Abstract
Background/Objective:
The main objective of this study is to evaluate the incremental cost-effectiveness (ICER) of the Cambridge hybrid closed-loop automated insulin delivery (AID) algorithm versus usual care for children and adolescents with type 1 diabetes (T1D).
Methods:
This multicenter, binational, parallel-controlled trial randomized 133 insulin pump using participants aged 6 to 18 years to either AID (n = 65) or usual care (n = 68) for 6 months. Both within-trial and lifetime cost-effectiveness were analyzed. Analysis focused on the treatment subgroup (n = 21) who received the much more reliable CamAPS FX hardware iteration and their contemporaneous control group (n = 24). Lifetime complications and costs were simulated via an updated Sheffield T1D policy model.
Results:
Within-trial, both groups had indistinguishable and statistically unchanged health-related quality of life, and statistically similar hypoglycemia, severe hypoglycemia, and diabetic ketoacidosis (DKA) event rates. Total health care utilization was higher in the treatment group. Both the overall treatment group and CamAPS FX subgroup exhibited improved HbA1C (−0.32%, 95% CI: −0.59 to −0.04; P = .02, and −1.05%, 95% CI: −1.43 to −0.67; P < .001, respectively). Modeling projected increased expected lifespan of 5.36 years and discounted quality-adjusted life years (QALYs) of 1.16 (U.K. tariffs) and 1.52 (U.S. tariffs) in the CamAPS FX subgroup. Estimated ICERs for the subgroup were £19 324/QALY (United Kingdom) and −$3917/QALY (United States). For subgroup patients already using continuous glucose monitors (CGM), ICERs were £10 096/QALY (United Kingdom) and −$33 616/QALY (United States). Probabilistic sensitivity analysis generated mean ICERs of £19 342/QALY (95% CI: £15 903/QALY to £22 929/QALY) (United Kingdom) and −$28 283/QALY (95% CI: −$59 607/QALY to $1858/QALY) (United States).
Conclusions:
For children and adolescents with T1D on insulin pump therapy, AID using the Cambridge algorithm appears cost-effective below a £20 000/QALY threshold (United Kingdom) and cost saving (United States).
Keywords: Cambridge algorithm, cost-effectiveness, closed-loop automated insulin delivery, type 1 diabetes, United Kingdom, United States
Introduction
Managing type 1 diabetes (T1D) is particularly challenging for children and adolescents. In the United Kingdom, only 10% of youth reach glycosylated hemoglobin (HbA1c) goals, 1 and in the United States, only 17% do so.2,3 Based on registry data, HbA1c levels are highest in adolescents aged 9 to 17 years. 2 Despite the increasing use of continuous glucose monitors (CGM) and insulin pumps, in the U.S. and United Kingdom average HbA1c levels have worsened in both children and adolescents over the last 10 years. Even in adults above 30 years old, only 30% achieve target HbA1c <53 mmol/mol (7.0%). 4 Preventing long-term microvascular and macrovascular complications, premature mortality, and associated health care costs requires sustained HbA1c control. 5
Recently, several commercial closed-loop automated insulin delivery (AID) systems have been approved for use in the United States and Europe. 4 Automated insulin delivery systems automatically adjust insulin delivery in response to sensed serum glucose. In clinical trials, they improve glycemic control and reduce hypoglycemia in children and adolescents with T1D.6-9 However, real-world studies on the earliest widely deployed AID system (MiniMed 670G) reported high discontinuation rates and declining auto-mode use for that system amongst children and adolescents with HbA1c levels above target. 10 Both the AID hardware (CGM and insulin pump) and the specific glucose-response algorithm contribute to clinical effectiveness.8,10-12 Reliability appears essential to sustained use.
The goal of this study was to evaluate the incremental cost-effectiveness (ICER) of the Cambridge hybrid AID algorithm versus usual care for children and adolescents with T1D and baseline HbA1c levels above the recommended target.
Research Design and Methods
Study Design
The underlying clinical trial employed an unblinded multicenter, binational (United States and United Kingdom), block-randomized, parallel design, comparing closed-loop AID (treatment) versus insulin pump therapy with or without CGM (control) over a 6-month period.13,14 Eligible participants had T1D diagnosed at least 12 months prior, current insulin pump therapy for at least 3 months, screening HbA1c values between 53 and 86 mmol/mol (7.0% and 10.0%), and ages between 6 and 18 years, inclusive. The trial implemented the Cambridge model predictive control algorithm (version 0.3.71) on two different hardware combinations, CamAPS FX and FlorenceM. 14 All participants (or parents for those under 12 years) completed surveys at baseline, 3, and 6 months assessing both their health-related quality of life (HRQoL) and non-study health care utilization. Additional participant contacts were also recorded, both device-related and unrelated. Personnel time for training and counseling participants was also estimated through staff surveys for treatment and control groups. The main outcome measure was the between-groups mean change in HbA1c at 6 months (after adjusting for baseline HbA1c and other covariates). Other (safety) outcomes measured included the frequency of severe hypoglycemia episodes, the frequency of diabetic ketoacidosis (DKA), and any other adverse or serious adverse events.
Further details of the clinical trial, including design, methods, and clinical outcomes, can be found in the main study report. 14
Cost-Effectiveness Analysis
Two cost-effectiveness analyses (CEAs) were conducted: a within-trial CEA using observed trial data, and a lifetime CEA using an updated and modified version of the Sheffield T1D model. 15 The within trial analysis adopted a payer perspective, whereas the lifetime analysis employed a health system perspective. Data analysis focused on clinical factors that would potentially influence the CEAs. An impact inventory and reporting checklist were prepared, per recommendations of the Second Panel on Cost-Effectiveness in Health and Medicine 16 and the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) 17 (Supplemental Tables S1 and S2).
Costs were reported in 2019 U.K. British pounds for the U.K. analysis and 2020 U.S. Dollars for the U.S. analysis. Total costs included all direct costs associated with AID device use, nonresearch clinical care provided by trial personnel, additional health care utilization, insulin use, and indirect costs associated with daily diabetes care time. Cost assumptions are summarized in Supplemental Tables S3 and S4. Time discounting of 3.5% per annum was used for both costs and health utilities.
For the within-study analysis, HRQoL was directly measured from participants at baseline, 3, and 6 months using both the CHU-9D 18 and EQ-5D-Y-3L. 19 Results were scored to estimate a univariate HRQoL using the validated U.K. adult tariffs18,20 and the corresponding U.S. adult EQ-5D tariff. 20 (Note that there was currently no validated child tariff for the EQ-5D.) 21
Long-term cost-effectiveness outcomes over the participants’ lifetimes were simulated using a modified version the Sheffield T1D policy model. 15 Model parameters for probability, utility, and cost (including parameter distributions and ranges for the probabilistic sensitivity analysis [PSA]) are presented in Supplemental Tables S5 to S8. A simulated population of 5000 was drawn for each run (treatment and control groups separately), with baseline mean characteristics using those of trial participants, and U.K. or U.S. population norms for young adult smoking, cholesterol, and blood pressure, as shown in Supplemental Table S5a and S5b. The base model and all sensitivity analyses were each run 30 times, and the results averaged. The PSA on the base case was run with 500 treatment and control iterations of the model parameters (Supplemental Tables S5-S8), for a total of 5 000 000 simulated patient lifetimes. All simulations were implemented using Simul8 2020 Professional (SIMUL8 Corp., Boston, MA).
Trial approval was obtained from both U.S. and U.K. ethics and regulatory authorities, including the East of England–Cambridge East Research Ethics Committee, the Jaeb Center for Health Research Institutional Review Board, the U.K. Medicines and Healthcare products Regulatory Agency, and the U.S. Food and Drug Administration. Trial safety was supervised by an independent data safety monitoring board. The clinical study was registered with clinicaltrials.gov (NCT02925299). Funding for the clinical trial and sole funding for the CEA was provided by the U.S. National Institutes of Health and National Institutes of Diabetes, Digestive and Kidney Diseases (grant no. UC4 DK108520).
Results
A total of 133 participants were randomized: 65 to the AID group and 68 to the control group. Baseline characteristics are summarized in Table 1. Two-thirds (67%) of those randomized were using a CGM device at enrolment. Ten participants withdrew following randomization (six AID and four control). In the AID group, four of the six withdrew prior to initiating AID and two withdrew later, due to device issues (one FlorenceM, one CamAPS FX). Of the 61 participants randomized to AID who completed AID training, 34 exclusively used FlorenceM for the duration of the study and 21 exclusively used CamAPS FX.
Table 1.
Study Participant Characteristics at Baseline, by Treatment Group.
| Characteristic | AID | Control |
|---|---|---|
| Number of subjects, N | 65 | 68 |
| Age, years (%) | 13.1 ± 2.6 | 12.8 ± 2.9 |
| 6-12 | 29 (45%) | 30 (44%) |
| 13-18 | 36 (55%) | 38 (56%) |
| Range | 7.5-18.4 | 6.3-18.4 |
| Gender, N (%) | ||
| Female | 37 (57) | 39 (57) |
| Male | 28 (43) | 29 (43) |
| Diabetes duration, years | 6.3 ± 3.3 | 6.6 ± 3.1 |
| Total daily insulin dose, U/kg/day | 0.93 ± 0.23 | 0.95 ± 0.24 |
| Continuous glucose monitor (CGM) use, N (%) | ||
| Current | 45 (69%) | 44 (65%) |
| In past, but not current | 12 (18%) | 14 (21%) |
| Never | 8 (12%) | 10 (15%) |
| BMI percentile | 60 ± 25 | 67 ± 25 |
| BMI z-score | 0.35 ± 0.86 | 0.58 ± 0.89 |
| Ethnicity, N (%) | ||
| White non-Hispanic | 60 (94%) | 53 (79%) |
| Black non-Hispanic | 0 (0) | 2 (3%) |
| Hispanic or Latino | 0 (0) | 4 (6%) |
| Asian | 3 (5%) | 4 (6%) |
| Native Hawaiian/Other Pacific Islander | 0 (0) | 1 (1%) |
| More than one race | 1 (2%) | 3 (4%) |
| Highest parent education level, N (%) | ||
| High school diploma or less | 7 (11%) | 2 (3%) |
| Associates degree or some College but no degree | 17 (28%) | 19 (30%) |
| Bachelor’s degree | 18 (30%) | 13 (20%) |
| Master’s degree | 8 (13%) | 15 (23%) |
| Doctoral or professional degree | 11 (18%) | 15 (23%) |
| Glycated hemoglobin at baseline, mmol/mol (%) | 66 ± 8 | 67 ± 8 |
| [8.2 ± 0.7] | [8.3 ± 0.8] | |
| Range (%) | 53-83 | 53-89 |
| [7.0-9.7] | [7.0-10.3] | |
| <64 mmol/mol (<8.0%), N (%) | 28 (43%) | 29 (43%) |
| 64 to <75 mmol/mol (8.0 to <9.0%), N (%) | 26 (40%) | 25 (37%) |
| ≥75 mmol/mol (≥9.0%), N (%) | 11 (17%) | 14 (21%) |
Only the CamAPS FX hardware proved sufficiently reliable to allow near continuous AID use, with a median use-time of 93%, versus 40% for FlorenceM. There were 98 device issues reported in the FlorenceM cohort (e.g., unit failure requiring replacement and/or reset), versus only four device issues reported in the CamAPS FX cohort. The adjusted mean improvement in HbA1c at 6 months for the combined CamAPS FX and FlorenceM treatment groups (versus usual care) was small, −0.32% (−3.5 mmol/mol); 95% confidence interval (CI): 0.04 to 0.59; P = .02.
Since only the CamAPS FX hardware proved reliable enough to implement in clinical practice, the economic analysis focused on estimating the incremental cost-effectiveness of that hardware iteration for the Cambridge predictive algorithm. Examining just the CamAPS FX treatment subgroup (n = 21) versus contemporaneous controls recruited in the same period (n = 24) showed an adjusted mean improvement in HbA1c at 6 months of 1.05% (11.5 mmol/mol), 95% CI 0.67 to 1.43, P < .001. The mean HbA1c measurements were 63 ± 10 mmol/mol (7.9% ± 0.9%) at baseline and 51 ± 6 mmol/mol (6.8% ± 0.5%) at 6 months for the treatment group versus 64 ± 6 mmol/mol (8.0% ± 0.6%) at baseline and 63 ± 8 mmol/mol (7.9% ± 0.8%) at 6 months for usual care. Baseline use of CGM, after adjusting for initial HbA1c, age, and other factors, did not appear independently correlated with HbA1c at 6 months.
For the within-study cost-effectiveness analysis, there was no statistically significant difference in HRQoL at baseline or 6 months using either EQ-5D or CHU-9D (Supplemental Table S9). The usual care group experienced 0.41 unscheduled, nonprotocol-related contacts per person year versus 5.21 in the overall treatment group and 1.92 in the CamAPS FX group (Supplemental Table S3).
A total of seven severe hypoglycemia events occurred (four AID and three control), and three DKA events (one prerandomization and two AID, and zero control). Another 23 reportable hyperglycemia events (not meeting criteria for DKA) also occurred (AID 11, control 12). None were associated with clinical sequelae. The rates of severe hypoglycemia and DKA per person year in the CamAPS FX treatment subgroup were not statistically distinguishable from those in the control group (Supplemental Table S3).
Since health care utilization and costs were higher in the treatment group, with no discernable improvement in directly measured HRQoL, no meaningful within-trial incremental cost-effectiveness ratio was calculable.
The mean lifetime ICER for the overall AID group (including both CamAPS FX and Florence M) was £51 278/quality-adjusted life year (QALY).
The long-term (lifetime) baseline results for the CamAPS FX subgroup are shown in Table 2. For the base case, the mean expected lifetime increased by 5.36 years. Estimated mean discounted QALYs increased by 1.161 using U.K. HRQoL values and 1.518 using U.S. values. Estimated mean total discounted lifetime costs increased by £22 182 using U.K. costs and decreased by $5949 using U.S. costs. The resulting ICER was £19 324/QALY for the United Kingdom and −$3,917/QALY for the United States.
Table 2.
Base-Case and Probabilistic Sensitivity Analysis (PSA), Lifetime CEA Results.
| Lifetime probability (%) | AID | Difference | Control |
|---|---|---|---|
| Base case | |||
| Blindness | 0.38% | −0.69% | 1.07% |
| Amputation | 9.27% | −4.61% | 13.89% |
| Death from end-stage renal disease (ESRD) | 19.22% | −15.74% | 34.96% |
| Death from myocardial infarction | 48.14% | 5.92% | 42.22% |
| Death from stroke | 2.69% | 0.27% | 2.43% |
| Death from heart failure | 1.15% | 0.30% | 0.85% |
| U.K. Base Case | |||
| Expected life–years | 54.32 | 5.36 | 48.96 |
| Discounted QALYs | 20.44 | 1.14 | 19.30 |
| Discounted total costs (GBP) | 239 162 | 22 182 | 216 980 |
| Incremental cost-effectiveness ratio (ICER), mean (95% CI) | 19 324 (19 291, 19 356) | ||
| U.K. PSA | |||
| Discounted QALYs, mean | 20.45 | 1.16 | 19.29 |
| Discounted total costs, mean (GBP) | 255 607 | 22 377 | 233 230 |
| ICER, mean (95% CI) | 19 342 (15 903, 22 929) | ||
| U.S. Base Case | |||
| Expected life–years | 54.28 | 5.19 | 49.09 |
| Discounted QALYs | 19.96 | 1.52 | 18.44 |
| Discounted total costs (USD) | 751 721 | −5 949 | 757 670 |
| ICER, mean 95% CI | −3917 (−5181, −2711) | ||
| U.S. PSA | |||
| Discounted QALYs, mean | 19.92 | 1.55 | 18.37 |
| Discounted total costs, mean (USD) | 854 508 | 22 377 | 898 280 |
| ICER, mean (95% CI) | −28 283 (−59 607, 1858) | ||
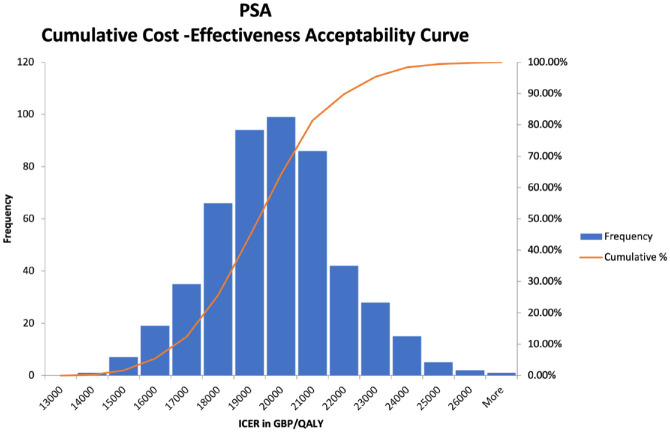
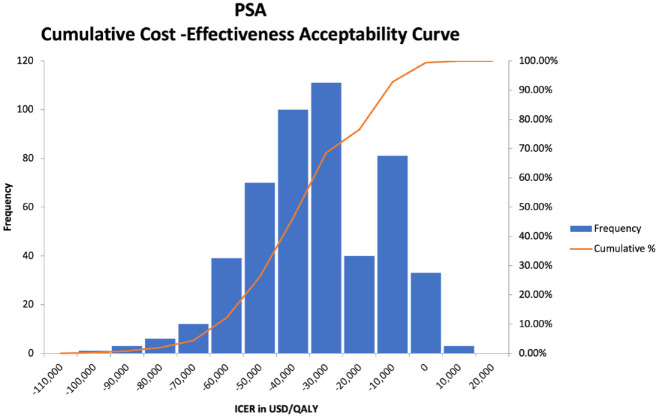
Probabilistic sensitivity analysis on the base case yielded very similar mean ICER results of £19 342/QALY (United Kingdom), with a 95% confidence interval range of £15 903/QALY to £22 929/QALY and −$28,283/QALY (United States), with a 95% confidence interval range of −$59 607/QALY to $1858/QALY. Figures 1 and 2 show scatter plots of all of the PSA model runs for the U.K. and U.S. cases, respectively, whereas Figures 3 and 4 show the frequency of results within specific ICER ranges, as well as the cumulative frequency of ICERs below a given threshold for the U.K. and U.S. cases. These account for uncertainty in the model’s input parameters by allowing those parameters to vary widely, hence the large scatter in results from individual model runs. The key point is that even most of the worst case results were within acceptable cost-effectiveness cutoffs.
Figure 1.
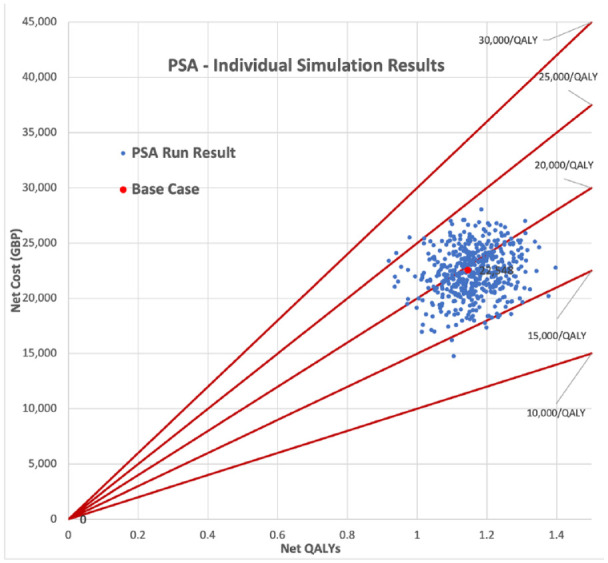
Probabilistic sensitivity analysis simulations full results (U.K. case).
Figure 2.
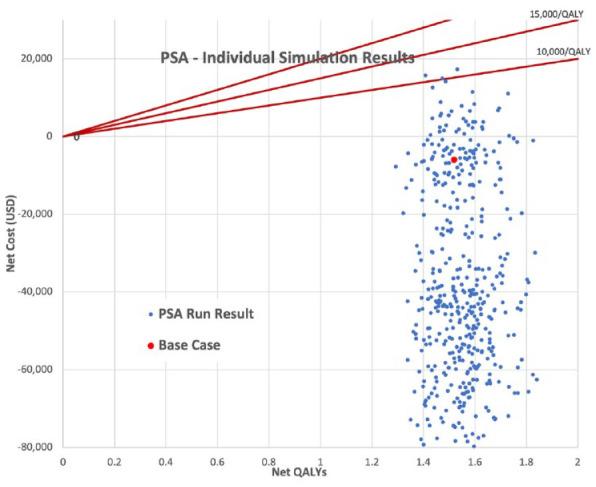
Probabilistic sensitivity analysis simulations full results (U.S. case).
Figure 3.
Probabilistic sensitivity analysis cumulative cost-effectiveness acceptability curve (U.K. case).
Figure 4.
Probabilistic sensitivity analysis cumulative cost-effectiveness acceptability curve (U.S. case).
The results of the one-way sensitivity analyses are shown in Table 3. Two key scenarios were considered, singly and combined. For patients already using compatible hardware (CGM and insulin pump), the only incremental cost was initial system training, plus the annual algorithm fee of £840/$1000, yielding much lower lifetime ICERs of £10 096/QALY and −$33 616/QALY. Incorporating the assumption that actual treatment effectiveness (decrease in HbA1c) would only be sustained at 60% of the trial result increased the lifetime ICERs to £32 897/QALY and $20 841/QALY. However, for patients already using compatible hardware, even a 60% sustained treatment effectiveness yielded ICERs of £18 674/QALY and −$30 847/QALY.
Table 3.
One-Way Sensitivity Analysis Scenario Results.
| (U.K. Case) | Average Cost (GBP) | Average QALYs | QALY Difference | Cost Difference (GBP) | ICER (GBP/QALY) |
|---|---|---|---|---|---|
| Base case—control | 216 980 | 19.30 | 1.14789 | 22,181 | 19 323 |
| Base case—treatment (CL) | 239 161 | 20.44 | |||
| Algorithm cost only—control | 216 980 | 19.30 | 1.15302 | 11,641 | 10 096 |
| Algorithm cost only—treatment (CL) | 228 621 | 20.45 | |||
| 60% Effectiveness—control | 216 980 | 19.30 | 0.74302 | 24,443 | 32 897 |
| 60% Effectiveness—treatment (CL) | 241 423 | 20.04 | |||
| Algorithm cost only and 60% effectiveness—control | 216 980 | 19.30 | 0.74302 | 13,875 | 18 674 |
| Algorithm cost only and 60% effectiveness—treatment (CL) | 230 855 | 20.04 | |||
| U.S. Case | Average Cost (USD) | Average QALYs | QALY Difference | Cost Difference (USD) | ICER (USD/QALY) |
| Base case—control | 757 670 | 18.44 | 1.51865 | −5949 | −3917 |
| Base case—treatment (CL) | 751 721 | 19.96 | |||
| Algorithm cost only—control | 757 670 | 18.44 | 1.51865 | −54,312 | −35 763 |
| Algorithm cost only—treatment (CL) | 703 358 | 19.96 | |||
| 60% Effectiveness—control | 757 670 | 18.44 | 1.04883 | 21,859 | 20 841 |
| 60% Effectiveness—treatment (CL) | 779 529 | 19.49 | |||
| Algorithm cost only and 60% effectiveness—control | 757 670 | 18.44 | 1.04883 | −31,514 | −30 047 |
| Algorithm cost only and 60% effectiveness—treatment (CL) | 726 156 | 19.49 |
Discussion
This health economic analysis, which used results from an unblinded multicenter, binational (United States and United Kingdom), block-randomized, parallel design clinical trial, indicated that the Cambridge hybrid AID algorithm was cost-effective versus insulin pump therapy with or without CGM (control) below a £20 000/QALY threshold (with a 75% probability) for the United Kingdom, and −$3917/QALY (i.e., both health improving and cost-saving or “dominant” in health economic terms with nearly 100% probability) for the United States. This was based on results from the treatment subgroup using the CamAPS FX hardware iteration; both devices in that iteration already have U.K. regulatory approval. For those already using both an insulin pump and CGM, the estimated U.K. ICER was only £10 096/QALY (and −$35 763/QALY for the United States), driven by the lower incremental annual cost of adding only the Cambridge algorithm.
Several recent international studies have also reported incremental cost-effectiveness of various hybrid AID systems.22-27 Those studies, spanning several European countries, Australia, and the United States, estimated ICERs for AID systems within a roughly comparable range. Almost all of those studies, like the one reported here, also adopted a health system, rather than societal perspective, which excludes indirect costs such as lost productivity. Five of those studies applied the IQVIA CORE Diabetes Model, which employs a similar modeling approach to the Sheffield model—Markov microsimulation with nested diabetes complication submodels. However, as should be evident just from the differing ICER results reported here for the U.S. and U.K. cases, even using identical disease model parameters will yield disparate results due to between-country differences in the local costs and health utility decrements associated with specific complications. For example, one study of six European countries using identical disease parameters yielded cost-effectiveness results that ranged from EUR 11 765 per QALY gained in Austria to EUR 43 963 per QALY gained in Italy. 27 Thus, between-country results will never be directly comparable.
The estimated increase in (undiscounted) life expectancy of 5.36 years was substantial, with an accompanying large decrease in T1D complications, save for cardiovascular deaths (which occurred at much later average ages in the treatment group). In the U.S. case, averting the high average cost of care and HRQOL decrement for end-stage renal disease (ESRD) actually yielded average net cost savings, even after future discounting. However, because of the longer expected lifespan in the treatment group, the estimated lifetime ICER in both U.K. and U.S. cases was very sensitive to the incremental annual cost of implementing the AID system. Reducing the net annual system cost in the United Kingdom by £399/year improved the ICER by more than £9000/QALY. Thus, reducing the net CGM hardware cost (for the device and supplies, less the savings on conventional blood glucose monitoring) could improve the baseline ICER (which assumed 65% CGM use) even further. While this analysis took a conservative approach to estimating the net CGM cost, other published studies suggest that net cost of adding CGM may be closer to £0.28,29 Regardless, implementing the Cambridge algorithm on existing hardware already appears highly cost-effective.
This analysis had several limitations. We deviated from the preplanned protocol in two significant ways. First, we did not calculate a within-trial ICER because there was no significant demonstrable improvement in HRQOL, or utilization and costs. A longer follow-up, or larger treatment group might have demonstrated greater improvement, especially for reducing complications such as severe hypoglycemia (requiring outside intervention) or DKA. Second, and more significantly, the ICER results presented here were based on the treatment subgroup which exclusively used the CamAPS FX hardware, which was relatively small (n = 21), with a relatively brief (6-month) study follow-up. While that subgroup analysis was post-hoc, there are clear clinical reasons why the results remain credible (much higher device use-time percentages, much better time in range). Other AID studies showed a similar link between auto mode use-time percentage and clinical outcomes.10,30 Statistical significance tests using analysis of covariance (ANCOVA) and multiple regression on the main HbA1c outcome, adjusted for other baseline factors, also suggest that the observed difference is unlikely due to chance (P < .001). Therefore, system reliability, and not other specifics of the hardware, appears to be the distinguishing factor for the differing effectiveness of the algorithm between platforms, making this subgroup the appropriate analytic choice.
The long-term cost-effectiveness model was also limited by available evidence. There have been no controlled studies on how improving HbA1c and other model clinical inputs long-term, starting in childhood, influence patients’ lifetime T1D complication rates. Even 30-year follow-up of the Diabetes Control Complications Trial (DCCT) mostly showed sustained risk improvement (“metabolic memory”) for the intensive-treatment group, despite their return to mean baseline HbA1c levels of about 8.0%, providing no evidence on the benefits of sustained long-term control starting in childhood.5,31,32 The actual long-term clinical benefits may well be larger than the model estimates. Lacking alternative evidence, the Sheffield model used in this study relied mainly on the original probability parameters, updating only the cost and utility parameters as appropriate. However, the PSA showed tight cost-effectiveness acceptability curves at the higher end, even assuming substantial parameter uncertainties.
Strengths of the study include the use of a well-validated, interrogatable health economic model. This allows comparison with cost-effectiveness results from other studies and facilitates outside confirmation. The model also conservatively incorporates a higher risk of hypoglycemia events (and lower risk of DKA) associated with lower average HbA1c, a trend toward which was seen in the randomized control trial (RCT). Regardless, the model proved reasonably robust to parameter uncertainties, which had a limited impact on the ICER results. The main clinical input, improvement in HbA1c, was based on a multicenter, binational study, enrolling patients with a wide range of baseline HbA1c levels, suggesting external generalizability. Finally, one-way sensitivity analysis also suggested robust results; even if the sustained HbA1c treatment effect were 60% of the observed value, the algorithm remains cost-effective for patients already using a CGM.
Conclusions
The key study conclusion is that the Cambridge AID algorithm appears cost-effective in both the U.S. and U.K. context. Based on a relatively small sample (n = 21) and 6-month follow-up, the algorithm safely generated significant sustained improvements in glycemic control and is lifetime cost-effective below a £20 000/QALY threshold in the U.K. case, and both health improving and cost saving in the U.S. case, compared to usual care for children and adolescents with T1D on insulin pump therapy. For those already using CGM, the algorithm appears cost-effective near a £10 000/QALY threshold for the United Kingdom.
Supplemental Material
Supplemental material, sj-docx-1-dst-10.1177_19322968241231950 for Cost-Effectiveness of Closed-Loop Automated Insulin Delivery Using the Cambridge Hybrid Algorithm in Children and Adolescents with Type 1 Diabetes: Results from a Multicenter 6-Month Randomized Trial by D. Steven Fox, Julia Ware, Charlotte K Boughton, Janet M. Allen, Malgorzata E Wilinska, Martin Tauschmann, Louise Denvir, Ajay Thankamony, Fiona Campbell, R. Paul Wadwa, Bruce A. Buckingham, Nikki Davis, Linda A. DiMeglio, Nelly Mauras, Rachel E. J. Besser, Atrayee Ghatak, Stuart A. Weinzimer, Lauren Kanapka, Craig Kollman, Judy Sibayan, Roy W. Beck, Korey K. Hood and Roman Hovorka in Journal of Diabetes Science and Technology
Acknowledgments
None.
Footnotes
Abbreviations: AID, automated insulin deliver; CEA, cost-effectiveness analysis; CGM, continuous glucose monitor; CHEERS, Consolidated Health Economic Evaluation Reporting Standards; CUA, cost utility analysis; DCCT, Diabetes Complications and Control Trial; DKA, diabetic ketoacidosis; ESRD, end-stage renal disease; HbA1c, percentage of glycosylated hemoglobin; HRQoL, health-related quality of life; ICER, incremental cost-effectiveness ratio; PSA, probabilistic sensitivity analysis; QALY, quality-adjusted life year; RCT, Randomized Control Trial; T1D, type 1 diabetes
Author Contributions: DSF designed the cost-effectiveness analysis. RH, MT, FC, RPW, BAB, LADM, SAW, CK, and RWB co-designed the clinical study. JMA, CKB, JW, MT, BAB, RB, FC, ND, AG, LADM, NM, AT, SAW, and RPW provided patient care and/or took samples. RWB was the medical monitor. DSF and RH wrote the manuscript. DSF, LK, CK, and MEW carried out or supported data analysis, including the statistical analyses. All authors critically reviewed the manuscript and contributed to the interpretation of the results. DSF takes responsibility for the integrity of the cost-effectiveness data and the accuracy of the analysis. All authors critically reviewed the paper prior to publication.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: MT reports receiving speaking and advisory fees from Eli Lilly, Novo Nordisk, Medtronic, and Abbott. RPW reports receiving grant support from MannKind, and Novo Nordisk, grant support and lecture fees from Dexcom, grant support, and advisory board fees from Eli Lilly, and grant support, travel support, and lecture fees from Tandem Diabetes Care. BAB reports receiving grant support, donated supplies, and advisory board fees from ConvaTec, grant support from Dexcom, grant support and honoraria from Insulet, grant support and advisory board fees from Medtronic MiniMed, and grant support from Tandem Diabetes Care. LADM reports grants from Medtronic. NM reports receiving grant support for devices from Medtronic. REJB reports receiving speaking honoraria from Eli Lilly and Springer Healthcare. SAW reports receiving consulting fees from Eli Lilly, Sanofi US Services, and Zealand, speaker honoraria from Dexcom, Insulet, Medtronic, and Tandem Diabetes Care, and grant support to his institution from Abbott and Medtronic. LK reports receiving grant support from Tandem Diabetes Care. CK reports receiving grant support from Tandem Diabetes Care. RWB reports receiving grant support and donated supplies from Abbott Diabetes Care, Ascensia Diabetes Care US, Beta Bionics, and Roche Diabetes Care, grant support, donated supplies, and consulting fees from Dexcom, Novo Nordisk, and Tandem Diabetes Care, grant support and consulting fees from Bigfoot Biomedical, and consulting fees from Eli Lilly and Insulet. RH reports receiving speaker honoraria from Eli Lilly, Dexcom and Novo Nordisk, receiving license fees from B. Braun and Medtronic; patents related to closed-loop, and being director at CamDiab. MEW reports patents related to closed-loop and being a consultant at CamDiab. JW reports receiving speaker honoraria from Ypsomed and Novo Nordisk. KKH reports receiving consulting fees from Cecelia Health and Havas Health. CKB reports receiving consultancy fees from CamDiab and speaker honoraria from Ypsomed, JMA, AT, JS, LD, FC, ND, AG, and DSF declare no competing financial interests exist.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The cost-effectiveness study and the clinical trial were funded by National Institutes of Health and National Institutes of Diabetes, Digestive and Kidney Diseases (grant no. UC4 DK108520). Additional support for the Artificial Pancreas work was provided by National Institute for Health Research Cambridge Biomedical Research Center and Wellcome Strategic Award (grant no. 100574/Z/12/Z). Abbott Diabetes Care supplied continuous glucose monitoring devices and receivers. Dexcom provided support for the development of the CamAPS FX system and supplied discounted continuous glucose monitoring devices. Medtronic provided support for the development of the FlorenceM system and supplied discounted continuous glucose monitoring devices, phone enclosures, pumps and consumables. The views expressed are those of the author(s) and not necessarily those of the funders.
Role of Funding Sources: NIDDK, Medtronic, and Dexcom representatives read the manuscript before submission. No sponsor had any role in the study design, data collection, data analysis, data interpretation, or writing of the report.
ORCID iDs: D. Steven Fox  https://orcid.org/0000-0002-5587-3226
https://orcid.org/0000-0002-5587-3226
Julia Ware  https://orcid.org/0000-0002-4497-0979
https://orcid.org/0000-0002-4497-0979
Charlotte K. Boughton  https://orcid.org/0000-0003-3272-9544
https://orcid.org/0000-0003-3272-9544
R. Paul Wadwa  https://orcid.org/0000-0002-4139-2122
https://orcid.org/0000-0002-4139-2122
Bruce A. Buckingham  https://orcid.org/0000-0003-4581-4887
https://orcid.org/0000-0003-4581-4887
Rachel E. J. Besser  https://orcid.org/0000-0002-4645-6324
https://orcid.org/0000-0002-4645-6324
Lauren Kanapka  https://orcid.org/0000-0003-4440-5168
https://orcid.org/0000-0003-4440-5168
Korey K. Hood  https://orcid.org/0000-0001-5730-7749
https://orcid.org/0000-0001-5730-7749
Roman Hovorka  https://orcid.org/0000-0003-2901-461X
https://orcid.org/0000-0003-2901-461X
Supplemental Material: Supplemental material for this article is available online.
References
- 1. National Paediatric Diabetes Audit. Annual Report 2018-2019: Care Processes and Outcomes. London, England: Royal College of Paediatrics and Child Health; 2020. [Google Scholar]
- 2. Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016-2018. Diabetes Technol Ther. 2019;21(2):66-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hermann JM, Miller KM, Hofer SE, et al. The Transatlantic HbA1c gap: differences in glycaemic control across the lifespan between people included in the US T1D Exchange Registry and those included in the German/Austrian DPV registry. Diabet Med. 2020;37(5):848-855. [DOI] [PubMed] [Google Scholar]
- 4. Leelarathna L, Choudhary P, Wilmot EG, et al. Hybrid D-loop therapy: where are we in 2021? Diabetes, Obesity Metabolism. 2020;23(3):655-660. [DOI] [PubMed] [Google Scholar]
- 5. Nathan DM, Bayless M, Cleary P, et al. Diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: advances and contributions. Diabetes. 2013;62(12):3976-3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bergenstal RM, Nimri R, Beck RW, et al. A comparison of two hybrid closed-loop systems in adolescents and young adults with type 1 diabetes (FLAIR): a multicentre, randomised, crossover trial. Lancet. 2021;397(10270):208-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Breton MD, Kanapka LG, Beck RW, et al. A randomized trial of closed-loop control in children with type 1 diabetes. N Engl J Med. 2020;383(9):836-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brown SA, Kovatchev BP, Raghinaru D, et al. Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med. 2019;381(18):1707-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tauschmann M, Bally HTL, Allen JM, et al. Closed-loop insulin delivery in suboptimally controlled type 1 diabetes: a multicentre, 12-week randomised trial. Yearbook of Paediatric Endocrinology. Lancet. 2019;392:1321-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lal RA, Basina M, Maahs DM, Hood K, Buckingham B, Wilson DM. One year clinical experience of the first commercial hybrid closed-loop system. Diabetes Care. 2019;42(12):2190-2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Messer LH, Berget C, Vigers T, et al. Real world hybrid closed-loop discontinuation: predictors and perceptions of youth discontinuing the 670G system in the first 6 months. Pediatr Diabetes. 2020;21(2):319-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kovatchev B, Anderson SM, Raghinaru D, et al. Erratum. Randomized controlled trial of mobile closed-loop control. Diabetes Care 2020;43:607-615. Diabetes Care. 2020;43(6):1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Musolino G, Allen JM, Hartnell S, et al. Assessing the efficacy, safety and utility of 6-month day-and-night automated closed-loop insulin delivery under free-living conditions compared with insulin pump therapy in children and adolescents with type 1 diabetes: an open-label, multicentre, multinational, single-period, randomised, parallel group study protocol. BMJ Open. 2019;9(6):e027856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ware J, Boughton CK, Allen JM, et al. Cambridge hybrid closed-loop algorithm in children and adolescents with type 1 diabetes: a multicentre 6-month randomised controlled trial. Lancet Digit Health. 2022;4(4):e245-e255. [DOI] [PubMed] [Google Scholar]
- 15. Thokala P, Kruger J, Brennan A, et al. Assessing the cost-effectiveness of Type 1 diabetes interventions: the Sheffield Type 1 Diabetes Policy Model. Diabet Med. 2014;31(4):477-486. [DOI] [PubMed] [Google Scholar]
- 16. Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses. JAMA. 2016;316(10):1093. [DOI] [PubMed] [Google Scholar]
- 17. Husereau D, Drummond M, Petrou S, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Euro J Health Economic. 2013;14(3):367-372. [DOI] [PubMed] [Google Scholar]
- 18. Stevens K. Valuation of the child health utility 9D Index. Pharmacoeconomics. 2012;30(8):729-747. [DOI] [PubMed] [Google Scholar]
- 19. EuroQol Group. EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199-208. [DOI] [PubMed] [Google Scholar]
- 20. Group E. EQ-5D-Y User Guide: How to apply, score, and present results from the EQ-5D-Y; 2020. https://www.unmc.edu/centric/_documents/EQ-5D-5L.pdf
- 21. Retra JGA, Essers BAB, Joore MA, Evers SMAA, Dirksen CD. Age dependency of EQ-5D-Youth health states valuations on a visual analogue scale. Health Qual Life Outcomes. 2020;18(1):386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Biskupiak JE, Ramos M, Levy CJ, et al. Cost-effectiveness of the tubeless automated insulin delivery system vs standard of care in the management of type 1 diabetes in the United States. J Manag Care Spec Pharm. 2023;29(7):807-817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Serné EH, Roze S, Buompensiere MI, Valentine WJ, De Portu S, de Valk HW. Cost-effectiveness of hybrid closed loop insulin pumps versus multiple daily injections plus intermittently scanned glucose monitoring in people with type 1 diabetes in the Netherlands. Adv Ther. 2022;39(4):1844-1856. [DOI] [PubMed] [Google Scholar]
- 24. Pease A, Callander E, Zomer E, et al. The cost of control: cost-effectiveness analysis of hybrid closed-loop therapy in youth. Diabetes Care. 2022;45(9):1971-1980. [DOI] [PubMed] [Google Scholar]
- 25. Jendle J, Pöhlmann J, de Portu S, Smith-Palmer J, Roze S. Cost-effectiveness analysis of the MiniMed 670G hybrid closed-loop system versus continuous subcutaneous insulin infusion for treatment of type 1 diabetes. Diabetes Technol Ther. 2019;21(3):110-118. [DOI] [PubMed] [Google Scholar]
- 26. Roze S, Buompensiere MI, Ozdemir Z, de Portu S, Cohen O. Cost-effectiveness of a novel hybrid closed-loop system compared with continuous subcutaneous insulin infusion in people with type 1 diabetes in the UK. J Med Econ. 2021;24(1):883-890. [DOI] [PubMed] [Google Scholar]
- 27. Jendle J, Buompensiere MI, Ozdemir Saltik AZ, et al. A European cost–utility analysis of the MiniMed™ 780G advanced hybrid closed-loop system versus intermittently scanned continuous glucose monitoring with multiple daily insulin injections in people living with type 1 diabetes. Diabetes Technol Ther. 2023;25(12):864-876. [DOI] [PubMed] [Google Scholar]
- 28. Roze S, Isitt J, Smith-Palmer J, Javanbakht M, Lynch P. Long-term cost-effectiveness of Dexcom G6 real-time continuous glucose monitoring versus self-monitoring of blood glucose in patients with type 1 diabetes in the U.K. Diabetes Care. 2020;43(10):2411-2417. [DOI] [PubMed] [Google Scholar]
- 29. Hellmund R, Weitgasser R, Blissett D. Cost calculation for a flash glucose monitoring system for UK adults with type 1 diabetes mellitus receiving intensive insulin treatment. Diabetes Res Clin Pract. 2018;138:193-200. [DOI] [PubMed] [Google Scholar]
- 30. Berget C, Messer LH, Vigers T, et al. Six months of hybrid closed loop in the real-world: an evaluation of children and young adults using the 670G system. Pediatr Diabetes. 2020;21(2):310-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nathan DM, DCCT/EDIC Research Group. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care. 2014;37(1):9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Diabetes Control and Complications Trial (DCCT) / Epidemiology of Diabetes Interventions and Complications (EDIC) Study Research Group. Mortality in type 1 diabetes in the DCCT/EDIC versus the general population. Diabetes Care. 2016;39(8):1378-1383.27411699 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-dst-10.1177_19322968241231950 for Cost-Effectiveness of Closed-Loop Automated Insulin Delivery Using the Cambridge Hybrid Algorithm in Children and Adolescents with Type 1 Diabetes: Results from a Multicenter 6-Month Randomized Trial by D. Steven Fox, Julia Ware, Charlotte K Boughton, Janet M. Allen, Malgorzata E Wilinska, Martin Tauschmann, Louise Denvir, Ajay Thankamony, Fiona Campbell, R. Paul Wadwa, Bruce A. Buckingham, Nikki Davis, Linda A. DiMeglio, Nelly Mauras, Rachel E. J. Besser, Atrayee Ghatak, Stuart A. Weinzimer, Lauren Kanapka, Craig Kollman, Judy Sibayan, Roy W. Beck, Korey K. Hood and Roman Hovorka in Journal of Diabetes Science and Technology