Abstract
Background:
Concerns regarding the ongoing opioid epidemic have led to heightened scrutiny of postoperative opioid prescribing patterns for common orthopedic surgical procedures. This study investigated patient- and procedure-specific risk factors for additional postoperative opioid rescue prescriptions following ambulatory cubital tunnel surgery.
Methods:
A retrospective review was performed of patients who underwent cubital tunnel surgery at 2 academic medical centers between June 1, 2015 and March 1, 2020. Patient demographics, comorbidities, prior opioid history, and surgical variables were recorded. The primary outcome was postoperative rescue opioid prescription. Univariate and bivariate statistical analyses were performed.
Results:
Two hundred seventy-four patients were included, of whom 171 (62%) underwent in situ ulnar nerve decompression and 103 (38%) underwent ulnar nerve decompression with anterior transposition. The median postoperative opioid prescription amount was 90 morphine equivalent units (MEU) for the total cohort, 77.5 MEU for in situ ulnar nerve decompression, and 112.5 MEU for ulnar nerve decompression with transposition. Twenty-two patients (8%) required additional rescue opioid prescriptions postoperatively. Female sex, fibromyalgia, chronic opioid use, chronic pain diagnosis, and recent opioid were associated with the need for additional postoperative rescue opioid prescriptions.
Conclusions:
While most patients do not require additional rescue opioid prescriptions after cubital tunnel surgery, chronic pain patients and patients with pain sensitivity syndromes are at risk for requiring additional rescue opioid prescriptions. For these high-risk patients, preoperative collaboration of a multidisciplinary team may be beneficial for developing a perioperative pain management plan that is both safe and effective.
Keywords: opioids, cubital tunnel syndrome, nerve, diagnosis, prescriptions, refills, chronic pain
Introduction
In recent years, increasing rates of opioid use disorder and opioid overdose deaths have led to legitimate concerns regarding an opioid crisis in the United States. Prescription opioid misuse, in particular, has increased, with the Centers for Disease Control and Prevention reporting more than 16 000 deaths attributable to prescription opioid overdoses in 2020 alone, as well as a 24% rate of prescription opioid involvement among all opioid overdoses. 1 These staggering figures have resulted in increased scrutiny of opioid prescribing patterns among health care providers. Orthopedic surgeons have previously been identified as the third highest opioid-prescribing specialty group among physicians in the United States and the highest opioid prescribers among surgical specialties.2,3 Prior studies have demonstrated that between 2010 and 2012, up to 13% of hand surgery patients continued to fill opioid prescriptions between 90 and 180 days after surgery, 4 and that patients undergoing ambulatory upper extremity procedures in 2014 were routinely prescribed 3 times the amount of opioid needed for adequate postoperative pain control. 5
In light of these concerns, prior studies have investigated factors associated with opioid refills6 -11 and prolonged opioid use,4,9,12 -16 after a variety of both orthopedic and nonorthopedic surgical procedures. These studies have identified numerous and sometimes contradictory potential risk factors, including both male and female sex, both younger and older age, preoperative substance use, mental health disorders, preoperative opioid use, preoperative chronic pain disorders, and larger initial postoperative opioid prescription. In the upper extremity–specific literature, previously identified risk factors for postoperative opioid refills and/or prolonged postoperative opioid use after upper extremity surgery include: younger age, female sex, lower income, medical comorbidities, mental health disorders, substance use, underweight body mass index (BMI), trauma-related surgery, and larger initial postoperative opioid prescription.4,8,10,11,17,18 However, most of these studies included only opioid-naïve patients and did not attempt to investigate the effect of chronic pain disorders or procedure-specific variables on postoperative opioid refills.
As such, to address these shortcomings, we identified cubital tunnel release as a common ambulatory upper extremity procedure that typically requires a low but non-zero amount of opioid-based postoperative analgesia, and through this study sought to investigate both patient- and procedure-based risk factors for requiring additional rescue opioid prescriptions after cubital tunnel surgery.
Materials and Methods
Patient Identification
Approval from the Institutional Review Board was obtained prior to initiating data collection. Patients who underwent cubital tunnel surgery at 2 academic medical centers over a 5-year period between June 1, 2015 and March 1, 2020 were identified by querying the hospital Research Patient Data Registry using the Current Procedural Terminology code 64718 (neuroplasty and/or transposition; ulnar nerve at elbow). Both patients who underwent in situ ulnar nerve decompression and patients who underwent ulnar nerve decompression with anterior (subcutaneous or submuscular) transposition were included. Exclusion criteria included: age less than 18 years, revision surgery, prior traumatic ulnar nerve injury, and additional concurrent surgical procedures. For patients who underwent bilateral ulnar nerve decompressions during the study period, only data from the first surgery were included to maintain the assumption of independent observations. The initial query yielded 967 patients. Six hundred thirteen patients who had additional concurrent surgical procedures, 51 patients who had revision surgery, 17 patients who had incomplete documentation, 10 patients who were less than 18 years old, and 2 patients with traumatic ulnar nerve lacerations were excluded from the study, resulting in a final cohort of 274 patients included in the study (Figure 1).
Figure 1.
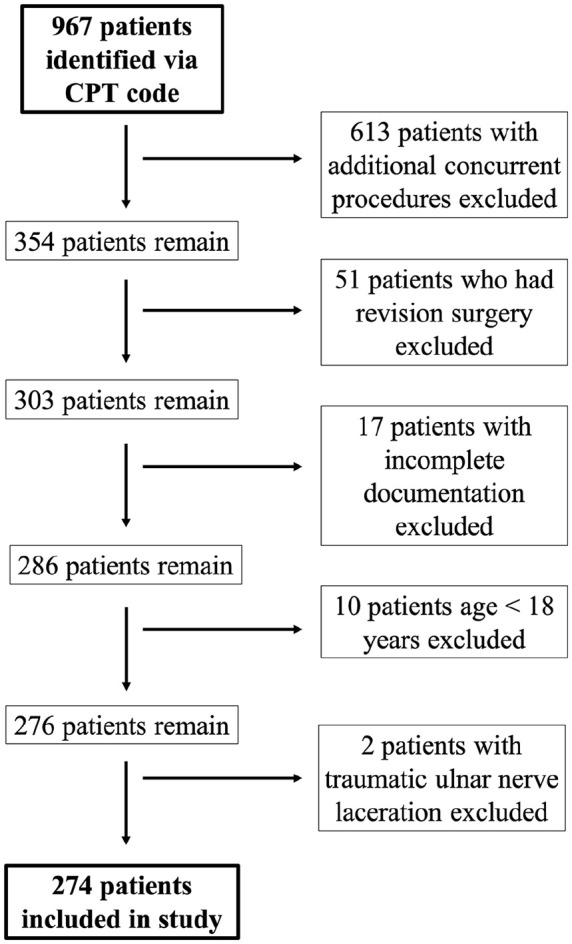
Study inclusion flowchart.
Note. CPT = Current Procedural Terminology.
Explanatory Variables
The patients’ medical records were retrospectively reviewed. Demographic information, medical comorbidities, opioid history, surgical details, and postoperative opioid prescription records were recorded. Patient-based explanatory variables included: age, BMI, Distressed Communities Index (DCI), sex, race, primary language, comorbidities including depression, anxiety, diabetes mellitus, and fibromyalgia, smoking status, upper extremity dominance, chronic opioid use, chronic pain, and recent opioid prescription. The DCI is a tool developed by the Economic Innovation Group for comparing socioeconomic status across zip codes 19 ; the tool comprises 7 metrics that assess education level, housing vacancies, employment rate, poverty rate, household income, changes in employment, and changes in business establishments, and has been shown to predict adverse outcomes after cardiac and vascular surgery.20,21 Chronic opioid use was defined as daily use of prescription opioids for at least 90 days, which was determined based on review of the medication list in the medical record; in our system, this list is routinely confirmed and updated at the time of each clinic visit and on the day of surgery in the preoperative area, and also includes the reported start date of the medication. Chronic pain was defined as having documentation of prior appointments with any chronic pain provider. Recent opioid prescription was defined as a record of an opioid prescription sent within the past 3 months prior to surgery. Perioperative explanatory variables included: surgical time, tourniquet time, initial postoperative opioid prescription in morphine equivalent units (MEU), type of decompression, anesthesia modality, and the use of a regional nerve block.
Response Variable
The primary study outcome was the need for an additional postoperative rescue opioid prescription, which was defined as a second opioid prescription sent by the surgical team after surgery for continued postoperative pain. In our system, all opioid prescriptions are sent electronically via our electronic medical record, enabling the capture of all possible postoperative refills sent by the surgical office.
Statistical Analysis
Univariate analysis was performed to calculate descriptive statistics for the cohort, including mean and standard deviation (SD) for continuous parametric variables, median and interquartile range (IQR) for continuous nonparametric variables, and percentages for categorical variables. All variables were analyzed using the available data, and missing data are shown in Tables 1 and 2. Bivariate analysis was performed to determine statistical associations of independent variables with the primary study outcome. Student t test was used for continuous parametric variables, Mann-Whitney U test was used for continuous nonparametric variables, and Fisher exact test was used for categorical variables. Due to the low rates of outcome events, multivariable logistic regression modeling was not performed. Statistical significance was defined as a P value less than .05.
Table 1.
Patient Characteristics of the Study Cohort (n = 274).
| Patient-based variable | Total cohort (n = 274) a |
|---|---|
| Age, y, mean (SD) | 50.0 (16.5) |
| Body mass index, kg/m2, median (IQR) | 26.9 (23.7-30.4) |
| Distressed Communities Index, median (IQR) | 23.7 (9.9-46.6) |
| Female sex, No. (%) | 109 (39.8) |
| Race, No. (%) | |
| American Indian or Alaska Native | 2 (0.8) |
| Asian | 5 (1.9) |
| Black or African American | 25 (9.6) |
| Hispanic | 8 (3.1) |
| White | 220 (84.6) |
| English-speaking, No. (%) | 265 (97.1) |
| Comorbidities, No. (%) | |
| Depression | 74 (27.0) |
| Anxiety | 67 (24.5) |
| Diabetes mellitus | 34 (12.4) |
| Fibromyalgia | 12 (4.4) |
| Current smoker, No. (%) | 37 (13.6) |
| Dominant upper extremity affected, No. (%) | 151 (57.2) |
| Chronic opioid use, No. (%) | 18 (6.6) |
| Chronic pain, No. (%) | 30 (11.0) |
| Recent opioid prescription, No. (%) | 33 (12.1) |
Note. SD = standard deviation; IQR = interquartile range.
Data were missing for the following explanatory variables (n = number of patients with available data): body mass index (n = 273), race (n = 260), language (n = 273), smoking status (n = 273), upper extremity dominance (n = 264), chronic opioid use (n = 273), recent opioid prescription (n = 273), chronic pain (n = 272).
Table 2.
Perioperative Parameters of the Study Cohort (n = 274).
| Procedure-based variable | Total cohort (n = 274) a |
|---|---|
| Surgical time, min, median (IQR) | 28 (19-44) |
| Tourniquet time, min, median (IQR) | 24 (17-36) |
| Initial postoperative opioid prescription (MEU), median (IQR) | 90 (75-150) |
| Type of decompression, No. (%) | |
| In situ decompression | 171 (62.4) |
| Submuscular transposition | 44 (16.1) |
| Subcutaneous transposition | 59 (21.5) |
| Anesthesia modality, No. (%) | |
| General anesthesia | 74 (27.1) |
| Sedation/monitored anesthesia care | 199 (72.9) |
| Regional nerve block, No. (%) | 237 (86.5) |
Note. SD = standard deviation; IQR = interquartile range; MEU = morphine equivalent units.
Data were missing for the following explanatory variables (n = number of patients with available data): surgical time (n = 264), tourniquet time (n = 213), initial postoperative opioid prescription (n = 253), anesthesia modality (n = 273).
Post hoc power calculation showed that, assuming an equal sample distribution of patients for a dichotomous variable, our study had greater than 80% power to detect a 12% absolute difference in the rate of rescue opioid prescriptions between groups.
Results
Patient Demographics and Perioperative Parameters
Two hundred seventy-four patients qualified for study inclusion. The mean age was 50.0 ± 16.5 years and 39.8% of patients were women. Thirty patients (11.0%) had chronic pain, 18 patients (6.6%) had chronic opioid use, and 33 patients (12.1%) had recent opioid prescriptions. One hundred and seventy-one patients (62.4%) underwent in situ ulnar nerve decompression, 44 patients (16.1%) underwent ulnar nerve decompression with submuscular transposition, and 59 patients (21.5%) underwent ulnar nerve decompression with subcutaneous transposition. Additional descriptive statistics for patient demographic variables and perioperative parameters are presented in Tables 1 and 2.
Initial Postoperative Opioid Prescriptions
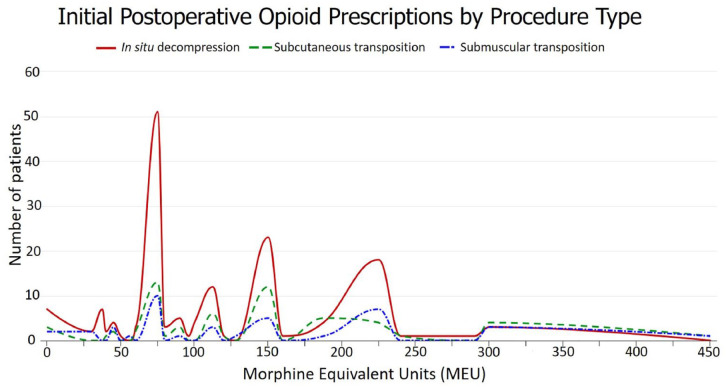
Postoperative opioid prescriptions were routinely written in the form of 5 mg oxycodone tablets; if a patient reported a contraindication to oxycodone, 2 mg hydromorphone tablets were substituted. Initial postoperative opioid prescription amounts ranged from 0 to 450 MEU (equivalent to 0-60 5 mg oxycodone tablets), with a mode of 75 MEU (equivalent to 10 5 mg oxycodone tablets). The distributions of initial postoperative opioid prescription amounts for the overall cohort and for each type of procedure are depicted graphically in Figures 2 and 3, respectively. The median opioid amount included in the initial postoperative prescription was 90 MEU (equivalent to 12 5 mg oxycodone tablets, IQR = 75-150) for the overall cohort, 77.5 MEU (IQR = 75-150) for patients who underwent in situ ulnar nerve decompression, 112.5 MEU (IQR = 75-168.75) for patients who underwent ulnar nerve decompression with subcutaneous transposition, and 112.5 MEU (IQR = 75-225) for patients who underwent ulnar nerve decompression with submuscular transposition. Female patients received a median of 90 MEU (IQR = 75-150), whereas male patients received a median of 100 MEU (IQR = 75-150).
Figure 2.
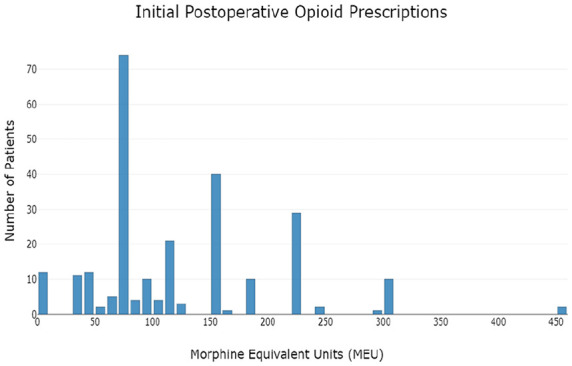
Initial postoperative opioid prescription amounts.
Figure 3.
Initial postoperative opioid prescriptions amounts by procedure type.
Additional Rescue Opioid Prescriptions
Twenty-two patients (8.0%) required additional rescue opioid prescriptions postoperatively. Nineteen patients received 1 additional prescription, 1 patient received 2 additional prescriptions, and 2 patients received 3 additional prescriptions. In the bivariate analysis of patient-based characteristics, female sex, fibromyalgia, chronic pain, chronic opioid use, and recent opioid prescription were associated with requiring an additional rescue opioid prescription. No procedure-specific characteristics were significantly associated with rescue opioid prescriptions, although as reported above, the initial prescriptions for in situ release differed from those for transpositions. Full results of bivariate analysis are presented in Table 3 (patient-specific characteristics) and Table 4 (perioperative parameters). Rescue prescription rates based on individual associated factors are presented in Table 5. Multivariate analysis was not performed due to the low rate of outcome events.
Table 3.
Characteristics of Patients Who Required and Did Not Require Additional Postoperative Opioid Prescription.
| Patient-based variable | No opioid refill (n = 252) | Opioid refill (n = 22) | P value |
|---|---|---|---|
| Age, y, mean (SD) | 50.1 (16.6) | 47.5 (14.9) | .7 |
| BMI, kg/m2, median (IQR) | 26.8 (23.7-30.7) | 27.2 (23.7-29.0) | .5 |
| Distressed Communities Index, median (IQR) | 24.7 (10.0-45.7) | 12.5 (6.1-45.3) | .2 |
| Female sex, No. (%) | 95 (37.7) | 14 (63.6) | <.05 |
| Race, No. (%) | .6 | ||
| American Indian or Alaska Native | 2 (0.8) | 0 (0.0) | |
| Asian | 5 (2.1) | 0 (0.0) | |
| Black or African American | 21 (8.8) | 4 (19.1) | |
| Hispanic | 8 (3.4) | 0 (0.0) | |
| White | 203 (84.9) | 17 (81.0) | |
| English-speaking, No. (%) | 243 (96.8) | 22 (100.0) | .9 |
| Comorbidities, No. (%) | |||
| Depression | 64 (25.4) | 10 (45.5) | .1 |
| Anxiety | 59 (23.4) | 8 (36.4) | .2 |
| Diabetes mellitus | 33 (13.1) | 1 (4.6) | .2 |
| Fibromyalgia | 8 (3.2) | 4 (18.2) | <.05 |
| Current smoker, No. (%) | 32 (12.7) | 5 (23.8) | .2 |
| Dominant upper extremity affected, No. (%) | 137 (56.4) | 14 (66.7) | .5 |
| Chronic opioid use, No. (%) | 13 (5.2) | 5 (22.7) | <.05 |
| Chronic pain, No. (%) | 24 (9.6) | 6 (27.3) | <.05 |
| Recent opioid prescription, No. (%) | 24 (9.6) | 9 (40.9) | <.05 |
Note. SD = standard deviation; IQR = interquartile range; BMI = body mass index. Bolded values indicate statistical significance with p < 0.05.
Table 4.
Perioperative Parameters of Patients Who Required and Did Not Require Additional Postoperative Opioid Prescription.
| Procedure-based variable | No opioid refill (n = 252) | Opioid refill (n = 22) | P value |
|---|---|---|---|
| Surgical time, min, median (IQR) | 28 (18-43) | 32 (24-67) | .1 |
| Tourniquet time, min, median (IQR) | 24 (17-35) | 33 (18-41) | .3 |
| Initial postoperative opioid prescription, MEU, median (IQR) | 90 (75-150) | 132 (75-225) | .1 |
| Type of decompression, No. (%) | .4 | ||
| In situ decompression | 160 (63.5) | 11 (50.0) | |
| Submuscular transposition | 39 (15.5) | 5 (22.7) | |
| Subcutaneous transposition | 53 (21.0) | 6 (27.3) | |
| Anesthesia modality, No. (%) | .4 | ||
| General anesthesia | 66 (26.2) | 8 (36.4) | |
| Sedation/monitored anesthesia care | 185 (73.4) | 14 (63.6) | |
| Regional nerve block, No. (%) | 218 (86.5) | 19 (86.4) | .9 |
Note. SD = standard deviation; IQR = interquartile range; MEU = morphine equivalent units.
Table 5.
Rates of Rescue Prescription by Associated Factor.
| Risk factor | Rate of rescue prescription, % |
|---|---|
| Total study cohort | 8.0 |
| Risk factors for rescue refill | |
| Female sex | 12.8 |
| Fibromyalgia | 33.3 |
| Chronic opioid use | 27.8 |
| Chronic pain | 20.0 |
| Recent opioid prescription | 27.3 |
Discussion
While the ongoing opioid epidemic has prompted attempts to investigate risk factors associated with postoperative opioid refills and prolonged postoperative opioid use in various surgical populations, studies investigating ambulatory upper extremity surgical procedures, nonopioid naïve patients, and procedure-based parameters are lacking. In this retrospective study of 274 patients who underwent ambulatory cubital tunnel surgery, we demonstrate that a notable minority of patients required additional postoperative rescue opioid prescriptions. In addition, we identified female sex, fibromyalgia, chronic pain, chronic opioid use, and recent opioid prescription as patient-related risk factors for requiring an additional rescue opioid prescription.
Eight percent of patients in our study required an additional rescue opioid prescription. In prior literature, reported rates of postoperative rescue opioid refills range from 0% to 50%,6 -11,22 -28 and from 0% to 30% in the ambulatory upper extremity–specific literature8,10,11,22,27,28; our value of 8% is well within this interval and comparable with a previously reported cubital tunnel surgery–specific rate of 11%. 8 The low but finite rate of rescue prescriptions suggests that existing prescribing patterns achieve adequate postoperative pain control in most patients undergoing ambulatory cubital tunnel surgery.
In the current literature, data are sparse regarding both patient- and procedure-based risk factors for inadequacy of initial postoperative opioid prescriptions after ambulatory upper extremity surgery. Prior studies have suggested that younger age, female sex, lower socioeconomic status, substance use, medical comorbidities, recent opioid prescriptions, and larger initial opioid prescription amount may increase risk for requiring a postoperative rescue opioid refill. These studies often excluded chronic pain patients and did not consider perioperative variables in their analyses.8,10,11 Our results corroborate prior findings that female sex and recent opioid prescription are statistically significant risk factors for requiring an additional postoperative rescue opioid prescription and also highlight fibromyalgia, chronic opioid use, and chronic pain as additional risk factors. Wyles et al 23 found that male patients were more likely to receive postoperative opioid prescriptions in excess of standardized amounts than female patients, and our data also demonstrate a higher initial postoperative median MEU for male patients compared with female patients; if male patients routinely receive larger postoperative opioid prescriptions than female patients, female patients may be more likely to require additional prescriptions when they exhaust their smaller initial prescriptions. In addition, female sex may also be acting as a proxy variable for other explanatory variables, including fibromyalgia; however, due to sample size limitations, we were unable to perform a multivariate analysis to identify independent risk factors. Importantly, our data should not be interpreted as indicating that all female patients should be considered likely to need additional opioids postoperatively. It is accepted that perioperative pain management can be challenging in patients with chronic pain, chronic opioid use, and/or pain sensitivity syndromes. Prior studies have found that patients with chronic pain and chronic opioid use report higher mean postoperative pain levels29 -31 and a slower resolution of postoperative pain compared with controls. 29 In addition, Rishel et al 32 previously found that chronic opioid users who decreased their opioid consumption in the 3 months immediately prior to surgery were less likely to continue filling opioid prescriptions 3 months after surgery. Furthermore, prior studies have demonstrated increased postoperative opioid consumption among patients with fibromyalgia,33,34 despite recommendations against chronic opioid use in this patient population.35,36 Interestingly, our study did not identify any associations between perioperative parameters and rescue opioid prescription risk, suggesting that patient-specific characteristics are more closely associated with additional prescription risk than procedure-specific factors. In addition, while prior studies have associated age,7 -9 socioeconomic status, 8 and smoking6,9 with risk of rescue refills, these variables did not show statistical significance in our study.
Our data should not be interpreted as supporting larger initial opioid prescriptions in patients who are identified as higher risk for requiring additional rescue opioid prescriptions. Other studies have identified larger initial opioid prescription as a risk factor itself for rescue opioid refills and prolonged postoperative opioid use.10,11,14 Rather, these patients can be identified as high risk preoperatively, and a distinct perioperative pain management plan that is both safe and effective can be developed. At times, collaboration among a pain specialist, the patient’s primary care physician, and the surgical team may be helpful.
In this study, the median initial opioid amount prescribed postoperatively was 77.5 MEU for in situ ulnar nerve decompression and 112.5 MEU for ulnar nerve decompression with transposition, and the most commonly prescribed amount was 75 MEU. In 2019, Hozack et al 37 investigated opioid consumption following cubital tunnel surgery and found that patients consumed a mean of 40.4 MEU after in situ release and a mean of 62.5 MEU after ulnar nerve transposition. Both of these values are substantially lower than the median prescription amounts seen in our cohort, highlighting either a population-specific difference or an opportunity to improve prescribing patterns to achieve a superior balance between adequate pain control and responsible opioid stewardship.
This study is not without limitations. Notably, as our data collection relied on records of opioid prescriptions sent to pharmacies, we were unable to identify patients who requested an additional prescription from our office but whose requests were denied. Furthermore, as we did not stratify patients by surgeon but rather examined departmental patterns, we cannot address whether prescribing patterns vary by surgeon. In addition, due to a low rate of our outcome of interest, we were unable to perform multivariate analysis. Sample size may have also limited our ability to determine statistical significance of some explanatory variables with more subtle effects on refill rates. Finally, as our study was limited to cubital tunnel surgery, the results may not necessarily be generalizable to other hand and upper extremity procedures.
Future studies on this topic could include larger sample sizes to enable both identification of additional risk factors and multivariate analysis, as well as investigations of surgeon-specific prescribing patterns and a wider breadth of upper extremity procedures. In addition, investigations of the number of opioids consumed throughout the postoperative course—rather than solely the amount prescribed—would also be beneficial for the refinement of safe and effective postoperative prescribing practices.
Conclusion
In this study, we found that 92% of patients do not require additional rescue opioid prescriptions after ambulatory cubital tunnel surgery. Chronic pain patients and patients with pain sensitivity syndromes are at risk for requiring additional rescue opioid prescriptions after exhausting their initial postoperative opioid prescription. These high-risk patients may benefit from the preoperative collaboration of a multidisciplinary team to develop a perioperative pain management plan that is both safe and effective.
Footnotes
Ethical Approval: This study was approved by our Institutional Review Board.
Statement of Human and Animal Rights: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5). The Institutional Review Board identified this study as exempt from requiring informed consent from subjects.
Statement of Informed Consent: No patient identifying information is contained within this article. The Institutional Review Board identified this study as exempt from requiring informed consent from subjects.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Carew Giberson-Chen  https://orcid.org/0000-0003-3149-7876
https://orcid.org/0000-0003-3149-7876
Phillip Grisdela  https://orcid.org/0000-0002-5325-3461
https://orcid.org/0000-0002-5325-3461
References
- 1. Centers for Disease Control and Prevention, National Center for Health Statistics. National Vital Statistics System, Mortality 1999-2020 on CDC WONDER Online Database, released in 2021. http://wonder.cdc.gov/ucd-icd10.html.
- 2. Morris BJ, Mir HR. The opioid epidemic: impact on orthopaedic surgery. J Am Acad Orthop Surg. 2015;23(5):267-271. [DOI] [PubMed] [Google Scholar]
- 3. Volkow ND, McLellan TA, Cotto JH, et al. Characteristics of opioid prescriptions in 2009. JAMA. 2011;305(13):1299-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Johnson SP, Chung KC, Zhong L, et al. Risk of prolonged opioid use among opioid-naïve patients following common hand surgery procedures. J Hand Surg Am. 2016;41(10):947-957. [DOI] [PubMed] [Google Scholar]
- 5. Kim N, Matzon JL, Abboudi J, et al. A prospective evaluation of opioid utilization after upper-extremity surgical procedures: identifying consumption patterns and determining prescribing guidelines. J Bone Joint Surg Am. 2016;98(20):e89. [DOI] [PubMed] [Google Scholar]
- 6. Sekhri S, Arora NS, Cottrell H, et al. Probability of opioid prescription refilling after surgery: does initial prescription dose matter? Ann Surg. 2018;268(2):271-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gifford ED, Hanson KT, Davila VJ, et al. Patient and institutional factors associated with postoperative opioid prescribing after common vascular procedures. J Vasc Surg. 2020;71(4):1347-1356.e11. [DOI] [PubMed] [Google Scholar]
- 8. Waljee JF, Zhong L, Hou H, et al. The use of opioid analgesics following common upper extremity surgical procedures: a national, population-based study. Plast Reconstr Surg. 2016;137(2):355e-364e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Okoli MU, Rondon AJ, Townsend CB, et al. Comprehensive analysis of opioid use after common elective outpatient orthopaedic surgeries. J Am Acad Orthop Surg Glob Res Rev. 2022;6(4):e21.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Runge W, Gabig AM, Karzon A, et al. Prolonged opioid use following distal radius fracture fixation: who is at risk and what are the consequences? J Hand Surg Glob Online. 2023;5(3):338-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Townsend CB, Ly JA, Judy R, et al. Larger perioperative opioid prescriptions lead to prolonged opioid use after hand and upper extremity surgery: a multicenter analysis. J Am Acad Orthop Surg Glob Res Rev. 2022;6(10):e22.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sun EC, Darnall BD, Baker LC, et al. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med. 2016;176(9):1286-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lawal OD, Gold J, Murthy A, et al. Rate and risk factors associated with prolonged opioid use after surgery: a systematic review and meta-analysis. JAMA Netw Open. 2020;3(6):e207367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gil JA, Gunaseelan V, DeFroda SF, et al. Risk of prolonged opioid use among opioid-naïve patients after common shoulder arthroscopy procedures. Am J Sports Med. 2019;47(5):1043-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Namba RS, Singh A, Paxton EW, et al. Patient factors associated with prolonged postoperative opioid use after total knee arthroplasty. J Arthroplasty. 2018;33(8):2449-2454. [DOI] [PubMed] [Google Scholar]
- 16. Bedard NA, DeMik DE, Dowdle SB, et al. Trends and risk factors for prolonged opioid use after unicompartmental knee arthroplasty. Bone Joint J. 2018;100-B(1 suppl. A):62-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shipp MM, Sanghavi KK, Kolm P, et al. Preoperative patient-reported data indicate the risk of prolonged opioid use after hand and upper extremity surgeries. J Hand Surg Am. 2022;47(11):1068-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Giladi AM, Shipp MM, Sanghavi KK, et al. Patient-reported data augment prediction models of persistent opioid use after elective upper extremity surgery. Plast Reconstr Surg. 2023;152(2):358e-366e. [DOI] [PubMed] [Google Scholar]
- 19. Economic Innovation Group Distressed Communities Index. http://eig.org/dci. Accessed November 27, 2023.
- 20. Charles EJ, Mehaffey JH, Hawkins RB, et al. Socioeconomic Distressed Communities Index predicts risk-adjusted mortality after cardiac surgery. Ann Thorac Surg. 2019;107(6):1706-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hawkins RB, Mehaffey JH, Charles EJ, et al. Socioeconomically Distressed Communities Index independently predicts major adverse limb events after infrainguinal bypass in a national cohort. J Vasc Surg. 2019;70(6):1985-1993. [DOI] [PubMed] [Google Scholar]
- 22. Earp BE, Silver JA, Mora AN, et al. Implementing a postoperative opioid-prescribing protocol significantly reduces the total morphine milligram equivalents prescribed. J Bone Joint Surg Am. 2018;100(19):1698-1703. [DOI] [PubMed] [Google Scholar]
- 23. Wyles CC, Hevesi M, Ubl DS, et al. Implementation of procedure-specific opioid guidelines: a readily employable strategy to improve consistency and decrease excessive prescribing following orthopaedic surgery. JB JS Open Access. 2020;5(1):e0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Landes EK, Leucht P, Tejwani NC, et al. Decreasing postoperative opioid prescriptions after orthopedic trauma surgery: the “lopioid” protocol. Pain Med. 2022;23(10):1639-1643. [DOI] [PubMed] [Google Scholar]
- 25. NO PAin Investigators; Gazendam A, Ekhtiari S, et al. Effect of a postoperative multimodal opioid-sparing protocol vs standard opioid prescribing on postoperative opioid consumption after knee or shoulder arthroscopy: a randomized clinical trial. JAMA. 2022;328(13):1326-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vaz KM, Huang PS, Copp SN. Standardized opioid prescription protocol reduces opioid consumption after total joint arthroplasty. J Am Acad Orthop Surg Glob Res Rev. 2019;3(12):e19.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dwyer CL, Soong M, Hunter A, et al. Prospective evaluation of an opioid reduction protocol in hand surgery. J Hand Surg Am. 2018;43(6):516-522.e1. [DOI] [PubMed] [Google Scholar]
- 28. Benavent KA, Altschul ND, Lincoln LF, et al. Patient satisfaction and opioid use with a postoperative opioid protocol after common hand procedures. J Hand Surg Glob Online. 2020;2(4):191-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chapman CR, Donaldson G, Davis J, et al. Postoperative pain patterns in chronic pain patients: a pilot study. Pain Med. 2009;10(3):481-487. [DOI] [PubMed] [Google Scholar]
- 30. Hina N, Fletcher D, Poindessous-Jazat F, et al. Hyperalgesia induced by low-dose opioid treatment before orthopaedic surgery: an observational case-control study. Eur J Anaesthesiol. 2015;32(4):255-261. [DOI] [PubMed] [Google Scholar]
- 31. Rapp SE, Ready BL, Nessly ML. Acute pain management in patients with prior opioid consumption: a case-controlled retrospective review. Pain. 1995;61(2):195-201. [DOI] [PubMed] [Google Scholar]
- 32. Rishel CA, Angst MS, Sun EC. Preoperative Opioid utilization patterns and postoperative opioid utilization: a retrospective cohort study. Anesthesiology. 2021;135(6):1015-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brummett CM, Janda AM, Schueller CM, et al. Survey criteria for fibromyalgia independently predict increased postoperative opioid consumption after lower-extremity joint arthroplasty: a prospective, observational cohort study. Anesthesiology. 2013;119(6):1434-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Janda AM, As-Sanie S, Rajala B, et al. Fibromyalgia survey criteria are associated with increased postoperative opioid consumption in women undergoing hysterectomy. Anesthesiology. 2015;122(5):1103-1111. [DOI] [PubMed] [Google Scholar]
- 35. Brummett CM, Clauw DJ. Fibromyalgia: a primer for the anesthesia community. Curr Opin Anaesthesiol. 2011;24(5):532-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Painter JT, Crofford LJ. Chronic opioid use in fibromyalgia syndrome: a clinical review. J Clin Rheumatol. 2013;19(2):72-77. [DOI] [PubMed] [Google Scholar]
- 37. Hozack BA, Abboudi J, Gallant G, et al. Prospective evaluation of opioid consumption following cubital tunnel decompression surgery. Hand (N Y). 2019;14(1):42-47. [DOI] [PMC free article] [PubMed] [Google Scholar]