Abstract
Advances in cancer diagnosis and treatment have substantially improved patient outcomes and survival in recent years. However, up to 75% of cancer patients and survivors, including those with non-central nervous system (non-CNS) cancers, suffer from “brain fog” or impairments in cognitive functions such as attention, memory, learning, and decision-making. While we recognize the impact of cancer-related cognitive impairment (CRCI), we have not fully investigated and understood the causes, mechanisms and interplays of various involving factors. Consequently, there are unmet needs in clinical oncology in assessing the risk of CRCI and managing patients and survivors with this condition in order to make informed treatment decisions and ensure the quality of life for cancer survivors. The state-of-the-art neuroimaging technologies, particularly clinical imaging modalities like magnetic resonance imaging (MRI) and positron emission tomography (PET), have been widely used to study neuroscience questions, including CRCI. However, in-depth applications of these functional and molecular imaging methods in CRCI and their clinical implementation for CRCI management are largely limited. This scoping review provides the current understanding of contributing neurological factors to CRCI and applications of the state-of-the-art multi-modal neuroimaging methods in investigating the functional and structural alterations related to CRCI. Findings from these studies and potential imaging-biomarkers of CRCI that can be used to improve the assessment and characterization of CRCI as well as to predict the risk of CRCI are also highlighted. Emerging issues and perspectives on future development and applications of neuroimaging tools to better understand CRCI and incorporate neuroimaging-based approaches to treatment decisions and patient management are discussed.
Keywords: Cancer survivors, Cognitive impairment, Brain functions, Chemotherapy, Radiation, Neuroimaging, Magnetic resonance imaging, Positron-Emission Tomography
Introduction
Progress in understanding cancer biology, the development of new drugs and targeted therapeutics, and the implementation of personalized care plans have substantially improved treatment efficacy and patient survival. The population of cancer survivors has increased to 18 million in the United States based on the 2022 report and is estimated to reach more than 22 million by 2030 [1]. However, a glaring unmet need is that current patient management and new therapeutic development often overlook and underestimate cancer and/or treatment-associated psychological and neurological impairments in patients across the course of their cancer trajectories, i.e., diagnosis, treatment, recovery, and survival [2]. It is reported that up to 75% of cancer patients experience varying degrees of cancer-related cognitive impairment (CRCI), highlighted with cognitive decline in attention, memory, learning, executive function, and decision-making [3]. These underlying neuropsychological difficulties experienced by cancer patients, commonly referred to as “brain fog” or “chemo-brain”, can disrupt patient adherence to treatment plans and limit their options to different treatments, resulting in decreased efficacy of treatments, lower quality of life during and after treatment, and eventually worse outcomes [3].
Despite such significant burdens in the clinical management of cancer patients and survivors, our understanding of the biological mechanisms of CRCI is still limited. The impact of neurological side effects from systemic treatment, especially chemotherapies, are recognized, but the causes and mechanisms are not well characterized and investigated. Interrogating intertwined contributing factors, requires systematic approaches enabled by tools capable of providing wholistic views of these conditions over the course of disease diagnosis and treatment. Worth noting is that current advancements in anti-cancer drugs and interventions primarily focus on targeting and managing primary tumors, often neglecting the systemic repercussions of treatment on the central nervous system (CNS). This oversight is partly due to the limited ability to determine whether a small amount of therapeutic agents or drugs can reach and accumulate in the brain, subsequently damaging brain tissue and its functions. The systemic treatments commonly used in chemotherapy and, most recently, immunotherapy are especially worrisome when given to patients with diverse health conditions and disease stages. This is because cancer patients, even those with non-central nervous system (non-CNS) cancers, may have a compromised blood brain barrier (BBB) due to comorbidities of pre-existing health conditions and inflammatory responses from treatment side effects [4]. The profound knowledge gap in this area, along with the increasing emphasis on personalized medicine, presents significant challenges in treating and caring for cancer patients while maintaining their quality of life, both during and years after treatment.
Currently, the diagnosis and evaluation of CRCI in oncology clinics primarily relies on self-reported symptoms from patients and sometimes by caregivers. A smaller subset of oncology patients may undergo clinical neuropsychological performance assessments to detect CRCI. Nevertheless, these tests were primarily designed for patients or individuals with neurological diseases or disorders, but not specifically for those with cancer [5].
On the other hand, there have been tremendous advances in the development and implementation of novel neuroimaging technologies in neuroscience research in the past two decades. The state-of-the-art neuroimaging modalities reveal brain structure, physiology, and functions with high spatial resolution, molecular and metabolic information as well as the changes associated with diseases and disorders. However, applications of these imaging tools in CRCI research and patient management are far behind.
In this review, we outline the current understanding on several cancer-associated effects on the brain and cognitive functions of cancer patients, focusing on non-CNS cancers, and their association with patient outcomes. We highlight examples of using clinically adoptable neuroimaging methods, including magnetic resonance imaging (MRI), x-ray computer-assisted tomography (CT), and positron emission tomography (PET), to investigate CRCI. Further applications of the current neuroimaging methods as well as the challenges and future development of this field are discussed, focusing on translating advanced imaging technologies and potential imaging biomarkers for practice-changing clinical applications in oncology.
Current understanding on contributing factors and mechanisms of CRCI
The cognitive changes and impairments in cancer patients and survivors can be classified into three categories: the effects of cancer itself, patient- and context-related vulnerabilities, and the impacts of therapeutic interventions. The onset and presentation of CRCI vary depending on physical, mental and health conditions of individual patients. Although many conditions can potentially contribute to CRCI, current research efforts and our knowledge on CRCI are focused on several specific areas.
Age and genetic predisposition
Aging and treatment-related cognitive decline share common mechanisms in cancer patients [6]. It is proposed that cancer and cancer treatment may accelerate normal aging due to increased DNA damage and reduced repair capacity (e.g., shortened telomere length) [6], which limits cognitive reserve and brain reorganization in cancer patients, as observed in older cancer populations [7]. One longitudinal study [8] revealed that older breast cancer patients treated with chemotherapy had poorer baseline cognitive reserve and more severe CRCI, particularly in processing speed, compared to those who did not receive chemotherapy and controls. In addition, age-related hormone level decline has been found to play a role in CRCI as anti-hormonal treatments can amplify hormone fluctuation-induced cognitive dysfunction in elderly cancer patients [9].
Several host germline single nucleotide polymorphisms (SNPs) are associated with CRCI [10, 11], including genes encoding apolipoprotein E (APOE), catechol-o-methyltransferase (COMT), and brain-derived neurotrophic factor (BDNF) [12]. Individuals with these specific SNPs are more susceptible to developing CRCI. Notably, the APOE ε4 genotype is a major risk factor for Alzheimer’s disease (AD) and age-related cognitive deficits. It negatively affects brain functions by altering lipid binding, boosting oxidative stress and inflammation, reducing the metabolism of neural progenitor cells, and damaging the BBB [13]. Patients with these allelic deficits in those alleles have been consistently found to be more vulnerable to CRCI or have poorer cognitive performance [14–17].
Cancer-induced neuroinflammation and treatment side effects
Most primary cancers are organ-specific, but they also affect overall health of patients, especially the immune system. Emerging evidence has shown that cancer may impair multiple cognitive functions through neuroinflammation [18, 19]. Tumor growth recruits various immune and neoplastic cells into the tissue stroma, increasing the levels of inflammatory cytokines [20]. This mechanism involves upregulated cytokines resulting from cancer-induced systemic inflammation, which can infiltrate the brain via receptor-mediated endocytosis or passive diffusion across the BBB [21]. Elevated cytokine levels activate immune cells in the meningeal and choroid plexus or stimulate inflammatory glial polarization, causing severe biochemical alterations in cognitive-functioning brain structures [22]. In cancer patients, these elevated inflammatory cytokines may disrupt the BBB and impair neural plasticity [23]. Brain-infiltrating immune cells, such as neutrophils [24], monocytes [25], can exacerbate neuroinflammation. A preclinical mouse model of pancreatic cancer cachexia, a condition of cancer-induced weight loss and metabolic disorder, showed that preventing immune cell infiltration into the brain reduces brain inflammation and disease progression, thereby improving cognitive functioning [26].
In general, all chemotherapy medications have been considered neurotoxic [27, 28]. Widely used chemotherapy agents, such as oxaliplatin and cisplatin, can enter both cancer cells and neuronal cells to form mitochondrial DNA (mtDNA) adducts in the brain [29]. Adriamycin or doxorubicin (DOX) can reduce glucose metabolism and cognition by affecting blood vessels in the hippocampus [30]. These medications mainly impact cognitive functions by suppressing neuron activities in the mitochondria [31–33]. Furthermore, combining chemotherapy and adjuvant hormone therapy has been reported to worsen CRCI [34]. A combination of chemotherapy and hormone therapy involving tamoxifen, exemestane, or anastrozole for breast cancer treatment has been linked to impair cognitive performance [35, 36]. Although the exact mechanism is still unclear, an age-dependent effect of tamoxifen on cognition and an endocrine imbalance in hypothalamo-pituitury-adrenal axis caused by decreased estrogen are thought to be contributing factors [37, 38]. Similarly, androgen deprivation therapy (ADT) has been found to negatively affect cognition in prostate cancer patients [39]. Retrospective studies suggest that reduced testosterone following ADT exposure may increase the risk of developing dementia and subsequent AD [40–42].
Targeted therapies and immunotherapies can cause CRCI due to their specific side effects. Recently, checkpoint inhibitors (CPI) targeting immune checkpoint proteins have been associated with CRCI [43, 44]. Although there are only sporadic reports, the underlying mechanism is thought to involve neuroinflammation resulting from changes in proinflammatory cytokine levels and increased microglia activation [45].
Psychological conditions
Although the exact mechanisms have not been fully defined, psychological disorders and symptoms such as depression, anxiety, insomnia, fatigue, apathy, pain, and general health-related comorbidities have been shown to contribute to CRCI [46–49]. An intriguing finding is that non-CNS cancer patients with lower treatment expectations, upon learning about potential side effects, reported lower self-assessed cognitive function and performed worse in neuropsychological tests [50]. This suggests that cognitive complaints may be more subjective, highlighting the need of more effective psychological interventions in cancer treatment [51–53].
Neuroimaging of cancer-related cognitive impairments
Various non-invasive imaging modalities, especially clinical imaging modalities in CT, MRI, ultrasound, PET, and integrated PET/MRI, have all been used for studying and visualizing brain structures and changes in normal and disease-affected conditions. These clinical imaging modalities are routinely used by cancer patients in their diagnosis, treatment assessment, and follow-up. Therefore, it is important to leverage the accessibility and capabilities of these imaging tools for investigating cancer related neurological and psychological changes. Given its exquisite soft tissue contrast and 3D deep tissue imaging at sub-millimeter resolution, along with its functional imaging capability, MRI stands out as the most powerful neuroimaging tool for obtaining morphological, physiological, and functional information. In addition, magnetic resonance spectroscopy (MRS) can provide metabolic and neurochemical information associated with cancer-induced alterations. On the other hand, PET provides highly sensitive biomarker-specific molecular imaging and metabolic information that are complementary with MRI and MRS for studying CRCI.
Cortical and volumetric changes revealed by MRI
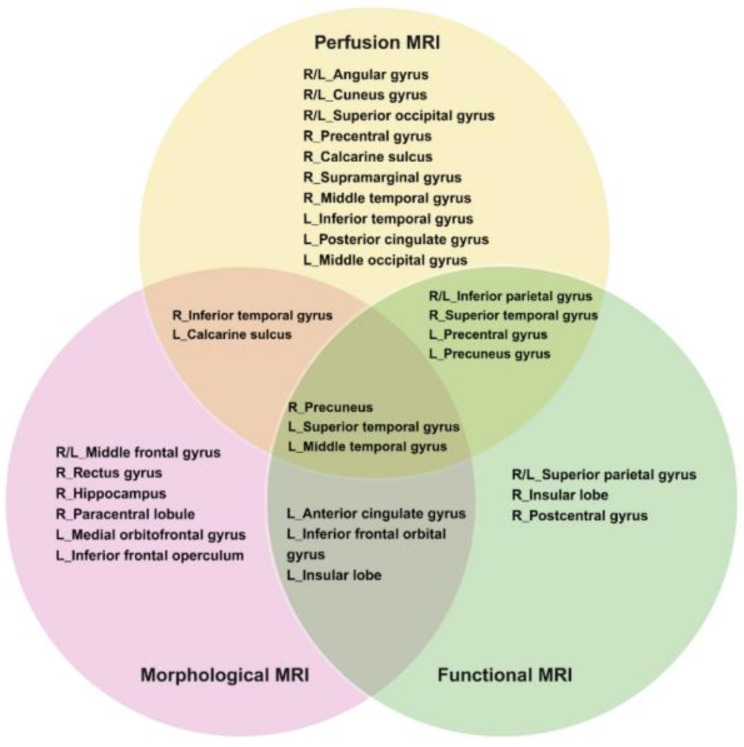
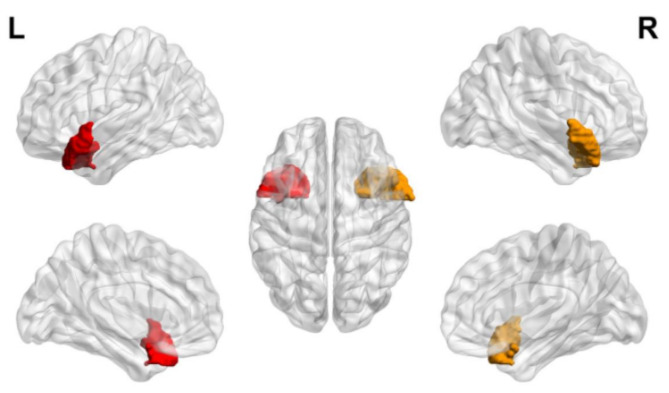
Whole-brain or regional structural alterations in the brain gray matter (GM) and white matter (WM) of cancer survivors after receiving chemotherapy can be quantified using high-resolution 3D T1-weighted imaging with the region of interest (ROI) or voxel-based morphometry (VBM) analysis. Early studies using ROI analysis found that breast cancer patients had a bilateral reduction of the hippocampi volume [54], indicating the high vulnerability of hippocampal structures to chemotherapy [55, 56]. Similar findings have been reported from subsequent VBM studies using high-resolution images to segment specific brain structures [57–65]. The cerebral volumetric loss in cancer survivors after chemotherapy is commonly associated with cognitive impairments such as attention, concentration, verbal memory, executive function, and overall function [58, 59, 62, 64, 65]. While chemo-affected brain areas differ across cancer survivors, significant structural changes are primarily found in the frontal and temporal regions of the brain. Other brain areas have also been identified in previous MRI studies [5, 66] and are illustrated in Fig. 1, including the right middle and superior frontal gyrus, parahippocampal gyrus, bilateral cerebellum, left lateral posterior parietal cortex, bilateral precuneus, left occipital cortex, sub-genual and anterior midcingulate cortex, inferior temporal gyrus, right paracingulate gyrus, left inferior frontal operculum, supramarginal gyrus, temporal area, right amygdala, caudate, and left hippocampal regions.
Fig. 1.
Alterations in Selected Brain Areas in Cancer Survivors. Brain areas that show changes in brain morphology, perfusion and activation after chemotherapy, and several brain areas reveal overlap between modalities. Morphological MRI includes diffusion and anatomical MRI examinations. Functional MRI (fMRI) includes task-related and resting-state fMRI examinations. Perfusion MRI includes arterial spin labelling (ASL) imaging. L = left, R = right Drawn based on the reference by Li & Caeyenberghs [67]. Created with BioRender.com
Studies also found that the chemo-related volumetric changes based on MRI measurements might manifest within a few months (typically one month) after cancer treatment [61, 68], which may persist for months [61, 69] or perhaps extend to years or even decades [62, 70]. These volumetric changes in the brain caused by chemotherapy may undergo a self-compensatory and reversible process [71]. Chemotherapy regimens, genes/SNPs, sex, age, hormone levels, and proinflammatory conditions can also exert such cortical structural change in chemotherapy treated cancer survivors [62, 72, 73].
Analyzing brain volumetric changes using deformation-based morphometry (DBM), another automated morphometric analysis enhanced over the standard VBM, revealed significant widespread WM enlargement and GM reduction in premenopausal breast cancer survivors after receiving chemotherapy for 5 ∼ 6 months [74]. The degree of WM expansion is age-dependent and can be correlated with the level of cognitive decline, linking this form of structural change with a protective mechanism against chemo-related neurotoxicity [74].
In addition to only measuring brain volumetric changes, changes in the cortical thickness and surface area have been found to be more sensitive imaging biomarkers for finding regional cortical deformation associated with aging and diseases. These measurements can be obtained by computing the distance between the WM boundary and the pial surface at each location of the cortex [75]. It is reported that non-CNS cancer survivors have reduced brain surface area and cortical thickness, especially in the frontal and temporal lobes [76]. For example, lung cancer survivors were found to have less cortical thickness in the areas of the frontal, temporal, and insular lobes three to eight months after chemotherapy [72]. Interestingly, although those affected areas are thought to be associated with neuropsychological performance, no statistically significant correlation between those morphological changes and cognitive functions was discovered [72, 77].
In recent years, cortical gyrification, a sensitive imaging marker in the developmental brain, has been used to identify cancer-induced cortical alterations [78, 79]. In a study of older breast cancer survivors 5 to 15 years after chemotherapy, more gyrification has been shown in the right fusiform, paracentral, precuneus, superior, middle temporal gyri, and left pars opercularis, while less gyrification was observed in the left superior parietal gyrus and left cuneus gyrus [80]. The increased right paracentral gyrification was negatively correlated with cognition composite scores for visual vocabulary and oral reading recognition based on past learning experiences [81], suggesting a long-term chemo-related cognitive impairments in older cancer survivors.
To further examine the potential mechanisms underlying the reported correlations between brain structures and cognitive performance in CRCI, more sophisticated graph theory-based network analysis has been used to identify disruptions in brain structural connectivity. It is observed that breast cancer survivors had lower small-world properties after chemotherapy compared to matched healthy controls [82]. Since altered brain topology has been associated with AD [83], breast cancer survivors having a higher risk of developing AD compared to healthy controls and chemotherapy-naïve patients [84], possibly due to declining efficiency and function in both regional and global networks.
Microscopic changes and disrupted connectivity revealed by diffusion-based MRI
Brain WM consists of a well-organized network of axon fibers or tracts that facilitates structural connections among various cortical areas, which are important for coordination of different cortical structures to perform complex cognitive tasks. Damages in WM tracts can impede neural impulse transmission in the cognitive performance [85]. In breast cancer patients, these changes can manifest as early as three months after treatment and then stabilize six months later [86].
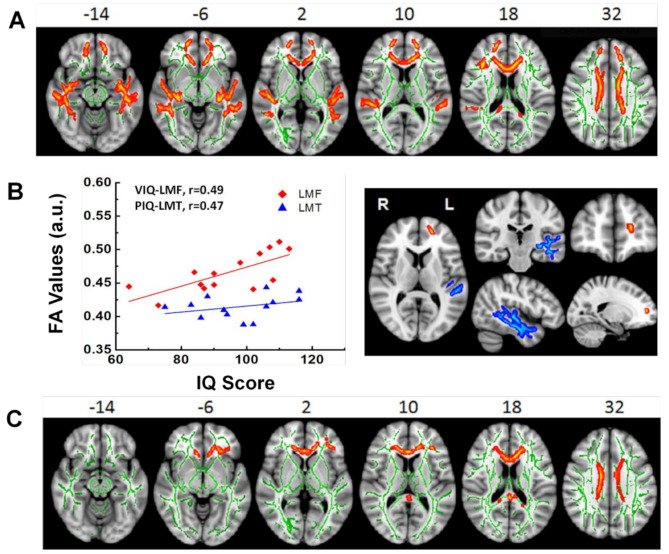
While WM hyperintensity on conventional T2-weighted imaging is the imaging feature of WM changes following chemotherapy [87], diffusion weighted imaging (DWI), and particularly diffusion tensor imaging (DTI), are employed to identify and describe the WM alterations at a microscopic level. Fractional anisotropy (FA) and diffusivity measurements (e.g., mean, radial, and axial diffusivity) are the most commonly used parameters derived from DWI and DTI to characterize the structural integrity of WM tracts. Patients who underwent chemotherapy exhibit extensive microscopic WM damages based on the measurements of these parameters [88, 89]. These WM changes mostly observed in the regions of corpus callosum, superior corona radiate, and fibers within the frontal, temporal, parietal, and occipital areas. They are coupled with increased water content within myelin and water diffusivity in axons compared with healthy controls and those cancer patients not receiving chemotherapy [90–92]. As an example shown in Fig. 2, adult individuals who survived childhood cerebellar brain tumors had regionally reduced FA, demonstrating persisting WM impairments years after treatment. These impairments were associated with disrupted cognitive performance that continues into adulthood, even decades after completing treatment during childhood [88, 89]. Although the association between WM microstructure degradation and cognitive dysfunction is well established, the results on the long-term effect over a decade or even decades following chemotherapy remain inconclusive [62, 70, 93, 94], requiring systematical and in-depth investigations in the future.
Fig. 2.
White Matter Changes in Adult Survivors of Childhood Brain Tumors. (A) Significant white matter (WM) differences of fractional anisotropy (FA) were found between survivors with and with no radiation treatment and healthy controls (HC). Clusters of significantly lower FA for survivor group were in orange and red. (B) The plot of statistically significant correlations between intellectual performance and the WM FA measured from the areas of left frontal pole (LFP) in red, right frontal pole (RFP) in blue and corpus callosum (CC) in green. The open symbols represent survivors with radiation treatment and solid symbol represent those with no radiation treatment. (C) Significant WM differences between survivors with no radiation treatment and HC. Clusters of significantly lower FA for survivor group were in orange and red. No statistically significant correlation between intellectual performance and the WM FA was found in these areas. WM skeleton (in green) was overlaid on a T1 weighted image. VIQ = verbal intelligence quotient, PIQ = performance intelligence quotient. a.u. = arbitrary units. R = right, L = left. Adopted from King et al. [88]
Additionally, graph theory-based analysis of WM-based structural network analysis has also been applied to investigate changes in structural connectivity in chemo-treated cancer patients to explain impaired memory and executive function in cancer survivors. Compared to healthy controls, breast cancer survivors who underwent chemotherapy exhibited significantly poorer cognitive performance and longer characteristic path lengths, defined as the average shortest path length between all pairs of nodes in the graph of WM tracts [91]. The prolonged path length indicates worse global integration as well as greater segregation within the structural network, which suggests a compensatory mechanism for this brain reorganization.
Altered activations and functional connectivity observed in BOLD-fMRI
Blood oxygen level-dependent functional MRI (BOLD-fMRI) detects and maps task-invoked activity during a neurophysiological event or resting state brain activation within the whole brain non-invasively. Functional connectivity (FC) analysis is usually used to study the functional coherence of anatomically separate brain regions over the whole brain space. The most popular methods for analyzing FC are the seed-based time series correlation analysis, independent component analysis (ICA), and graph theory-based analysis.
The first fMRI study on CRCI was conducted by Ferguson and colleagues [95]. They compared the activation map of working memory between a breast cancer patient 22 months after chemotherapy and her monozygotic twin sister. They found the cancer patient with CRCI exhibited increased levels of activation in the frontal and parietal regions, which was explained by the compensatory recruitment of a broader neural network to accomplish cognitive performance comparable to that of her unaffected twin. However, more cross-sectional [68, 96] and longitudinal [97] studies comparing pre- and post-treatment showed chemo-treated survivors with rather suppressed activation in regions, such as the hippocampus, precuneus, cingulate gyrus, prefrontal, premotor, and temporal cortices as shown in Fig. 3. These functional alterations are usually accompanied by worse cognitive performance [98, 99]. The reduced activation was interpreted as: (1) diminished baseline functional activation patterns caused by cancer and treatment [100]; (2) insufficient compensation for the functional damage after chemotherapy [101]; and (3) physical and psychological stresses when coping with the disease, which accelerate or worsen these cognitive dysfunctions [102].
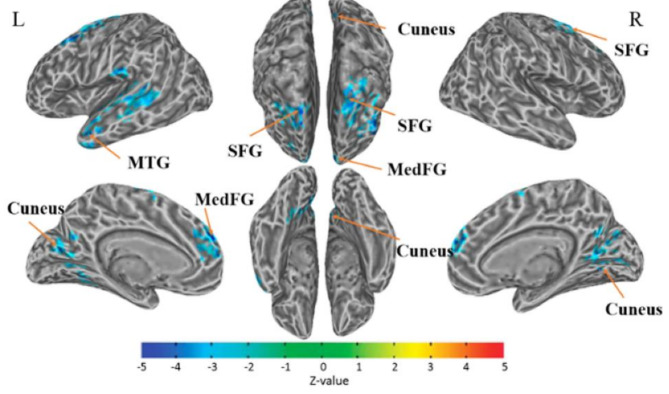
Fig. 3.
fMRI Detected Functional Connectivity Changes in Cancer Survivors. Demonstration of the lower connectivity areas with the anterior cingulate cortex (ACC) determined by the seed-based method. The color represents the degree of correlation (z-value) between functional connectivity strength. The altered connectivity areas included left superior frontal gyrus (L_SFG), left medial frontal gyrus (L_MedFG), left middle temporal gyrus (L_MTG), left cuneus, right cuneus, and right superior frontal gyrus (R_SFG). L = left hemisphere; R = right hemisphere. The threshold was set to P ≤ 0.05 (corrected with the Monte Carlo method). Adopted from Miao, et al. [103]
Regarding functional networks, FC analysis showed reduced connectivity in regions within the default mode network (DMN) [104], central executive network (CEN) [103, 105], and salience network (SN) [105] in chemo-treated cancer survivors [106, 107]. Within the DMN, altered functional activation in the precuneus has been linked to the types of cognitive impairments in CRCI, including episodic memory retrieval, visuospatial imagery, and self-referential processing [98]. The possible reason is that the functional centrality and higher metabolic demands during brain activation may render the precuneus less resistant to diseases, aging processes [108], and chemotherapy neurotoxicity [109, 110]. Another study on chemo-treated multiple myeloma patients [105] found that cognition-related frontal-parietal regions within the CEN and SN were more vulnerable to chemo-induced neurotoxicity. Furthermore, a large-scale FC analysis of breast cancer patients [111] revealed that altered FC between DMN and other subnetworks may be more sensitive to cognitive changes in the chemo-treated patients, suggesting the importance of evaluating integration and segregation of functional networks in assessing patients for possible CRCI.
In order to find cancer-related changes in topological features of the neural network such as small-worldness, modularity, centrality, and regional network parameters, the graph theory-based brain network analysis is commonly used [112]. This approach defines vertices (“nodes”) as brain regions, and connections (“edges”) as structural and functional connectivity between regions to evaluate brain network efficiency and organization based on fMRI data [113]. In this case, small-world networks have the shortest path between pairs of nodes and the fewest edges, representing high efficiency, optimized organization, and stable topology for signal processing [114, 115]. The parameter of hubness refers to nodes with high nodal centrality interconnecting nodes between different modules or in the same module extensively [116]. It is found that chemo-treated breast cancer patients have compromised small-worldness and network efficiency in the frontotemporal areas [82]. Interestingly, these functional network changes resemble those observed in AD patients [83], suggesting the higher risk of developing cognitive dysfunction or AD related dementia in those chemotherapy-treated patients compared to chemotherapy-naïve patients and healthy controls [84]. Similarly, reduced nodal and global network efficiency have been reported in breast cancer survivors with cognitive deficits five years after chemotherapy [117].
Collectively, changes in functional networks, along with structural connectivity, can serve as an indicator for early diagnosis and prediction of CRCI. Similar to the recovery of structural changes, regional FC usually can recover, in some extents, one year after chemotherapy [77, 100]. In breast cancer patients, FC recovery can be seen as early as one week after chemotherapy, which continues further six months later and even returns to baseline levels eventually [111]. However, self-reported memory deficits arise a month to one year later [61], suggesting that CRCI-related brain network changes appear late in the development of CRCI. Moreover, a study on network-based topological changes in breast cancer patients demonstrated that pre-existing neurological deficits made individuals more vulnerable to the effects of chemotherapy, other adjuvant treatment and even normal aging [118]. Therefore, it is important for oncologists to consider these new information and conditions to select proper treatments with low risks of causing CRCI.
Molecular and metabolic imaging of neurochemical changes
Neuroinflammation and neurotoxicity caused by cancer and chemotherapy can affect neurochemistry and brain metabolism, which can be investigated by various non-invasive and clinically available oncology imaging methods like PET, single-photon emission computed tomography (SPECT), and MRS [119] (Fig. 4). However, biochemical and metabolic alterations in CRCI are understudied compared to structural and functional changes reported from aforementioned MRI studies, thus presenting opportunities for future studies and clinical applications.
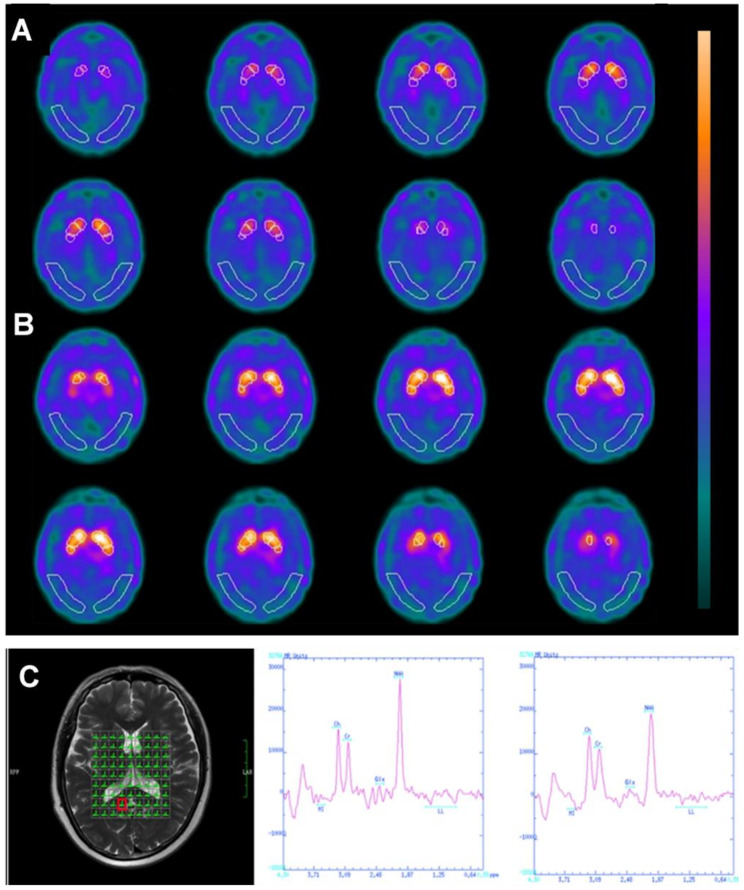
Fig. 4.
Metabolic Changes Shown in PET and MRS. Volumes of interest (VOIs) superimposed on images of a 62-year-old chemobrain patient received TRODAT-1 scan (A) and an age-matched control subject (B). Target region VOIs include the entire striatum, head of caudate nucleus, entire putamen, anterior putamen, and posterior putamen. Compared to the paired control (B), patients’ images (A) show decreased striatal uptake. Adopted from Vitor et al. [125]. (C) an example of the volumes of interest for multivoxel magnetic resonance spectroscopy (MRS) (Left) and the attained spectra (Middle and Right) from a breast cancer patient. The region of interest was placed in the right posterior cingulate cortex, and significantly lower NAA values were shown after treatment (Right) compared with before chemotherapy (Middle). Adopted from Tong et al. [126]
SPECT and PET are the most prevalent nuclear medicine techniques that use radioactive tracers or substrates to examine tissue metabolism and neurological function before and after chemotherapy [120]. 99m-Technetium-hexamethyl propylene amine oxime (99mTc-HMPAO) and 99mTc-labeled tropane derivative (99mTc-TRODAT-1) are SPECT tracers that have been used for studying CRCI. The former is a neutral lipophilic agent used to measure cerebral blood flow [121], while the latter is a dopamine transporter (DAT) radioligand applied to study neuronal degeneration [122]. 99mTc-HMPAO SPECT imaging enables the detection of chemotherapy-induced brain microvascular damage [123] and identifies diffusely heterogeneous hypoperfusion as an early imaging indication of neurotoxicity in children with leukemia [124]. Using 99mTc-TRODAT-1 imaging, a significantly reduced striatal uptake was found in breast cancer survivors with CRCI, suggesting CRCI-related neurotoxicity specific to the basal ganglia [125].
One of the most recognized physiological features of the brain is the high level of glucose metabolism driven by synaptic activity. However, post-chemotherapy lymphoma patients with CRCI have been shown to have reduced 18F-fluorodeoxyglucose (18F-FDG) uptake in cognition-related areas of prefrontal cortices, cerebellum, medial cortices, and limbic areas at resting state [127] or during performing tasks [128]. The observed reduction in glucose metabolism was negatively correlated with chemotherapy cycles and positively correlated with post-treatment time [129]. To study neuroinflammation in CRCI, probes targeting the translocator protein (TSPO) have been used [130]. TSPO is a transmembrane protein in the mitochondrial membrane that is expressed at a low level in healthy brains but substantially overexpressed when microglia are activated in neuroinflammation. Using TSPO targeted 18F-labeled N, N-diethyl-2-(2-[4-(2-fluoroethoxy)phenyl]-5,7-dimethylpyrazolo[1,5-α]pyrimidine-3-yl)acetamide (18F-DPA-714), PET-MR imaging showed that patients treated with epirubicin combined with cyclophosphamide and paclitaxel had a higher probe uptake than chemotherapy-naïve ones and healthy controls, revealing chemotherapy caused neuroinflammation [131].
The concentration of neuronal metabolites such as N-acetyl aspartate (NAA), creatine (Cr), choline (Cho), glutamate, myo-inositol (mI), lactate, and GABA can be measured by MRS on most clinical MRI scanners [132]. The lower NAA concentration in the disease-affected brain indicates losing neurons resulting from degenerations, injuries, or other damages, which can lead to reduced neuronal activity or malfunction [132]. mI is a precursor of inositol triphosphate and phosphatidylinositol, serving neuronal excitability and neurotransmitter release [133], which strongly correlates to neuroinflammation [134]. Moreover, the increase in the Cho level is an indicator of increased membrane turnover when cell membranes break down rapidly, as seen in aggressively growing tumors with high cellularity and CNS neuroinflammation [119, 135]. Breast cancer survivors exhibited elevated levels of Cho and mI in the brain after chemotherapy, which were correlated to reduced executive function and memory ability [136]. The possible explanation is that increased mI is increased to reduce inflammatory effects mediated by cytokines [137], while the increased Cho may result from the compensatory release of free choline for acetylcholine deficiency in membrane maintenance [138].
Imaging of dysfunctions in BBB and the glymphatic system
BBB is a complex structural and functional barrier composed of endothelial cells, pericytes, the basement membrane, and the astrocyte end-foot [139]. The normal BBB keeps most macromolecules and neurotoxins out of the CNS and maintains steady brain uptake of nutrition and regulatory molecules while protecting the brain and its environment from exposure to harmful substances. Systemic inflammation due to development of cancer and chemo-, radiation treatment can increase BBB permeability by disrupting BBB components and functions, allowing more chemotherapy compounds to reach the brains of cancer patients. However, the precise impact of cancer development on the BBB, as well as the specific types and treatment methods, remain unclear.
Increasing efforts have been made to non-invasively investigate the events and processes of BBB disruption using various modalities, including PET, dynamic contrast-enhanced MRI (DCE-MRI) with injection of a contrast agent, and arterial spin labeling (ASL) without using exogenous contrast agents [140] but using magnetically labeled arterial blood water protons as an endogenous tracer instead of injected exogenous contrast agents. PET assesses BBB function by measuring brain uptake of PET tracers of 11C-butanol and 15O-H2O. It has higher sensitivity, spatial resolution, and fast scan time. However, using imaging tracers with an extremely short half-life (i.e., 15O, 2 min; 11C, 20 min) limits its use in oncology clinics for assessing BBB and CRCI [141]. On the other hand, DCE or DSC MRI using blood-pool small molecule contrast agents is commonly performed on cancer patients for diagnosis and treatment monitoring. Thus, there is a growing interest and technical development effort in leveraging DCE or DSC MRI for studying BBB integrity. The parameters used for describing BBB permeability are the volume transfer constant of Ktrans obtained from analyzing DCE-MRI data and the water exchange (Kw) calculated from ASL [141]. DCE-MRI uses gadolinium-induced signal changes when blood crosses the BBB to quantify BBB permeability but is insensitive to minor and early BBB dysfunction [142]. Based on DCE-MRI-derived Ktrans, increased BBB permeability has been found in patients with advanced lung cancer without CNS metastases [4]. Moreover, observed BBB damage, as shown in Fig. 5, was associated with cognitive dysfunction of visuospatial and executive functions, and delayed recall [143]. ASL imaging is normally used for measuring blood flow in studying brain perfusion in different conditions and brain activations when blood flow increases in the activated regions. However, it can be also used to study water exchange across BBB. ASL imaging is more sensitive to minor BBB damage than DCE or DSC MRI but with lower signal-to-noise ratio [141, 144]. ASL imaging was shown to be able to reveal compensatory hyperperfusion early in chemo-treated breast cancer patients after receiving chemotherapy. The high water change rate across BBB evidenced by hyperperfusion is correlated to worse pre-treatment neuropsychological performance, suggesting chemotherapy-induced damage preferentially occurring in regions with less cognitive reserve [145].
Fig. 5.
DCE-MRI Detected BBB Disruption in Lung Cancer Patients without Brain Metastases. Compared with healthy controls, the Ktrans of bilateral temporal gyrus were increased in lung cancer patients, mainly in patients with advanced lung cancer (P = 0.042). Adopted from Zhang et al. [143]
More recently, the glymphatic system, a unique system of perivascular channels made up of astroglial cells, was discovered and shown to be responsible for waste removal from the CNS, particularly the brain. In the context of CRCI, the structure and functions of glymphatic system, as well as their changes during the courses of cancer development and treatment are new subjects of investigation for their possible roles in managing neurotoxicity and inflammatory responses induced by cancer treatment, especially chemotherapy. The impaired glymphatic system may impede the delivery of hazardous chemical waste, leading to long-term neurotoxicity and cognitive impairment in cancer patients [141].
Perspectives and conclusion
As new discoveries and advanced cancer therapies continue to improve the outcome of cancer treatment, ensuring the good quality of life for cancer survivors becomes increasingly important. However, our understanding of CRCI and approaches towards managing such conditions are still limited. The state-of-the-art neuroimaging modalities are well suited for studying CRCI in a variety of cancer populations with opportunities to expand their applications and implementation in radiology practices in the multidisciplinary care of cancer patients. In this context, their advantages and limitations need to be considered during development of novel applications for specific biological and physiological variables involved in CRCI.
Emerging evidence has demonstrated the essential roles of systemic inflammation and neurotoxicity, individual health conditions, treatment side effects, and cognitive dysfunction in alterations in brain structure, function, and metabolism of cancer patients and survivors. Various health comorbidities need to be examined for their possible roles in the development of cognitive dysfunction. Breast cancer patients with cardiovascular disease or diabetes have been found to show a higher rate of cognitive impairments than controls before and after systemic treatment [91]. It is recognized that chronic inflammation during cancer development prior to and during treatment can contribute to CRCI [32]. However, the investigation and understanding of the underlying mechanisms need to be carried out in conjunction with studying the natural history of the chronic conditions and the specific treatment applied to the individual patients for their chronic disease and cancer. Given that many cancers are more prevalent in older populations, it is of great interest to study the CRCI along with aging processes and aging-related diseases and disorders that affect these populations. It is likely that the aging-related diseases and disorders can accelerate the development and progression of CRCI and vice versa. The disease mechanisms and comorbidities shared by dementia, cancer, and aging should be considered and investigated systematically with rigorous multimodal quantitative approaches and multidisciplinary collaborations.
Future studies in CRCI should also focus on how emerging new treatments and drugs may affect patients neurologically in terms of both short- and long-term outcomes. With the increased use and success of biomarker-targeted treatments and immunotherapies, more patients will benefit from these practice-changing interventions. However, their side effects and risk of causing CRCI need to be better understood together with our improvement in understanding of neurobiology, physiology, and functions, such as the properties and changes of the BBB and glymphatic system under different conditions. Understandably, due to current limitations in proper tools and methods for in-depth investigations, there is very little knowledge and data on whether they can be affected by cancer and cancer treatment.
Furthermore, it is crucial that CRCI among cancer survivors be investigated and managed within the specific resources and experiences of patients and survivors. There are diverse ranges and types of variables that need to be taken into account, such as challenges to healthcare and basic life resources (clean air, water, housing, and energy), quality and level of education, socioeconomic status, medical insurance status, language barriers, including race or ethnicity, and social behavior and attitude towards specific demographic groups [146]. Until now, these factors and their confounding effects have rarely been considered in CRCI studies, and there have been recent calls to action to better understand social determinants of health when examining cancer outcomes [147]. Future studies including these essential variables are necessary, but the complexity and dynamic nature of social determinants of health must be taken into account at the forefront of neuroimaging literature as well. This is particularly important when considering current studies and the methods used for recruitment, the wide variety of clinical assessment tools employed for CRCI, and the limited representative normative data available for the measures despite a growing diverse and multiracial/ethnic/language society.
As we recognize the great need for better understanding complex and inter-related contributing conditions in CRCI and subjective, repeatable, and quantitative measurements of CRCI, it becomes apparent that the current available cancer imaging modalities in oncology are well poised to make a valuable impact in this area. The future development of neuroimaging methods for CRCI should be first focused on: (1) developing robust imaging protocols, especially with MRI-based approaches, such as DCE-MRI, DTI, and MRS, that can be integrated into the imaging studies in patient standard care; (2) developing a toolbox or system that uses neuroimaging measurements of CRCI with improved performance, i.e., sensitivity and specificity for diagnosis of CRCI with demonstrated validity compared to those in current clinical practice; (3) taking advantage of multimodal imaging capabilities of mapping brain structures and functions, especially PET and MRI, to cover the broad range of biological and psychological mechanisms underlying CRCI. Ultimately, building optimized multimodal neuroimaging with data fusion models for predicting CRCI at the individual level will enhance the standard of care of cancer patients and survivors.
Naturally, imaging methods can also be applied to enhance preclinical studies of the neurotoxicity and pharmacokinetics of new chemo- or immunological drugs and other novel therapeutics, especially in the context of their effects on the brain. For example, emerging new methods for BBB imaging will enable investigating BBB permeability and/or dysfunctional transcellular transport in the conditions associated with CRCI, although less attention has been given to this field currently. Thus, innovative imaging approaches with comprehensive and longitudinal research designs will help understand the dynamic progression of CRCI and establish neuroimaging-guided or informed treatment decision and clinical management in order to make a practice-changing impact on patient care in the future.
Abbreviations
- CRCI
Cancer-related cognitive impairments
- MRI
Magnetic resonance imaging
- PET
Positron emission tomography
- BBB
Blood brain barrier
- CNS
Central neurological system
- SNP
Single nucleotide polymorphism
- APOE
Apolipoprotein E
- COMT
Catechol-O-methyltransferase
- BDNF
Brain-derived neurotrophic factor
- AD
Alzheimer disease
- GABA
Gamma-aminobutyric acid
- mtDNA
The mitochondrial DNA
- ROS
Reactive oxygen species
- DOX
Doxorubicin
- ADT
Androgen deprivation therapy
- CPI
Checkpoint inhibitor
- VEGF
Vascular endothelial growth factor
- KPS
Karnofsky performance status
- PROMIS
Patient-reported outcomes measurement information system
- ICCTF
International cancer and cognition task force
- MRS
Magnetic resonance spectroscopy
- GM
Grey matter
- WM
White matter
- ROI
Region of interest
- VBM
Voxel-based morphometry
- fMRI
Functional magnetic resonance imaging
- DCE-MRI
Dynamic contrast-enhanced magnetic resonance imaging
- ASL
Arterial spin labelling
- DBM
Deformation-based morphometry
- DWI
Diffusion weighted imaging
- DTI
Diffusion tensor imaging
- ADC
Apparent diffusion constant
- FA
Fractional anisotropy
- HC
Healthy controls
- BOLD-fMRI
Blood oxygen level dependent functional MRI
- FC
Functional connectivity
- ICA
Independent component analysis
- DMN
Default mode network
- CEN
Central executive network
- SN
Salience network
- SPECT
Single-photon emission computed tomography
- 99mTc-HMPAO
Technetium-99m hexamethyl propylene amine oxime:
- 99mTc-TRODAT-1
Technetium-99m-labeled tropane derivative
- DAT
Dopamine transporter
- 18F-FDG
18 F-fluorodeoxyglucose
- TSPO
Translocator protein
- 18F-DPA-714
18 F-labeled N, N-diethyl-2-(2-[4-(2-fluoroethoxy) phenyl]-5,7-dimethylpyrazolo[1,5-α] pyrimidine-3-yl) acetamide)
- NAA
N-acetyl aspartate
- Cr
Creatine
- Cho
Choline
- mI
Myo-inositol
Author contributions
All authors contributed to the manuscript preparation and critical revisions. H. M. identified the directions and focus of the research. Q. G. and H. M. wrote the first draft of the manuscript. L.W., T.K., H.C., L.Z. and J.N. gave constructive comments on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This work is supported in part by a grant from the National Institutes of Health (R01CA169937 to HM).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Ethics approval for human subject research and consent to participate are not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Weaver KE, Forsythe LP, Reeve BB, Alfano CM, Rodriguez JL, Sabatino SA, et al. Mental and physical health-related quality of life among U.S. cancer survivors: population estimates from the 2010 National Health interview survey. Cancer Epidemiol Biomarkers Prev. 2012;21(11):2108–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henneghan AM, Van Dyk K, Kaufmann T, Harrison R, Gibbons C, Heijnen C, et al. Measuring self-reported Cancer-related cognitive impairment: recommendations from the Cancer Neuroscience Initiative Working Group. J Natl Cancer Inst. 2021;113(12):1625–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horowitz TS, Suls J, Trevino M. A call for a Neuroscience Approach to Cancer-related cognitive impairment. Trends Neurosci. 2018;41(8):493–6. [DOI] [PubMed] [Google Scholar]
- 4.Zhang DF, Ma H, Yang GJ, Zhang ZP, He YF, Feng MY, et al. Blood-brain barrier and brain structural changes in lung cancer patients with non-brain metastases. Front Oncol. 2022;12:1015011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lange M, Joly F, Vardy J, Ahles T, Dubois M, Tron L, et al. Cancer-related cognitive impairment: an update on state of the art, detection, and management strategies in cancer survivors. Ann Oncol. 2019;30(12):1925–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mandelblatt JS, Hurria A, McDonald BC, Saykin AJ, Stern RA, VanMeter JW, et al. Cognitive effects of cancer and its treatments at the intersection of aging: what do we know; what do we need to know? Semin Oncol. 2013;40(6):709–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the silver tsunami: prevalence trajectories and Comorbidity Burden among Older Cancer survivors in the United States. Cancer Epidemiol Biomarkers Prev. 2016;25(7):1029–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahles TA, Saykin AJ, McDonald BC, Li Y, Furstenberg CT, Hanscom BS, et al. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: impact of age and cognitive reserve. J Clin Oncol. 2010;28(29):4434–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu LM, Amidi A. Cognitive impairment following hormone therapy: current opinion of research in breast and prostate cancer patients. Curr Opin Support Palliat Care. 2017;11(1):38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez BD, Jim HS, Booth-Jones M, Small BJ, Sutton SK, Lin HY, et al. Course and predictors of cognitive function in patients with prostate Cancer receiving androgen-deprivation therapy: a controlled comparison. J Clin Oncol. 2015;33(18):2021–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kautiainen R, Aleksonis H, King TZ. A systematic review of host genomic variation and neuropsychological outcomes for Pediatric Cancer survivors. Neuropsychol Rev. 2023;33(1):278–306. [DOI] [PubMed] [Google Scholar]
- 12.Oppegaard KR, Armstrong TS, Anguera JA, Kober KM, Kelly DL, Laister RC, et al. Blood-based biomarkers of cancer-related cognitive impairment in non-central nervous system cancer: a scoping review. Crit Rev Oncol Hematol. 2022;180:103822. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez HR, Varma A, Flowers SA, Rebeck GW. Cancer chemotherapy related cognitive impairment and the impact of the Alzheimer’s disease risk factor APOE. Cancers (Basel). 2020;12(12):3842. [DOI] [PMC free article] [PubMed]
- 14.Harrison RA, Rao V, Kesler SR. The association of genetic polymorphisms with neuroconnectivity in breast cancer patients. Sci Rep. 2021;11(1):6169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng H, Li W, Gan C, Zhang B, Jia Q, Wang K. The COMT (rs165599) gene polymorphism contributes to chemotherapy-induced cognitive impairment in breast cancer patients. Am J Transl Res. 2016;8(11):5087–97. [PMC free article] [PubMed] [Google Scholar]
- 16.Savitz J, Solms M, Ramesar R. The molecular genetics of cognition: dopamine, COMT and BDNF. Genes Brain Behav. 2006;5(4):311–28. [DOI] [PubMed] [Google Scholar]
- 17.Small BJ, Rawson KS, Walsh E, Jim HS, Hughes TF, Iser L, et al. Catechol-O-methyltransferase genotype modulates cancer treatment-related cognitive deficits in breast cancer survivors. Cancer. 2011;117(7):1369–76. [DOI] [PubMed] [Google Scholar]
- 18.Williams AM, Shah R, Shayne M, Huston AJ, Krebs M, Murray N, et al. Associations between inflammatory markers and cognitive function in breast cancer patients receiving chemotherapy. J Neuroimmunol. 2018;314:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schagen SB, Tsvetkov AS, Compter A, Wefel JS. Cognitive adverse effects of chemotherapy and immunotherapy: are interventions within reach? Nat Rev Neurol. 2022;18(3):173–85. [DOI] [PubMed] [Google Scholar]
- 20.Seruga B, Zhang H, Bernstein LJ, Tannock IF. Cytokines and their relationship to the symptoms and outcome of cancer. Nat Rev Cancer. 2008;8(11):887–99. [DOI] [PubMed] [Google Scholar]
- 21.Wardill HR, Mander KA, Van Sebille YZ, Gibson RJ, Logan RM, Bowen JM, et al. Cytokine-mediated blood brain barrier disruption as a conduit for cancer/chemotherapy-associated neurotoxicity and cognitive dysfunction. Int J Cancer. 2016;139(12):2635–45. [DOI] [PubMed] [Google Scholar]
- 22.Dias-Carvalho A, Ferreira M, Ferreira R, Bastos ML, Sa SI, Capela JP, et al. Four decades of chemotherapy-induced cognitive dysfunction: comprehensive review of clinical, animal and in vitro studies, and insights of key initiating events. Arch Toxicol. 2022;96(1):11–78. [DOI] [PubMed] [Google Scholar]
- 23.Krasnow SM, Knoll JG, Verghese SC, Levasseur PR, Marks DL. Amplification and propagation of interleukin-1beta signaling by murine brain endothelial and glial cells. J Neuroinflammation. 2017;14(1):133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zenaro E, Pietronigro E, Della Bianca V, Piacentino G, Marongiu L, Budui S, et al. Neutrophils promote Alzheimer’s disease-like pathology and cognitive decline via LFA-1 integrin. Nat Med. 2015;21(8):880–6. [DOI] [PubMed] [Google Scholar]
- 25.Varvel NH, Neher JJ, Bosch A, Wang W, Ransohoff RM, Miller RJ, et al. Infiltrating monocytes promote brain inflammation and exacerbate neuronal damage after status epilepticus. Proc Natl Acad Sci U S A. 2016;113(38):E5665–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burfeind KG, Zhu X, Norgard MA, Levasseur PR, Huisman C, Buenafe AC, et al. Circulating myeloid cells invade the central nervous system to mediate cachexia during pancreatic cancer. Elife. 2020;9:e54095. [DOI] [PMC free article] [PubMed]
- 27.Hoeffner EG. Central Nervous System complications of oncologic therapy. Hematol Oncol Clin North Am. 2016;30(4):899–920. [DOI] [PubMed] [Google Scholar]
- 28.Briones TL, Woods J. Chemotherapy-induced cognitive impairment is associated with decreases in cell proliferation and histone modifications. BMC Neurosci. 2011;12:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zajaczkowska R, Kocot-Kepska M, Leppert W, Wrzosek A, Mika J, Wordliczek J. Mechanisms of Chemotherapy-Induced Peripheral Neuropathy. Int J Mol Sci. 2019;20(6):1451. [DOI] [PMC free article] [PubMed]
- 30.Seigers R, Timmermans J, van der Horn HJ, de Vries EF, Dierckx RA, Visser L, et al. Methotrexate reduces hippocampal blood vessel density and activates microglia in rats but does not elevate central cytokine release. Behav Brain Res. 2010;207(2):265–72. [DOI] [PubMed] [Google Scholar]
- 31.Parikh S. The neurologic manifestations of mitochondrial disease. Dev Disabil Res Rev. 2010;16(2):120–8. [DOI] [PubMed] [Google Scholar]
- 32.Wang C, Zhao Y, Wang L, Pan S, Liu Y, Li S, et al. C-phycocyanin mitigates cognitive impairment in Doxorubicin-Induced Chemobrain: impact on Neuroinflammation, oxidative stress, and Brain mitochondrial and synaptic alterations. Neurochem Res. 2021;46(2):149–58. [DOI] [PubMed] [Google Scholar]
- 33.Weymann KB, Wood LJ, Zhu X, Marks DL. A role for orexin in cytotoxic chemotherapy-induced fatigue. Brain Behav Immun. 2014;37:84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wagner LI, Gray RJ, Sparano JA, Whelan TJ, Garcia SF, Yanez B, et al. Patient-reported cognitive impairment among women with early breast Cancer randomly assigned to endocrine therapy alone Versus Chemoendocrine Therapy: results from TAILORx. J Clin Oncol. 2020;38(17):1875–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schilder CM, Seynaeve C, Linn SC, Boogerd W, Beex LV, Gundy CM, et al. Self-reported cognitive functioning in postmenopausal breast cancer patients before and during endocrine treatment: findings from the neuropsychological TEAM side-study. Psychooncology. 2012;21(5):479–87. [DOI] [PubMed] [Google Scholar]
- 36.Bender CM, Merriman JD, Gentry AL, Ahrendt GM, Berga SL, Brufsky AM, et al. Patterns of change in cognitive function with anastrozole therapy. Cancer. 2015;121(15):2627–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schilder CM, Seynaeve C, Beex LV, Boogerd W, Linn SC, Gundy CM, et al. Effects of tamoxifen and exemestane on cognitive functioning of postmenopausal patients with breast cancer: results from the neuropsychological side study of the tamoxifen and exemestane adjuvant multinational trial. J Clin Oncol. 2010;28(8):1294–300. [DOI] [PubMed] [Google Scholar]
- 38.Merriman JD, Von Ah D, Miaskowski C, Aouizerat BE. Proposed mechanisms for cancer- and treatment-related cognitive changes. Semin Oncol Nurs. 2013;29(4):260–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McGinty HL, Phillips KM, Jim HS, Cessna JM, Asvat Y, Cases MG, et al. Cognitive functioning in men receiving androgen deprivation therapy for prostate cancer: a systematic review and meta-analysis. Support Care Cancer. 2014;22(8):2271–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jayadevappa R, Chhatre S, Malkowicz SB, Parikh RB, Guzzo T, Wein AJ. Association between Androgen Deprivation Therapy Use and diagnosis of dementia in men with prostate Cancer. JAMA Netw Open. 2019;2(7):e196562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Plata-Bello J, Plata-Bello A, Perez-Martin Y, Fajardo V, Concepcion-Massip T. Androgen deprivation therapy increases brain ageing. Aging. 2019;11(15):5613–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nelson CJ, Lee JS, Gamboa MC, Roth AJ. Cognitive effects of hormone therapy in men with prostate cancer: a review. Cancer. 2008;113(5):1097–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Joly F, Heutte N, Duclos B, Noal S, Leger-Hardy I, Dauchy S, et al. Prospective evaluation of the impact of antiangiogenic treatment on cognitive functions in metastatic renal Cancer. Eur Urol Focus. 2016;2(6):642–9. [DOI] [PubMed] [Google Scholar]
- 44.Ng T, Cheung YT, Ng QS, Ho HK, Chan A. Vascular endothelial growth factor inhibitors and cognitive impairment: evidence and controversies. Expert Opin Drug Saf. 2014;13(1):83–92. [DOI] [PubMed] [Google Scholar]
- 45.McGinnis GJ, Friedman D, Young KH, Torres ER, Thomas CR Jr., Gough MJ, et al. Neuroinflammatory and cognitive consequences of combined radiation and immunotherapy in a novel preclinical model. Oncotarget. 2017;8(6):9155–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vardy JL, Dhillon HM, Pond GR, Rourke SB, Bekele T, Renton C, et al. Cognitive function in patients with Colorectal Cancer who do and do not receive chemotherapy: a prospective, longitudinal, controlled study. J Clin Oncol. 2015;33(34):4085–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim YK, Na KS, Myint AM, Leonard BE. The role of pro-inflammatory cytokines in neuroinflammation, neurogenesis and the neuroendocrine system in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2016;64:277–84. [DOI] [PubMed] [Google Scholar]
- 48.Zhu B, Dong Y, Xu Z, Gompf HS, Ward SA, Xue Z, et al. Sleep disturbance induces neuroinflammation and impairment of learning and memory. Neurobiol Dis. 2012;48(3):348–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hampson JP, Zick SM, Khabir T, Wright BD, Harris RE. Altered resting brain connectivity in persistent cancer related fatigue. Neuroimage Clin. 2015;8:305–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schagen SB, Das E, Vermeulen I. Information about chemotherapy-associated cognitive problems contributes to cognitive problems in cancer patients. Psychooncology. 2012;21(10):1132–5. [DOI] [PubMed] [Google Scholar]
- 51.Biglia N, Bounous VE, Malabaila A, Palmisano D, Torta DM, D’Alonzo M, et al. Objective and self-reported cognitive dysfunction in breast cancer women treated with chemotherapy: a prospective study. Eur J Cancer Care (Engl). 2012;21(4):485–92. [DOI] [PubMed] [Google Scholar]
- 52.Castellon SA, Ganz PA, Bower JE, Petersen L, Abraham L, Greendale GA. Neurocognitive performance in breast cancer survivors exposed to adjuvant chemotherapy and tamoxifen. J Clin Exp Neuropsychol. 2004;26(7):955–69. [DOI] [PubMed] [Google Scholar]
- 53.Krolak D, Collins B, Weiss L, Harris C, Van der Jagt R. Cognitive function and its relationship to other psychosocial factors in lymphoma survivors. Support Care Cancer. 2017;25(3):905–13. [DOI] [PubMed] [Google Scholar]
- 54.Eberling JL, Wu C, Tong-Turnbeaugh R, Jagust WJ. Estrogen- and tamoxifen-associated effects on brain structure and function. NeuroImage. 2004;21(1):364–71. [DOI] [PubMed] [Google Scholar]
- 55.Ranganath C, Ritchey M. Two cortical systems for memory-guided behaviour. Nat Rev Neurosci. 2012;13(10):713–26. [DOI] [PubMed] [Google Scholar]
- 56.Poppenk J, Moscovitch M. A hippocampal marker of recollection memory ability among healthy young adults: contributions of posterior and anterior segments. Neuron. 2011;72(6):931–7. [DOI] [PubMed] [Google Scholar]
- 57.McDonald BC, Conroy SK, Ahles TA, West JD, Saykin AJ. Gray Matter reduction associated with systemic chemotherapy for breast cancer: a prospective MRI study. Breast Cancer Res Treat. 2010;123(3):819–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Amidi A, Agerbaek M, Wu LM, Pedersen AD, Mehlsen M, Clausen CR, et al. Changes in cognitive functions and cerebral grey matter and their associations with inflammatory markers, endocrine markers, and APOE genotypes in testicular cancer patients undergoing treatment. Brain Imaging Behav. 2017;11(3):769–83. [DOI] [PubMed] [Google Scholar]
- 59.McDonald BC, Conroy SK, Smith DJ, West JD, Saykin AJ. Frontal gray matter reduction after breast cancer chemotherapy and association with executive symptoms: a replication and extension study. Brain Behav Immun. 2013;30(Suppl0):S117–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jenkins V, Thwaites R, Cercignani M, Sacre S, Harrison N, Whiteley-Jones H, et al. A feasibility study exploring the role of pre-operative assessment when examining the mechanism of ‘chemo-brain’ in breast cancer patients. Springerplus. 2016;5:390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lepage C, Smith AM, Moreau J, Barlow-Krelina E, Wallis N, Collins B, et al. A prospective study of grey matter and cognitive function alterations in chemotherapy-treated breast cancer patients. Springerplus. 2014;3:444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de Ruiter MB, Reneman L, Boogerd W, Veltman DJ, Caan M, Douaud G, et al. Late effects of high-dose adjuvant chemotherapy on white and gray matter in breast cancer survivors: converging results from multimodal magnetic resonance imaging. Hum Brain Mapp. 2012;33(12):2971–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Simo M, Vaquero L, Ripolles P, Gurtubay-Antolin A, Jove J, Navarro A, et al. Longitudinal brain changes Associated with prophylactic cranial irradiation in Lung Cancer. J Thorac Oncol. 2016;11(4):475–86. [DOI] [PubMed] [Google Scholar]
- 64.de Ruiter MB, Deardorff RL, Blommaert J, Chen BT, Dumas JA, Schagen SB, et al. Brain gray matter reduction and premature brain aging after breast cancer chemotherapy: a longitudinal multicenter data pooling analysis. Brain Imaging Behav. 2023;17(5):507–18. [DOI] [PMC free article] [PubMed]
- 65.Feng Y, Zhang XD, Zheng G, Zhang LJ. Chemotherapy-induced brain changes in breast cancer survivors: evaluation with multimodality magnetic resonance imaging. Brain Imaging Behav. 2019;13(6):1799–814. [DOI] [PubMed] [Google Scholar]
- 66.Nekhlyudov L, Campbell GB, Schmitz KH, Brooks GA, Kumar AJ, Ganz PA, et al. Cancer-related impairments and functional limitations among long-term cancer survivors: gaps and opportunities for clinical practice. Cancer. 2022;128(2):222–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li M, Caeyenberghs K. Longitudinal assessment of chemotherapy-induced changes in brain and cognitive functioning: a systematic review. Neurosci Biobehav Rev. 2018;92:304–17. [DOI] [PubMed] [Google Scholar]
- 68.Correa DD, Root JC, Kryza-Lacombe M, Mehta M, Karimi S, Hensley ML, et al. Brain structure and function in patients with ovarian cancer treated with first-line chemotherapy: a pilot study. Brain Imaging Behav. 2017;11(6):1652–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Billiet T, Emsell L, Vandenbulcke M, Peeters R, Christiaens D, Leemans A, et al. Recovery from chemotherapy-induced white matter changes in young breast cancer survivors? Brain Imaging Behav. 2018;12(1):64–77. [DOI] [PubMed] [Google Scholar]
- 70.Koppelmans V, de Groot M, de Ruiter MB, Boogerd W, Seynaeve C, Vernooij MW, et al. Global and focal white matter integrity in breast cancer survivors 20 years after adjuvant chemotherapy. Hum Brain Mapp. 2014;35(3):889–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Inagaki M, Yoshikawa E, Matsuoka Y, Sugawara Y, Nakano T, Akechi T, et al. Smaller regional volumes of brain gray and white matter demonstrated in breast cancer survivors exposed to adjuvant chemotherapy. Cancer. 2007;109(1):146–56. [DOI] [PubMed] [Google Scholar]
- 72.Lv P, Ma G, Chen W, Liu R, Xin X, Lu J, et al. Brain morphological alterations and their correlation to tumor differentiation and duration in patients with lung cancer after platinum chemotherapy. Front Oncol. 2022;12:903249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Daniel E, Deng F, Patel SK, Sedrak MS, Kim H, Razavi M, et al. Cortical thinning in chemotherapy-treated older long-term breast cancer survivors. Brain Imaging Behav. 2023;17(1):66–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Blommaert J, Schroyen G, Vandenbulcke M, Radwan A, Smeets A, Peeters R, et al. Age-dependent brain volume and neuropsychological changes after chemotherapy in breast cancer patients. Hum Brain Mapp. 2019;40(17):4994–5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97(20):11050–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 1999;9(2):179–94. [DOI] [PubMed] [Google Scholar]
- 77.McDonald BC, Van Dyk K, Deardorff RL, Bailey JN, Zhai W, Carroll JE, et al. Multimodal MRI examination of structural and functional brain changes in older women with breast cancer in the first year of antiestrogen hormonal therapy. Breast Cancer Res Treat. 2022;194(1):113–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Spalthoff R, Gaser C, Nenadic I. Altered gyrification in schizophrenia and its relation to other morphometric markers. Schizophr Res. 2018;202:195–202. [DOI] [PubMed] [Google Scholar]
- 79.Madan CR. Age-related decrements in cortical gyrification: evidence from an accelerated longitudinal dataset. Eur J Neurosci. 2021;53(5):1661–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Daniel E, Deng F, Patel SK, Sedrak MS, Kim H, Razavi M, et al. Altered gyrification in chemotherapy-treated older long-term breast cancer survivors. Brain Behav. 2024;14(8):e3634. [DOI] [PMC free article] [PubMed]
- 81.Doucet GE, Hamlin N, Kruse JA, Taylor BK, Poirel N. Link between fluid/crystallized intelligence and global/local visual abilities across adulthood. Conscious Cogn. 2022;106:103429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hosseini SM, Koovakkattu D, Kesler SR. Altered small-world properties of gray matter networks in breast cancer. BMC Neurol. 2012;12:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Supekar K, Menon V, Rubin D, Musen M, Greicius MD. Network analysis of intrinsic functional brain connectivity in Alzheimer’s disease. PLoS Comput Biol. 2008;4(6):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kesler SR, Rao V, Ray WJ, Rao A, Alzheimer’s Disease Neuroimaging I. Probability of Alzheimer’s disease in breast cancer survivors based on gray-matter structural network efficiency. Alzheimers Dement (Amst). 2017;9:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201(3):637–48. [DOI] [PubMed] [Google Scholar]
- 86.Brown MS, Stemmer SM, Simon JH, Stears JC, Jones RB, Cagnoni PJ, et al. White matter disease induced by high-dose chemotherapy: longitudinal study with MR imaging and proton spectroscopy. AJNR Am J Neuroradiol. 1998;19(2):217–21. [PMC free article] [PubMed] [Google Scholar]
- 87.Stemmer SM, Stears JC, Burton BS, Jones RB, Simon JH. White matter changes in patients with breast cancer treated with high-dose chemotherapy and autologous bone marrow support. AJNR Am J Neuroradiol. 1994;15(7):1267–73. [PMC free article] [PubMed] [Google Scholar]
- 88.King TZ, Wang L, Mao H. Disruption of White Matter Integrity in Adult survivors of Childhood Brain tumors: correlates with long-term intellectual outcomes. PLoS ONE. 2015;10(7):e0131744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Na S, Li L, Crosson B, Dotson V, MacDonald TJ, Mao H, et al. White matter network topology relates to cognitive flexibility and cumulative neurological risk in adult survivors of pediatric brain tumors. Neuroimage Clin. 2018;20:485–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Deprez S, Amant F, Yigit R, Porke K, Verhoeven J, Van den Stock J, et al. Chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning in breast cancer patients. Hum Brain Mapp. 2011;32(3):480–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li TY, Chen VC, Yeh DC, Huang SL, Chen CN, Chai JW, et al. Investigation of chemotherapy-induced brain structural alterations in breast cancer patients with generalized q-sampling MRI and graph theoretical analysis. BMC Cancer. 2018;18(1):1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Simo M, Root JC, Vaquero L, Ripolles P, Jove J, Ahles T, et al. Cognitive and brain structural changes in a lung cancer population. J Thorac Oncol. 2015;10(1):38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stouten-Kemperman MM, de Ruiter MB, Koppelmans V, Boogerd W, Reneman L, Schagen SB. Neurotoxicity in breast cancer survivors >/=10 years post-treatment is dependent on treatment type. Brain Imaging Behav. 2015;9(2):275–84. [DOI] [PubMed] [Google Scholar]
- 94.Kesler SR, Watson CL, Blayney DW. Brain network alterations and vulnerability to simulated neurodegeneration in breast cancer. Neurobiol Aging. 2015;36(8):2429–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ferguson RJ, McDonald BC, Saykin AJ, Ahles TA. Brain structure and function differences in monozygotic twins: possible effects of breast cancer chemotherapy. J Clin Oncol. 2007;25(25):3866–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang L, Apple AC, Schroeder MP, Ryals AJ, Voss JL, Gitelman D, et al. Reduced prefrontal activation during working and long-term memory tasks and impaired patient-reported cognition among cancer survivors postchemotherapy compared with healthy controls. Cancer. 2016;122(2):258–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pergolizzi D, Root JC, Pan H, Silbersweig D, Stern E, Passik SD, et al. Episodic memory for visual scenes suggests compensatory brain activity in breast cancer patients: a prospective longitudinal fMRI study. Brain Imaging Behav. 2019;13(6):1674–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Saward JB, Ellis EG, Cobden AL, Caeyenberghs K. Mapping cognitive deficits in cancer patients after chemotherapy: an activation likelihood estimation meta-analysis of task-related fMRI studies. Brain Imaging Behav. 2022;16(5):2320–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.de Ruiter MB, Reneman L, Boogerd W, Veltman DJ, van Dam FS, Nederveen AJ, et al. Cerebral hyporesponsiveness and cognitive impairment 10 years after chemotherapy for breast cancer. Hum Brain Mapp. 2011;32(8):1206–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kesler SR, Adams M, Packer M, Rao V, Henneghan AM, Blayney DW, et al. Disrupted brain network functional dynamics and hyper-correlation of structural and functional connectome topology in patients with breast cancer prior to treatment. Brain Behav. 2017;7(3):e00643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.McDonald BC, Conroy SK, Ahles TA, West JD, Saykin AJ. Alterations in brain activation during working memory processing associated with breast cancer and treatment: a prospective functional magnetic resonance imaging study. J Clin Oncol. 2012;30(20):2500–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Menning S, de Ruiter MB, Veltman DJ, Koppelmans V, Kirschbaum C, Boogerd W, et al. Multimodal MRI and cognitive function in patients with breast cancer prior to adjuvant treatment–the role of fatigue. Neuroimage Clin. 2015;7:547–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Miao H, Li J, Hu S, He X, Partridge SC, Ren J, et al. Long-term cognitive impairment of breast cancer patients after chemotherapy: a functional MRI study. Eur J Radiol. 2016;85(6):1053–7. [DOI] [PubMed] [Google Scholar]
- 104.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. [DOI] [PubMed] [Google Scholar]
- 105.Correa DD, Vachha BA, Baser RE, Koch A, Wong P, Gohel S, et al. Neuroimaging and neurocognitive outcomes in older patients with multiple myeloma treated with chemotherapy and autologous stem cell transplantation. Cancers (Basel). 2023;15(18):4484. [DOI] [PMC free article] [PubMed]
- 106.Dumas JA, Makarewicz J, Schaubhut GJ, Devins R, Albert K, Dittus K, et al. Chemotherapy altered brain functional connectivity in women with breast cancer: a pilot study. Brain Imaging Behav. 2013;7(4):524–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bromis K, Gkiatis K, Karanasiou I, Matsopoulos G, Karavasilis E, Papathanasiou M, et al. Altered Brain Functional Connectivity in Small-Cell Lung Cancer patients after Chemotherapy Treatment: a resting-state fMRI study. Comput Math Methods Med. 2017;2017:1403940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Klaassens BL, van Gerven JMA, van der Grond J, de Vos F, Moller C, Rombouts S. Diminished posterior Precuneus Connectivity with the default Mode Network differentiates normal aging from Alzheimer’s Disease. Front Aging Neurosci. 2017;9:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mounier NM, Abdel-Maged AE, Wahdan SA, Gad AM, Azab SS. Chemotherapy-induced cognitive impairment (CICI): an overview of etiology and pathogenesis. Life Sci. 2020;258:118071. [DOI] [PubMed] [Google Scholar]
- 110.Kesler SR, Blayney DW. Neurotoxic effects of Anthracycline- vs nonanthracycline-based chemotherapy on cognition in breast Cancer survivors. JAMA Oncol. 2016;2(2):185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Feng Y, Wang YF, Zheng LJ, Shi Z, Huang W, Zhang LJ. Network-level functional connectivity alterations in chemotherapy treated breast cancer patients: a longitudinal resting state functional MRI study. Cancer Imaging. 2020;20(1):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Petrella JR. Use of graph theory to evaluate brain networks: a clinical tool for a small world? Radiology. 2011;259(2):317–20. [DOI] [PubMed] [Google Scholar]
- 113.Bullmore ET, Bassett DS. Brain graphs: graphical models of the human brain connectome. Annu Rev Clin Psychol. 2011;7:113–40. [DOI] [PubMed] [Google Scholar]
- 114.Sporns O, Honey CJ. Small worlds inside big brains. Proc Natl Acad Sci U S A. 2006;103(51):19219–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lago-Fernandez LF, Huerta R, Corbacho F, Siguenza JA. Fast response and temporal coherent oscillations in small-world networks. Phys Rev Lett. 2000;84(12):2758–61. [DOI] [PubMed] [Google Scholar]
- 116.Power JD, Schlaggar BL, Lessov-Schlaggar CN, Petersen SE. Evidence for hubs in human functional brain networks. Neuron. 2013;79(4):798–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bruno J, Hosseini SM, Kesler S. Altered resting state functional brain network topology in chemotherapy-treated breast cancer survivors. Neurobiol Dis. 2012;48(3):329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kesler SR, Rao A, Blayney DW, Oakley-Girvan IA, Karuturi M, Palesh O. Predicting Long-Term Cognitive Outcome following breast Cancer with pre-treatment resting state fMRI and Random Forest Machine Learning. Front Hum Neurosci. 2017;11:555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Quarantelli M. MRI/MRS in neuroinflammation: methodology and applications. Clin Transl Imaging. 2015;3(6):475–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chiaravalloti A, Filippi L, Pagani M, Schillaci O. Functional imaging of Chemobrain: usefulness of Nuclear Medicine in the fog coming after Cancer. J Nucl Med. 2023;64(4):508–14. [DOI] [PubMed] [Google Scholar]
- 121.Prosser AMJ, Tossici-Bolt L, Kipps CM. The impact of regional (99m)Tc-HMPAO single-photon-emission computed tomography (SPECT) imaging on clinician diagnostic confidence in a mixed cognitive impairment sample. Clin Radiol. 2020;75(9):714. e7- e14. [DOI] [PubMed] [Google Scholar]
- 122.Yamamoto R, Takenoshita N, Inagawa Y, Kato H, Kaneshiro K, Kamiya T, et al. Association between longitudinal changes in striatal dopamine transporter uptake and clinical features of dementia with Lewy bodies. Psychogeriatrics. 2023;23(6):1036–42. [DOI] [PubMed] [Google Scholar]
- 123.Carlson BW, Craft MA, Carlson JR, Razaq W, Deardeuff KK, Benbrook DM. Accelerated vascular aging and persistent cognitive impairment in older female breast cancer survivors. Geroscience. 2018;40(3):325–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vera P, Rohrlich P, Stievenart JL, Elmaleh M, Duval M, Bonnin F, et al. Contribution of single-photon emission computed tomography in the diagnosis and follow-up of CNS toxicity of a cytarabine-containing regimen in pediatric leukemia. J Clin Oncol. 1999;17(9):2804–10. [DOI] [PubMed] [Google Scholar]
- 125.Vitor T, Kozasa EH, Bressan RA, Lacerda SS, Campos Neto GC, Batista IR, et al. Impaired brain dopamine transporter in chemobrain patients submitted to brain SPECT imaging using the technetium-99m labeled tracer TRODAT-1. Ann Nucl Med. 2019;33(4):269–79. [DOI] [PubMed] [Google Scholar]
- 126.Tong T, Lu H, Zong J, Lv Q, Chu X. Chemotherapy-related cognitive impairment in patients with breast cancer based on MRS and DTI analysis. Breast Cancer. 2020;27(5):893–902. [DOI] [PubMed] [Google Scholar]
- 127.Schroyen G, Schramm G, Van Weehaeghe D, Leenaerts N, Vande Casteele T, Blommaert J, et al. Cerebral glucose changes after chemotherapy and their relation to long-term cognitive complaints and fatigue. Front Oncol. 2022;12:1021615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Silverman DH, Dy CJ, Castellon SA, Lai J, Pio BS, Abraham L, et al. Altered frontocortical, cerebellar, and basal ganglia activity in adjuvant-treated breast cancer survivors 5–10 years after chemotherapy. Breast Cancer Res Treat. 2007;103(3):303–11. [DOI] [PubMed] [Google Scholar]
- 129.Baudino B, D’Agata F, Caroppo P, Castellano G, Cauda S, Manfredi M, et al. The chemotherapy long-term effect on cognitive functions and brain metabolism in lymphoma patients. Q J Nucl Med Mol Imaging. 2012;56(6):559–68. [PubMed] [Google Scholar]
- 130.Filippi L, Schillaci O, Palumbo B. Neuroimaging with PET/CT in chronic traumatic encephalopathy: what nuclear medicine can do to move the field forward. Expert Rev Mol Diagn. 2022;22(2):149–56. [DOI] [PubMed] [Google Scholar]
- 131.Schroyen G, Blommaert J, van Weehaeghe D, Sleurs C, Vandenbulcke M, Dedoncker N, et al. Neuroinflammation and its Association with Cognition, neuronal markers and peripheral inflammation after chemotherapy for breast Cancer. Cancers (Basel). 2021;13(16):4198. [DOI] [PMC free article] [PubMed]
- 132.Buonocore MH, Maddock RJ. Magnetic resonance spectroscopy of the brain: a review of physical principles and technical methods. Rev Neurosci. 2015;26(6):609–32. [DOI] [PubMed] [Google Scholar]
- 133.Kesler S, Janelsins M, Koovakkattu D, Palesh O, Mustian K, Morrow G, et al. Reduced hippocampal volume and verbal memory performance associated with interleukin-6 and tumor necrosis factor-alpha levels in chemotherapy-treated breast cancer survivors. Brain Behav Immun. 2013;30(Suppl0):S109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chiappelli J, Hong LE, Wijtenburg SA, Du X, Gaston F, Kochunov P, et al. Alterations in frontal white matter neurochemistry and microstructure in schizophrenia: implications for neuroinflammation. Transl Psychiatry. 2015;5(4):e548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Huang BY, Kwock L, Castillo M, Smith JK. Association of choline levels and tumor perfusion in brain metastases assessed with proton MR spectroscopy and dynamic susceptibility contrast-enhanced perfusion weighted MRI. Technol Cancer Res Treat. 2010;9(4):327–37. [DOI] [PubMed] [Google Scholar]
- 136.Kesler SR, Watson C, Koovakkattu D, Lee C, O’Hara R, Mahaffey ML, et al. Elevated prefrontal myo-inositol and choline following breast cancer chemotherapy. Brain Imaging Behav. 2013;7(4):501–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Arefhosseini S, Roshanravan N, Asghari S, Tutunchi H, Ebrahimi-Mameghani M. Expression of inflammatory genes, WBC-derived inflammatory biomarkers and liver function indices: effects of myo-inositol supplementation in obese patients with NAFLD. J Funct Foods. 2023;104:105524.
- 138.Haroon E, Chen X, Li Z, Patel T, Woolwine BJ, Hu XP, et al. Increased inflammation and brain glutamate define a subtype of depression with decreased regional homogeneity, impaired network integrity, and anhedonia. Transl Psychiatry. 2018;8(1):189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Knox EG, Aburto MR, Clarke G, Cryan JF, O’Driscoll CM. The blood-brain barrier in aging and neurodegeneration. Mol Psychiatry. 2022;27(6):2659–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Harris WJ, Asselin MC, Hinz R, Parkes LM, Allan S, Schiessl I, et al. In vivo methods for imaging blood-brain barrier function and dysfunction. Eur J Nucl Med Mol Imaging. 2023;50(4):1051–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Moyaert P, Padrela BE, Morgan CA, Petr J, Versijpt J, Barkhof F, et al. Imaging blood-brain barrier dysfunction: a state-of-the-art review from a clinical perspective. Front Aging Neurosci. 2023;15:1132077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Varatharaj A, Liljeroth M, Darekar A, Larsson HBW, Galea I, Cramer SP. Blood-brain barrier permeability measured using dynamic contrast-enhanced magnetic resonance imaging: a validation study. J Physiol. 2019;597(3):699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhang DF, Li ZH, Zhang ZP, He YF, Shang BL, Xu XF, et al. Cognitive changes are associated with increased blood-brain barrier leakage in non-brain metastases lung cancer patients. Brain Imaging Behav. 2023;17(1):90–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Telischak NA, Detre JA, Zaharchuk G. Arterial spin labeling MRI: clinical applications in the brain. J Magn Reson Imaging. 2015;41(5):1165–80. [DOI] [PubMed] [Google Scholar]
- 145.Nudelman KN, Wang Y, McDonald BC, Conroy SK, Smith DJ, West JD, et al. Altered cerebral blood flow one month after systemic chemotherapy for breast cancer: a prospective study using pulsed arterial spin labeling MRI perfusion. PLoS ONE. 2014;9(5):e96713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Franco-Rocha OY, Lewis KA, Longoria KD, De La Torre Schutz A, Wright ML, Kesler SR. Cancer-related cognitive impairment in racial and ethnic minority groups: a scoping review. J Cancer Res Clin Oncol. 2023;149(13):12561–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Peterson RK, King TZ. A systematic review of pediatric neuropsychological outcomes with proton versus photon radiation therapy: a call for equity in access to treatment. J Int Neuropsychol Soc. 2023;29(8):798–811. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.