan DH, et al. A clinical and immunologic study of blood transfusion and postoperative bacterial infection in spinal surgery. Transfusion. 1992;32:517-524. [DOI] [PubMed] [Google Scholar]
- 18.Popovsky MA, Davenport RD. Transfusion-related acute lung injury: femme fatale? Transfusion. 2001;41:312-315. [DOI] [PubMed] [Google Scholar]
- 19.Nie Z, Ma W, Hu J. Models to predict the probability for intraoperative RBC transfusion during lumbar spinal stenosis and femoral fracture surgeries in aged patients. Transfus Apher Sci 2021;60:103277. [DOI] [PubMed] [Google Scholar]
- 20.De la Garza Ramos R, Hamad MK, Ryvlin J, et al. An artificial neural network model for the prediction of perioperative blood transfusion in adult spinal deformity surgery. J Clin Med. 2022;11:4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pennington Z, Ehresman J, Molina CA, et al. A novel predictive model of intraoperative blood loss in patients undergoing elective lumbar surgery for degenerative pathologies. Spine J. 2020;20:1976-1985. [DOI] [PubMed] [Google Scholar]