Abstract
Background
The objective of this study is to compare the measles immunoglobulin G (IgG) and rubella IgG levels in patient groups with mild and severe COVID-19 disease and reveal the possible relationship.
Methods
This study was conducted among COVID-19-confirmed patients over 18, under 65 years of age. This study involved 75 participants- divided into two groups. The first group usually comprised asymptomatic patients who did not require hospitalization (n=43), and the second group consisted of patients who had diffuse pneumonia on thoracic CT and required hospitalization (n=32).
Results
Anti-measles and anti-rubella IgG titers were detected to be higher in the group with severe disease compared to the group with mild disease (p=0.001 and p=0.001, respectively). The analyses were repeated by taking n=27 in Group 1 and n=27 in Group 2, which were similar in terms of age, gender and number. In the analysis performed without any age difference between the groups, no significant difference was found between the two groups in terms of Anti Measles IgG antibody titers (p=0.068). However, Anti Rubella antibody titers were found to be higher in the group with severe COVID-19 disease than in those with mild disease (p=0.03). Regardless of the severity of the disease, there was a positive correlation between Anti Rubella and Anti Measles IgG antibody titers and age (p=<0.001 Spearman’s rho 0.517; p=0.008 Spearman’s rho 0.304, respectively).
Conclusion
We believe that the pre-existing Anti-Rubella IgG antibodies in the patient may increase in parallel with the patient’s viral load by recognizing the common macrodomain of SARS-CoV-2 and Rubella viruses. The common macrodomain of SARS-CoV-2 and Rubella viruses is also present in the attenuated rubella virus used in the MMR vaccine4. In this case, we predict that previously administered MMR vaccine may be protective for COVID-19 patients. disease compared to those with mild disease.
Keywords: COVID-19, measles, rubella, antibody, SARS-CoV-2, immunoglobulin G
Introduction
The clinical course of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection may be asymptomatic, or it may result in respiratory failure, pneumonia, and death.1 The world continues to witness the rapid transformation of the SARS-CoV-2 virus with the Omicron variants (BA.1, BA.2, BA.3, BA.4, and BA.5) of the SARS-CoV-2 virus. The continuous change of Omicron variants brings about considerable challenges in establishing herd immunity for SARS-CoV-2.2 However, vaccines help significantly reduce disease severity.3 The fact that COVID-19 vaccines and effective antiviral drugs are still inaccessible in middle- and low-income countries and inequality in access to vaccines increase the risk of COVID-19 infection in low-income countries, mostly in Africa, and the likelihood of the emergence of new variants of the SARS-CoV-2 virus.2,3 Studies have reported that COVID-19 infection is severe, especially in the elderly and adult population with a single comorbidity, while the disease progresses with mild symptoms in young people and the pediatric age group.4–6 Likewise, it has been stated in the literature that children are less likely to be infected in other epidemics with viruses from the same family as the SARS-CoV-2 virus, such as severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS).7 A reason for this difference may be associated with the more recent immunization of children with multiple vaccines such as Bacillus Calmette–Guérin (BCG), Diphtheria-Pertussis and Tetanus (DPT), hepatitis B, polio, and Measles-Mumps-Rubella (MMR).8 Furthermore, some vaccines, such as BCG, may provide cross-protection against other pathogens not related to Mycobacterium tuberculosis.9 The BCG vaccine has been shown to provide non-specific protection against pathogens such as Candida albicans, Staphylococcus aureus, and various respiratory viruses.10–13 The said type of immunity refers to a non-specific immune response mediated by innate immune cells such as monocytes, macrophages, and natural killer (NK) cells.14,15 In addition to the non-specific immune response present in vaccines, what makes the MMR vaccine interesting in COVID-19 infection is the similarities and identities between the SARS-CoV-2 virus and measles, mumps, and rubella viruses. It has been demonstrated that the SARS-CoV-2 virus and the rubella virus have 29% amino acid sequence identity, suggesting that they have the same protein coat in the macro domain. The region in question is also present in the attenuated rubella virus used in the MMR vaccine.16 SARS-CoV-2 spike glycoproteins are viral membrane fusion proteins with structural similarities to the fusion proteins of the measles virus. Likewise, the 30 amino acid sequence is similar between the spike glycoprotein of the SARS-CoV-2 virus and the measles fusion glycoprotein.17 Furthermore, a strong correlation was revealed between the magnitudes of T-cell responses to SARS-CoV-2 (Spike-S1 and Nucleocapsid) and MMR vaccine proteins.18 For the above-mentioned reasons, cross-reactive antibodies against the measles and rubella viruses can also be induced in COVID-19 infection. In this antibody response, it is expected that cross-protection antibodies will be induced due to the presence of identical amino acids and non-specific immune response will increase with other vaccines such as BCG due to the strong correlation between the magnitudes of T-cell responses to SARS-CoV-2 and MMR vaccine proteins.19 Due to this effect, an increase in measles and rubella IgG antibodies can be expected in the serum of COVID-19 patients. SARS-CoV-2 antibody titer values also vary depending on the patient’s viral load and the extent and magnitude of the T-cell response to the virus. The disease severity increases with the increasing viral load and magnitude of the T-cell response.20,21 Because of the amino acid identity of respiratory diseases such as measles and rubella with COVID-19 and the strong correlation between the magnitudes of T-cell responses to SARS-CoV-2 and MMR vaccine proteins, MMR vaccines may be effective in combating COVID-19, especially in low-income countries where the access to SARS-CoV-2 virus vaccines is difficult. In 1971, Merck licensed the MMR vaccine for use in the USA.22 The MMR vaccine was added to the Republic of Turkey’s Ministry of Health in 2006. The first dose of vaccine is given at the age of 1 year, and the second dose given at the age of 4–6 years.22 In our country, between 2009 and 2019, the single-dose MMR vaccination rate varied between 94–98% depending on the year, while the two-dose MMR vaccination rate varied between 85–98% depending on the year.²³ Due to the presence of identical amino acid sequences between the rubella and measles viruses and the SARS-CoV-2 virus, pre-existing rubella and measles IgG antibodies may be induced in COVID-19 patients due to cross-reactivity, and these induced antibodies can be detected to be higher in patients with severe disease than in those with less severe disease, depending on viral load, as in the SARS-CoV-2 antibody response. The objective of this study is to compare the measles immunoglobulin G (IgG) and rubella IgG levels in patient groups with mild and severe COVID-19 disease and reveal the possible relationship.
Method
Study of Design and Participants
This study is a prospective, observational, single-center case-control trial. The patients included in the study were selected from among the patients who presented to the COVID-19 outpatient clinic of Samsun University Samsun Training and Research Hospital between May 1 and September 1, 2020. Both oropharyngeal and nasopharyngeal swabs were taken from patients with any symptom and/or symptoms of COVID-19 infection, from individuals who had suspected contact with a patient diagnosed with COVID-19 infection, or from patients who would be routinely operated on before the operation. The swabs were taken to the laboratory, and the SARS-CoV-2 real-time reverse transcriptase polymerase chain reaction (rRT-PCR) test was studied using the SARS-CoV-2 RT-qPCR detection kit (Bioeksen, Istanbul, Turkey). All patients over 18, under 65 years of age, who were not pregnant, and who did not have any chronic disease with positive test results were included in the study. All diseases of the patients registered in our hospital’s automation system of the Ministry of Health of the Republic of Turkey and all previously prescribed drugs were evaluated in terms of the presence of chronic disease. The presence of chronic disease such as hypertension, diabetes mellitus, thyroid disease, hyperlipidemia, renal disease, cardiovascular disease, hematological disease, malignancies, and lung diseases such as asthma/chronic obstructive pulmonary disease was determined, and such patients were excluded from the study.
The study’s purpose and procedures were explained to each patient. After oral and written information about the study procedures was provided, the participants were asked to sign a written informed consent form for participation. Confidentiality throughout the study was ensured. Our study was performed in line with the declarations of Helsinki.
The presence of pulmonary involvement in the patients was evaluated by the thoracic computed tomography (CT) imaging method. Since the period between May 1 and September 1, 2020, when patients were selected for our study, was the first wave of the pandemic, in other words, the first period of the pandemic, the chest computerized tomography (CT) imaging method was applied to every patient with suspected COVID-19 or contact with COVID-19 in our hospital. Moreover, between these dates, hemogram, C-reactive protein (CRP), d-dimer, and ferritin tests were studied for each patient in our hospital in terms of the availability of laboratory findings, important in determining the hospitalization criteria according to the COVID-19 treatment algorithm of the Republic of Turkey Ministry of Health.
Selection of the Patient and Control Groups
The patients were divided into two subgroups according to the disease severity: asymptomatic-mild and severe COVID-19 cases. Patients with mild or asymptomatic COVID-19 infection who did not require treatment and follow-up in the hospital were classified as group 1, while patients with moderate or severe viral pneumonia consistent with COVID-19 infection on thoracic CT imaging and requiring hospitalization were classified as group 2.
Inclusion criteria for group 1 were patients whose nasopharyngeal swab samples were confirmed as positive by the SARS-CoV-2 real-time reverse transcriptase polymerase chain reaction (rRT-PCR) test, without viral pneumonia on thoracic CT or findings compatible with COVID-19 infection. Inclusion criteria for group 2 were patients with the laboratory-confirmed diagnosis of COVID-19 (with positive SARS-CoV-2 rRT-PCR test results).
Enzyme-Linked Immunosorbent Assay (ELISA)
In addition to routine blood tests, 5 cc venous blood samples were collected from each patient to detect anti-measles IgG and anti-rubella IgG antibody titers. Venous blood samples were taken from group 1 patients at the time of admission to the hospital and from group 2 patients during the first two days of hospitalization, and the blood was centrifuged at 3000 rpm for 10 minutes, separated into sera, and stored in Eppendorf tubes at −80 °C until the tests were performed. Measles and rubella IgG levels were studied with the enzyme-linked immunosorbent assay (ELISA) test kit following the manufacturer’s recommendations (Anti-Measles Virus IgG ELISA Euroimmun, Lübeck, Germany; Anti-Rubella Virus IgG ELISA Euroimmun, Lübeck, Germany). The results were quantitatively evaluated in an ELISA reader (Tecan Infinite M200 Pro, Austria) by creating a standard curve according to the absorbance and concentration values of the calibrators used in the commercial kit. The antibody results were expressed as international units per milliliter (IU/mL). According to the manufacturer’s protocol, when measles IgG titers were < 8 IU/mL, they were considered seronegative; when they were between ≥8 and <11 IU/mL, they were considered borderline; when they were ≥11 IU/mL, they were considered seropositive. According to the manufacturer’s protocol, when rubella IgG titers were < 200 IU/mL, they were accepted as seronegative; when they were between ≥200 and <275 IU/mL, they were accepted as borderline; when they were ≥275 IU/mL, they were accepted as seropositive.
Statistical Analysis
The statistical program “SPSS 20.0 (Statistical Package for the Social Sciences, SPSS Inc., Chicago, Illinois)”, “Jamovi project (2020), Jamovi (Version 1.8.1) [Computer Software] (retrieved from https://www.jamovi.org), and JASP (Version 0.14.1.0) (retrieved from https://jasp-stats.org) were used for statistical analysis. The normal distribution of numerical variables was analyzed by the Shapiro–Wilk, Kolmogorov–Smirnov, and Anderson-Darling tests. Descriptive data were presented as mean ± standard deviation (SD) and median, minimum-maximum values for continuous variables depending on their distribution. Number and percentage were employed for categorical variables. Differences between Anti Measles and Anti Rubella antibody titers in age groups were assessed by Kruskal Wallis test. Post-hoc analysis of Kruskal Wallis test was performed using Dunn test for significant.
In the comparison of two independent groups, Student’s t-test (independent samples t-test) was used when numerical variables were normally distributed. The Mann–Whitney U-test was applied for the non-normally distributed variables. To compare differences between categorical variables by groups, Pearson’s chi-square and Fisher’s exact tests were used in 2×2 tables, and the Fisher-Freeman-Halton test was used in RxC tables. All comparisons found not to be normally distributed were performed with a Spearman’s rank correlation coefficient, and the obtained rho values are presented here. Spearman correlation test was conducted to investigate whether independent variables such as age and laboratory values such as CRP, d-dimer, lymphocyte count and the median of anti-measles/rubella IgG antibody titers impact the length of hospital stay, and rho values were determined as the correlation coefficient. The level of significance (p-value) was accepted as <0.05 in all statistical analyses.
We compared the measles and rubella IgG titers of the patients enrolled in the study with the length of hospital stay for each individual in both the severe and mild disease groups. Using a one-tailed test, it was determined that a sample size of 26 per group would provide 95% power for the study, with alpha set at 0.05 and correlation value set at 0.50. The minimum required sample size was achieved in both groups (n=43 in Group 1, n=32 in Group 2). Sample sizing was performed using SAS software, version 9.2 (SAS Institute, Cary, NC).
Results
There were 1433 individuals with positive SARS-CoV-2 rRT-PCR test results from the nasopharyngeal swab samples taken from the patients who presented to our hospital between May 1, 2020, and September 1, 2020. Of the patients, 26.94% (n=386) were over 65 years of age. Of the remaining 1047 individuals, 34.4% (n=360) had an additional comorbid disease. No findings suggesting pulmonary involvement of COVID-19 disease were found on thoracic CT in 40.9% (n=281) of 687 people under 65 years of age who did not have any comorbid disease. Of these patients without pulmonary involvement, 70.8% (n=198) had two or more symptoms/signs. Of the 83 patients who had no or only one symptom, 40 refused to participate in the study. Forty-three patients who gave consent to participate in the study were accepted as the group with mild disease and were enrolled in the study.
Of 406 individuals with pulmonary involvement on thoracic CT (5.7% had mild unilateral involvement on tomography; 56.8% had bilateral mild or moderate pulmonary involvement), 152 had bilateral severe pulmonary involvement compatible with viral pneumonia on thoracic CT. Thirty-two patients who accepted to participate in the study were enrolled in the study as the second group, ie, the group with severe disease.
Comparison of the Groups’ Demographic Findings, Clinical Features, and Laboratory Values
Whereas the mean age of the group with mild disease was 34.7 ± 13.2 years, the mean age of the group with severe disease was 47.2 ± 10.6 years. The difference between them was statistically significant (p<0.001) (Table 1).
Table 1.
Demographic and Clinical Characteristics of the Study Groups
| Group 1 (n=43) | Group 2 (n=32) | P value | |
|---|---|---|---|
| Age (years) | 34.7 ± 13.2 | 47.2 ± 10.6 | <0.001* |
| Age groups | |||
| 50–65 | 8 (40) | 12 (60) | 0.117* |
| <50 | 35 (63.6) | 20 (36.4) | |
| Gender | |||
| Female | 12 (27.9) | 11 (34.4) | 0.728** |
| Male | 31 (72.1) | 21 (65.6) | |
| Presence of symptoms | 6 (14.0) | 32 (100.0) | <0.001** |
| Type of symptoms | |||
| Fever | 0 (0.0) | 24 (75.0) | 0.001** |
| Fatigue | 1 (16.7) | 5 (15.6) | 0.999** |
| Cough | 2 (33.3) | 16 (50.0) | 0.663** |
| Dyspnea | 0 (0.0) | 18 (56.2) | 0.021** |
| Diarrhea | 2 (33.3) | 5 (15.6) | 0.302** |
| Nausea and vomiting | 0 (0.0) | 4 (12.5) | 0.999** |
| Others | 1 (16.7) | 6 (18.8) | 0.999** |
| Outcome | 0.427** | ||
| Survival | 43 (100.0) | 31 (96.9) | |
| Mortality | 0 (0.0) | 1 (3.1) |
Notes: Bold numbers emphasize significant p values *independent samples t-test **Pearson’s Chi-Square, Fisher’s Exact, or Fisher-Freeman-Halton test.
Patients were categorized into five-year age groups. They were assessed for Anti Measles and Anti Rubella IgG antibody titers, regardless of COVID-19 severity.There was no statistically significant difference between age groups in terms of Anti Rubella IgG antibody titers (p=0.135). A statistically significant difference was observed between age groups in terms of Anti Measles antibody titers (p=0.002). In the post-hoc analysis, a statistically significant difference was found between the 18–23 age group and the 60–65 age group (p=0.047). (Table 2).
Table 2.
Comparison of the Anti-Measles and Anti Rubella IgG Titers Between the Age Groups
| Age Groups | n | Mean Rank | p value | |
|---|---|---|---|---|
| Measles IgG titers (IU/mL) | 18–23 | 13 | 21,38 | 0.002 |
| 24–29 | 5 | 20,00 | ||
| 30–35 | 13 | 30,04 | ||
| 36–41 | 8 | 40,56 | ||
| 42–47 | 12 | 48,21 | ||
| 48–53 | 8 | 45,44 | ||
| 54–59 | 9 | 49,00 | ||
| 60–65 | 7 | 53,43 | ||
| Rubella IgG titers (IU/mL) | 18–23 | 13 | 32,08 | 0.135 |
| 24–29 | 5 | 24,10 | ||
| 30–35 | 13 | 37,58 | ||
| 36–41 | 8 | 31,25 | ||
| 42–47 | 12 | 34,17 | ||
| 48–53 | 8 | 45,38 | ||
| 54–59 | 9 | 44,94 | ||
| 60–65 | 7 | 56,64 |
Upon classifying the patients as over and under 50 years of age, the number of patients over 50 years of age was similar between the groups (p=0.117). Sex distribution was similar between the groups (p=0.728). Table 1 contains the demographic characteristics of the patient groups included in the study.
Of the patients in the mild disease group, 86% did not have any symptoms. One patient had fatigue, two patients had only mild cough, two patients had diarrhea, and one patient had myalgia. The symptoms of the patients in this group were mild, and the symptoms improved in one to two day during the follow-up.
All patients in the severe disease group were symptomatic. The most common complaints of the patients were fever (n=24, 75%) and dyspnea (n=18, 56%). Whereas 22 patients (68.8%) were hospitalized in the ward, 10 patients (31.2%) were treated in the intensive care unit. The mean oxygen saturation value of the patients at the time of admission to the hospital was 91% (62.0–97.0), and the mean length of hospital stay of the patients in this group was ten days (5.0–21.0). Furthermore, the overall mortality rate was 3.1%. Table 1 represents the clinical characteristics of the patient groups included in the study.
While ferritin, CRP, and D-dimer values were detected to be higher in the severe disease group than in the mild disease group, lymphocyte counts were found to be lower (ferritin p=0.003, CRP p<0.001, D-dimer p<0.001, p< 0.001, respectively). The differences in laboratory values between the groups were statistically significant (Table 3).
Table 3.
Comparison of the Laboratory Investigations Between the Groups
| Parameters | Group 1 (n=43) | Group 2 (n=32) | p value |
|---|---|---|---|
| Measles IgG titers (IU/mL) | 711.0 [0.0–5000.0] | 2575.0 [97.0–5000.0] | 0.001* |
| Measles IgG | |||
| Negative | 9 (20.9) | 2 (6.2) | 0.103** |
| Positive | 34 (79.1) | 30 (93.8) | |
| Rubella IgG titers (IU/mL) | 47.0 [0.0–171.0] | 99.5 [26.0–200.0] | 0.001* |
| Rubella IgG | |||
| Negative | 3 (7.0) | 0 (0.0) | 0.256** |
| Positive | 40 (93.0) | 32 (100.0) | |
| Lymphocyte count (109/L) | 2.2 [0.9–4.6] | 1.2 [0.1–4.3] | <0.001* |
| Ferritin (ng/mL) | 106.0 [5.8–440.0] | 405.0 [33.0–2000.0] | 0.003* |
| C-reactive protein (mg/L) | 2.4 [0.3–31.0] | 44.0 [1.7–243.0] | <0.001* |
| D-Dimer (ng/mL) | 190.0 [20.0–1250.0] | 400.0 [190.0–16,600.0] | <0.001* |
Notes: Bold numbers emphasize significant p values *Mann–Whitney U-test **Pearson’s Chi-Square or Fisher’s exact test.
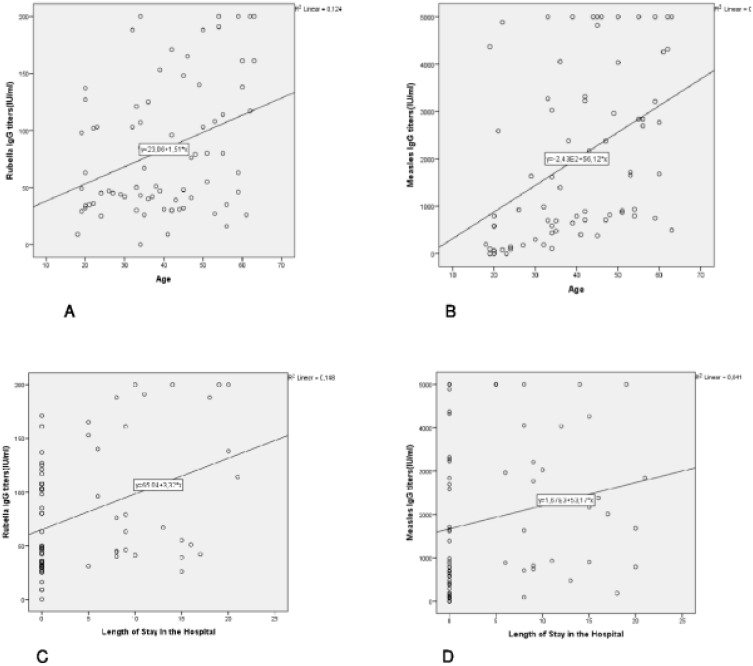
In the severe disease group, no significant correlation was revealed between the length of hospital stay and the patients’ age and laboratory values (Anti-measles and anti-rubella IgG titers, lymphocyte count, ferritin, CRP, and D-Dimer) variables (Table 4 and Figure 1).
Table 4.
Correlation Between Numerical Parameters and the Length of Hospital Stay in Group 2 Patients
| Parameters | Spearman’s rho | p value | |
|---|---|---|---|
| Length of hospital stay (days) vs. | – Age | 0.326 | 0.079 |
| – Measles IgG titers | −0.206 | 0.276 | |
| – Rubella IgG titers | 0.150 | 0.428 | |
| – Lymphocyte count | −0.044 | 0.816 | |
| – Ferritin | 0.123 | 0.617 | |
| – C-reactive protein | −0.060 | 0.753 | |
| – D-Dimer | 0.213 | 0.329 |
Figure 1.
(A) Correlation between Anti Rubella IgG and age. (B) Correlation between Anti Measles IgG and age. (C) Correlation between anti Rubella IgG and the length of hospital stay in Group 2. (D) Correlation between anti Measles IgG and the length of hospital stay in Group 2.
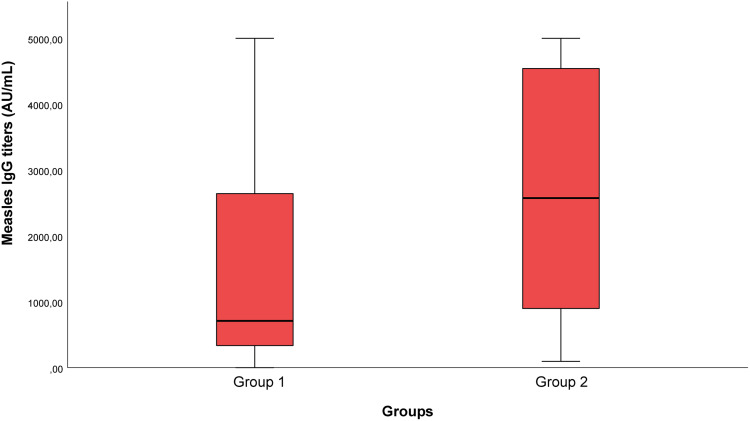
While anti-measles IgG seropositivity of the patients in the mild disease group was 79.1%, this rate was 93.8% in the severe disease group. No significant difference was revealed between the groups in terms of measles IgG seropositivity rates (p=0.103). Whereas the median values of measles IgG antibody titers were 711 IU/mL [0.0–5000.0] in the mild disease group, they were 2575 IU/mL [97.0–5000.0] in the severe disease group. The measles IgG antibody titers of the severe disease group were higher. The difference between the two groups was statistically significant (p=0.001) (Table 3 and Figure 2).
Figure 2.
Measles IgG titers in Groups 1 and 2.
When the patients were classified as over 50 years old and under 50 years old, anti-measles IgG antibody seropositivity was 100% in patients over 50 years of age, and measles antibody seropositivity was 80% in patients under 50 years of age (p=0.031). The difference between the groups was found to be statistically significant. The median value of anti-measles IgG antibody titers was 2733 IU/mL (475.0–5000.0) in patients over 50 years of age (n=20), and the median value of measles IgG antibody titers was 818 IU/mL (0.0–5000.0) in patients under 50 years of age (n=55). The difference between the groups in terms of anti-measles IgG antibody titers was significant (p=0.014). Table 5 contains the anti-measles IgG seropositivity rates and titer values of the groups over 50 years old and under 50 years of age.
Table 5.
Comparison of the Laboratory Investigations Between the Over and Under 50 Years Patient Groups
| Parameters | Group >50 Years (n=20) | Group ≤50 Years (n=55) | p value | |||
|---|---|---|---|---|---|---|
| Measles IgG titers (IU/mL) | 2733 [475.0–5000.0] | 818 [0.0–5000.0] | 0.014* | |||
| Measles IgG | ||||||
| Negative | 0 (0.0) | 11 (20.0) | 0.031** | |||
| Positive | 20 (100.0) | 44 (80.0) | ||||
| Rubella IgG titers (IU/mL) | 111 [16.0–200.0] | 48 [0.0–200.0] | 0.029* | |||
| Rubella IgG | ||||||
| Negative | 0 (0.0) | 3 (5.5) | 0.560** | |||
| Positive | 20 (100.0) | 52 (94.5) | ||||
| Group 1 (n=8) | Group 2 (n=12) | p value | Group 1 (n=35) | Group 2 (n=20) | p value | |
| Measles IgG titers (IU/mL) | 2207.50 [494.0–5000.0] | 2805.5 [748.0–5000.0] | 0.624* | 644.0 [0.0–5000.0] | 2377 [97.0–5000.0] | 0.004* |
| Rubella IgG titers (IU/mL) | 80.0 [16.0–161.0] | 149.50 [26.0–200.0] | 0.082* | 45 [0.0–171.0] | 77.50 [31.0–200.0] | 0.012* |
Notes: Bold numbers emphasize significant p value *Mann–Whitney U-test **Pearson’s Chi-Square or Fisher’s exact test.
Anti-Rubella IgG Antibody Results
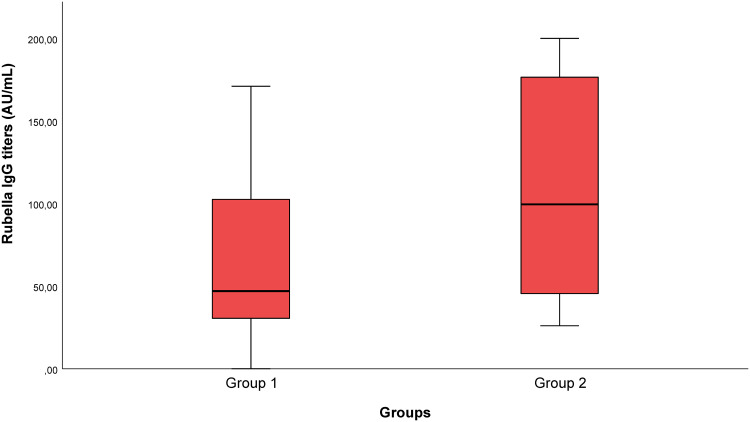
Whereas anti-rubella IgG seropositivity was 93.0% in the mild disease group, it was 100% in the severe disease group (p=0.256). While the median values of anti-rubella IgG titers were 47.0 IU/mL [0.0–171.0] in the mild disease group, they were determined as 99.5 IU/mL [26.0–200.0] in the severe disease group. The anti-rubella IgG titers of the severe disease group were higher. The difference between the two groups was statistically significant (p=0.001) (Table 3 and Figure 3).
Figure 3.
Rubella IgG titers in Groups 1 and 2.
Upon categorizing the patients as over and under 50 years of age, anti-rubella IgG seropositivity was 100% in patients over 50 years of age, and it was 94.5% in patients under 50 years of age (p=0.560). The median value of anti-rubella IgG titers in patients over 50 years of age (n=20) was 111 IU/mL (16.0–200.0), and the median value of rubella IgG titers was 48 IU/mL (0.0–200.0) in patients under 50 years of age (n=55). The difference between the groups in terms of anti-rubella IgG titers was found to be significant (p=0.029) (Table 5).
The levels of Anti Rubella and Anti Measles IgG antibodies were found to have a positive correlation with age, irrespective of the disease severity (p=<0.001, Spearman’s rho 0.517; p=0.008, Spearman’s rho 0.304, respectively). The correlation graphs illustrating the relationship between age and antibody levels can be seen in Figure 3.
Because the mean ages were not similar between the groups, a new analysis was performed to exclude the age factor. When the patients were grouped by age in 5-year periods, there was a significant difference between the 18–23 age group and the 60–65 age group in terms of Anti Measles IgG antibody titers. There were 7 patients aged 60–65. 5 of the patients had severe disease, while 2 had mild disease. There were 13 patients in the 18–23 age group, and all of the patients had mild disease. The 18–23 and 60–65 age groups were excluded from the study to determine if the difference in antibody titers between the groups could be attributed to age. The groups were similar in terms of sample size, age and gender. The minimum sample size required in our study was 26. The minimum sample size required in both groups was n=27 for Group 1 and n=27 for Group 2. The mean age of Group 1 was 40.04±9.032, and the mean age of Group 2 was 44.67±9.401. There was no statistically significant difference in terms of mean age between the groups (p=0.071). While 47.6% of Group 1 was female and 51.5% was male, 52.4% of Group 2 was female and 48.5% was male (p=0.78). After analyzing the data, no significant difference was found between the two groups in terms of Anti-Measles IgG antibody titers (p=0.068). The Anti-Measles IgG antibody titer in Group 1 was 9228 (min 109, max 5000), while it was 2373 (min 97, max 5000) in Group 2. However, the levels of Anti Rubella IgG antibodies were higher in the group with severe disease. The difference in anti-Rubella IgG antibody levels between the two groups was statistically significant (p=0.003). The median anti-Rubella IgG antibody titer value in Group 1 was 45 (range: 0 to 171), while in Group 2 it was 79 (range: 31 to 200).
Discussion
The relationship between the measles, mumps, and rubella (MMR) vaccine and COVID-19 is still not clear. There is a systematic review published to this end.1 However, the results were presented as a qualitative review since it was impossible to conduct a meta-analysis due to the heterogeneity of the control and study groups included in this research differences in results, and insufficient data availability with different study designs. In this study, a total of 11 studies, 8 of which directly mentioned the impact of the MMR vaccine on COVID-19, were included in the qualitative synthesis. A quasi-experimental study reported the positive impact of the MMR vaccine on reducing the severity of COVID-19 disease.23 A genomic data analysis study reported that rubella IgG titers increased with the increasing severity of COVID-19 disease.16 In the mentioned systematic review, data up to November 28, 2020, were analyzed, and two more controlled randomized trials investigating the relationship between COVID-19 and the MMR vaccine have been performed since this date.18,19,23 One of the controlled randomized trials stated higher measles IgG antibodies in COVID-19 patients compared to individuals who had never had the disease, ¹9 while rubella IgG titers were reported to be lower in COVID-19 patients compared to individuals who had never had the disease.24
Our study is one of the pioneering studies investigating the relationship between measles IgG and rubella IgG titers and the disease severity in which patients with comorbid diseases and older than 65 years of age among COVID-19 patients were excluded from the study and in which the pulmonary involvement of patients with thoracic CT images and thus the severity of COVID-19 disease were evaluated objectively. In the present study, we detected anti-rubella IgG titers as 47.0 IU/mL in the mild disease group and as 99.5 IU/mL in the severe disease group. The difference between the two groups was statistically significant (p=0.001). We revealed that the anti-rubella IgG titer values of the severe disease group were higher than the mild disease group. Whereas the median anti-measles IgG titer values were 711 IU/mL in the mild disease group, they were 2575 IU/mL in the severe disease group. The difference between the two groups was statistically significant (p=0.001). We detected that the anti-measles IgG titers median values of the severe disease group were higher.
Sumbul et al25 compared the rubella and measles IgG titers of COVID-19 patients and those who had never had the disease. They reported lower rubella IgG titers in COVID-19 patients in comparison with individuals who had never had the disease. However, while this study reported the mean age of the control group as 51.5 years, the mean age of the patient group was reported as 42.0 years. In the present study, when we grouped the patients as over 50 years of age and under 50 years of age, anti-measles IgG and anti-rubella IgG antibody titers were revealed to be higher in the patient group over 50 years of age than in the group under 50 years of age (p=0.014 and p=0.029, respectively). Rubella vaccines were licensed for the first time in the United States of America in 1969. Before the rubella vaccine was licensed, rubella was a common childhood disease.26 Patients older than 50 years coincided with the periods of rubella epidemics, they were more likely to be exposed to wild virus. Considering that each interaction with the wild virus could potentially boost patients’ immune response and increase antibody levels.
In the present study, when we grouped the patients as over 50 years of age and under 50 years of age, anti-measles IgG and anti-rubella IgG antibody titers were revealed to be higher in the patient group over 50 years of age than in the group under 50 years of age (p=0.014 and p=0.029, respectively).
Gold et al24 could not identify any correlation between measles and rubella antibody titers and disease severity. Similarly, we could not detect a relationship between measles antibody titers and disease severity. On the contrary, we detected higher measles and rubella antibody titers in patients with severe disease than those with mild disease. It has been stated in the literature that additional comorbid diseases increase the severity of COVID-19 disease.27,28 The study by Gold et al presented no data on the exclusion of additional comorbid diseases.29 In this study, we excluded patients with additional comorbid diseases. Thus, we attempted to exclude the factors that we thought might affect the disease severity in the study. Furthermore, the disease severity scores in this study are different from those in our study. In our scoring, each patient was evaluated with thoracic CT in terms of whether there was pulmonary involvement and the severity of pulmonary involvement.
Young et al compared the rubella IgG antibody titers of patients with severe COVID-19 infection hospitalized in the intensive care unit with the rubella IgG antibody titers of patients hospitalized in the ward with milder COVID-19 infection and identified that the rubella IgG antibody titers of patients hospitalized in the intensive care unit were higher than the group with milder disease.16 Similar to the said study, we revealed the anti-rubella IgG antibody titer values of the severe disease group to be higher compared to the mild disease group, and the difference between the two groups was statistically significant (p=0.001). Young et al16 demonstrated the presence of 29% amino acid sequence identity in the surface-exposed macrodomains of the SARS-CoV-2 and rubella virus, suggesting that this macrodomain could be recognized by antibodies developed against the rubella virus. Moreover, they presented data showing that patients with SARS-CoV2 infection had increased rubella IgG levels to a level consistent with secondary rubella infection. Similar to this study, we found that anti-Rubella antibody titers were higher in the group with severe COVID-19 disease compared to those with mild disease. Like Young et al,16 we believe that the macrodomain common to these two viruses might be recognized by antibodies previously developed against the rubella virus during SARS-CoV-2 infection.
Finally, Hassani et al compared COVID-19 patients and the control group comprising healthy individuals in terms of measles, rubella, and mumps IgG antibodies and determined higher measles IgG antibody titers in COVID-19 patients in comparison with the control group. In this study, they included patients who had recovered from COVID-19.¹9 But in our study samples were taken from these patients during their illness. It’s important to note that since the groups were different, the study may have produced different results.
The group with severe disease was older. Since this group coincided with the periods of measles and rubella epidemics, they were more likely to be exposed to wild viruses. Considering that each interaction with the wild virus could potentially boost patients’ immune response and increase antibody levels, a new group was formed without any age discrepancies, and the analyses were repeated. There was no significant difference found between the two groups in terms of Anti-Measles IgG antibody titers (p=0.068). Based on these data, it was concluded that the difference in the Anti-Measles IgG antibody titer values between the severe disease group and the mild disease group could be due to age.
However, in the analysis performed without considering age differences between the groups, higher Anti Rubella antibody titers were found in the group with severe COVID-19 disease compared to those with mild disease. The difference between the two groups was statistically significant (p=0.03). In our initial analysis, we found that the difference in Anti Rubella IgG antibody titer values between the group with severe disease and the group with mild disease was not related to age. Young et al demonstrated that there is a 29% amino acid sequence identity between the SARS-CoV-2 virus and the Rubella virus.16 This suggests that they share the same protein fold in the macro domain which is also present in the attenuated rubella virus used in the MMR vaccine.
In the severe disease group, no significant relationship was found between the duration of hospital stay and laboratory values (Anti Measles and Anti Rubella IgG titers, lymphocyte count, ferritin, CRP and D-Dimer). No correlation was found between the duration of hospital stay and Anti Measles and Anti Rubella antibody titers. However, the study was conducted during the early period of the COVID-19 pandemic in our country, when COVID-19 cases were just beginning to emerge. Patients admitted to our hospital were not discharged until their SARS-CoV-2 rRT-PCR test results were negative. Therefore, we believe that there might not be a correlation between the duration of hospital stay and antibody titers.
Since the patient’s age and comorbid diseases were not taken into account, the viral load emerges as one of the most crucial factors in determining the severity of COVID-19 disease. Han et al30 detected high SARS-CoV-2 viral loads in patients treated in the intensive care unit. A higher antigen load, in general, triggers higher antibody titers in patients.30 Our findings show that there may be a specific increase in rubella immunoglobulin G (IgG) antibody titers in COVID-19 patients. The SARS-CoV-2 viral load was not studied in our study. Nevertheless, we predict that antibody titers may be higher due to cross-reaction with rubella because of amino acid similarity. In our study, we did not take age and comorbid diseases into account when assessing disease severity. We hypothesize that the presence of pre-existing Anti Rubella IgG antibodies in the patient might increase as a secondary infection, in line with the patient’s viral load. This could happen due to the recognition of the common macrodomain of SARS-CoV-2 virus and Rubella virus, and may be detected at higher levels in the group with severe disease.
The current study has some limitations. The sample size was small in both groups with severe and mild disease. Due to the early stages of the COVID-19 pandemic, there were a limited number of patients hospitalized for severe COVID-19 pneumonia who did not have any other health conditions and were under 65 years of age in the study. Hence, the number of groups with mild and severe disease was not equal.
Titrations of mumps IgG antibodies, a component of the MMR vaccine, were not included in the study. Moreover, the vaccination history of the patients was not questioned. Therefore, the measured antibodies are likely to be due to natural infection as well as vaccination. SARS-CoV-2 PCR Ct (Threshold Cycle) values, indicating the patients’ SARS-CoV-2 viral load, could not be reached, and, therefore, a direct comparison of viral load could not be made between the severe disease group and the mild disease group. In the study, blood was collected from the patients during the COVID-19 infection, and blood was not taken before the infection.
As a result, there are studies in the literature that yield conflicting results concerning the relationship between the measles, mumps, and rubella (MMR) vaccine and the severity of COVID-19 infection, and the relationship in question has not been revealed in the literature. ³² In our study, we identified that rubella antibody titers were higher in patients with severe disease compared to individuals with mild disease. These findings suggest that previous MMR vaccination might provide protection for patients with COVID-19. Further research is required on this subject.
Acknowledgments
We would like to express our gratitude to Zeynep Karatas, who contributed to the current study, and Health Sciences University Samsun Training and Research Hospital Medical Specialization Education Board.
Funding Statement
This research received no external funding.
Ethics Approval
The present research was conducted at our hospital with the approval of the Health Sciences University Samsun Training and Research Hospital Clinical Research Ethics Committee [Date: 29.06.2020 and Decision no: 2020/15]. The study was also approved by the Ministry of Health of the Republic of Turkey.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declared no conflict of interest concerning this article.
References
- 1.Taheri Soodejani M, Basti M, Tabatabaei SM, Rajabkhah K. Measles, mumps, and rubella (MMR) vaccine and COVID-19: a systematic review. Int J Mol Epidemiol Genet. 2021;12(3):35–39. [PMC free article] [PubMed] [Google Scholar]
- 2.Araf Y, Akter F, Tang YD, et al. Omicron variant of SARS-CoV-2: genomics, transmissibility, and responses to current COVID-19 vaccines. J Med Virol. 2022;94(5):1825–1832. doi: 10.1002/jmv.27588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferré VM, Peiffer-Smadja N, Visseaux B, Descamps D, Ghosn J, Charpentier C. Omicron SARS-CoV-2 variant: what we know and what we don’t. Anaesth Crit Care Pain Med. 2022;41(1):100998. doi: 10.1016/j.accpm.2021.100998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anbarasu A, Ramaiah S, Livingstone P. Vaccine repurposing approach for preventing COVID 19: can MMR vaccines reduce morbidity and mortality? Hum Vaccin Immunother. 2020;16(9):2217–2218. doi: 10.1080/21645515.2020.1773141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145(6):1–12. doi: 10.1542/peds.2020-0702 [DOI] [PubMed] [Google Scholar]
- 6.Okyay RA, Sahin AR, Aguinada RA, Tasdogan AM. Why are children less affected by COVID19? Could there be an overlooked bacterial co-Infection? EJMO. 2020;4(1):104–105. doi: 10.14744/ejmo.2020.40743 [DOI] [Google Scholar]
- 7.Salman S, Salem ML. Routine childhood immunization may protect against COVID-19. Med Hypotheses. 2020;140:109689. doi: 10.1016/j.mehy.2020.109689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamborsky J, Kroger A, Wolfe C. Epidemiology and Prevention of Vaccine-Preventable Diseases: The Pink Book: Course Textbook. 13th ed. Washington: Public Health Foundation; 2015. [Google Scholar]
- 9.Covian C, Retamal-Diaz A, Bueno SM, Kalergis AM. Could BCG vaccination induce protective trained immunity for SARS-CoV-2? Front Immunol. 2020;11:1–7. doi: 10.3389/fimmu.2020.00970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kleinnijenhuis J, Quintin J, Preijers F, et al. Bacille CalmetteGuerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci USA. 2012;109(43):17537–17542. doi: 10.1073/pnas.1202870109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kleinnijenhuis J, Quintin J, Preijers F, et al. Long-lasting effects of BCG vaccination on both heterologous Th1/ Th17 responses and innate trained immunity. J Innate Immun. 2014;6(2):152–158. doi: 10.1159/000355628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bueno SM, González PA, Cautivo KM, et al. Protective T cell immunity against respiratory syncytial virus is efficiently induced by recombinant BCG. Proc Natl Acad Sci USA. 2008;105(52):20822–20827. doi: 10.1073/pnas.0806244105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cautivo KM, Bueno SM, Cortes CM, Wozniak A, Riedel CA, Kalergis AM. Efficient lung recruitment of respiratory syncytial virus specific Th1 cells induced by recombinant bacillus Calmette-Guerin promotes virus clearance and protects from infection. J Immunol. 2010;185(12):7633–7645. doi: 10.4049/jimmunol.0903452 [DOI] [PubMed] [Google Scholar]
- 14.Netea MG, Quintin J, Van Der Meer JW. Trained immunity: a memory for innate host defense. Cell Host Microbe. 2011;9(5):355–361. doi: 10.1016/j.chom.2011.04.006 [DOI] [PubMed] [Google Scholar]
- 15.Sanchez-Ramon S, Conejero L, Netea MG, Sancho D, o P, Subiza JL. Trained immunity-based vaccines: a new paradigm for the development of broad-spectrum anti-infectious formulations. Front Immunol. 2018;9:1–11. doi: 10.3389/fimmu.2018.02936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Young A, Neumann B, Fernandez R, et al. Homologous protein domains in SARS-CoV-2 and measles, mumps and rubella viruses: preliminary evidence that MMR vaccine might provide protection against COVID- 19. MedRxiv 2020. doi: 10.1101/2020.04.10.20053207 [DOI] [Google Scholar]
- 17.Sidiq KR, Sabir DK, Ali SM, Kodzius R. Does early childhood vaccination protect against COVID-19? Front Mol Biosci. 2020;7:120. doi: 10.3389/fmolb.2020.00120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mysore V, Cullere X, Settles ML, et al. Protective heterologous T cell immunity in COVID-19 induced by MMR and Tdap vaccine antigens. bioRxiv. 2021. doi: 10.1101/2021.05.03.441323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hassani D, Amiri MM, Maghsood F, et al. Does prior immunization with measles, mumps, and rubella vaccines contribute to the antibody response to COVID-19 antigens? Iran J Immunol. 2021;18(1):47–53. doi: 10.22034/iji.2021.87990 [DOI] [PubMed] [Google Scholar]
- 20.Jarjour NN, Masopust D, Jameson SC. T Cell Memory: understanding COVID-19. Immunity. 2021;54(1):14–18. doi: 10.1016/j.immuni.2020.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peng Y, Mentzer AJ, Liu G, et al.; Oxford Immunology Network Covid-19 Response T cell Consortium; ISARIC4C Investigators. Broad and strong memory CD4+ and CD8+ T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat Immunol. 2020;21(11):1336–1345. doi: 10.1038/s41590-020-0782-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goodson JL, Seward JF. Measles 50 years after use of measles vaccine. Infect Dis Clin North Am. 2015;29(4):725–743. doi: 10.1016/j.idc.2015.08.001 [DOI] [PubMed] [Google Scholar]
- 23.Orhon FS. An Overview of the Extended Immunization Program. Osmangazi med J. 2020;6–14. doi: 10.20515/otd.681563 [DOI] [Google Scholar]
- 24.Gold JE, Baumgartl WH, Okyay RA, et al. Analysis of measles-mumps-rubella (MMR) titers of recovered COVID-19 patients. mBio. 2020;11(6):e02628–20. doi: 10.1128/mBio.02628-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sumbul B, Sumbul HE, Okyay RA, et al. Is there a link between pre-existing antibodies acquired due to childhood vaccinations or past infections and COVID-19? A case control study. PeerJ. 2021;9:e10910. doi: 10.7717/peerj.10910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Preblud SR, Serdula MK, JA F Jr, Hinman AR. From the center for disease control. Current status of rubella in the United States, 1969-1979. J Infect Dis. 1980;142(5):776–779. doi: 10.1093/infdis/142.5.776 [DOI] [PubMed] [Google Scholar]
- 27.Itoh M, Okuno Y, Hotta H. Comparative analysis of titers of antibody against measles virus in sera of vaccinated and naturally infected Japanese individuals of different age groups. J Clin Microbiol. 2002;40(5):1733–1738. doi: 10.1128/JCM.40.5.1733-1738.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nayir T, Nazlican E, Sahin M, Kara F, Emine A. Effects of immunization program on morbidity and mortality rates of vaccine-preventable diseases in Turkey. Turk J Med Sci. 2020;50(8):1909–1915. doi: 10.3906/sag-2008-177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han M, Xu M, Zhang Y, et al. Assessing SARS-CoV-2 RNA levels and lymphocyte/T cell counts in COVID-19 patients revealed initial immune status as a major determinant of disease severity. Med Microbiol Immunol. 2020;209(6):657–668. doi: 10.1007/s00430-020-00693-z [DOI] [PMC free article] [PubMed] [Google Scholar]