Abstract
Background
Post-stroke delirium affects between 24% and 43% of patients, and negatively impacts patient outcomes. Recently, research attention has been on preventive interventions for delirium, with melatonin receptor agonists and orexin receptor antagonists reported to be effective in preventing delirium in intensive care unit patients. However, the efficacy of these agents in preventing post-stroke delirium remain unclear. This study examined the efficacy of ramelteon, suvorexant, and lemborexant in preventing post-stroke delirium symptoms in patients with stroke.
Methods
A retrospective survey of medical records was conducted for patients with stroke aged > 75 years at Kyoto University Hospital from October 2021 to March 2023. Patients who received ramelteon, suvorexant, or lemborexant on admission and the following day were classified into the consecutive administration group, whereas those who did not were classified into the non-consecutive administration group. The primary outcome was an increase in the number of positive items in the delirium screening tool over 7 days.
Results
Of the 104 patients, 33 and 71 were in the consecutive and non-consecutive administration groups, respectively. Fewer patients in the consecutive administration group had an increase in the number of positive items than in the other group (6% vs. 21%). Patients in the consecutive administration group significantly less often had an increase in the number of positive items in the delirium screening tool (P = 0.05; hazard ratio, 0.27; 95% confidence interval, 0.10–0.75).
Conclusions
This study revealed that early administration of a melatonin receptor agonist or orexin receptor antagonists may effectively prevent post-stroke delirium in older patients.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40780-024-00397-z.
Keywords: Stroke, Delirium, Melatonin receptor agonist, Orexin receptor antagonist
Background
Stroke is the second leading cause of death worldwides, as well as the third leading cause of death and disability combined [1]. Furthermore, this condition is one of the major causes of death in older adult patients, with nearly one-third of all strokes occurring in patients aged 75 years and older [2, 3], and more than half of those patients experiencing reduced mobility [3]. Therefore, with an aging population, the treatment of stroke in older individuals is increasingly vital. In the pharmacological treatment of stroke, initiating antiplatelet therapy with aspirin within 48 h of onset is effective in improving long-term outcomes [4–6]. Furthermore, initiating rehabilitation as early as possible, particularly intensive rehabilitation within 24 h of onset, significantly improves functional disabilities [7]. Consequently, prompt initiating of both pharmacological treatment and rehabilitation following stroke onset is essential for improving outcomes.
Stroke is a common cause of delirium [8]. The incidence of delirium in the acute phase following stroke ranges from 24 to 43% of cases [9–14], with a meta-analysis utilizing a random-effects approach placing the rate at 26% (95% confidence interval [CI]: 19–33%) [9]. Delirium is an independent risk factor for death and the later onset of dementia [15]. It also presents a patient safety concern, as it can lead to dangerous behaviors and falls, while also interfering with adherence to treatment of the underlying disease [16] and rehabilitation. These may hamper early initiation of pharmacotherapy and rehabilitation after stroke onset, resulting in worsening outcomes. Therefore, it is crucial to implement appropriate prophylaxis and interventions for delirium promptly.
In a randomized controlled trial and a retrospective observational study for patients in intensive care units, ramelteon (a melatonin receptor agonist) [17], suvorexant [18], and lemborexant [19] (orexin receptor antagonists) administered nightly to older patients admitted for acute care were reported to prevent the onset of delirium in patients. However, these reports included various diseases, such as infection, heart failure, and stroke, and the effectiveness of these sleep medications in patients with stroke has not been verified. In daily practice, these medications are commonly utilized for sleep management following the onset of stroke because approximately 20 to 40% of patients with stroke have sleep-wake disorders [20]. We determined that it is possible to investigate the effects of ramelteon, suvorexant, and lemborexant on post-stroke delirium in older patients based on the medical records. In this study, we retrospectively investigated the effects of these three kinds of sleep medications on prevention of delirium symptoms.
Methods
Patients
Patients with stroke, aged 75 years or older, admitted for acute care to the Department of Neurology, Kyoto University Hospital between October 2021 and March 2023 were retrospectively analyzed. The patients followed up to one week after admission. Patients who were transferred to another hospital, died within a week of admission, or could not be evaluated due to communication difficulties arising from stroke-related symptoms, such as impaired consciousness, were excluded.
Patients were categorized into two groups: a consecutive administration group and a non-consecutive administration group. The consecutive administration group consisted of patients who received ramelteon, suvorexant, and lemborexant on the day of admission and the following before sleep, because of complaints of insomnia. In contrast, the non-consecutive administration group consisted of patients who were not treated with a melatonin receptor agonist or orexin receptor antagonist on admission or the following day. Previous studies have highlighted the importance of initiating treatment within 48 h of stroke onset [4–6], making it essential to investigate the effect of early intervention on preventing delirium. Additionally, prolonged use of hypnotics is associated with an increased risk of adverse events, such as falls, suggesting the importance of evaluating their short-term efficacy. Therefore, in this study, patients were divided into these two groups based on a 2-day drug administration period. The consecutive administration group also included patients who continued using hypnotics for more than 3 days depanding on their sleep status.
Assessment of delirium
Delirium was assessed by doctors or nurses using the Delirium Screening Tool (DST) [21, 22], which is based on the Delirium Rating Scale (DRS) [23]. The DST has a sensitivity of 98% and has been verified as a useful screening tool. The DST comprises 11 items, belonging to the following categories: (A) level of consciousness, arousal, and environmental awareness (seven items), (B) change in cognition (two items), and (C) change in symptoms (two items). If patients were positive for any item in category A, then patients were evaluated for category B items. If patients were positive for any item in category B, then they were evaluated for category C items. In this study, we investigated an increase in the number of items from category A, which all patients were evaluated for, including a sense of reality, decrease in activity, excitement, mood fluctuation, sleep–awakening level, delusion and hallucination. Given that DST evaluations are conducted according to standardized manuals, the likelihood of variability in results among evaluators is minimized. It has been reported that more than 90% of patients who develop delirium after a stroke do so within 4 days of onset [12]. To cover this period, a 7-day observation period was set in this study.
Variables and measurement
In addition to the DST, information on age, sex, cognitive impairment, and other delirium risk factors, history of delirium, medications associated with an increased risk of delirium, and regular alcohol use were extracted from the medical records. The medications were only listed if they were administered to two or more patients. Adverse events, including self-extraction of the intravascular tube, falls, somnolence, drowsiness, lightheadness, dizziness, hepatic impairment, and renal impairment attributed to sleep medications and delirium, were investigated during the first seven days following admission. In this study, hepatic impairment was defined as ≥ 5 × upper limit of normal (ULN) elevation in alanine transaminase (ALT), ≥ 2 × ULN elevation in alkaline phosphatase (ALP) or ≥ 3 × ULN elevation in ALT and simultaneous elevation of total bilirubin (TBL) concentration exceeding 2 × ULN [24], and renal impairment was defined as serum creatinine levels that were 1.5 × the basesline, an increase of ≥ 0.3 mg/dL or urine volume ≤ 0.5 ml/kg/h for 6 h [25].
Statistical analysis
Kaplan-Meier curves were used to estimate the time to increase in the number of positive items in category A of DST over 7 days. The log rank test (Mantel-Cox) as well as hazard ratio with 95% confidence intervals (CIs) were used to compare the two survival curves. Classified data from two independent populations were compared using the Fisher’s exact test. All data were analyzed using GraphPad Prism (version 9.5.0, GraphPad Software, San Diego, CA, USA). Statistical significance was set at a two-sided P value of < 0.05.
Results
Baseline characteristics
A total of 104 patients were eligible for this study; 33 patients were categorized into the consecutive administration group and 71 into the non-consecutive administration group. Table 1 shows the patients’ characteristics information at admission. Patients in the consecutive administration group were significantly more likely to have a history of delirium and cognitive decline than those in the non-consecutive administration group, both of which have been identified as risk factors for the development of delirium. No significant differences were observed in other risk factors between the groups. In the consecutive administration group, 20 patients were treated with a melatonin receptor agonist, 3 were treated with an orexin receptor antagonist, and 10 with a combination of both. Only 4 patients had been using these sleep medications prior to admission, while most patients commenced treatment after admission. Benzodiazepine receptor agonist treatment was continued in two patients, while its use was discontinued in three patients on admission. Of the two patients who continued treatment, both ramelteon and lemborexant were additionally prescribed in one patient, while the other was prescribed suvorexant. In the non-consecutive administration group, 7 out of 14 patients discontinued benzodiazepine receptor agonists, while 8 patients continued treatment at the same dose. While benzodiazepines are well-known risk factors for delirium, there was no difference in the frequency of their use between the two groups in this study. Admission blood laboratory values are presented in Table S1; however, complete data could not be obtained for all eligible patients.
Table 1.
Baseline characteristics
| Characteristics | Consecutive administration group | Non-consecutive administration group | p value |
|---|---|---|---|
| All patients, n | 33 | 71 | |
| Age, median (min-max), year | 84 (75–96) | 81 (75–94) | |
| Male, n (%) | 19 (57.6) | 39 (54.9) | 0.83 |
| Risk factor of delirium, n (%) | |||
| Dementia | 6 (18.2) | 7 (9.8) | 0.34 |
| Cognitive decline | 17 (51.5) | 21 (29.6) | 0.048 |
| History of delirium | 6 (18.2) | 3 (4.2) | 0.027 |
| Habitual use of alcohol | 3 (9.1) | 9 (12.7) | 0.74 |
| Prescribed medication | |||
| Benzodiazepine receptor agonist | 5 (15.2) | 14 (19.7) | 0.79 |
| Opioid | 1 (3.0) | 1 (1.4) | 0.54 |
| Corticosteroid | 2 (6.1) | 4 (5.6) | > 0.99 |
| Histamine-2 receptor antagonist | 2 (6.1) | 3 (4.2) | 0.65 |
| Antiseizure medication | 1 (3.0) | 1 (1.4) | 0.54 |
| Antiparkinsonism agent | 2 (6.1) | 1 (1.4) | 0.24 |
| Hypnotics added upon admission, n | |||
| Ramelteon 8 mg | 30 | - | - |
|
Lemborexant 2.5 mg 5 mg |
3 8 |
- | - |
|
Suvorexant 10 mg 15 mg |
1 1 |
- | - |
| DST positive items at day 0, n | |||
| Abnormal sense of reality | 1 | 0 | 0.31 |
| Decreased activity | 0 | 1 | > 0.99 |
| Excitement | 3 | 1 | 0.093 |
| Mood fluctuations | 1 | 0 | 0.31 |
| Sleep-wake rhythms | 4 | 4 | 0.26 |
| Delusions | 1 | 0 | 0.31 |
| Hallucinations | 0 | 0 | - |
Baseline parameters were collected on admission. P values reflect the difference between the consecutive administration group and the non-consecutive administration group at beseline using the Fisher’s exact test
Delirium assessment
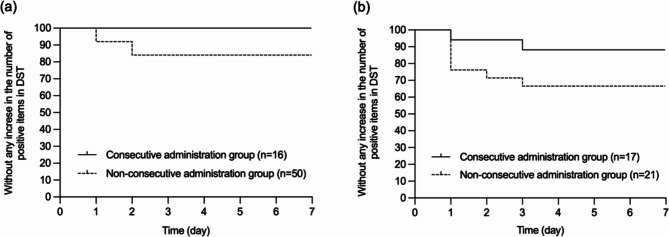
Two patients (6.1%) in the consecutive administration group and 15 patients (21.1%) in the non-consecutive administration group had an increase in the number of positive items from category A of the DST compared to their status at admission. Of the two patients in the consecutive administration group, one was treated with suvorexant, while the other was treated with a combination of ramelteon and lemborexant. Figure 1 shows the Kaplan-Meier curves for the time to increase in the number of positive items in category A of DST over 7 days. The log-rank test (Mantel-Cox) showed that significantly less in patients in the consecutive administration group had an increase in the number of positive items in the category A of DST than in those in the non-consecutive administration group (P = 0.05, hazard ratio: 0.27, 95% CI: 0.10–0.75). The same analysis was performed for the two groups with or without cognitive decline in cognitive function at the time of admission (Fig. 2). Irrespective of cognitive decline status, fewer patients in the consecutive administration group had an increase in the number of positive items in the category A of DST than those in the non-consecutive administration group (Fig. 2a: P = 0.11, 95% CI: 0.09–1.20, Fig. 2b: P = 0.09).
Fig. 1.
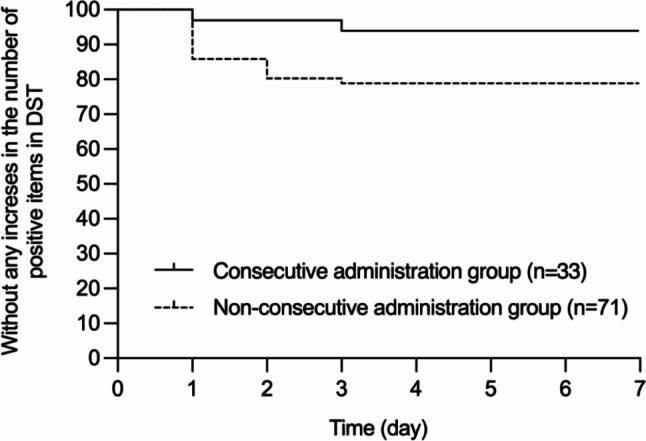
Time to increase in the number of positive items of category A in the Delirium Screening Tool. Kaplan-Meier curves were used to estimate the time to increase in the number of positive items in category A of the Delirium Screening Tool (DST) over 7 days. The log rank test (Mantel-Cox) as well as hazard ratio with 95% confidence intervals (CIs) were used to compare the two survival curves. (P = 0.05; hazard ratio, 0.27; 95% confidence interval, 0.10–0.75)
Fig. 2.
Time to increase in the number of positive items in category A in the Delirium Screenig Tool in the patients without or with Cognitive Decline. Kaplan-Meier curves were used to estimate the time to increase in the number of positive items in category A of the Deliruim Screening Tool (DST) in the patients without (a) or with (b) cognitive decline over 7 days. The log rank test (Mantel-Cox) as well as hazard ratio with 95% confidence intervals (CIs) were used to compare the two survival curves. (Fig. 2a: P = 0.11, 95% confidence interval, 0.09–1.20; Fig. 2b: P = 0.09)
Sleep-awakening level from the DST
Since this study focused on sleep medications, the “Sleep-awakening level” item, depending on the presence or absence of a sleep disorder, from the DST was analyzed. In the consecutive administration group, four patients (12.1%) were positive for the “Sleep-awakening level” item on day 0, and one patient (3.0%) was positive on day 3. Conversely, in the non-consecutive administration group, four patients (5.6%) were positive on day 0 and nine patients (12.7%) were positive on day 3. Furthermore, two patients in the consecutive administration group and 12 in the non-consecutive administration group exhibited an increase in items other than the “sleep-awakening level” (Table S2).
Safety
The adverse events associated with sleep medication use and delirium are presented in Table 2. No significant differences were observed in these adverse events between the two groups. However, self-extraction of intravascular tube resulting from delirium occurred more frequently in the consecutive administration group (15.2%) than in the non-consecutive administration group (7.0%).
Table 2.
Comparison of adverse events associated with sleep medication use and delirium
| Adverse events, n (%) | Consecutive administration group n = 33 |
Non-consecutive administration group n = 71 |
p value |
|---|---|---|---|
| Somnolence | 6 (18.2) | 11 (15.5) | 0.78 |
| Drowsiness | 1 (3.0) | 2 (2.8) | > 0.99 |
| Lightheadedness | 8 (24.2) | 15 (21.1) | 0.80 |
| Dizziness | 0 (0) | 4 (5.6) | 0.30 |
| Hepatic impairment | 0 (0) | 1 (1.4) | > 0.99 |
| Renal impairment | 2 (6.1) | 2 (2.8) | 0.58 |
| Self-extraction of intravascular tube | 5 (15.2) | 5 (7.0) | 0.28 |
Discussion
This study suggests that the use of a melatonin receptor agonist or orexin receptor antagonists during the first 2 days after admission could potentially prevent symptoms of delirium. It is noteworthy that patients in the consecutive administration group who had more risk factors for delirium, including cognitive decline and history of delirium [8, 26, 27], exhibited less symptoms of delirium. It is also possible that cognitive decline and a history of delirium influenced the decision to prescribe sleep medications, as there is a strong association between cognitive decline and sleep disturbances in older adults [28]. In this study, although these medication were administered for insomnia, they may indirectly contributed to the prevention of delirium. These findings align with those observed in patients from previously reported studies [17, 18, 29, 30], suggesting that the use of melatonin receptor agonist or orexin receptor antagonists could potentially lower the risk of delirium.
Notably, the number of patients who tested positive for the DST “Sleep-Awakening Level” item decreased in the consecutive administration group but increased in the non-consecutive administration group. Due to the limited sample size of this study, a comprehensive analysis of the relationship between Sleep-Awakening Level item disturbances and delirium was not feasible. However, sleep medication appeared to improve insomnia, a known predisposing factor for delirium [26]. Furthermore, the positive results for items other than “Sleep-Awakening Level” in the non-consecutive administration group suggested that the results are related to delirium as well as improved sleep control. Thus, it potentially decrease the risk of delirium. This preliminary observation highlights the need for further research on the effect of sleep management on the incidence of delirium in patients with stroke.
This retrospective study revealed that 21% of patients in the non-consecutive administration group who did not receive the melatonin receptor agonist or orexin receptor antagonist for two days had an increase in the number of positive items of DST. This incidence aligns closely with that reported in a previous meta-analysis (26%) [9], though a direct comparison with previous studies is challenging due to differences in assessment methods. Additionally, the timing of increase in the number of positive items observed in this study mirrors that reported in prior research [12], with all cases manifesting within four days. These results suggest that this DST-based assessment serves as a valid tool for detection of delirium, rendering the findings of this study as having significant importance.
Sleep-wake rhythm disturbances may play a role in the pathological mechanisms underlying delirium, and could also be a contributing factor [31]. Consequently, effective management of sleep through the administration of melatonin receptor agonists and orexin receptor antagonists is considered highly beneficial in the prevention of delirium. Furthermore, evidence indicates that inflammatory cytokines are also involved in the pathogenesis of delirium [32]. A study on patients with delirium found that interleukin-6 (IL-6) levels were significantly lower in patients treated with melatonin than in those receiving a placebo [33]. Additionally, increased serum orexin levels have been reported in patients with delirium [34]. These findings suggest that melatonin receptor agonists and orexin receptor antagonists may contribute to the prevention of delirium through improved sleep management and pharmacological effects. However, the present study did not assess inflammatory cytokines or other relevant serum factors, and further research is required to elucidate these underlying molecular mechanisms.
Considering the risk of adverse events associated with sleep medication [35], there were concerns regarding the older adult participants in this study. In particular, orexin receptor antagonists may increase the risk of falls [36] and should be prescribed with caution, especially in this population. However, no significant difference was observed in the frequency of adverse events between the consecutive and non-consecutive administration group. One potential explanation for this outcome is that the treatment protocol for cerebral infraction often includes behavioral restrictions following the initiation of treatment [37], which could have suppressed the occurrence of behavior-related adverse events, such as falls. This investigation focused on the short-term sleep medication (limited to two days post-admission). To our knowledge, only one small randomized control trial has been reported on strategies for the prevention of delirium in the acute phase of stroke [38]. Our findings suggest that the short-term use of a melatonin receptor antagonist and orexin receptor agonists in patients with stroke may be safe and could potentially contribute to preventing further episode of delirium. However, delirium is often associated with long-term consequences, such as an increased risk of dementia and mortality [15]. Therefore, further research is necessary to determine the long-term effects of a melatonin receptor agonist or orexin receptor antagonists in patients following a stroke.
The limitations of this study are as follows. First, this study was a single-center, retrospective study. Therefore, it was not possible to generalize the patient backgrounds, which resulted in differences in items such as cognitive decline and history of delirium. Conversely, the singular nature of the study site ensured uniformity in the hospitalization environment and a cadre of healthcare providers, potentially suppressing the variance in factors known to influence delirium outcomes. Second, the sample size in this study was relatively small. A post-hoc analysis of statistical power based on the study findings (hazard ratio = 0.27, total number of events over both groups = 17, alpha = 0.05, using the R package “powerSurvEpi”) showed a low statistical power of 0.44. The sample size required to guarantee a statistical power of 0.8 is approximately 250. Therefore, future studies should employ a larger sample size to more effectively assess the impact of the study drug. Due to the limited sample size, it was not feasible to analyze the data by grouping patients based on the specific drug administered. Given that melatonin receptor agonist and orexin receptor antagonists have distinct pharmacological mechanisms, their effects on delirium prevention may also differ. Consequently in line with previous studies, we analyzed a melatonin receptor agonist and orexin receptor antagonists as a single group in this study. Third, it should be noted that a widely accepted rating scale such as the DRS-R-98 [39] was not employed in assessing delirium. Therefore, the incidence and severity of delirium could not be assessed accurately. Nevertheless, as previously mentioned, the findings of this study are deemed significant because of their validity in comparison with past research findings [12, 17–19] regarding the efficacy of those sleep medication in preventing delirium and the timing of its onset. Additional research utilizing scales such as the DRS-R-98 [39] is required to further validate of these results.
Conclusion
In this retrospective and single-center analysis, we demonstrated that administering a melatonin receptor agonist or orexin receptor antagonists during the first 2 days of hospital admission can effectively reduce the symptoms of delirium in older patients with stroke.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the physicians, pharmacists, and nurses for their assistance with and care for the patient. We would like to thank Editage (www.editage.jp) for English language editing.
Abbreviations
- DST
Delirium Screening Tool
- ULN
Upper limit of normal
- ALT
Alanine transaminase
- ALP
Alkaline phosphatase
- TBL
Total bilirubin
Author contributions
YM and YS conceived and designed the study, drafted the manuscript, and analyzed the data. YM and Y Kojima obtained patient data. DH, NK, MH, MK, HE, KI, Y Katsube, NI, SN, MT, and TT critically reviewed the manuscript.TM obtained feedback from an expert in neurology. N Sugawara, HK, and N Sugita provided feedback as experts in psychiatry. All authors participated in drafting the discussion of the results and reviewed the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for profit sectors.
Data availability
Not applicable.
Declarations
Ethics approval and consent to participate
This study was performed in accordance with the Ethical Guidelines for Life Science and Medical Reseach Involving Human Subjects, and was reviewed by the Ethics Committee of the Kyoto University Graduate School and Faculty of Medicine (approval number, R0545-2).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the global burden of Disease Study 2019. Lancet Neurol. 2021;20:795–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lindley RI. Stroke prevention in the very elderly. Stroke. 2018;49:796–802. [DOI] [PubMed] [Google Scholar]
- 3.Tsao CW, Aday AW, Almarzooq ZI, Anderson CAM, Arora P, Avery CL, et al. Heart disease and stroke statistics-2023 update: a report from the American Heart Association. Circulation. 2023;147:e93–621. [DOI] [PubMed] [Google Scholar]
- 4.Chen ZM, Sandercock P, Pan HC, Counsell C, Collins R, Liu LS, et al. Indications for early aspirin use in acute ischemic stroke: a combined analysis of 40 000 randomized patients from the Chinese Acute Stroke Trial and the International Stroke Trial. On behalf of the CAST and IST collaborative groups. Stroke. 2000;31:1240–9. [DOI] [PubMed] [Google Scholar]
- 5.Sandercock P, Gubitz G, Foley P, Counsell C. Antiplatelet therapy for acute ischaemic stroke. Cochrane Database Syst Rev. 2003;2:CD000029. [DOI] [PubMed]
- 6.Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernhardt J, Dewey H, Thrift A, Collier J, Donnan G. A very early rehabilitation trial for stroke (AVERT): phase II safety and feasibility. Stroke. 2008;39:390–6. [DOI] [PubMed] [Google Scholar]
- 8.Lipowski ZJ. Delirium (acute confusional states). JAMA. 1987;258:1789–92. [PubMed] [Google Scholar]
- 9.Carin-Levy G, Mead GE, Nicol K, Rush R, van Wijck F. Delirium in acute stroke: screening tools, incidence rates and predictors: a systematic review. J Neurol. 2012;259:1590–9. [DOI] [PubMed] [Google Scholar]
- 10.Hénon H, Lebert F, Durieu I, Godefroy O, Lucas C, Pasquier F, et al. Confusional state in stroke: relation to preexisting dementia, patient characteristics, and outcome. Stroke. 1999;30:773–9. [DOI] [PubMed] [Google Scholar]
- 11.Kostalova M, Bednarik J, Mitasova A, Dušek L, Michalcakova R, Kerkovsky M, et al. Towards a predictive model for post-stroke delirium. Brain Inj. 2012;26:962–71. [DOI] [PubMed] [Google Scholar]
- 12.Mc Manus J, Pathansali R, Hassan H, Ouldred E, Cooper D, Stewart R, et al. The evaluation of delirium post-stroke. Int J Geriatr Psychiatry. 2009;24:1251–6. [DOI] [PubMed] [Google Scholar]
- 13.Miu DK, Yeung JC. Incidence of post-stroke delirium and 1-year outcome. Geriatr Gerontol Int. 2013;13:123–9. [DOI] [PubMed] [Google Scholar]
- 14.Sheng AZ, Shen Q, Cordato D, e Zhang YY, Yin Chan DK. Delirium within three days of stroke in a cohort of elderly patients. J Am Geriatr Soc. 2006;54:1192–8. [DOI] [PubMed] [Google Scholar]
- 15.Witlox J, Eurelings LS, de Jonghe JF, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. 2010;304:443–51. [DOI] [PubMed] [Google Scholar]
- 16.Thingstad P, Askim T, Beyer MK, Bråthen G, Ellekjær H, Ihle-Hansen H, et al. The Norwegian cognitive impairment after stroke study (Nor-COAST): study protocol of a multicentre, prospective cohort study. BMC Neurol. 2018;18:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hatta K, Kishi Y, Wada K, Takeuchi T, Odawara T, Usui C, et al. Preventive effects of ramelteon on delirium: a randomized placebo-controlled trial. JAMA Psychiatry. 2014;71:397–403. [DOI] [PubMed] [Google Scholar]
- 18.Hatta K, Kishi Y, Wada K, Takeuchi T, Ito S, Kurata A, et al. Preventive effects of suvorexant on delirium: a randomized placebo-controlled trial. J Clin Psychiatry. 2017;78:e970–9. [DOI] [PubMed] [Google Scholar]
- 19.Matsuoka A, Tobita S, Sogawa R, Shinada K, Murakawa-Hirachi T, Shimanoe C, et al. Evaluation of suvorexant and lemborexant for the prevention of delirium in adult critically ill patients at an advanced critical care center: a single-center, retrospective, observational study. J Clin Psychiatry. 2022;84:22m14471. [DOI] [PubMed] [Google Scholar]
- 20.Bassetti CL. Sleep and stroke. Semin Neurol. 2005;25:19–32. [DOI] [PubMed] [Google Scholar]
- 21.Machida I, Aoki T, Kohduki K, Kishi Y, Hoaka T. Development of delirium screening tool. Jpn J Gen Hosp Psychiatry. 2003;15:150–5. [in Japanese]. [Google Scholar]
- 22.Hashimoto Y, Kano O, Ebihara S. Noise pareidolia test for predicting delirium in hospitalized older patients with cognitive decline. Geriatr Gerontol Int. 2022;22:883–8. [DOI] [PubMed] [Google Scholar]
- 23.Trzepacz PT, Baker RW, Greenhouse J. A symptom rating scale for delirium. Psychiatry Res. 1988;23:89–97. [DOI] [PubMed] [Google Scholar]
- 24.European Association for the Study of the Liver. EASL Clinical Practice guidelines: drug-induced liver injury. J Hepatol. 2019;70:1222–61. [DOI] [PubMed] [Google Scholar]
- 25.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179–84. [DOI] [PubMed] [Google Scholar]
- 26.Saxena S, Lawley D. Delirium in the elderly: a clinical review. Postgrad Med J. 2009;85:405–13. [DOI] [PubMed] [Google Scholar]
- 27.Schor JD, Levkoff SE, Lipsitz LA, Reilly CH, Cleary PD, Rowe JW, et al. Risk factors for delirium in hospitalized elderly. JAMA. 1992;267:827–31. [PubMed] [Google Scholar]
- 28.Spira AP, Gamaldo AA, An Y, Wu MN, Simonsick EM, Bilgel M, et al. Self-reported sleep and β-amyloid deposition in community-dwelling older adults. JAMA Neurol. 2013;70(12):1537–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu YC, Tseng PT, Tu YK, Hsu CY, Liang CS, Yeh TC, et al. Association of delirium response and safety of pharmacological interventions for the management and prevention of delirium: a network meta-analysis. JAMA Psychiatry. 2019;76:526–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hatta K, Kishi Y, Wada K, Takeuchi T, Hashimoto N, Suda K, et al. Real-world effectiveness of ramelteon and suvorexant for delirium prevention in 948 patients with delirium risk factors. J Clin Psychiatry. 2019;81:19m12865. [DOI] [PubMed] [Google Scholar]
- 31.Watson PL, Ceriana P, Fanfulla F. Delirium: is sleep important? Best Pract Res Clin Anaesthesiol. 2012;26(3):355–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan CK, Song Y, Greene R, Lindroth H, Khan S, Rios G, et al. Meta-analysis of ICU delirium biomarkers and their alignment with the NIA-AA Research Framework. Am J Crit Care. 2021;30(4):312–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cho JH, Bhutani S, Kim CH, Irwin MR. Anti-inflammatory effects of melatonin: a systematic review and meta-analysis of clinical trials. Brain Behav Immun. 2021;93:245–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sateia MJ, Buysse DJ, Krystal AD, Neubauer DN, Heald JL. Clinical practice Guideline for the Pharmacologic Treatment of Chronic Insomnia in adults: an American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2017;13(2):307–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Albrecht S, Ihmsen H, Hering W, Geisslinger G, Dingemanse J, Schwilden H, et al. The effect of age on the pharmacokinetics and pharmacodynamics of midazolam. Clin Pharmacol Ther. 1999;65:630–9. [DOI] [PubMed] [Google Scholar]
- 36.Ishibashi Y, Nishitani R, Shimura A, Takeuchi A, Touko M, Kato T, et al. Non-GABA sleep medications, suvorexant as risk factors for falls: case-control and case-crossover study. PLoS ONE. 2020;15(9):e0238723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jauch EC, Saver JL, Adams HP Jr, Bruno A, Connors JJ, Demaerschalk BM, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870–947. [DOI] [PubMed] [Google Scholar]
- 38.Rice KL, Bennett MJ, Berger L, Jennings B, Eckhardt L, Fabré-LaCoste N, et al. A pilot randomized controlled trial of the feasibility of a Multicomponent Delirium Prevention intervention Versus Usual Care in Acute Stroke. J Cardiovasc Nurs 2. 2017;32(1):E1–10. [DOI] [PubMed] [Google Scholar]
- 39.Trzepacz PT, Mittal D, Torres R, Kanary K, Norton J, Jimerson N. Validation of the Delirium Rating Scale-revised-98: comparison with the Delirium Rating Scale and the cognitive test for Delirium. J Neuropsychiatry Clin Neurosci. 2001;13:229–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.