Abstract
Advances in diabetes technologies have enabled automated insulin delivery (AID) systems, which have demonstrated benefits to glycemia, psychosocial outcomes, and quality of life for people with type 1 diabetes (T1D). Despite the many demonstrated benefits, AID systems come with their own unique challenges: continued user attention and effort, barriers to equitable access, personal costs vs benefits, and integration of the system into daily life. The purpose of this narrative review is to identify challenges and opportunities for supporting uptake and onboarding of AID systems to ultimately support sustained AID use. Setting realistic expectations, providing comprehensive training, developing willingness to adopt new treatments and workflows, upskilling of diabetes team members, and increasing flexibility of care to tailor care to individual needs, preferences, lifestyle, and personal goals will be most effective in facilitating effective, widespread, person-centered implementation of AID systems.
Keywords: automated insulin delivery, type 1 diabetes, education, health care delivery, psychosocial
Introduction
The current landscape of diabetes management includes tools that are unlike anything previously offered, as diabetes technologies become more automated, widespread, and recommended as standard care. Diabetes technologies (insulin pumps or continuous subcutaneous insulin infusion [CSII] and continuous glucose monitoring [CGM]), previously able to be used only in isolation, now utilize advanced features to function as a unit: the automated insulin delivery (AID) system. Automated insulin delivery systems have demonstrated substantial benefits for short- and long-term health outcomes. Randomized controlled trials (RCTs) and real-life observational studies have linked AID system use with a reduction in HbA1c levels and a corresponding increase in time in range (TIR) across the lifespan: among infants, children, adolescents, adults, and pregnant individuals with diabetes.1-7 Automated insulin delivery systems have further demonstrated safety for those at increased risk of hypoglycemia 8 with reductions in severe hypoglycemia and ketoacidosis. 5
Automated insulin delivery systems have also demonstrated benefits to quality of life. 9 Automated insulin delivery users and parents of children using AID report improved sleep due to reductions in overnight alarms and increased TIR overnight.10-12 Positive effects of using an AID system have been observed for several diabetes-related person-reported outcomes (PROs) including diabetes-specific quality of life, treatment satisfaction, treatment self-efficacy, hypoglycemia fear, diabetes distress, and family conflict. 4,9,10,13-16
Yet, even the most advanced technologies are not without their challenges to access, use, and integrate into one’s life. Automated insulin delivery systems are not hands-off. Individuals living with type 1 diabetes (T1D) must still engage in daily self-management and navigate the associated diabetes-specific behavioral, emotional, and psychosocial burdens that AID systems do not eliminate from the lived experience of diabetes. Automated insulin delivery systems demand user effort and interaction to be effective, requiring users to maintain and troubleshoot 2 active devices, count and enter carbohydrates, and announce exercise. Management-related challenges evolve for people with T1D as treatment technologies advance. It will be important to develop targeted ways to support people with T1D in these challenges so that more people may experience the benefits that come with uptake and sustained use of advanced technologies. To this end, the goals of this narrative review are to (1) describe emerging challenges to supporting the uptake and onboarding of AID systems; (2) explore existing efforts and recommendations to promote effective uptake and onboarding to support optimal, continued use of AID systems; and (3) to present future directions to support more widespread person-centered AID use in both clinical and research settings.
Challenges in Uptake and Onboarding of Automated Insulin Delivery Systems
As of 2023, around 1 million people had adopted AID systems, a small fraction of the >530 million with diabetes worldwide. 17 Several factors influence adoption of AID, including those related to the opportunity and equity, accessibility, and perceived value. 18 Although this review will focus on the latter two categories, it is important to acknowledge that AID systems are not readily available to all people with T1D, limiting opportunity and equity. Social determinants of health (SDOH), societal factors, and inequity within the health care system impact AID adoption and use. A key challenge in understanding how to best support AID uptake and use is in our ability to serve a more heterogeneous population of people with T1D. Participants in clinical trials have not always been representative of the larger population with T1D. 19 Understanding the unique barriers to AID use for marginalized or underserved groups is necessary to promote widespread, equitable access and sustained use. Furthermore, the structural inequities within the health care system impact AID uptake. For example, insurance coverage and reimbursement are key factors which can influence AID uptake, given that cost and insurance coverage have been cited as the biggest barriers to diabetes technology uptake. 20 The “cost” of opting to use diabetes technology extends beyond finances to include costs to time, society, and relationships as major considerations of technology uptake and continued use. 21
Outside of systemic barriers, people still face barriers in accessibility. Health care providers (HCPs) can serve as gatekeepers to technology access, either intentionally or inadvertently. Health care providers may lack sufficient education and training needed to AID uptake and onboarding and may not offer it to their patients. The recent JDRF Pathway to Choice survey, which aimed to increase knowledge of and access to T1D technologies, reported a leading barrier to device uptake as: “My clinician/nurse has not recommended it to me.” 22 Furthermore, HCPs may hold attitudes and concerns about diabetes technology and prerequisites for system users that may influence their decision to offer it to people living with diabetes, even when not evidence-based. 23 For example, those who struggle with carbohydrate counting have traditionally not been viewed as “ideal” candidates for AID; however, evidence has demonstrated that simplified bolusing strategies can lead to benefits from AID for these individuals. 24 Similarly, some HCPs may feel that newly diagnosed individuals should learn the basics of diabetes management first before adopting AID; this assumption has been contradicted by RCT evidence of AID benefits for new-onset T1D. 25 Finally, HCP implicit bias regarding race, ethnicity, and/or insurance type (public versus private) has been shown to play a role in decision-making about offering diabetes technology. 26
The perceived value of AID for the person living with T1D may have a number of valid usability-related concerns about AID that affect uptake and utilization, such as wearability, pain or skin irritation, physical appearance, unwanted social attention, trust, accuracy, and other concerns.27-31 Using an AID system requires wearing both a CGM and insulin pump on the body, and any challenges with continually wearing or using either device would negatively affect attitudes toward adoption or continued use of AID systems. 28 Pain, skin reactions, site failures, and devices falling off early due to adhesive issues are critical to address and manage for someone to be willing to adopt and continue to use the two devices required for an AID system.32-35 Furthermore, uptake and onboarding onto a new AID system may demand more time and attention for T1D, at least in the short term, given the potentially time-consuming process of initially obtaining supplies and then learning to use an AID system. Some people with T1D may be reluctant to put their trust in an algorithm to make insulin-dosing decisions due to worries and concerns over CGM inaccuracy and potential negative outcomes. Automated insulin delivery systems are not “plug-and-play” 36 ; system users still need to be willing to count carbohydrates, announce physical activity, deliver insulin boluses, address hypo- and hyperglycemia, change infusion sets, carry back-up supplies, and monitor the system to be able to detect issues such as site failures. These burdens may feel costly to those considering adopting AID for their diabetes management. In fact, discontinuation has been documented in past systems due to several factors relating to frustration with operating the system, including burden of alarms and the time-consuming nature of keeping the system working.37,38
Finally, given the number of options available today which may differ in functions, wear time, cost, insurance coverage and algorithms, choosing a system may present an added burden; more research is needed around device decision-making given the emerging nature of this barrier. Lack of access to relevant, timely information and education to enable informed decision-making is an additional barrier to uptake for individual users.
Supporting Automated Insulin Delivery Uptake and Onboarding to Promote Sustained Use
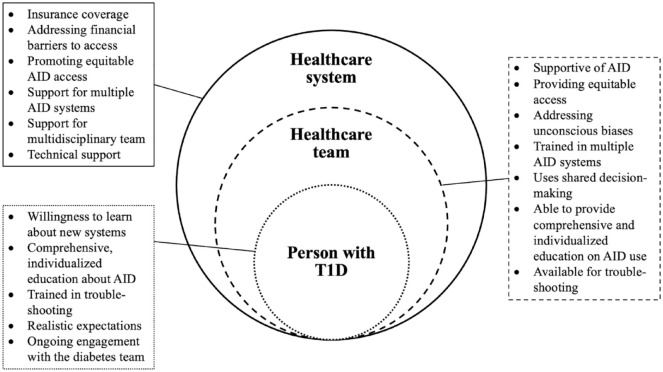
There are a wide variety of barriers to AID use, and people with T1D may experience any combination of these challenges in the present era. By supporting person-centered decision-making in uptake, health care teams may be able to promote sustained and effective use of AID systems going forward. In Figure 1, we present recommendations for the health care system, the health care team, and the person with T1D for effective implementation of AID systems, with a focus on the latter two categories.
Figure 1.
Elements for supporting the effective use of AID systems for the person with T1D, the health care team, and the health care system.
Promoting Equitable Access Within the Health Care System
In the context of the health care system, greater support for insurance coverage and reimbursement for AID systems will be a critical prerequisite to promoting greater uptake and acceptance of this technology. 17 A promising recent development is that in response to the wealth of RCT data and a real-world evaluation of outcomes associated with AID,38,39 the UK National Institute for Clinical Excellence (NICE) deemed AID a cost-effective intervention. Furthermore, the American Diabetes Association (ADA) Standards of Care now advise that AID systems “should be offered for diabetes management to youth and adults with type 1 diabetes . . . who are capable of using the device safely (either by themselves or with a caregiver).” 40 The German health care system also now recommends AID system initiation at diagnosis. 41 To ensure that AID access is equitable, more research will be needed to understand the specific challenges for uptake and ongoing use in marginalized groups (eg, those from deprived backgrounds, with language barriers, learning difficulties, with frailty, eating disorders or mental health challenges). This work will be crucial in current and future attempts to embrace AID systems as a tool in overcoming SDOH that have historically negatively impacted diabetes management.
Upskilling Health Care Teams
Wide rollout of AID will require buy-in from health care teams working with people living with T1D. The recent international consensus statement on AID use recognized the importance of ensuring equitable access through addressing health care professionals’ unconscious biases about individual, family, and psychological attributes required to use AID. 5 Furthermore, qualitative work has identified the need for training, mentorship, expert advice, and access to 24-hour technical support to upskill HCPs with less familiarity with the technology. 42 Furthermore, HCPs need training on multiple AID systems, how to interpret system data, how to adjust and optimize settings, and how to deliver AID-based T1D care in a standardized way.42,43 Beyond this, HCPs also need training in navigating finances related to AID use, psychosocial challenges, and more. 44 Given the benefits of AID in pregnancy, HCPs will also need specialized training on the use during the antenatal and postnatal periods. Wider use in pregnancy will need upskilling of providers and staff and timely access to technical support. 45 Importantly, many of the reviewed barriers related to health care teams and people with T1D emphasize psychosocial or behavioral challenges to AID use. Thus, it requires HCPs to participate in psychoeducation around AID use in addition to an information-focused approach. Psychoeducational topics may include psychosocial challenges to effective AID use, efforts to understand the life context of the person with diabetes, the need for balanced patient education, motivational interviewing skills, and effective communication techniques when interpreting data (eg, strengths-based language, avoidance of blame and shame).
Individualized Automated Insulin Delivery System Selection for Each Person Living With Type 1 Diabetes
Increased attention toward individual concerns, preferences, and priorities of the person living with T1D is imperative for effective uptake and onboarding which leads to sustained use. Shared decision-making between HCPs and people with T1D will be an important element of promoting effective uptake and ongoing use. Older adults, for example, may have specific concerns related to visual impairment or dexterity, and it is important that individual needs and preferences are addressed when selecting diabetes devices.46,47 Given the unique features of each AID system, people with T1D may consider other important aspects of the system that would benefit their lives, including size, tubing, insertion process, mobile phone compatibility, comfort, alert sounds, integration into activity routines, and more. These options allow individuals to identify features that not only help them to manage diabetes, but to reduce the bodily, emotional, mental, and social burdens of diabetes. Recent work has suggested a focus on personalized treatment options when considering diabetes technologies, with HCPs assisting people with diabetes in identifying the features, glycemic goals, affordability, preferences, support services, and limitations that would be the best fit for the individual. 48 People with T1D will naturally engage in their own personal cost-benefit analysis prior to and during device use 49 ; diabetes management already requires significant effort and attention, and people affected must ensure that new treatment options are beneficial to them. Online resources such as DiabetesWise and DTN-UK have aimed to address this critical gap by providing information about available device options and associated benefits of AID.50,51 The reduced burden from AID combined with support for the choice of system to meet individual needs will hopefully support ongoing, long-term use.
Balancing Standardized and Tailored Onboarding Education
International consensus has determined the need for “a rigorous, comprehensive, consistent, and structured education curriculum for all AID systems.” 5 AID onboarding programs should both contain standard elements and be tailored to each individual user, as onboarding needs will differ depending on each person’s T1D management regimen prior to AID initiation, comfort with new technology, and other factors. For example, someone who is already using an insulin pump and CGM may require less training than someone who is newly diagnosed, or is new to CSII and/or CGM. 5
Automated insulin delivery clinical training programs conducted to date have been delivered over multiple formats (eg, in-person, video conference, phone calls) and provide initial education and ongoing support (eg, for adjustments to insulin dosing).52,53 Components of onboarding education programs include instruction on how the system works and expectation-setting; benefits of AID; setting up the system; bolusing, hypoglycemia, and correction doses in the context of AID; responding to alerts; and troubleshooting the system.52,53 Realistic expectations at onboarding and beyond may facilitate ongoing use of the system, as people better understand how it will function in various situations and the degree of effort required from them. Those with unrealistic expectations may experience disappointment, frustration, or discontinuation of AID. 29 Furthermore, new adopters of AID may benefit from guidance on customizing alerts and alarms to fit with their personal priorities and lifestyle and to balance safety considerations while minimizing the potential for developing alarm fatigue. 36 Because physical activity is recommended for people with T1D to benefit cardiovascular and overall health, AID onboarding education ought to incorporate strategies for optimizing the AID system for exercise (both planned and unplanned). 54 Effective AID education may need to be split up into multiple visits, particularly if delivering onboarding support to newly diagnosed individuals. 42
Finally, there are also circumstances that may require further detailed and unique training. For example, AID use during pregnancy may require specific ongoing education and support to optimize settings as insulin requirements increase with gestation, in an attempt to maintain >70% time in the pregnancy glucose target range.55,56 More research is needed to develop onboarding and continuing resources for effective AID use leading up to, during, and after pregnancy. Furthermore, more tailored resources may be beneficial and may need further research and development, for AID onboarding support in other specific contexts such as in people who experience fear of hypoglycemia; parents of very young children, and other situations.
Support for Continuous Use of Automated Insulin Delivery Systems
For many users, a challenge of sustained diabetes technology use is identifying ongoing benefit during different points in their life. As technology options and capabilities advance, user expectations may expand beyond solely glycemic benefits to include psychosocial or person-specific benefits. The AID users will benefit from continued, flexible, and adaptable support from their HCPs in the face of changing life demands and new technological developments.
Challenges with engaging in AID require validation and exploration from HCPs. In some cases, further education and support may be necessary throughout the duration of technology use. For example, additional AID-specific support may be needed during key developmental transitions such as when adolescents take over diabetes management responsibilities from parents. A qualitative analysis identified five psychological constructs to include in positive psychology interventions to help adolescents adjust to AID systems: knowledge and education, identity and sense of responsibility, positive affect and gratitude, social support, and trust in the system. 57 The involvement of a multidisciplinary team and, in particular, behavioral health providers with expertise in diabetes, is particularly valuable for both AID support and overall diabetes care. Others may encounter challenges with age or new life demands where their prior AID routine no longer works for them. The HCPs need to discuss these issues in detail and assist in problem-solving these situations, to help the individual once again utilize AID in a way that is perceived as more beneficial than burdensome. Ongoing access to support and education around troubleshooting system issues is necessary, 58 as this may reduce burden and empower people with diabetes to feel they can manage their AID system, thus avoiding burnout around system use. The HCPs may be able to better identify and address AID challenges through repeated, holistic assessment of needs in managing diabetes, adapting recommendations over the lifespan, offering support for gaps in care, and working with the person with T1D to provide specific skills or knowledge needed to better engage in management. 46 Challenges with AID use may not always be reflective of the AID system itself, but rather, of an individual’s own preferences, priorities, and stressors at that point in their lives.
Finally, although AID systems offer both glycemic and psychosocial benefit for many, we cannot assume they are the right “fit” for everyone at any point in time. A recent review of the psychological implications of AID systems identified a variety of reasons for discontinued use, including device-specific frustrations (eg, wear or accuracy issues), supply issues, discouragement with the system, greater workload than anticipated, life intrusions, and other life stressors. 59 If AID challenges cannot be resolved with education, support, and problem-solving, it is imperative that HCPs respect the individual’s decision to take a break from their AID system. The HCPs ought to support a range of diabetes treatment options and to privilege the perspective and preferences of the person living with T1D who knows their health, barriers, and resources best. For some, a break from devices may serve as a needed respite before they restart. For others, discontinuation may feel like the best option until they can resolve the other challenges or demands getting in the way of use.
Conclusion
Current AID systems are a major milestone in the pathway to improving health outcomes and quality of life for people with T1D. However, barriers to uptake, onboarding, and sustained use remain. A multisystemic approach that addresses the gaps in the health care system and health care team and provides individualized support to the person with T1D is likely the most effective way to promote widespread, equitable, effective, and sustained use of AID systems. Efforts to facilitate a positive experience with uptake and onboarding will likely support continued use; more research is needed to develop high-quality, evidence-based programs to support AID uptake, onboarding, and continued use that can be tailored to individual needs. Comprehensive, balanced education, realistic expectations, a teamwork approach between the person with T1D and their HCP, and adaptive, ongoing support are needed for both HCPs and people with T1D. The individual experiences and needs of people who take responsibility for their diabetes 24/7/365 should be the focus of a holistic approach to diabetes care with AID systems.
Footnotes
Abbreviations: AID, automated insulin delivery; CGM, continuous glucose monitoring; CSII, continuous subcutaneous insulin infusion; HbA1c, glycated hemoglobin A1c; HCP, health care provider; PRO, person-reported outcomes; RCT, randomized controlled trial; TIR, time in range; T1D, type 1 diabetes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: KL has received research funding from Abbott, NovoNordisk, Roche Diabetes Care, and Sanofi-Aventis; she has received speaker honoraria from Allpressan, AstraZeneca, BioMarin, Chiesi, Insulet, Lilly Deutschland, Medtronic, Menarini Berlin Chemie, Merck, MSD SHARP & DOHME, NovoNordisk, and Sanofi-Aventis. EGW has received personal fees from Abbott, AstraZeneca, Dexcom, Eli Lilly, Embecta, Insulet, Medtronic, Novo Nordisk, Roche, Sanofi, Sinocare, and Ypsomed and research support from Abbott, Embecta, Insulet, Novo Nordisk, and Sanofi. MLT and PC report no conflict of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: MLT and PVC are supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award numbers K23DK119470 and K23DK137024, respectively. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ORCID iDs: Molly L. Tanenbaum  https://orcid.org/0000-0003-4222-4224
https://orcid.org/0000-0003-4222-4224
Persis V. Commissariat  https://orcid.org/0000-0002-6964-1223
https://orcid.org/0000-0002-6964-1223
References
- 1. Brown SA, Kovatchev BP, Raghinaru D, et al. Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med. 2019;381(18):1707-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. DeSalvo DJ, Bode BW, Forlenza GP, et al. Glycemic outcomes persist for up to 2 years in very young children with the Omnipod(®) 5 automated insulin delivery system. Diabetes Technol Ther. 2024;26(6):383-393. doi: 10.1089/dia.2023.0506. [DOI] [PubMed] [Google Scholar]
- 3. Bisio A, Gonder-Frederick L, McFadden R, et al. The impact of a recently approved automated insulin delivery system on glycemic, sleep, and psychosocial outcomes in older adults with type 1 diabetes: a pilot study. J Diabetes Sci Technol. 2022;16(3):663-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hood KK, Garcia-Willingham N, Hanes S, et al. Lived experience of CamAPS FX closed loop system in youth with type 1 diabetes and their parents. Diabetes Obes Metab. 2022;24:2309-2318. doi: 10.1111/dom.14815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Phillip M, Nimri R, Bergenstal RM, et al. Consensus recommendations for the use of automated insulin delivery technologies in clinical practice. Endocr Rev. 2023;44(2):254-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ware J, Allen JM, Boughton CK, et al. Hybrid closed-loop with faster insulin aspart compared with standard insulin aspart in very young children with type 1 diabetes: a double-blind, multicenter, randomized, crossover study. Diabetes Technol Ther. 2023;25(6):431-436. doi: 10.1089/dia.2023.0042. [DOI] [PubMed] [Google Scholar]
- 7. Lee TT, Collett C, Bergford S, et al. Automated insulin delivery in women with pregnancy complicated by type 1 diabetes. N Engl J Med. 2023;389(17):1566-1578. [DOI] [PubMed] [Google Scholar]
- 8. Anderson SM, Buckingham BA, Breton MD, et al. Hybrid closed-loop control is safe and effective for people with type 1 diabetes who are at moderate to high risk for hypoglycemia. Diabetes Technol Ther. 2019;21(6):356-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schipp J, Hendrieckx C, Braune K, et al. Psychosocial outcomes among users and nonusers of open-source automated insulin delivery systems: multinational survey of adults with type 1 diabetes. J Med Internet Res. 2023;25:e44002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sherr JL, Heinemann L, Fleming GA, et al. Automated insulin delivery: benefits, challenges, and recommendations. A consensus report of the Joint Diabetes Technology Working Group of the European Association for the Study of Diabetes and the American Diabetes Association. Diabetes Care. 2022;45(12):3058-3074. [DOI] [PubMed] [Google Scholar]
- 11. Malone SK, Matus AM, Flatt AJ, et al. Prolonged use of an automated insulin delivery system improves sleep in long-standing type 1 diabetes complicated by impaired awareness of hypoglycemia. J Diabetes Sci Technol. 2023. doi:10.1177/19322968231182406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cobry EC, Bisio A, Wadwa RP, Breton MD. Improvements in parental sleep, fear of hypoglycemia, and diabetes distress with use of an advanced hybrid closed-loop system. Diabetes Care. 2022;45(5):1292-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ng SM, Katkat N, Day H, Hubbard R, Quinn M, Finnigan L. Real-world prospective observational single-centre study: hybrid closed loop improves HbA1c, time-in-range and quality of life for children, young people and their carers. Diabet Med. 2022;39(7):e14863. [DOI] [PubMed] [Google Scholar]
- 14. Franceschi R, Mozzillo E, Di Candia F, et al. A systematic review on the impact of commercially available hybrid closed loop systems on psychological outcomes in youths with type 1 diabetes and their parents. Diabet Med. 2023;40(9):e15099. [DOI] [PubMed] [Google Scholar]
- 15. Farrington C. Psychosocial impacts of hybrid closed-loop systems in the management of diabetes: a review. Diabet Med. 2018;35(4):436-449. [DOI] [PubMed] [Google Scholar]
- 16. Rankin D, Kimbell B, Hovorka R, Lawton J. Adolescents’ and their parents’ experiences of using a closed-loop system to manage type 1 diabetes in everyday life: qualitative study. Chronic Illn. 2022;18(4):742-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lingen K, Maahs D, Bellini N, Isaacs D. Removing barriers, bridging the gap, and the changing role of the health care professional with automated insulin delivery systems. Diabetes Technol Ther. 2024;26(suppl 3):45-52. [DOI] [PubMed] [Google Scholar]
- 18. Walker AF, Hood KK, Gurka MJ, et al. Barriers to technology use and endocrinology care for underserved communities with type 1 diabetes. Diabetes Care. 2021;44(7):1480-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Addala A. Making a good thing even better: expanding access and applicability of automated insulin delivery systems to benefit all youth with type 1 diabetes. Diabetes Care. 2023;46(12):2126-2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tanenbaum ML, Hanes SJ, Miller KM, Naranjo D, Bensen R, Hood KK. Diabetes device use in adults with type 1 diabetes: barriers to uptake and potential intervention targets. Diabetes Care. 2017;40(2):181-187. doi: 10.2337/dc16-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Addala A, Suttiratana SC, Wong JJ, et al. Cost considerations for adoption of diabetes technology are pervasive: a qualitative study of persons living with type 1 diabetes and their families. Diabet Med. 2021;38(10):e14575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. JDRF. Pathway to choice. Updated August 18, 2023. Accessed March 22 2024. https://jdrf.org.uk/pathway-to-choice/.
- 23. Farrington C, Murphy HR, Hovorka R. A qualitative study of clinician attitudes towards closed-loop systems in mainstream diabetes care in England. Diabet Med. 2020;37(6):1023-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Petrovski G, Campbell J, Pasha M, et al. Simplified meal announcement versus precise carbohydrate counting in adolescents with type 1 diabetes using the MiniMed 780G advanced hybrid closed loop system: a randomized controlled trial comparing glucose control. Diabetes Care. 2023;46(3):544-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Boughton CK, Allen JM, Ware J, et al. Closed-loop therapy and preservation of C-peptide secretion in type 1 diabetes. N Engl J Med. 2022;387(10):882-893. [DOI] [PubMed] [Google Scholar]
- 26. Odugbesan O, Mungmode A, Rioles N, et al. Increasing continuous glucose monitoring use for non-Hispanic Black and Hispanic people with type 1 diabetes: results from the T1D exchange quality improvement collaborative equity study. Clin Diabetes. 2024;42(1):40-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Read M, Henshaw KN, Zaharieva DP, et al. “Empowering us”: a community-led survey of real-world perspectives of adults with type 1 diabetes using insulin pumps and continuous glucose monitoring to manage their glucose levels. Diabetes Res Clin Pract. 2023;202:110830. [DOI] [PubMed] [Google Scholar]
- 28. Naranjo D, Suttiratana SC, Iturralde E, et al. What end users and stakeholders want from automated insulin delivery systems. Diabetes Care. 2017;40(11):1453-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Iturralde E, Tanenbaum ML, Hanes SJ, et al. Expectations and attitudes of individuals with type 1 diabetes after using a hybrid closed loop system. Diabetes Educ. 2017;43(2):223-232. doi: 10.1177/0145721717697244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Barnard KD, Pinsker JE, Oliver N, Astle A, Dassau E, Kerr D. Future artificial pancreas technology for type 1 diabetes: what do users want. Diabetes Technol Ther. 2015;17(5):311-315. [DOI] [PubMed] [Google Scholar]
- 31. Tanenbaum ML, Iturralde E, Hanes SJ, et al. Trust in closed loop systems: a qualitative study of perspectives of experienced system users. J Health Psychol. 2020;24(4):429-438. doi: 10.1177/1359105317718615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Messer LH, Berget C, Beatson C, Polsky S, Forlenza GP. Preserving skin integrity with chronic device use in diabetes. Diabetes Technol Ther. 2018;20(suppl 2):S254-S264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Christensen MO, Berg AK, Rytter K, et al. Skin problems due to treatment with technology are associated with increased disease burden among adults with type 1 diabetes. Diabetes Technol Ther. 2019;21(4):215-221. [DOI] [PubMed] [Google Scholar]
- 34. Pleus S, Ulbrich S, Zschornack E, Kamann S, Haug C, Freckmann G. Documentation of skin-related issues associated with continuous glucose monitoring use in the scientific literature. Diabetes Technol Ther. 2019;21(10):538-545. [DOI] [PubMed] [Google Scholar]
- 35. Cameli N, Silvestri M, Mariano M, Messina C, Nisticò SP, Cristaudo A. Allergic contact dermatitis, an important skin reaction in diabetes device users: a systematic review. Dermatitis. 2022;33(2):110-115. [DOI] [PubMed] [Google Scholar]
- 36. Boughton CK, Hartnell S, Allen JM, Fuchs J, Hovorka R. Training and support for hybrid closed-loop therapy. J Diabetes Sci Technol. 2022;16(1):218-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Messer LH, Berget C, Vigers T, et al. Real world hybrid closed-loop discontinuation: predictors and perceptions of youth discontinuing the 670G system in the first 6 months. Pediatr Diabetes. 2020;21(2):319-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Crabtree TS, Griffin TP, Yap YW, et al. Hybrid closed-loop therapy in adults with type 1 diabetes and above-target HbA1c: a real-world observational study. Diabetes Care. 2023;46(10):1831-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. NICE. Hybrid closed loop systems for managing blood glucose levels in type 1 diabetes. NICE Technology Appraisal TA943. Updated December 19, 2023. Accessed March 3 2024, https://www.nice.org.uk/guidance/ta943. [Google Scholar]
- 40. Diabetes technology: standards of care in diabetes–2024. Diabetes Care. 2024;47(suppl 1):S126-S144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Holder M, Kapellen T, Ziegler R, et al. Diagnostik, Therapie und Verlaufskontrolle des Diabetes mellitus im Kindes-und Jugendalter. Diabetol Stoffwechs. 2023;18(suppl 2):S148-S161. [Google Scholar]
- 42. Kimbell B, Rankin D, Ashcroft NL, et al. What training, support, and resourcing do health professionals need to support people using a closed-loop system? A qualitative interview study with health professionals involved in the closed loop from onset in type 1 diabetes (CLOuD) trial. Diabetes Technol Ther. 2020;22(6):468-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Leelarathna L, Choudhary P, Wilmot EG, et al. Hybrid closed-loop therapy: where are we in 2021. Diabetes Obes Metab. 2021;23(3):655-660. [DOI] [PubMed] [Google Scholar]
- 44. Patil SP, Albanese-O’Neill A, Yehl K, Seley JJ, Hughes AS. Professional competencies for diabetes technology use in the care setting. Sci Diabetes Self Manag Care. 2022;48(5):437-445. doi: 10.1177/26350106221120889. [DOI] [PubMed] [Google Scholar]
- 45. Rankin D, Hart RI, Kimbell B, et al. Rollout of closed-loop technology to pregnant women with type 1 diabetes: healthcare professionals’ views about potential challenges and solutions. Diabetes Technol Ther. 2023;25(4):260-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cristello Sarteau A, Muthukkumar R, Smith C, et al. Supporting the “lived expertise” of older adults with type 1 diabetes: an applied focus group analysis to characterize barriers, facilitators, and strategies for self-management in a growing and understudied population. Diabet Med. 2024;41(1):e15156. doi: 10.1111/dme.15156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Allen NA, Litchman ML. Using diabetes technology in older adults. Diabetes Digital Health. 2020:131-143. [Google Scholar]
- 48. Zahid M, Dowlatshahi S, Kansara AH, Sadhu AR. The evolution of diabetes technology–options toward personalized care. Endocr Pract. 2023;29(8):653-662. doi: 10.1016/j.eprac.2023.04.007. [DOI] [PubMed] [Google Scholar]
- 49. Tanenbaum ML, Commissariat PV. Barriers and facilitators to diabetes device adoption for people with type 1 diabetes. Curr Diab Rep. 2022;22(7):291-299. doi: 10.1007/s11892-022-01469-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wong JJ, Addala A, Hanes SJ, et al. DiabetesWise: an innovative approach to promoting diabetes device awareness. J Diabetes. 2023;15(7):597-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Choudhary P, Wilmot E. Choosing your Hybrid Closed Loop System. DTN-UK. Accessed July 29, 2024, https://abcd.care/dtn/resource/current/choosing-your-hybrid-closed-loop-system. [Google Scholar]
- 52. Berget C, Thomas SE, Messer LH, et al. A clinical training program for hybrid closed loop therapy in a pediatric diabetes clinic. J Diabetes Sci Technol. 2020;14(2):290-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Berget C, Sherr JL, DeSalvo DJ, et al. Clinical implementation of the Omnipod 5 automated insulin delivery system: key considerations for training and onboarding people with diabetes. Clin Diabetes. 2022;40(2):168-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zaharieva DP, Morrison D, Paldus B, Lal RA, Buckingham BA, O’Neal DN. Practical aspects and exercise safety benefits of automated insulin delivery systems in type 1 diabetes. Diabetes Spectr. 2023;36(2):127-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Farrington C, Stewart Z, Hovorka R, Murphy H. Women’s experiences of day-and-night closed-loop insulin delivery during type 1 diabetes pregnancy. J Diabetes Sci Technol. 2018;12(6):1125-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Benhalima K, Jendle J, Beunen K, Ringholm L. Automated insulin delivery for pregnant women with type 1 diabetes: where do we stand? J Diabetes Sci Technol. 2024. doi: 10.1177/19322968231223934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kruger S, Deacon E, van Rensburg E, Segal D. Identification of psychological constructs for a positive psychology intervention to assist with the adjustment to closed loop technology among adolescents living with type 1 diabetes. Front Psychol. 2023;14:1273586. doi: 10.3389/fpsyg.2023.1273586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lewis DM, Hussain S. Practical guidance on open source and commercial automated insulin delivery systems: a guide for healthcare professionals supporting people with insulin-requiring diabetes. Diabetes Ther. 2022;13(9):1683-1699. doi: 10.1007/s13300-022-01299-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nefs G. The psychological implications of automated insulin delivery systems in type 1 diabetes care. Front Clin Diabetes Healthc. 2022;3:846162. doi: 10.3389/fcdhc.2022.846162. [DOI] [PMC free article] [PubMed] [Google Scholar]