Abstract
Fatigue surrounding hemodialysis treatments is a common and often debilitating symptom that impacts patients’ quality of life. Intra-dialytic fatigue develops or worsens immediately prior to hemodialysis and persists through the dialysis treatment. Little is known about associated risk factors or pathophysiology, although it may relate to a classical conditioning response. Post-dialysis fatigue (PDF) develops or worsens after hemodialysis and may persist for hours. There is no consensus on how to measure PDF. Estimates for prevalence range from 20-86%, likely due to variation in methods of ascertainment and participant characteristics. Several hypotheses seek to explain the pathophysiology of PDF, including inflammation, hypothalamic-pituitary-adrenal axis dysregulation, and osmotic and fluid shifts, but none is currently supported by compelling or consistent data. PDF is associated with several clinical factors, including cardiovascular and hemodynamic effects of the dialysis procedure, laboratory abnormalities, depression, and physical inactivity. Clinical trials have reported hypothesis-generating data about the utility of cold dialysate, frequent dialysis, clearance of large middle molecules, treatment of depression, and exercise as potential treatments. Existing studies were often limited by sample size, lack of a control group, observational design, or short intervention duration. Robust studies are needed to establish the pathophysiology and management of this important symptom.
Keywords: fatigue, hemodialysis, post-dialysis fatigue, intra-dialytic fatigue
INTRODUCTION
Patients with end-stage kidney disease (ESKD) receiving hemodialysis (HD) suffer a high burden of symptoms during and after dialysis treatments that adversely impact their quality of life. One of the most common, distressing, and debilitating symptoms is fatigue.1 Fatigue is a complex phenomenon that involves physical, psychological, and emotional components.2 Although unrelenting chronic fatigue is common among patients with ESKD, there are two additional patterns of fatigue in this patient population related to the timing of dialysis sessions: (1) intra-dialytic fatigue (IDF) that develops or worsens immediately before the dialysis session and persists for the duration of the treatment;3 and (2) post-dialysis fatigue (PDF) that develops or worsens after the end of the dialysis session and may persist for hours.4 The aim of this review is to consolidate known information about the measurement, epidemiology, pathophysiology, and potential management of IDF and PDF in patients with ESKD on maintenance HD.
DIFFERENTIATING IDF AND PDF FROM CHRONIC FATIGUE
It is controversial whether IDF and PDF should be considered unique entities or as parts of a cumulative experience chronic fatigue.5 For affected patients, these nuances about the timing of fatigue may be less important than the degree to which they are limited in participation in their daily lives.5 Clinically, it may be difficult to distinguish between IDF, PDF, and chronic fatigue in patients who experience some overlap between these loosely defined categories. Consequently, much of the existing research about fatigue in this patient population either ascertained chronic fatigue or did not specify the timing of symptoms relative to the HD treatment.5-7 Such studies may encompass IDF and PDF without identifying them specifically. However, the pathogenesis of fatigue in patients with kidney disease is likely multifactorial but remains poorly understood,2 and the timing of fatigue relative to the HD treatment may offer some insights into mechanisms and potential treatments. Fatigue that develops or worsens during or after the HD treatment raises the question of whether factors related to dialysis itself may contribute to the increased symptom burden for some patients. Ultimately, whether IDF and PDF either have unique underlying contributing factors or represent heterogeneous manifestations of generalized fatigue, summarizing and organizing what is known about these entities may generate new hypotheses to more effectively manage fatigue in patients with ESKD.
INTRA-DIALYTIC FATIGUE (IDF)
Each HD session is a physiologically and psychologically demanding event that affects mental well-being, metabolism, cardiovascular function, and perfusion. IDF is characterized by fatigue that develops or worsens immediately prior to the start of the HD treatment and persists for the duration of the treatment.3 Fatigue is the most common symptom during the HD procedure, reported by 60-80% of patients,8,9 and appears to be more severe immediately before or during the HD procedure than on non-dialysis days.3,10,11 To further assess the relationship of fatigue severity with the timing of HD, one study showed that fatigue increased significantly during the HD treatment as compared to one hour before the treatment (Figure 1a). This pattern was particularly pronounced in individuals without depressive symptoms, in whom symptoms of fatigue increased significantly from one hour prior to HD to both immediately prior to HD and immediately after the HD treatment (Figure 1b). Individuals with depressive symptoms, on the other hand, reported persistently high levels of fatigue on the dialysis day that were not temporally associated with the HD procedure (Figure 1b).3
Figure 1.
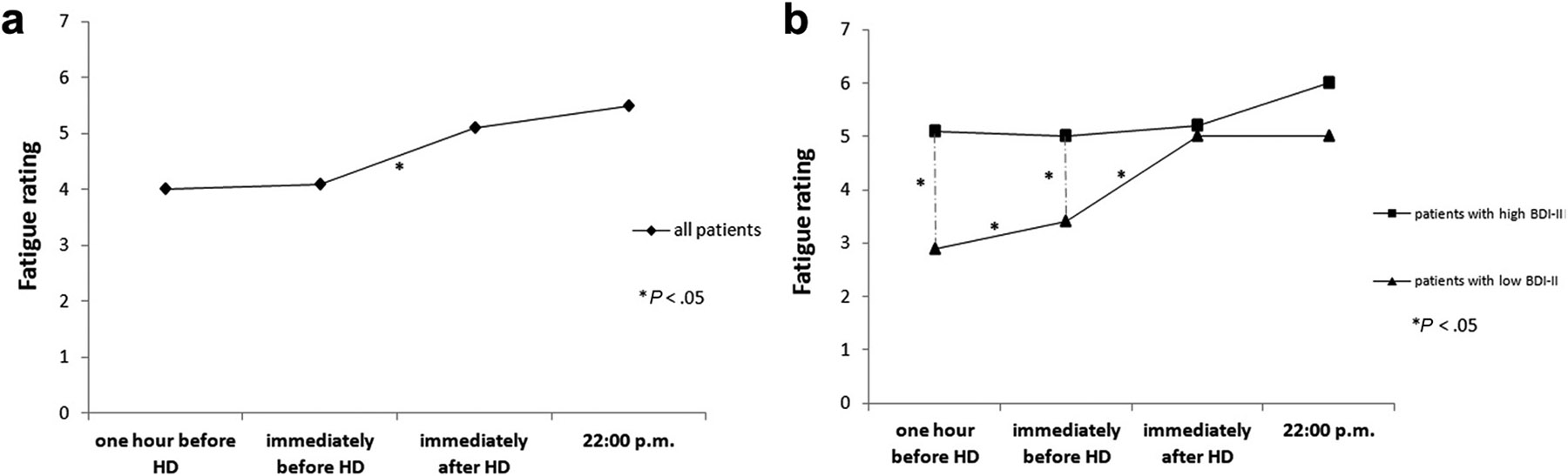
Course of fatigue on a HD treatment day (a) and its relation to depressive symptoms (b). Reproduced from Brys, et al.3 with permission of the copyright holder (Elsevier).
Abbreviations: BDI-II, Beck Depression Inventory-II; HD, hemodialysis
Data that patient-reported fatigue was higher immediately before HD than on non-dialysis days suggests a possible classical conditioning response to the HD procedure or environment.3,10,11 A similar phenomenon has been described in patients with breast cancer, whose experience of fatigue in anticipation of chemotherapy treatments is associated with their experience of fatigue during previous chemotherapy sessions.12 Other studies have also supported the important role of illness beliefs and behaviors in a patient’s experience of fatigue, although less is known about how these factors may affect IDF specifically.13
At this time, few studies have evaluated IDF, its risk factors, or its pathophysiology. Further research should investigate physical and psychological components to further elucidate this question of a classical conditioning response or other factors that may contribute to IDF.
POST-DIALYSIS FATIGUE (PDF)
Fatigue occurring after dialysis has been better studied than IDF. In qualitative studies, patients have identified PDF as a unique and debilitating form of fatigue, with a consistent theme across studies of feeling “worn out” or “exhausted” after treatment, and the need to “collapse” after HD, with gradual recovery just in time to return for another HD treatment and develop fatigue again.14
Measurement
There is currently no consensus on how to define or measure PDF (Table 1).15-22 One of the more consistently used measures quantifies PDF by the duration, frequency, and intensity of fatigue.15-18,22-24 The most commonly used measure is time to recovery from dialysis (TIRD), a validated indirect measure of PDF in which patients report the time required to recover from an HD session.20,25-31 To address the heterogeneity of existing fatigue measures, the Standardized Outcomes in Nephrology-Hemodialysis (SONG-HD) work group developed and validated a three-item measure to consistently assess fatigue in research and clinical practice.5,6 Patients are asked if, in the last week, they felt tired, lacked energy, or if fatigue limited their usual activities.6 Notably, this measure does not distinguish PDF, IDF, and chronic fatigue, but reflects a cumulative experience of fatigue. Existing measurement instruments assess symptom burden retrospectively, so may overestimate fatigue due to recall bias.32 Novel fatigue assessment methods combining a validated patient-reported outcome measure for fatigue with a real-time digital experience sampling methodology may overcome this limitation and provide more nuanced insight in the relationships of fatigue with clinical, psychological, and behavioral factors, but such tools need to be further studied.32
Table 1.
Measures of PDF
| References | Measure | Benefits | Limitations |
|---|---|---|---|
| Lindsay, et al. 200625 Awuah, et al. 201328 Bossola, et al. 201327 Rayner, et al. 201429 Hussein, et al. 201731 Davenport, et al. 201830 Alvarez, et al. 20208 Brys, et al. 202026 Guerraoui, et al. 202120 |
|
|
|
| Sklar, et al. 199617 Sklar, et al. 199816 Sklar, et al. 199915 Gordon, et al. 201118 Bossola, et al. 201823 Bossola, et al. 202024 Bossola, et al. 202322 |
|
|
|
| Dubin, et al. 201319 |
|
|
|
| Guerraoui, et al. 202120 |
|
|
|
| Brys, et al. 202032 |
|
|
|
Abbreviations: PDF, post-dialysis fatigue; TIRD, time to recovery from dialysis
Prevalence and Characteristics
Prevalence estimates of PDF range from 20% to 86% (Figure 2a).17-24 Variation in estimates are likely related to different inclusion criteria, ascertainment methods, or definitions of fatigue between studies. For example, in the study reporting the lowest prevalence of 20%, fatigue was only defined as severe fatigue,19 whereas four other studies that used the same ascertainment methods presented consistent results.17,22-24
Figure 2. Prevalence of PDF and TIRD.
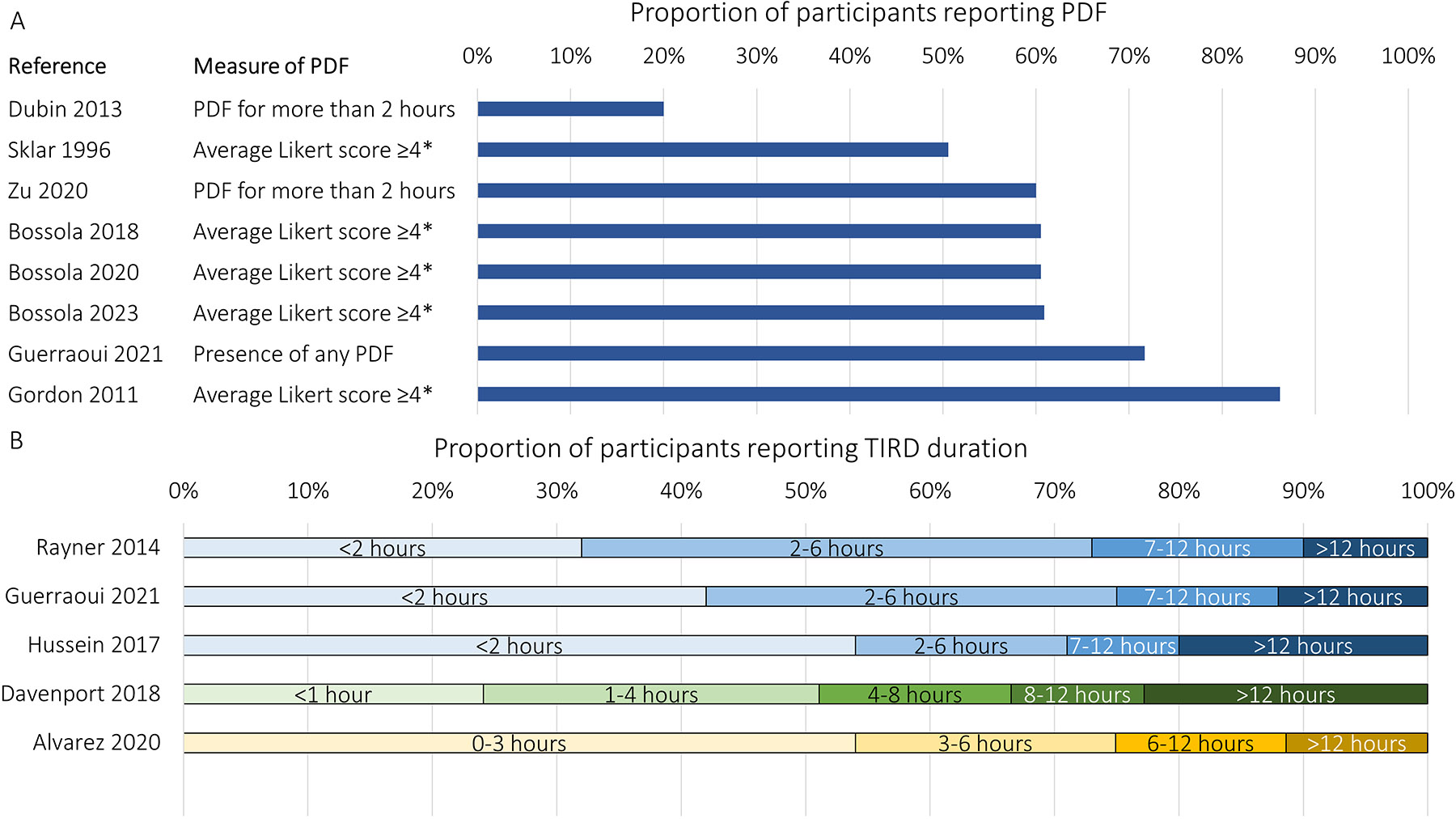
The prevalence of PDF has varied between studies from 20% to 86% depending on how PDF was defined and ascertained (A). The proportion of patients reporting TIRD of various lengths has also varied between studies, with as many as 22.8% reporting needing >12 hours to recover from an HD treatment (B).
*In these studies, patients were asked to report on 5-point Likert scales the duration, frequency, and intensity of fatigue. The patient was considered to have PDF if the average of these was ≥4 points.
Abbreviations: HD, hemodialysis; PDF, post-dialysis fatigue; TIRD, time to recovery from dialysis
With respect to TIRD, one study reported that 79% with PDF recovered within 4 hours, with a mean ± SD TIRD of 206 ±199 minutes.27 Another reported a similar mean TIRD but with greater variance between individuals, 246 ±451 minutes.28 In the Dialysis Outcomes and Practice Patterns Study (DOPPS), 32% of patients reported recovery time shorter than 2 hours, 41% reported 2-6 hours, 17% reported 7-12 hours, and 10% reported longer than 12 hours (Figure 2b).29 Each hour of increased recovery time after dialysis was associated with a 3% increased risk of hospitalization and a 5% increased risk of death.29 Not all studies use the same time cutoffs for TIRD, limiting comparison between studies (Figure 2b).8,20,29-31
Pathogenesis
The causes of fatigue after HD are not clearly understood, although some studies have reported factors associated with PDF or TIRD (Figure 3). Essentially, a few hypotheses have been proposed.
Figure 3. Factors associated with PDF.
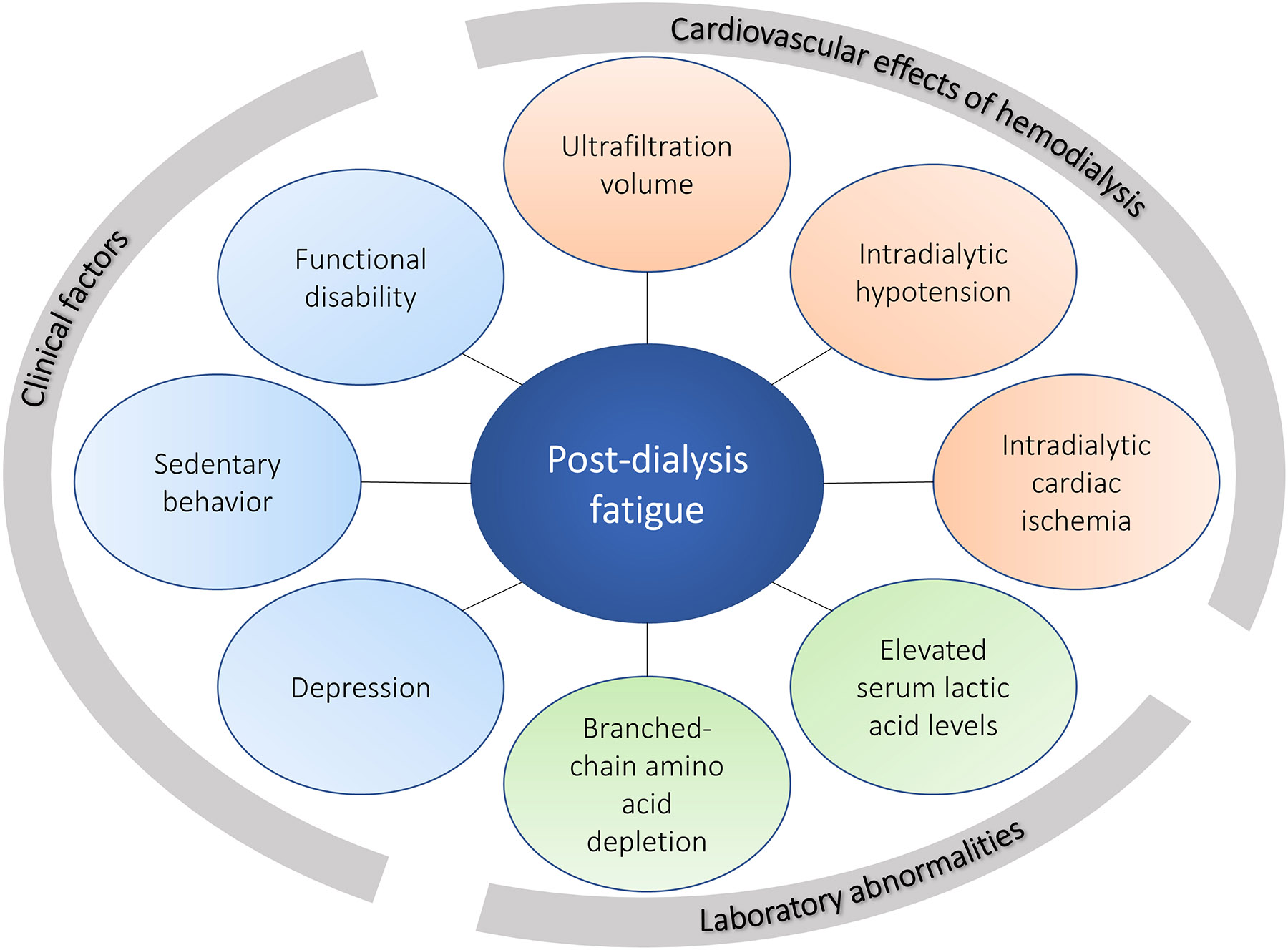
Factors associated with increased PDF can be categorized as the cardiovascular and hemodynamic effects of HD, laboratory abnormalities, and clinical factors.
Abbreviations: HD, hemodialysis; PDF, post-dialysis fatigue
The first is release of inflammatory cytokines such as interleukin (IL)-1, IL-6, IL-10, and tumor necrosis factor-alpha (TNF-α) during and after HD. Data to support this are mixed. One study showed that intradialytic elevation of TNF-α was significantly greater in the PDF group compared to the non-PDF group.33 Conversely, a crossover study showed that although TNF-α levels increased more after HD with a bioincompatible membrane compared to a biocompatible membrane, PDF was not affected, indicating that alterations in TNF-α alone are not sufficient to cause PDF.16 Another study showed no relationship between pre-dialysis, post-dialysis, or change in IL-1β, IL-6, or TNF-α with TIRD, but did find an unexpected relationship between elevated pre-dialysis levels of anti-inflammatory IL-10 and increased TIRD, which warrants further exploration.26
The second hypothesis relates to dysregulation of the hypothalamic-pituitary-adrenal axis, which is a key neuroendocrine system that adapts to emotional, physical, chemical, and immune stressors. These pathways are often disrupted in chronic illnesses associated with fatigue. Elevated cortisol has been associated with fatigue in patients with cancer, multiple sclerosis, and chronic obstructive pulmonary disease, although this relationship is not well studied in patients with kidney disease.34 It is unclear at this time whether the HD procedure impacts these systems in such a way that may contribute to PDF.
Third, some have proposed that rapid shifts in osmolality and fluid during HD may contribute to PDF and other dialysis-associated symptoms.15,18 Existing studies to evaluate this reported inconsistent results. One study showed that the reduction in osmolarity during HD did not differ between patients with and without PDF.33 Studies comparing PDF after dialysis against high-sodium and low-sodium dialysate arrived at conflicting conclusions, though these were often limited by small sample sizes.35-37 Furthermore, changes in brain density and ventricular size were similar in patients receiving standard or rapid dialysis.38 However, other studies reported that longer TIRD was associated with longer HD treatments,29 lower ultrafiltration rate,39 and lower dialysate sodium concentration29,40, which argues against this hypothesis.
Associated Factors
In the setting of these conflicting and limited data, no clear hypothesis currently explains the pathogenesis of PDF. Observational studies identified associated factors that suggest potential intervenable phenomena to improve PDF (Table 2). These can be organized into cardiovascular effects of HD, laboratory abnormalities, and clinical factors (Figure 3).
Table 2.
Studies evaluating factors associated with PDF or TIRD
| Reference | Study Design |
Participants | N | PDF measure | Factors associated with increased PDF | ||
|---|---|---|---|---|---|---|---|
| Cardiovascular effects of HD | Laboratory abnormalities |
Clinical factors | |||||
| Sklar, et al. 199617 | Prospective cohort | Prevalent ESKD on HD at a single center | 85 | Average ≥4 for PDF duration, frequency, and intensity on 5-point Likert scales | Individuals with fatigue had lower pre-HD and post-HD systolic and DBP | Depressive symptoms were higher in those with than without PDF, 11.6±8.0 vs. 7.8±6.3, P=0.02 | |
| Lindsay, et al. 200625 | Prospective cohort | Patients receiving short daily HD, nocturnal HD, or conventional HD | 45 | TIRD | Self-reported dialysis stress, Pearson r=0.35, P<0.001 Self-reported hypotension, Pearson r=0.33, P<0.001 |
Disease stress, Pearson r=0.37, P<0.001 Psychosocial stress, Pearson r=0.33, P<0.001 Lower engagement in active social-leisure activities, Pearson r=−0.17, P=0.002 |
|
| Gordon, et al. 201118 | Baseline data from the Nandrolone and Exercise Trial | Prevalent ESKD on HD for ≥3 months at HD units associated with one academic center in the United States | 58 | Average of PDF duration, frequency, and intensity on 5-point Likert scales | Lower physical activity measured by accelerometer, β=−0,025, P=0.003 | ||
| Bossola, et al. 201327 | Cross-sectional | Prevalent ESKD on HD for ≥6 months at a single HD unit in Italy | 100 | TIRD | Depressive symptoms, β=16.10 (SE 4.82), P=0.001 IADL score, Spearman r= −0.29, P=0.02 |
||
| Dubin, et al. 201319 | Prospective cohort | Prevalent ESKD on HD at 3 hospitals in the United States | 40 | PDF for more than 2 hours | Change in wall motion abnormality score on echocardiogram was associated with PDF, RR 1.9 (95% CI 1.4, 2.6), P<0.001 | Depression, RR 3.4 (95% CI 1.3, 9.0), P=0.01; became non-significant when adjusting for blood pressure and ultrafiltration | |
| Lopes, et al. 201447 | Cross-sectional | Prevalent ESKD on HD in Brazil (PROHEMO) | 800 | TIRD | Depressive symptoms were higher in those with TIRD <60 vs. 0 minutes, 16.48±9.33 vs. 11.51±8.73, P<0.001. No difference in depressive symptoms for TIRD <60, 60-240, or >240 minutes. Poorer health-related quality of life in domains of physical functioning, general health, energy/vitality, social functioning, and cognition |
||
| Rayner, et al. 201429 | Prospective cohort | Random sample of patients on maintenance HD from 12 countries (DOPPS) | 6,040 | TIRD | Higher intradialytic weight loss Ultrafiltration rate 5-15 mL/min with longer TIRD than <5 mL/min or >15 mL/min | Dialysate sodium <140 mEq/L vs. 140 mEq/L, aOR 1.34 (95% CI 1.11, 1.61) | Depression, Spearman r=0.22 across categories of TIRD Fewer ADLs, r=−0.27 |
| Hussein, et al. 201731 | Cross-sectional | Prevalent ESKD on HD for ≥60 days | 2,689 | TIRD | Ultrafiltration rate ≥13 mL/kg/hr vs. <10 mL/kg/hr, OR 1.28 (95% CI 1.06, 1.54), P=0.01 Higher post-dialysis weight, OR 1.07 (95% CI 1.03, 1.11), P=0.001 per 10 kg gain Missed dialysis sessions, OR 1.08 (95% CI 1.02, 1.14), P=0.0001 Higher pre-HD SBP, OR 1.05 (95% CI 1.01, 1.08), P=0.02 per 10 mmHg increase |
Lower serum albumin, OR 0.82 (95% CI 0.74, 0.91), P=0.0001 per 0.5 g/dL increase | |
| Bossola, et al. 201823 | Cross-sectional | Prevalent ESKD on HD for ≥1 year at five HD units in Italy | 271 | Average ≥4 for PDF duration, frequency, and intensity on 5-point Likert scales | ADL score was higher in those without PDF than those with PDF, 5.5±1.1 vs. 5.1±1.6, P=0.009 | ||
| Bossola, et al. 201849 | Cross-sectional | Prevalent ESKD on HD for ≥1 year at five HD units in Italy | 271 | Average ≥4 for PDF duration, frequency, and intensity on 5-point Likert scales | Lower dialysate temperature, OR 0.27 (95% CI 0.11, 0.67), P=0.005 | Serum albumin, OR 2.78 (95% CI 1.34, 5.75), P=0.006 | PDF severity, intensity, duration, and frequency were all more severe in those with low ADL scores. PDF was associated with ADL score, OR 0.47 (95% CI 0.23, 0.95), P=0.04 |
| Davenport, et al. 201830 | Prospective cohort | Prevalent ESKD on HD for ≥3 months at five HD units in the United Kingdom | 701 | TIRD | Depressive symptoms as measured by the BDI-II (P<0.001) or the PHQ-9 (P<0.001) History of depression (P<0.001) Use of antidepressants (P=0.001) Urine output < cupful (P=0.01) Prior transplant status (P=0.02) |
||
| Bossola, et al. 201939 | Prospective cohort | Prevalent ESKD on HD for ≥1 year at five HD units in Italy | 210 | TIRD | Lower ultrafiltration rate associated with longer TIRD, OR 1.11 (95% CI 1.04, 1.22), P=0.04 | Functional disability (need for assistance in at least 2 ADLs), OR 0.50 (0.27, 0.94), P=0.03 | |
| Debnath, et al. 202046 | Cross-sectional | Prevalent ESKD on HD for ≥6 months | 114 | Brief Fatigue Inventory filled out between hours 3 and 4 of the HD session | Lower post-HD plasma branched-chain amino acids and leucine and isoleucine associated with fatigue severity, interference, and global fatigue | ||
| Zu, et al. 202021 | Cross-sectional | Prevalent ESKD on HD for ≥6 months at a single HD unit in China | 115 | PDF for more than 2 hours | Intradialytic hypotension, OR 3.82 (95% CI 1.33, 10.98) Higher ultrafiltration rate, OR 1.14 (95% CI 1.02, 1.28) |
Higher post-HD lactic acid level, OR 2.47 (95% CI 1.13, 5.40) | |
| Brys, et al. 202148 | Prospective cohort | Prevalent ESKD on HD at one HD units in the Netherlands | 40 | Presence of momentary fatigue on a 7-point Likert scale assessed at several time points per day | Negative affect (β=0.23, 95% CI 0.08, 0.38) Being at home (β=2.37, 95% CI 1.86, 2.88) Being alone (β=2.36, 95% CI 1.84, 2.89) Poor sleep quality (β=−0.12, 95% CI −0.18, −0.06) |
||
| Debnath, et al. 202110 | Prospective cohort | Prevalent ESKD on HD for ≥6 months at 2 HD units in the US | 115 | Brief Fatigue Inventory filled out during the first hour of the HD session and again ~24 hours later | Depressive symptoms associated with fatigue severity, interference, and global fatigue on both dialysis days and non-dialysis days (P<0.0001 except for fatigue severity on the dialysis day, P<0.05) | ||
| Ozen, et al. 202143 | Cross-sectional | Prevalent ESKD on HD at a single HD unit in Turkey | 86 | TIRD | Intradialytic hypotension was associated with TIRD above the median, OR 3.14 (95% CI 1.13, 8.77) | ||
| Elsayed, et al. 202240 | Cross-sectional | Prevalent ESKD on HD for ≥3 months at HD units associated with a University Hospital system | 191 | TIRD | More missed dialysis sessions, Spearman r=0.27, P<0.001 Ultrafiltration rate, r=−0.18, P=0.01 Lower dialysate sodium, r=−0.20, P=0.006 Faster dialysate flow, r=0.23, P=0.001 Lower post-HD blood pressure, r=−0.19, P=0.007 |
Lower phosphate, r=−0.18, P=0.01 Lower albumin, r-0.14, P=0.05 Higher malnutrition inflammation score, r=0.24, P=0.001 |
Poorer quality of life scores, including effect of kidney disease, r-0.31, P<0.001; burden of kidney disease, r=−0.27, P<0.001; physical composite, r=−0.23, P=0.001; and mental composite, r=−0.35, P<0.001 |
| Bossola, et al. 202322 | Cross-sectional | Prevalent ESKD on HD for ≥1 year at five HD units in Italy | 335 | Average ≥4 for PDF duration, frequency, and intensity on 5-point Likert scales | Lower ADL score, OR 0.79 (95% CI 0.65, 0.99) | ||
Abbreviations: ADL, activity of daily living; BDI-II, Beck Depression Inventory II; CI, confidence interval; DBP, diastolic blood pressure; DOPPS, Dialysis Outcomes and Practice Patterns Study; HD, hemodialysis; IADL, instrumental activities of daily living; OR, odds ratio; PDF, post-dialysis fatigue; PHQ-9, Patient Health Questionnaire; PROHEMO, Prospective Study of the Prognosis of Hemodialysis Patients; RR, relative risk; SBP, systolic blood pressure; TIRD, time to recovery from dialysis
Cardiovascular effects of HD include higher ultrafiltration volume, intradialytic hypotension, and intradialytic cardiac ischemia. Larger fluid shifts may be associated with PDF, such that longer reported TIRD was associated with higher intradialytic weight loss.29,40 However, TIRD showed a U-shaped association with ultrafiltration rate, such that a rate <5 mL/min or >15 mL/min was associated with shorter TIRD compared to an ultrafiltration rate of 5-15 mL/min.29 Other studies also showed that longer TIRD was associated with lower ultrafiltration rate39,40 and higher post-dialysis weight,31 raising the question of whether factors leading to inability to adequately remove fluid on dialysis may contribute to PDF.
Hypoperfusion during the dialysis procedure may produce fatigue. In one study, higher post-dialysis lactic acid levels were associated with PDF.21 This may indicate relative tissue ischemia during dialysis, which can be seen in the setting of fluid shifts, hypotension, and other cardiovascular effects of HD.41 Intradialytic cardiac ischemia, referred to as myocardial stunning and identified by regional wall motion abnormalities on echocardiogram during and shortly after HD, was associated with fatigue.19 Intradialytic hypotension, one of the most common complications of hemodialysis, increases the risk of heart, brain, and bowel ischemia and may contribute to elevated serum lactic acid levels.21,41,42 However, an association between intradialytic hypotension and PDF has not been clearly established due to conflicting data.17,19,23,31,43
Depletion of branched-chain amino acids during HD may also contribute to PDF by impacting central nervous system neurotransmitter biology.44 Branched-chain amino acids compete with tryptophan for movement into the central nervous system. Higher levels of 5-hydroxytryptamine (a metabolite of tryptophan) cause central fatigue.45 In one study, an association was found between lower post-dialysis plasma levels of branched-chain amino acids and PDF.46
Depressive symptoms and a history of depression have been found in several studies to be associated with PDF and TIRD.3,10,17,19,27,29,30,47 This may be in part due to the inclusion of fatigue as one diagnostic criterion for depression.2 However, other evidence suggests that depressed mood may worsen after episodes of fatigue, suggesting that a temporal relationship may exist between these two symptoms.48
Finally, sedentary behavior and functional disability are also associated with PDF and longer TIRD.18,25,49 This is consistent with the common observation that chronic fatigue in patients on HD is associated with poorer physical functioning.50 These relationships have been primarily identified in cross-sectional analyses, so it remains unclear to what degree poor physical functioning may lead to fatigue or vice versa.
Management
Several studies have evaluated treatments for PDF or to reduce TIRD, primarily as one outcome among several dialysis-associated symptoms. Studied interventions can be categorized as changes in the HD prescription, treatment of depression, and exercise (Box 1). Many of these studies employed small sizes, had short intervention duration, or were performed prior to modern dialysis techniques, which limits interpretation (Table 3).
Box 1. Interventions that have been studied to improve PDF or TIRD.
| Modifications to dialysate composition or temperature |
| Increased dialysate tonicity |
| High dialysate sodium |
| Sodium modeling protocols |
| Glucose-enriched dialysate |
| Cool dialysate temperature |
| Other modifications to hemodialysis prescription |
| Increased HD frequency |
| Increased clearance of middle molecules via HDF or expanded dialysis |
| Cognitive Behavioral Therapy |
| Physical activity |
| Intra-dialytic exercise |
| Exercise on non-dialysis days |
| Other interventions |
| Steroids |
| Erythropoietin stimulating agents |
| Sacubitril/valsartan |
| Acupuncture and acupressure |
| Carnitine supplementation |
Abbreviations: HDF, hemodiafiltration; PDF, post-dialysis fatigue; TIRD, time to recovery from hemodialysis
Table 3.
Studies evaluating interventions for PDF or TIRD
| Reference | Study Design | N | Intervention | Control | Total Duration |
Fatigue Results |
|---|---|---|---|---|---|---|
| High dialysate sodium | ||||||
| Sadowski, et al. 199336 | Multiple crossover trial | 16 | 3 sodium modeling protocols to decrease dialysate sodium from 148 to 138 mEq/L | Constant dialysate sodium 138 mEq/L | 8 weeks | Higher odds of fatigue improvement with sodium modeling (combined data from 3 sodium modeling programs), OR 3.6 (95% CI 2.0, 6.4) |
| Levin, et al. 199637 | Crossover RCT | 16 | Ramped hypertonic sodium dialysis | Standard dialysis | 6 weeks | 56% in the intervention group reported improved energy for recreational activities |
| Sang, et al. 199735 | Crossover RCT | 23 | 2 protocols (stepwise and linear) for dialysate sodium ramping from 155 to 140 mEq/L | Constant dialysate sodium 140 mEq/L | 6 weeks | No difference in fatigue presence in the 12 hours after HD. Fatigue the day after dialysis was lower in the control group than the stepwise protocol (P=0.003) but not different than the linear protocol (P=0.08). |
| Basile, et al. 200159 | Non-randomized prospective interventional study | 19 | Real time sodium modeling and ultrafiltration control via biofeedback mechanism | Constant dialysate conductivity | Variable, 14-30 months | Higher PDF in the sodium modeling group (6.2±0.2 vs. 4.3±0.1, P<0.0001). |
| Glucose-enriched dialysate | ||||||
| Leski, et al. 197961 | Randomized multiple crossover | 10 | Dialysate with 400 mg/dL glucose | Glucose-free dialysate | 4 weeks | PDF decreased from 0.75±0.77 at baseline to 0.50±0.72 after glucose-enriched dialysate, P<0.01. |
| Raju, et al. 198260 | Crossover, unclear if randomized | 17 | Dialysate with 200 mg/dL glucose | Glucose-free dialysate | Unclear | Decrease in PDF frequency in glucose-enriched dialysate group |
| Raimann, et al. 201062 | Crossover RCT | 30 | Dialysate with 200 mg/dL glucose | Dialysate with 100 mg/dL glucose | 6 weeks | More severe fatigue in the 200 mg/dL group among those with DM (5.0±1.0 vs. 4.2±1.1, P<0.05) but no difference in those without DM (3.5±1.9 vs. 3.0±1.6, P=0.23) |
| Cool dialysate | ||||||
| Ayoub, et al. 200466 | Non-randomized crossover trial (cool dialysate followed by standard) | 10 | 35.0°C dialysate | 36.5°C dialysate | 2 weeks | 8 (80%) felt more energetic with cooler dialysate |
| Azar, et al. 200963 | Non-randomized crossover trial (standard dialysate followed by cool) | 50 | 35.0°C dialysate | 37.0°C dialysate | 2 weeks | 76% reported feeling more energetic with cool dialysate. TIRD 1.4±0.9 hours in 35°C group vs. 9.9±6.3 in 37°C group, P<0.001. |
| Teruel, et al. 200664 | Non-randomized crossover trial (standard dialysate followed by cool) | 31 | 35.5°C dialysate | 37.0°C dialysate | 2 weeks | PDF was lower in the cool dialysate group than the standard dialysate group, mean (SD) 1.3 (1.0) in the standard group vs. 1.0 (0.9) in the cool group, P<0.05. |
| Sajadi, et al. 201665 | Crossover RCT | 46 | 35.5°C dialysate | 37.0°C dialysate | 2 weeks | Fatigue score decreased 31.3% from baseline with cool dialysate. Behavioral, emotional, cognitive, and sensory fatigue domains improved from baseline in cool dialysate group. |
| Garg, et al. 202267 | Pragmatic cluster RCT | 15,413 | Personalized cooler dialysate temperature | 36.5°C dialysate | 4 years | Fatigue (timing relative to HD not specified) assessed among 445 participants. No difference between groups, OR 0.81 (95% CI 0.56, 1.18). |
| HD frequency or duration | ||||||
| Maduell, et al. 200369 | Single arm trial | 8 | Transition from on-line HDF 3 times per week to short daily online HDF | None | 6 months | From baseline to 4 weeks PDF scores decreased for intensity (1.88±1.2 vs. 0.38±0.7, P<0.01) and duration (1.75±1.4 vs. 0.25±0.5, P<0.01). |
| Jaber, et al. 201068 | Prospective cohort | 239 | Transition to at-home HD 6 days per week | None | 12 months | TIRD decreased from baseline (473 minutes [IQR 385, 561]) to month 4 (240 minutes [IQR 172, 308], P<0.001) and month 12 (237 minutes [IQR 168, 306], P<0.001) |
| Garg, et al. 201770 | Parallel arm RCT | 245 | 6 days per week HD | 3 days per week HD | 12 months | Greater improvement in TIRD from baseline to 12 months in the frequent HD arm, between-groups difference −84 minutes (95% CI −89, −80, P<0.0001). |
| Davenport, et al. 201971 | Retrospective cohort | 709 | Incremental HD* | Standard HD | 12 months | Higher odds of TIRD <1 hour (OR 1.49 [95% CI 1.01, 2.18]) and <4 hours (OR 1.58 [95% CI 1.14, 2.19]) in the incremental HD group. No difference in TIRD < 8 hours or <12 hours. |
| Clearance of middle molecules | ||||||
| Karkar, et al. 201573 | Parallel arm RCT | 72 | High efficiency post-dilution on-line HDF | High-flux HD | 24 months | Improved PDF in on-line HDF arm, 61±18 vs. 10±9, P<0.0001. |
| Smith, et al. 201772 | Crossover RCT | 100 | Postdilution HDF | HD | 16 weeks | No difference in TIRD, median 47.5 minutes (IQR 0, 240) for HDF vs. 30 minutes (0, 210) for HD, P=0.9. |
| Bolton, et al. 202174 | Retrospective cohort | 58 | Implementation of expanded dialysis** | None | 12 months | Shorter median (IQR) TIRD from baseline (210 minutes [7.5, 600]) to 6 months (60 minutes [0, 210], P=0.002) and 12 months (105 [0, 180], P=0.001]). |
| CBT | ||||||
| Mehrotra, et al. 201975 | Parallel arm RCT | 120 | CBT | Sertraline | 12 weeks | Fatigue improved from baseline to week 12 in both groups and was more favorable at week 12 in the sertraline group than the CBT group (between-groups effect estimate of 10.2 points [95% CI 1.3, 19.0], P=0.02]. |
| Picariello, et al. 202176 | Parallel arm feasibility RCT | 24 | Tailored CBT self-management intervention aimed at fatigue | Wait list | 3 months | Standardized mean difference in fatigue severity was 0.81 (95% CI −0.67, 2.29), favoring the intervention group. |
| Exercise and physical activity | ||||||
| Malagoni, et al. 200880 | Non-randomized trial | 31 | Walking sessions on non-dialysis days | Control with no intervention | 6 months | From baseline to 6 months the exercise group had improvement in PDF score (2.8±1.4 vs. 2.3±1.6, P<0.05) and TIRD (3.4±2.8 hours vs. 2.6±3.1 hours, P<0.05), but there was no significant difference between groups at 6 months. |
| Devagourou, et al. 202179 | Non-randomized trial | 64 | Low intensity intra-dialytic exercises | Usual care | 6 weeks | At 6 weeks fatigue was lower in the exercise group than the control group (13.1±4.9 vs. 19.2±5.0, P=0.001. |
| Grigoriou, et al. 202178 | Single arm trial | 20 | Supervised intra-dialytic combined aerobic and resistance exercise | None | 9 months | From baseline to 9 months there was a decrease in PDF severity (1.7±0.6 vs. 1.3±0.6, P<0.05) and duration (1.8±0.7 vs. 1.1±0.8, P<0.05), but there was no change in PDF frequency. |
Incremental dialysis refers to individualizing HD prescription by decreasing dialysis time based on the patient’s residual kidney function. Standard dialysis refers to dialysis prescriptions that did not account for residual kidney function.
Expanded dialysis refers to the use of medium cut-off membranes designed to increase the clearance of large middle molecules.
Abbreviations: CBT, cognitive behavioral therapy; CI, confidence interval; ESA, erythropoietin stimulating agent; HDF, hemodiafiltration; IQR, interquartile range; OR, odds ratio; PDF, post-dialysis fatigue; RCT, randomized controlled trial; TIRD, time to recovery from hemodialysis
Dialysate composition and temperature
Since the 1970s, high dialysate sodium concentration has been considered as an intervention to improve PDF as a method to minimize osmotic shifts.52-58 In the 1990s, a small multiple crossover trial showed that exponential, linear, and stepwise sodium modeling protocols to decrease dialysate sodium concentration from 148 to 138 mEq/L over the course of the treatment resulted in lower rates of fatigue between HD sessions compared to a control dialysate with a constant sodium concentration of 138 mEq/L.36 Another crossover trial that compared ramped hypertonic sodium dialysis to standard dialysis showed that 9 of 16 (56%) participants reported improved energy for recreational activities on the hypertonic dialysis protocol.37 Another trial showed the opposite effect: that dialysate sodium ramping from 155 to 140 mEq/L had a trend toward higher fatigue in the 12 hours after HD compared to a constant dialysate sodium concentration of 140 mEq/L.35 Another trial in 19 individuals showed higher PDF after HD using a biofeedback mechanism for real-time sodium modeling and ultrafiltration rate control compared to treatments with constant dialysate conductivity.59 It is unknown whether adequately powered trials using modern dialysis techniques and equipment would support the use of sodium modeling to address PDF. There are also other concerns about the consequences of sodium modeling, including weight gain and thirst, that limit its use.
As an alternative means to decrease osmotic shifts, two small studies evaluated whether dialysate enriched with glucose may decrease the frequency or severity of PDF. These studies, conducted in 1979 (N=10) and 1982 (N=17), showed lower PDF after dialysis using glucose-enriched dialysate compared to glucose-free dialysate.60,61 Only one more recent study has been published on this topic, although it did not distinguish between PDF and chronic fatigue. Using a randomized crossover design to compare dialysate glucose of 100 mg/dL to 200 mg/dL in 30 patients, the study reported that higher glucose dialysate was associated with more severe fatigue among those with diabetes mellitus, but there was no difference among those without diabetes.62 These results appear to conflict with those of the older trials and argue against the osmotic shift theory of PDF.
Small trials reported that decreasing dialysate temperature to 35-35.5°C rather than the standard temperature of 37°C may favorably impact PDF.63-66 In one clinical trial, 8 of the 10 participants reported feeling more energetic after dialysis with 35°C dialysate compared to 36.5°C dialysate, although severity of PDF was not measured.66 Another randomized crossover study identified that various dimensions of fatigue, including behavioral, emotional, cognitive, and sensory domains, all improved from baseline after dialysis with cold dialysate, but not with 37°C dialysate.65 In addition to showing favorable effects on PDF and TIRD, in crossover trials low dialysate temperature was also associated with higher blood pressure,63-66 fewer intradialytic hypotensive episodes,66 lower heart rate,63,64 higher clearance,63 and higher ultrafiltration.66 Although it had been hypothesized that these hemodynamic and diffusive effects may explain the improvement in PDF, this has not been clearly demonstrated. Furthermore, the MyTEMP large pragmatic trial showed no difference in tiredness between the cool and standard dialysis groups, but failed to demonstrate hemodynamic or cardiovascular benefit of lower dialysate temperature and was poorly tolerated by many participants, limiting its potential utility.67
HD frequency and duration
Increased HD frequency may also favorably impact PDF. One observational cohort study of patients transitioning to at-home HD 6 days per week showed that TIRD decreased significantly at month 4 and month 12 as compared to baseline.68 Another single-arm study transitioned patients on thrice weekly on-line hemodiafiltration to short daily on-line hemodiafiltration and showed that daily treatments were associated with a dramatic improvement in the intensity and duration of PDF.69 In the Frequent Hemodialysis Network studies, 6 days per week HD led to a greater improvement in TIRD from baseline to 12 months compared to 3 days per week HD.70 Such short daily treatments typically involved decreased total fluid and solute shifts per treatment, but it remains unclear if these factors underlie the improvement seen in PDF.
One prospective observational cohort from the United Kingdom evaluated patients at five HD centers, four of which provided “standard HD” that did not account for residual kidney function, and one of which provided “incremental dialysis,” in which they individualized HD prescriptions by decreasing dialysis time based on the patient’s residual kidney function.71 The incremental dialysis group had a higher odds of TIRD less than 1 hour and less than 4 hours, suggesting that such individualized dialysis prescriptions may merit further investigation.
Clearance of middle molecules
Studies have shown conflicting results as to whether hemodiafiltration, which is intended to increase clearance of middle molecules such as inflammatory cytokines, may decrease PDF as compared to HD. One crossover study of 100 patients showed no difference.72 Another parallel arm randomized trial found that high efficiency post-dilution on-line hemodiafiltration improved PDF compared to high-flux HD.73 This study continued treatment for 24 months, which is longer than most other trials of dialysis interventions that evaluated PDF and may account for time-dependent treatment effects.
Conducting dialysis with newer medium cut-off membranes designed to increase the clearance of large middle molecules is referred to as expanded dialysis. One retrospective cohort study showed that after implementation of expanded dialysis at their center, patients reported shorter TIRD as soon as 6 months after baseline.74 This analysis excluded the 33% of participants who died or dropped out of the study by 12 months, which limits interpretation of the results.
Treatment of depression and other psychological interventions
Only one clinical trial, A Trial of Sertraline vs. Cognitive Behavioral Therapy For End-Stage Renal Disease Patients with Depression (ASCEND), evaluated the effect of treatment of depression with either sertraline or cognitive behavioral therapy (CBT) on fatigue among 120 patients on maintenance HD.75 Fatigue improved from baseline to week 12 in both groups. Fatigue scores were more favorable at week 12 in the sertraline group (mean score 53.0 [95% CI 45.7, 60.3]) as compared to the CBT group (mean score 39.2 [95% CI 33.4, 44.9]), with a between-groups effect estimate of 10.2 (95% CI 1.3, 19.0), P=0.02. In interpreting these results, it must be considered that fatigue was slightly more favorable at baseline in the sertraline group (mean score 36.0 [95% CI 30.6, 41.4]) than in the CBT group (mean score 28.4 [95% CI 23.0, 33.9]).75 This trial had no untreated arm to determine whether these interventions had a more favorable effect on fatigue than a control condition, and did not distinguish PDF from chronic fatigue. Another randomized feasibility trial of CBT vs. wait list for fatigue in patients on HD showed moderate to large treatment effects in favor of CBT for fatigue.76 Results of a trial evaluating the effect of a flexible intervention involving CBT and pharmacotherapy on the symptom cluster of depression, fatigue, and pain are eagerly anticipated.77
Exercise and Physical Activity
Small clinical trials have shown a benefit of physical activity for improving PDF in patients on maintenance HD. Interventions have included intra-dialytic exercise78,79 and walking sessions on non-dialysis days.80 Important considerations exist when implementing an exercise regimen. Fatigue itself is a common reason people may decline to participate in physical activity.81,82 A focus group also highlighted the importance of patients’ choice of exercise modality and timing, the importance of ensuring the safety of the HD vascular access during exercise, and a desire for achievable regimens.82 Optimizing participation may require the presence of a trainer; one study of intradialytic exercise showed that when a kinesiologist was not present, participants were far more likely to refuse the session and report they were too fatigued to participate.81 The authors concluded that the presence of a professional may be a motivating factor to improve adherence to exercise interventions.
Other interventions
A few additional interventions have been studied for fatigue in patients on maintenance HD with some success. Several trails have evaluated the effects of erythropoiesis stimulating agents on fatigue in patients on maintenance hemodialysis, supporting overall that management of anemia with these agents may improve fatigue.83 One small clinical trial in 29 patients showed that individuals receiving the anabolic steroid nandrolone for 6 months had a decrease in self-reported fatigue from baseline, while the placebo group had no change in fatigue.84 One small single-arm study showed that sacubitril/valsartan improved fatigue and quality of life compared to baseline in addition to improving cardiovascular parameters.85 Several studies have shown that acupuncture or acupressure may be effective for reducing fatigue in patients on maintenance HD.86-89 Some studies have also evaluated the effect of carnitine supplementation on fatigue without evidence to support its efficacy.90 These studies generally evaluated fatigue globally without discerning PDF from chronic fatigue.
SUMMARY AND CONCLUSIONS
Despite being a high priority for patients, fatigue temporally associated with maintenance HD treatments is an under-investigated phenomenon among patients with ESKD. The most frequently used measure to quantify PDF is TIRD, but otherwise consistent measures of IDF and PDF are lacking. Despite the importance of these symptoms to patients, the pathogenesis and management remain poorly understood due to small, older studies, lack of control groups, largely observational data, short intervention durations, and inability to elucidate underlying pathophysiology. Overall, data suggest that hemodynamic and osmotic effects of the HD treatment itself as well as psychological factors may contribute to fatigue, but causes and treatments of IDF and PDF have not been definitively identified. Because of the multifactorial nature and variability of fatigue patterns between patients, treatments will likely need to be tailored to each individual. In the modern era with innovation in dialysis delivery as an expressly stated priority, improvement in patients’ experience of fatigue surrounding dialysis treatments should be further studied to improve understanding of the underlying mechanisms and to develop effective therapeutic strategies.
ACKNOWLEDGEMENTS
The opinions expressed reflect those of the authors and not necessarily those of the Department of Veterans Affairs or the NIDDK.
FUNDING
LPG is supported by a VA Clinical Sciences Research & Development Career Development Award (IK2CX002368). This work was supported in part by the Houston VA Health Services Research & Development Center for Innovations grant (CIN13-413). SH is supported by grants 3R01DK124379-03S1 and R01DK124379 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). MB and ADHB declare no funds received for this work. The funding sources did not have a role in defining the content of this manuscript.
Footnotes
DISCLOSURES
Authors have nothing to disclose.
References
- 1.de Rooij ENM, Meuleman Y, de Fijter JW, et al. Symptom Burden before and after Dialysis Initiation in Older Patients. Clin J Am Soc Nephrol. Dec 2022;17(12):1719–1729. doi: 10.2215/CJN.09190822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gregg LP, Bossola M, Ostrosky-Frid M, Hedayati SS. Fatigue in CKD: Epidemiology, Pathophysiology, and Treatment. Clin J Am Soc Nephrol. Sep 2021;16(9):1445–1455. doi: 10.2215/CJN.19891220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brys ADH, Lenaert B, Van Heugten CM, Gambaro G, Bossola M. Exploring the Diurnal Course of Fatigue in Patients on Hemodialysis Treatment and Its Relation With Depressive Symptoms and Classical Conditioning. J Pain Symptom Manage. May 2019;57(5):890–898 e4. doi: 10.1016/j.jpainsymman.2019.02.010 [DOI] [PubMed] [Google Scholar]
- 4.Bossola M, Tazza L. Postdialysis Fatigue: A Frequent and Debilitating Symptom. Semin Dial. May 2016;29(3):222–7. doi: 10.1111/sdi.12468 [DOI] [PubMed] [Google Scholar]
- 5.Ju A, Unruh M, Davison S, et al. Establishing a Core Outcome Measure for Fatigue in Patients on Hemodialysis: A Standardized Outcomes in Nephrology-Hemodialysis (SONG-HD) Consensus Workshop Report. Am J Kidney Dis. Jul 2018;72(1):104–112. doi: 10.1053/j.ajkd.2017.12.018 [DOI] [PubMed] [Google Scholar]
- 6.Ju A, Teixeira-Pinto A, Tong A, et al. Validation of a Core Patient-Reported Outcome Measure for Fatigue in Patients Receiving Hemodialysis: The SONG-HD Fatigue Instrument. Clin J Am Soc Nephrol. Nov 6 2020;15(11):1614–1621. doi: 10.2215/CJN.05880420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jhamb M, Weisbord SD, Steel JL, Unruh M. Fatigue in patients receiving maintenance dialysis: a review of definitions, measures, and contributing factors. Am J Kidney Dis. Aug 2008;52(2):353–65. doi: 10.1053/j.ajkd.2008.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alvarez L, Brown D, Hu D, Chertow GM, Vassalotti JA, Prichard S. Intradialytic Symptoms and Recovery Time in Patients on Thrice-Weekly In-Center Hemodialysis: A Cross-sectional Online Survey. Kidney Med. Mar-Apr 2020;2(2):125–130. doi: 10.1016/j.xkme.2019.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caplin B, Kumar S, Davenport A. Patients’ perspective of haemodialysis-associated symptoms. Nephrol Dial Transplant. Aug 2011;26(8):2656–63. doi: 10.1093/ndt/gfq763 [DOI] [PubMed] [Google Scholar]
- 10.Debnath S, Rueda R, Bansal S, Kasinath BS, Sharma K, Lorenzo C. Fatigue characteristics on dialysis and non-dialysis days in patients with chronic kidney failure on maintenance hemodialysis. BMC Nephrol. Mar 27 2021;22(1):112. doi: 10.1186/s12882-021-02314-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lenaert B, Boddez Y, Vlaeyen JWS, van Heugten CM. Learning to feel tired: A learning trajectory towards chronic fatigue. Behav Res Ther. Jan 2018;100:54–66. doi: 10.1016/j.brat.2017.11.004 [DOI] [PubMed] [Google Scholar]
- 12.Bovbjerg DH, Montgomery GH, Raptis G. Evidence for classically conditioned fatigue responses in patients receiving chemotherapy treatment for breast cancer. J Behav Med. Jun 2005;28(3):231–7. doi: 10.1007/s10865-005-4659-9 [DOI] [PubMed] [Google Scholar]
- 13.Picariello F, Moss-Morris R, Macdougall IC, Chilcot AJ. The role of psychological factors in fatigue among end-stage kidney disease patients: a critical review. Clin Kidney J. Feb 2017;10(1):79–88. doi: 10.1093/ckj/sfw113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobson J, Ju A, Baumgart A, et al. Patient Perspectives on the Meaning and Impact of Fatigue in Hemodialysis: A Systematic Review and Thematic Analysis of Qualitative Studies. Am J Kidney Dis. Aug 2019;74(2):179–192. doi: 10.1053/j.ajkd.2019.01.034 [DOI] [PubMed] [Google Scholar]
- 15.Sklar A, Newman N, Scott R, Semenyuk L, Schultz J, Fiacco V. Identification of factors responsible for postdialysis fatigue. Am J Kidney Dis. Sep 1999;34(3):464–70. doi: 10.1016/s0272-6386(99)70073-9 [DOI] [PubMed] [Google Scholar]
- 16.Sklar AH, Beezhold DH, Newman N, Hendrickson T, Dreisbach AW. Postdialysis fatigue: lack of effect of a biocompatible membrane. Am J Kidney Dis. Jun 1998;31(6):1007–10. doi: 10.1053/ajkd.1998.v31.pm9631846 [DOI] [PubMed] [Google Scholar]
- 17.Sklar AH, Riesenberg LA, Silber AK, Ahmed W, Ali A. Postdialysis fatigue. Am J Kidney Dis. Nov 1996;28(5):732–6. doi: 10.1016/s0272-6386(96)90256-5 [DOI] [PubMed] [Google Scholar]
- 18.Gordon PL, Doyle JW, Johansen KL. Postdialysis fatigue is associated with sedentary behavior. Clin Nephrol. May 2011;75(5):426–33. [PubMed] [Google Scholar]
- 19.Dubin RF, Teerlink JR, Schiller NB, Alokozai D, Peralta CA, Johansen KL. Association of segmental wall motion abnormalities occurring during hemodialysis with post-dialysis fatigue. Nephrol Dial Transplant. Oct 2013;28(10):2580–5. doi: 10.1093/ndt/gft097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guerraoui A, Prezelin-Reydit M, Kolko A, et al. Patient-reported outcome measures in hemodialysis patients: results of the first multicenter cross-sectional ePROMs study in France. BMC Nephrol. Oct 30 2021;22(1):357. doi: 10.1186/s12882-021-02551-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zu Y, Lu X, Yu Q, Yu L, Li H, Wang S. Higher Postdialysis Lactic Acid Is Associated with Postdialysis Fatigue in Maintenance of Hemodialysis Patients. Blood Purif. 2020;49(5):535–541. doi: 10.1159/000505612 [DOI] [PubMed] [Google Scholar]
- 22.Bossola M, Monteburini T, Parodi E, et al. Post-dialysis fatigue: Comparison of bicarbonate hemodialysis and online hemodiafiltration. Hemodial Int. Jan 2023;27(1):55–61. doi: 10.1111/hdi.13058 [DOI] [PubMed] [Google Scholar]
- 23.Bossola M, Marzetti E, Di Stasio E, et al. Prevalence and associated variables of post-dialysis fatigue: Results of a prospective multicentre study. Nephrology (Carlton). Jun 2018;23(6):552–558. doi: 10.1111/nep.13059 [DOI] [PubMed] [Google Scholar]
- 24.Bossola M, Di Stasio E, Monteburini T, et al. Intensity, Duration, and Frequency of Post-Dialysis Fatigue in Patients on Chronic Haemodialysis. J Ren Care. Jun 2020;46(2):115–123. doi: 10.1111/jorc.12315 [DOI] [PubMed] [Google Scholar]
- 25.Lindsay RM, Heidenheim PA, Nesrallah G, Garg AX, Suri R, Daily Hemodialysis Study Group London Health Sciences C. Minutes to recovery after a hemodialysis session: a simple health-related quality of life question that is reliable, valid, and sensitive to change. Clin J Am Soc Nephrol. Sep 2006;1(5):952–9. doi: 10.2215/CJN.00040106 [DOI] [PubMed] [Google Scholar]
- 26.Brys A, Stasio ED, Lenaert B, et al. Peridialytic serum cytokine levels and their relationship with postdialysis fatigue and recovery in patients on chronic haemodialysis - A preliminary study. Cytokine. Nov 2020;135:155223. doi: 10.1016/j.cyto.2020.155223 [DOI] [PubMed] [Google Scholar]
- 27.Bossola M, Di Stasio E, Antocicco M, Silvestri P, Tazza L. Variables associated with time of recovery after hemodialysis. J Nephrol. Jul-Aug 2013;26(4):787–92. doi: 10.5301/jn.5000198 [DOI] [PubMed] [Google Scholar]
- 28.Awuah KT, Afolalu BA, Hussein UT, Raducu RR, Bekui AM, Finkelstein FO. Time to recovery after a hemodialysis session: impact of selected variables. Clin Kidney J. Dec 2013;6(6):595–8. doi: 10.1093/ckj/sft120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rayner HC, Zepel L, Fuller DS, et al. Recovery time, quality of life, and mortality in hemodialysis patients: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. Jul 2014;64(1):86–94. doi: 10.1053/j.ajkd.2014.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davenport A, Guirguis A, Almond M, et al. Postdialysis recovery time is extended in patients with greater self-reported depression screening questionnaire scores. Hemodial Int. Jul 2018;22(3):369–376. doi: 10.1111/hdi.12642 [DOI] [PubMed] [Google Scholar]
- 31.Hussein WF, Arramreddy R, Sun SJ, Reiterman M, Schiller B. Higher Ultrafiltration Rate Is Associated with Longer Dialysis Recovery Time in Patients Undergoing Conventional Hemodialysis. Am J Nephrol. 2017;46(1):3–10. doi: 10.1159/000476076 [DOI] [PubMed] [Google Scholar]
- 32.Brys ADH, Stifft F, Van Heugten CM, Bossola M, Gambaro G, Lenaert B. Unraveling Fatigue in Hemodialysis Patients: Comparing Retrospective Reports to Real-Time Assessments With an mHealth Experienced Sampling Method. J Pain Symptom Manage. Dec 2020;60(6):1100–1108 e2. doi: 10.1016/j.jpainsymman.2020.06.042 [DOI] [PubMed] [Google Scholar]
- 33.Dreisbach AW, Hendrickson T, Beezhold D, Riesenberg LA, Sklar AH. Elevated levels of tumor necrosis factor alpha in postdialysis fatigue. Int J Artif Organs. Feb 1998;21(2):83–6. [PubMed] [Google Scholar]
- 34.Matura LA, Malone S, Jaime-Lara R, Riegel B. A Systematic Review of Biological Mechanisms of Fatigue in Chronic Illness. Biol Res Nurs. Jul 2018;20(4):410–421. doi: 10.1177/1099800418764326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sang GL, Kovithavongs C, Ulan R, Kjellstrand CM. Sodium ramping in hemodialysis: a study of beneficial and adverse effects. Am J Kidney Dis. May 1997;29(5):669–77. doi: 10.1016/s0272-6386(97)90118-9 [DOI] [PubMed] [Google Scholar]
- 36.Sadowski RH, Allred EN, Jabs K. Sodium modeling ameliorates intradialytic and interdialytic symptoms in young hemodialysis patients. J Am Soc Nephrol. Nov 1993;4(5):1192–8. doi: 10.1681/ASN.V451192 [DOI] [PubMed] [Google Scholar]
- 37.Levin A, Goldstein MB. The benefits and side effects of ramped hypertonic sodium dialysis. J Am Soc Nephrol. Feb 1996;7(2):242–6. doi: 10.1681/ASN.V72242 [DOI] [PubMed] [Google Scholar]
- 38.Basile C, Miller JD, Koles ZJ, Grace M, Ulan RA. The effects of dialysis on brain water and EEG in stable chronic uremia. Am J Kidney Dis. Jun 1987;9(6):462–9. doi: 10.1016/s0272-6386(87)80072-0 [DOI] [PubMed] [Google Scholar]
- 39.Bossola M, Di Stasio E, Monteburini T, et al. Recovery Time after Hemodialysis Is Inversely Associated with the Ultrafiltration Rate. Blood Purif. 2019;47(1-3):45–51. doi: 10.1159/000492919 [DOI] [PubMed] [Google Scholar]
- 40.Elsayed MM, Zeid MM, Hamza OMR, Elkholy NM. Dialysis recovery time: associated factors and its association with quality of life of hemodialysis patients. BMC Nephrol. Sep 1 2022;23(1):298. doi: 10.1186/s12882-022-02926-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.MacEwen C, Sutherland S, Daly J, Pugh C, Tarassenko L. Relationship between Hypotension and Cerebral Ischemia during Hemodialysis. J Am Soc Nephrol. Aug 2017;28(8):2511–2520. doi: 10.1681/ASN.2016060704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seong EY, Zheng Y, Winkelmayer WC, Montez-Rath ME, Chang TI. The Relationship between Intradialytic Hypotension and Hospitalized Mesenteric Ischemia: A Case-Control Study. Clin J Am Soc Nephrol. Oct 8 2018;13(10):1517–1525. doi: 10.2215/CJN.13891217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ozen N, Cepken T, Tosun B. Do biochemical parameters and intradialytic symptoms affect post-dialysis recovery time? A prospective, descriptive study. Ther Apher Dial. Dec 2021;25(6):899–907. doi: 10.1111/1744-9987.13624 [DOI] [PubMed] [Google Scholar]
- 44.Deferrari G, Garibotto G, Robaudo C, Ghiggeri GM, Tizianello A. Brain metabolism of amino acids and ammonia in patients with chronic renal insufficiency. Kidney Int. Oct 1981;20(4):505–10. doi: 10.1038/ki.1981.168 [DOI] [PubMed] [Google Scholar]
- 45.Blomstrand E. Amino acids and central fatigue. Amino Acids. 2001;20(1):25–34. doi: 10.1007/s007260170063 [DOI] [PubMed] [Google Scholar]
- 46.Debnath S, Lorenzo C, Bansal S, et al. Branched-Chain Amino Acids Depletion during Hemodialysis Is Associated with Fatigue. Am J Nephrol. 2020;51(7):565–571. doi: 10.1159/000507839 [DOI] [PubMed] [Google Scholar]
- 47.Lopes GB, Silva LF, Pinto GB, et al. Patient’s response to a simple question on recovery after hemodialysis session strongly associated with scores of comprehensive tools for quality of life and depression symptoms. Qual Life Res. Oct 2014;23(8):2247–56. doi: 10.1007/s11136-014-0666-z [DOI] [PubMed] [Google Scholar]
- 48.Brys ADH, Stifft F, Van Heugten CM, Bossola M, Gambaro G, Lenaert B. mHealth-based experience sampling method to identify fatigue in the context of daily life in haemodialysis patients. Clin Kidney J. Jan 2021;14(1):245–254. doi: 10.1093/ckj/sfaa124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bossola M, Di Stasio E, Sirolli V, et al. Prevalence and Severity of Postdialysis Fatigue Are Higher in Patients on Chronic Hemodialysis With Functional Disability. Ther Apher Dial. Dec 2018;22(6):635–640. doi: 10.1111/1744-9987.12705 [DOI] [PubMed] [Google Scholar]
- 50.O’Sullivan D, McCarthy G. An exploration of the relationship between fatigue and physical functioning in patients with end stage renal disease receiving haemodialysis. J Clin Nurs. Nov 2007;16(11C):276–84. doi: 10.1111/j.1365-2702.2007.01965.x [DOI] [PubMed] [Google Scholar]
- 51.Bossola M, Picca A, Monteburini T, et al. Post-dialysis fatigue and survival in patients on chronic hemodialysis. J Nephrol. Dec 2021;34(6):2163–2165. doi: 10.1007/s40620-021-01141-8 [DOI] [PubMed] [Google Scholar]
- 52.Stewart WK, Fleming LW, Manuel MA. Benefits obtained by the use of high sodium dialysate during maintenance haemodialysis. Proc Eur Dial Transplant Assoc. 1972;9:111–8. [PubMed] [Google Scholar]
- 53.Van Stone JC, Cook J. Decreased postdialysis fatigue with increased dialysate sodium concentration. Proc Clin Dial Transplant Forum. Nov 18-20 1978;8:152–6. [PubMed] [Google Scholar]
- 54.Ogden DA. A double blind crossover comparison of high and low sodium dialysis. Proc Clin Dial Transplant Forum. Nov 18-20 1978;8:157–65. [PubMed] [Google Scholar]
- 55.Redaelli B, Sforzini S, Bonoldi G, et al. Hemodialysis with “adequate” sodium concentration in dialysate. Int J Artif Organs. May 1979;2(3):133–40. [PubMed] [Google Scholar]
- 56.Shimizu AG, Taylor DW, Sackett DL, et al. Reducing patient morbidity from high-efficiency hemodialysis: a double-blind crossover trial. Trans Am Soc Artif Intern Organs. 1983;29:666–8. [PubMed] [Google Scholar]
- 57.Daugirdas JT, Al-Kudsi RR, Ing TS, Norusis MJ. A double-blind evaluation of sodium gradient hemodialysis. Am J Nephrol. 1985;5(3):163–8. doi: 10.1159/000166927 [DOI] [PubMed] [Google Scholar]
- 58.Barre PE, Brunelle G, Gascon-Barre M. A randomized double blind trial of dialysate sodiums of 145 mEq/L, 150 mEq/L, and 155 mEq/L. ASAIO Trans. Jul-Sep 1988;34(3):338–41. [PubMed] [Google Scholar]
- 59.Basile C, Giordano R, Vernaglione L, et al. Efficacy and safety of haemodialysis treatment with the Hemocontrol biofeedback system: a prospective medium-term study. Nephrol Dial Transplant. Feb 2001;16(2):328–34. doi: 10.1093/ndt/16.2.328 [DOI] [PubMed] [Google Scholar]
- 60.Raju SF, White AR, Barnes TT, Smith PP, Kirchner KA. Improvement in disequilibrium symptoms during dialysis with low glucose dialyzate. Clin Nephrol. Sep 1982;18(3):126–9. [PubMed] [Google Scholar]
- 61.Leski M, Niethammer T, Wyss T. Glucose-enriched dialysate and tolerance to maintenance hemodialysis. Nephron. 1979;24(6):271–3. doi: 10.1159/000181734 [DOI] [PubMed] [Google Scholar]
- 62.Raimann JG, Kruse A, Thijssen S, et al. Fatigue in hemodialysis patients with and without diabetes: results from a randomized controlled trial of two glucose-containing dialysates. Diabetes Care. Sep 2010;33(9):e121. doi: 10.2337/dc10-1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Azar AT. Effect of dialysate temperature on hemodynamic stability among hemodialysis patients. Saudi J Kidney Dis Transpl. Jul 2009;20(4):596–603. [PubMed] [Google Scholar]
- 64.Teruel JL, Martins J, Merino JL, et al. [Temperature dialysate and hemodialysis tolerance]. Nefrologia. 2006;26(4):461–8. Temperatura del bano y tolerancia a la hemodialisis. [PubMed] [Google Scholar]
- 65.Sajadi M, Gholami Z, Hekmatpou D, Soltani P, Haghverdi F. Cold Dialysis Solution for Hemodialysis Patients With Fatigue: a Cross-over Study. Iran J Kidney Dis. Sep 2016;10(5):319–324. [PubMed] [Google Scholar]
- 66.Ayoub A, Finlayson M. Effect of cool temperature dialysate on the quality and patients’ perception of haemodialysis. Nephrol Dial Transplant. Jan 2004;19(1):190–4. doi: 10.1093/ndt/gfg512 [DOI] [PubMed] [Google Scholar]
- 67.My Twc. Personalised cooler dialysate for patients receiving maintenance haemodialysis (MyTEMP): a pragmatic, cluster-randomised trial. Lancet. Nov 12 2022;400(10364):1693–1703. doi: 10.1016/S0140-6736(22)01805-0 [DOI] [PubMed] [Google Scholar]
- 68.Jaber BL, Lee Y, Collins AJ, et al. Effect of daily hemodialysis on depressive symptoms and postdialysis recovery time: interim report from the FREEDOM (Following Rehabilitation, Economics and Everyday-Dialysis Outcome Measurements) Study. Am J Kidney Dis. Sep 2010;56(3):531–9. doi: 10.1053/j.ajkd.2010.04.019 [DOI] [PubMed] [Google Scholar]
- 69.Maduell F, Navarro V, Torregrosa E, et al. Change from three times a week on-line hemodiafiltration to short daily on-line hemodiafiltration. Kidney Int. Jul 2003;64(1):305–13. doi: 10.1046/j.1523-1755.2003.00043.x [DOI] [PubMed] [Google Scholar]
- 70.Garg AX, Suri RS, Eggers P, et al. Patients receiving frequent hemodialysis have better health-related quality of life compared to patients receiving conventional hemodialysis. Kidney Int. Mar 2017;91(3):746–754. doi: 10.1016/j.kint.2016.10.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Davenport A, Guirguis A, Almond M, et al. Comparison of characteristics of centers practicing incremental vs. conventional approaches to hemodialysis delivery - postdialysis recovery time and patient survival. Hemodial Int. Jul 2019;23(3):288–296. doi: 10.1111/hdi.12743 [DOI] [PubMed] [Google Scholar]
- 72.Smith JR, Zimmer N, Bell E, Francq BG, McConnachie A, Mactier R. A Randomized, Single-Blind, Crossover Trial of Recovery Time in High-Flux Hemodialysis and Hemodiafiltration. Am J Kidney Dis. Jun 2017;69(6):762–770. doi: 10.1053/j.ajkd.2016.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Karkar A, Abdelrahman M, Locatelli F. A Randomized Trial on Health-Related Patient Satisfaction Level with High-Efficiency Online Hemodiafiltration versus High-Flux Dialysis. Blood Purif. 2015;40(1):84–91. doi: 10.1159/000381255 [DOI] [PubMed] [Google Scholar]
- 74.Bolton S, Gair R, Nilsson LG, Matthews M, Stewart L, McCullagh N. Clinical Assessment of Dialysis Recovery Time and Symptom Burden: Impact of Switching Hemodialysis Therapy Mode. Patient Relat Outcome Meas. 2021;12:315–321. doi: 10.2147/PROM.S325016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mehrotra R, Cukor D, Unruh M, et al. Comparative Efficacy of Therapies for Treatment of Depression for Patients Undergoing Maintenance Hemodialysis: A Randomized Clinical Trial. Ann Intern Med. Mar 19 2019;170(6):369–379. doi: 10.7326/M18-2229 [DOI] [PubMed] [Google Scholar]
- 76.Picariello F, Moss-Morris R, Norton S, et al. Feasibility Trial of Cognitive Behavioral Therapy for Fatigue in Hemodialysis (BReF Intervention). J Pain Symptom Manage. Jun 2021;61(6):1234–1246 e5. doi: 10.1016/j.jpainsymman.2020.10.005 [DOI] [PubMed] [Google Scholar]
- 77.Roumelioti ME, Steel JL, Yabes J, et al. Rationale and design of technology assisted stepped collaborative care intervention to improve patient-centered outcomes in hemodialysis patients (TACcare trial). Contemp Clin Trials. Oct 2018;73:81–91. doi: 10.1016/j.cct.2018.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Grigoriou SS, Krase AA, Karatzaferi C, et al. Long-term intradialytic hybrid exercise training on fatigue symptoms in patients receiving hemodialysis therapy. Int Urol Nephrol. Apr 2021;53(4):771–784. doi: 10.1007/s11255-020-02711-8 [DOI] [PubMed] [Google Scholar]
- 79.Devagourou A, Sharma KK, Yadav RK, Gupta VP, Kalaivani M. An experimental study to evaluate the effect of low-intensity intradialytic exercises on serum urea, creatinine, and fatigue of chronic kidney disease patients undergoing hemodialysis. Saudi J Kidney Dis Transpl. Sep-Oct 2021;32(5):1253–1259. doi: 10.4103/1319-2442.344744 [DOI] [PubMed] [Google Scholar]
- 80.Malagoni AM, Catizone L, Mandini S, et al. Acute and long-term effects of an exercise program for dialysis patients prescribed in hospital and performed at home. J Nephrol. Nov-Dec 2008;21(6):871–8. [PubMed] [Google Scholar]
- 81.Parker K, Bennett PN, Tayler C, Lee C, MacRae J. Reasons for Nonparticipation in a Sustained Hemodialysis Intradialytic Exercise Program. J Ren Nutr. Jul 2021;31(4):421–426. doi: 10.1053/j.jrn.2020.11.010 [DOI] [PubMed] [Google Scholar]
- 82.Ju A, Scholes-Robertson N, Johnson DW, et al. Patient-led identification and prioritization of exercise interventions for fatigue on dialysis: a workshop report. Clin Kidney J. Mar 2021;14(3):831–839. doi: 10.1093/ckj/sfz200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Johansen KL, Finkelstein FO, Revicki DA, et al. Systematic review of the impact of erythropoiesis-stimulating agents on fatigue in dialysis patients. Nephrol Dial Transplant. Jun 2012;27(6):2418–25. doi: 10.1093/ndt/gfr697 [DOI] [PubMed] [Google Scholar]
- 84.Johansen KL, Mulligan K, Schambelan M. Anabolic effects of nandrolone decanoate in patients receiving dialysis: a randomized controlled trial. JAMA. Apr 14 1999;281(14):1275–81. doi: 10.1001/jama.281.14.1275 [DOI] [PubMed] [Google Scholar]
- 85.Wang B, Wang GH, Ding XX, et al. Effects of Sacubitril/Valsartan on resistant hypertension and myocardial work in hemodialysis patients. J Clin Hypertens (Greenwich). Mar 2022;24(3):300–308. doi: 10.1111/jch.14422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim KH, Kim TH, Kang JW, et al. Acupuncture for symptom management in hemodialysis patients: a prospective, observational pilot study. J Altern Complement Med. Aug 2011;17(8):741–8. doi: 10.1089/acm.2010.0206 [DOI] [PubMed] [Google Scholar]
- 87.Eglence R, Karatas N, Tasci S. The effect of acupressure on the level of fatigue in hemodialysis patients. Altern Ther Health Med. Nov-Dec 2013;19(6):23–31. [PubMed] [Google Scholar]
- 88.Tsai MY, Wu CH, Huang YC, et al. Treatment of intradialytic hypotension with an herbal acupoint therapy in hemodialysis patients: A randomized pilot study. Complement Ther Med. Jun 2018;38:67–73. doi: 10.1016/j.ctim.2018.04.007 [DOI] [PubMed] [Google Scholar]
- 89.Kim KH, Lee MS, Kim TH, Kang JW, Choi TY, Lee JD. Acupuncture and related interventions for symptoms of chronic kidney disease. Cochrane Database Syst Rev. Jun 28 2016;2016(6):CD009440. doi: 10.1002/14651858.CD009440.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nishioka N, Luo Y, Taniguchi T, et al. Carnitine supplements for people with chronic kidney disease requiring dialysis. Cochrane Database Syst Rev. Dec 6 2022;12(12):CD013601. doi: 10.1002/14651858.CD013601.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]


