Abstract
Ovarian cancer is a leading cause of death from gynecological cancers worldwide. Platinum-based chemotherapy provides the cornerstone of the medical management. In first line and subsequent relapses, maintenance strategies are offered to prolong intervals between lines of chemotherapy. Current maintenance options involve bevacizumab and poly ADP-ribose polymerase inhibitors, but these lines of therapy can only be used once in the disease course. Patients in first or second platinum sensitive relapse after poly ADP-ribose polymerase inhibitors and bevacizumab represent an area of unmet medical need. This academic sponsored, international Phase II randomized trial is evaluating the combination of a therapeutic cancer vaccine (OSE2101) with anti-PD1 (pembrolizumab) as maintenance therapy, in patients with platinum-sensitive recurrence regardless of number of prior lines and no progression after platinum-based chemotherapy.
Clinical Trial Registration: NCT04713514 (ClinicalTrials.gov)
Keywords: : anti PD1, maintenance, OSE2101, pembrolizumab, platinum-sensitive, recurrent ovarian cancer, vaccine
Tweetable Abstract
Ongoing Phase II study randomizing vaccine OSE2101 +/- Pembrolizumab vs supportive care as maintenance in platinum-sensitive recurrent ovarian cancer.
Plain Language Summary
Phase II study evaluating vaccine OSE2101 +/- Pembrolizumab vs supportive care as maintenance in patients with recurrent platinum-sensitive ovarian cancer.
Ovarian cancer often becomes a long-term condition that needs ongoing treatment. After chemotherapy, women usually receive additional treatments to keep the cancer under control. There are only two approved treatments for this: bevacizumab administered every 3 weeks, and poly ADP-ribose polymerase inhibitors (PARP) inhibitors, which are pills that women can take if their cancer responds to platinum-based chemotherapy. However, if women have already been treated with both bevacizumab and a PARP inhibitor, there are no approved options left to maintain control of the cancer after chemotherapy.
A new potential treatment, called OSE2101, is being studied: it is a vaccine designed to activate the body’s immune cells to fight against cancer. The TEDOVA study is looking at how well this vaccine works in women with ovarian cancer who have already been treated with both bevacizumab and a PARP inhibitor and whose cancer is responding to platinum-based chemotherapy again.
Participants are randomly assigned to one of three groups after their chemotherapy: standard supportive care, OSE2101 vaccine alone and the combination of OSE2101 vaccine with pembrolizumab.
The goal of the TEDOVA study is to see if the combination of the OSE2101 vaccine and pembrolizumab can better control the cancer and to make sure the treatments are safe. The study is being run by ARCAGY-GINECO and is currently recruiting patients in France, Germany and Belgium. The study will continue to accept new patients until the end of 2024, and it is registered on ClinicalTrial.gov under the number NCT04713514.
Plain language summary
Article highlights.
Advanced epithelial ovarian cancer
Most patients diagnosed with ovarian cancer (OC) have no evidence of disease after standard treatment with debulking surgery and platinum-based chemotherapy. Long-term survival depends on the recurrence of the disease, the delay between the last platinum-based chemotherapy defining the platinum-sensibility which is a major prognostic factor.
Study rationale
Women with platinum-sensible relapse (more than 6 months after the last platinum received) will be managed as patients with a chronic disease requiring iterative lines of platinum-based chemotherapy. After several cycles of treatment, chemotherapy is usually stopped and one of the major priorities is to extend platinum-free interval proposing maintenance strategies, as targeted therapy bevacizumab or poly ADP-ribose polymerase inhibitors (PARPi). When those lines of therapy have been received during previously, they cannot be reuse. That is the reason of developing new therapies and associations as maintenance therapy.
TEDOVA study
TEDOVA is a Phase II, randomized, open-label, multicenter study assessing the efficacy of vaccine OSE2101 + anti PD-1 pembrolizumab as maintenance therapy in women with platinum-sensible relapse of OC. We included women with HLA-A2 phenotype (~45% of the general population) with platinum sensitive OC regardless of the number of prior lines of platinum-based chemotherapy. Patients must have been previously treated with bevacizumab and a PARPi, or have a contra-indication.
End points
The primary end point is to evaluate the progression free survival of maintenance OSE2101 alone or in combination with PD1 inhibitor.
The secondary end points are to compare the best overall response rate for patients with measurable disease at randomization, the safety and tolerability, the time to subsequent first and second treatments and the overall survival.
Key eligibility
Eligible patients were aged ≥18 years of age with histologically proven non-mucinous epithelial OC, positive HLA-A2 phenotype, and platinum sensitive OC regardless of the number of prior lines of platinum-based chemotherapy. Patients must have been previously treated with bevacizumab and a PARPi, or have a contraindication.
Study procedures
A total of 180 patients with an HLA-A2 positive phenotype will be randomized into three study arms in a 1:1:2 ratio: Arm A (n = 45) will receive observation/best supportive care, Arm B (n = 45) will receive OSE2101 and Arm C (n = 90) will receive OSE2101 plus pembrolizumab. Patients will be followed until disease progression, intolerance, withdrawal of consent, or for up to 2 years.
Statistics
- One-sided log-rank tests will be conducted in a predefined sequence:
-
○H1: Compare Arm C (OSE2101 + pembrolizumab) with Arm A (best supportive care).
-
○If H1 shows a statistically significant difference, then proceed to:
-
○H2: Compare Arm C (OSE2101 + pembrolizumab) with Arm B (OSE2101).
-
○If both H1 and H2 show statistically significant differences, then proceed to:
-
○H3: Compare Arm B (OSE2101) with Arm A (best supportive care).
-
○A total of 121 events (progressions or deaths) is required to perform the first test of H1.
-
○
1. Introduction
1.1. Background & rationale
The prognosis of ovarian cancer (OC) varies by stage. Type I epithelial OCs are considered to be relatively indolent and genetically stable, while Type II epithelial OCs are thought to be biologically aggressive from the beginning, with a tendency to metastasize early [1]. High-grade serous – accounting for approximately 75% of epithelial OCs – develop according to the type II pathway and present p53 and BRCA mutations. These advanced stage has a poor prognosis [2] with most patients unfortunately relapsing after primary management. Initial treatment of advanced OC includes surgery and platinum-based chemotherapy (CT). These treatments can be followed by a maintenance strategy consisting of poly ADP-ribose polymerase inhibitors (PARPi) alone, anti-angiogenic bevacizumab alone or the combination of bevacizumab and PARPi [3]. In case of relapse, OC is managed as a chronic disease with iterative lines of chemotherapy.
BRCA1/2 germline mutations are the strongest known genetic risk factors for epithelial OCs and are found in 6–15% of women diagnosed with that disease. BRCA1/2 mutated carriers respond better than noncarriers to platinum-based chemotherapies. This yields greater survival, even though the disease is generally diagnosed at a later stage and higher grade [4]. One other major prognostic factor is the platinum-free interval (PFI) defined as the time between the last platinum CT and recurrence, which is important to interpret in combination with other specific clinical and molecular factors [5]. Current options for platinum-sensitive patients include bevacizumab and/or PARPi FDA-approved Olaparib as maintenance after platinum-based chemotherapy regardless of BRCA/HRD status, if they have not been previously administered [6–10]. Thus, women with OC presenting with platinum sensitive relapse after prior bevacizumab and PARP inhibition represent a crucial unmet medical need.
Immune therapies (mainly, checkpoint inhibitors) have opened new opportunities in cancer therapy [11]. Unfortunately, in the case of OC, previous studies targeting checkpoints inhibitors Programmed Death Ligand-1 (PD-L1) or Programmed Death-1 (PD-1) have been disappointing [12–14], partly explained by the low levels of endogenous tumor specific cytotoxic T cells or low mutation load [15]. In particular, ovarian tumors are frequently described as immunogenically ‘cold’, characterized by a highly immune-tolerant microenvironment. Pembrolizumab is a humanized monoclonal antibody therapy that targets the cell surface receptor PD-1, thus inhibiting its interaction with PD-L1 and PD-L2 [16,17]. The PD-1 receptor-ligand interaction is a major pathway hijacked by tumors to suppress immune control [11,18,19]. Pembrolizumab is currently under investigation in several studies of patients with platinum-sensitive recurrent OC (see Supplementary Table S1). Previous Phase II KEYNOTE-100 study in recurrent OC [14] included 285 women with one to three prior lines of treatment and a PFI between 3 and 12 months and 86 women with four to six prior lines and a PFI ≥3 months. Pembrolizumab was administered as a single-agent, and showed modest activity with 7.4 and 9.9% objective response rates respectively.
A recent therapeutic cancer vaccine OSE2101 is under clinical investigation in several localizations. OSE2101 is designed to activate cytotoxic T-lymphocytes (CTL) against five tumor-associated antigens highly expressed in non-small-cell lung cancer but also OC: carcinoembryonic antigen; p53; HER-2/neu and melanoma antigens 2 and 3 (Figure 1) [20,21]. OSE2101 is composed of 10 synthetic peptides of which 9 derive from these tumor-associated antigens, that have been modified to increase HLA and/or T cell receptor binding affinity and one pan-DR epitope. The pan-DR epitope is a rationally designed helper T-lymphocyte epitope included to augment the magnitude and duration of CTL responses.
Figure 1.
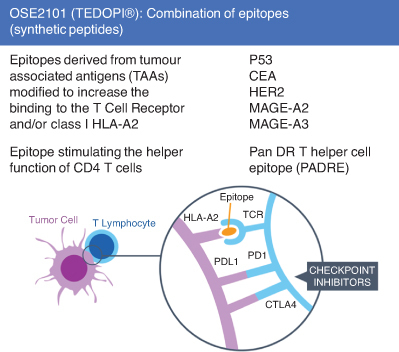
Description of OSE2101 (Tedopi®) combination of epitopes. Adjuvant (Montanide ISA 51™) is entrapping all epitopes in ISE2101 in a form amenable for efficient uptake and processing/presentation by antigen presenting cells through subcutaneous route.
CEA: Carcinoembryonic Antigen; CTLA4: Cytotoxic T-lymphocyte-associated protein 4; HER2: Human Epidermal Growth Factor Receptor 2; HLA-A2: Human Leucocyte Antigen A2; P53: Protein Tumor 53; PD1:Programmed Death 1; PDL1:Programmed Death Ligand 1; MAGE-A2: Melanoma A2 Antigen; MAGE-A3: Melanoma A3 Antigen; TCR: T Cell Receptor.
Each CTL epitope is restricted by HLA-A2. which is expressed by approximately 45% of the general population as well as cancer patients [22].
The innovation is to associate epitopes designed and modified chemically of different tumor associated antigens in the same vaccine.
Immunogenicity of OSE2101 is under investigation in maintenance among patients with locally advanced/metastatic pancreatic ductal adenocarcinoma (NCT03806309). It has also been previously published in a NSLCL Phase II study [23]. In the ATALANTE-1 study [24] including 118 randomized patients with HLA-A2 positive advanced non-small-cell lung cancer with secondary resistance to immunotherapy, OSE2101 monotherapy was associated with an improved overall survival in the secondary resistance to immunotherapy subgroup (11.1 vs 7.5 months) and better quality of life (vs standard of care chemotherapy). Safety data in ATALANTE-1 are also reassuring with 11.4% of treatment-related severe (not higher than grade 3) adverse event (TRAEs) (vs 35.1% in standard of care group). No grade 4 or 5 TRAE were observed.
Combination of vaccines containing multi-epitopes with immunotherapy is a recent era of personalized immunotherapy with tumor-specific neoantigens. The general mechanism of action is the recruitment and activation (interferon γ production) of tumor specific CD8+ T cells [25,26]. Immunoinformatics and bioinformatics approaches are promising strategies under investigation [27,28] including in OC [29] to find potential epitopes for a multi-epitope vaccine [30].
Associating a multi-epitope vaccine to an immune checkpoint inhibitor has the potential to change immunogenically ‘cold’ ovarian tumors into ‘hot’ tumors and more effectively harness a T cell-mediated immune response against tumor-associated antigens or tumor-specific neoantigens [31].
Within this landscape, the randomized, Phase II TEDOVA/GINECO-ov244b/ENGOT-ov58 will evaluate the association of the therapeutic cancer vaccine OSE2101 with or without the anti-PD1 antibody pembrolizumab as maintenance therapy, in patients with platinum-sensitive recurrence, with prior PARPi and bevacizumab exposure, who had no disease progression after platinum-based chemotherapy. The TEDOVA trial is sponsored by ARCAGY-GINECO, and is currently recruiting in France, Germany and Belgium. The first patient was randomized in August 2021. As of November 2023, 105 patients have been randomized. The duration of the inclusion period is estimated of around 36 months.
2. Objectives
2.1. Primary objective
The primary objective is to evaluate the progression-free survival (PFS) benefit according to RECIST 1.1 of maintenance OSE2101 alone or in combination with PD1 inhibitor after platinum-based CT in relapsed OC.
2.2. Secondary objectives
To compare the best overall response rate for patients with measurable disease at randomization.
Safety and tolerability, in terms of AEs (with a particular focus on immune related), deaths, laboratory data, vital signs and electrocardiogram.
Time to subsequent first and second treatments (TTST-1 and TTST-2).
Overall survival
2.3. Trial design (Figure 2)
Figure 2.
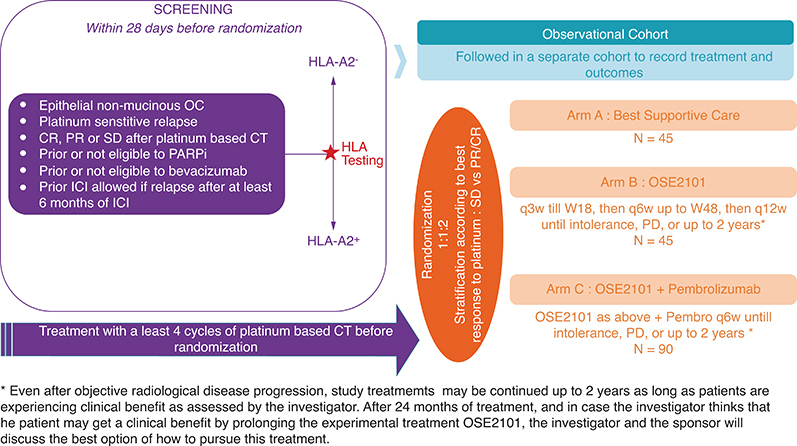
Study design.
CR: Complete response; CT: Chemotherapy; OC: Ovarian cancer; PARPi: Poly ADP-ribose polymerase inhibitors; PD: Progression disease; PR: Partial response; q3w: Every 3 weeks; SD: Stable disease; W18: Week 18.
This international multicenter study is a randomized Phase II open-label three arms trial, comparing best supportive care vs OSE2101 vs OSE2101 in combination with Pembrolizumab as maintenance treatment for patients with platinum sensitive relapsed OC, regardless of the number of prior lines. Patients must have been previously treated with bevacizumab and a PARPi, or have a contraindication, or not be eligible to these treatments (i.e., no approval for PARPi treatment in low grade OC). After positive prescreening for HLA A2 and at least four cycles of platinum-based chemotherapy, patients with complete response (CR), partial response (PR) or stable disease (SD) will be randomized (1:1:2) in one of three arms: best supportive care, OSE2101 monotherapy or OSE2101 in combination with pembrolizumab.Treatment will be administrated until progression, intolerance or up to 2 years from the first dose of study treatment.
3. Materials & methods
A total of 180 patients with HLA-A2 positive phenotype will be randomized using an Interactive Web Response System according to the best response to platinum therapy stratification factor (SD vs PR/CR)
The three study arms in 1:1:2 ratio are described in Figure 2:
Arm A (n = 45): Observation/best supportive care
Arm B (n = 45): OSE2101: every 3 weeks until week 18, then every 6 weeks up to week 48, then every 12 weeks until disease progression, intolerance, patient withdrawal of consent or up to 2 years
Arm C (n = 90): OSE2101 + Pembrolizumab: OSE2101 same schedule as arm B plus pembrolizumab IV every 6 weeks until disease progression, intolerance, patient withdrawal of consent or up to 2 years
HLA-A2 negative patients will be followed, as study participants, in a separate observational cohort to record treatment and outcomes.
3.1. Participant timeline
Inclusion began in Q2 2021 for 3 years with an estimated date of last patient included in Q3 2024. The estimated date of end of treatment and study is Q4 2025, which corresponds to the date of disease progression according to RECIST1.1 of last patient.
3.2. Eligibility criteria
Eligibility criteria are developed in Table 1.
Table 1.
Key inclusion and exclusion criteria in TEDOVA protocol.
| Key inclusion criteria | Key exclusion criteria |
|---|---|
| 1. Written informed consent 2. Histologically proven non-mucinous epithelial ovarian cancer 3. Positive HLA-A2 phenotype 4. Age ≥18 years 5. ECOG Performance Status 0–1 6. Platinum sensitive ovarian cancer regardless of the number of prior lines of platinum-based chemotherapy. Patient must have received at least four infusions of platinum during the last line of platinum-based chemotherapy 7. Previously treated with a PARP inhibitor or not eligible to PARPi 8. Previously treated with bevacizumab or contra-indication to bevacizumab 9. Patient may have received prior immune checkpoint inhibitor (ICI), and had a relapse after receiving the ICI without concomitant chemotherapy for at least 6 months 10. Randomization must be within 8 weeks of the last dose of chemotherapy 11. Adequate organ function: white blood cell ≥3000/mm3; neutrophils ≥1500/mm3; platelets ≥100 × 103/mm3; hemoglobin ≥9 g/dl; ALT and AST ≤2.5 × ULN; total bilirubin ≤1.5 × ULN; serum creatinine ≤1.5 × ULN or creatinine clearance ≥40 mL/min (using Cockcroft–Gault formula) 12. Archival or fresh tumor tissue must be available for evaluating relevant biomarkers |
1. Contra-indications or severe hypersensitivity (Grade 3 or higher) to immune therapies 2. Ongoing immunotherapy 3. Use of systemic corticosteroids (>10 mg/day equivalent prednisone), not stopped at least 7 days before study treatment start 4. Use of interferons, interleukins or live vaccine within 30 days prior to the first dose of study drug 5. Prior cancer vaccine therapy 6. Eligible for cytoreductive surgery at the time of inclusion 7. Clinical, radiological or biological progression (according Gynaecologic Cancer Intergroup criteria) at the end of last chemotherapy 8. Prior radiotherapy within 2 weeks of start of study intervention 9. Active autoimmune disease that has required systemic treatment in the past 2 years (corticosteroids or immunosuppressive drugs) 10. History of serious adverse reactions to any vaccine 13. History of other malignancies (except for basal cell or squamous cell carcinoma of the skin or carcinoma in situ of the cervix or other in situ cancer considered as cured) unless the patient has been free of the disease for at least 5 years. 11. Immune-deficient status 12. History of any chronic hepatitis 13. Uncontrolled or significant cardiovascular disease 14. Uncontrolled brain metastases 15. Life expectancy of less than 12 weeks 16. Pregnant or breastfeeding women |
3.3. Recruitment
After documented signed informed consent, patients undergo blood sampling for HLA-A2 genotyping before inclusion, determined by PCR performed centrally by a certified lab. The informed consent includes the possibility to collect clinical data in patient with HLA-A2 phenotype negative. Only patients with HLA-A2 positive phenotype who have completed at least four cycles of platinum chemotherapy and are free of progression will be randomized.
3.4. Randomization
Patients are included in the three arms of the study with several assessments performed at each study visit (Figure 3) and tumor imaging assessment every 12 weeks.
Figure 3.
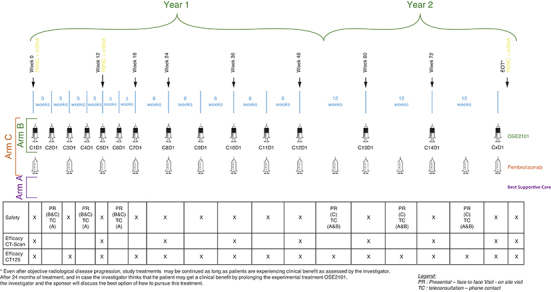
Study follow-up.
3.5. OSE2101 vaccine administration
OSE2101 vaccine is a 1 ml emulsion injected by subcutaneous administration. It is advisable to inject the vaccine preferably in the same area or in a site drained by the same lymph nodes (e.g., at anterior side of the thigh or in shoulder of the nondominant arm). Avoid injection into the thighs in patients who have had pelvic and aortic lymph node dissection. Patients have to be monitored for at least 5 h after each injection for reaction after injection including cytokine release syndrome (CRS) using CRS Severity (CTC-AE v5.0) grading scheme. OSE2101 vaccine is administered every 3 weeks for seven doses, then every 6 weeks for the remainder of year one, then every 12 weeks until progression, limiting toxicity, patient withdrawal or up to 2 years.
In Arm C, OSE2101 vaccine will be administered before the perfusion of pembrolizumab.
Dose reductions are not allowed. In case of NCI-CTCAE Grade 3 severe adverse effect, the definitive cessation of treatment has to be considered.
Antipyretics have to be administered prior to study drug administration (1 h before injection and every 6 h for the first 2–3 days), ketoprofen 100 mg twice a day and an antihistamine day 1. In addition, topical steroid cream is proposed at the site of injection.
3.6. Pembrolizumab administration
Pembrolizumab 400 mg is administered at the same time as OSE2101, as an intravenous infusion over 30 min, every 6 weeks until progression, limiting toxicity, patient withdrawal or up to 2 years. Immune-related adverse events (AEs) may occur shortly after the first dose or several months after the last dose of pembrolizumab/combination treatment, including pneumonitis, colitis, liver toxicity, diabetes, hypothyroidism or myocarditis. Participants may not have any dose modifications (no change in dose or schedule) of pembrolizumab in this study. Most immune-related AEs were reversible and could be managed with interruptions of pembrolizumab/combination treatment, administration of corticosteroids and/or other supportive care.
3.7. Statistical methods & sample size determination
This proof-of-concept trial aims to assess the efficacy of two investigational treatments (Arms B and C) compared with best supportive care (Arm A) as the control group. The primary end point is PFS, measured from randomization to disease progression. Sample size calculations were conducted using SAS 9.4 power analysis software.
The trial is designed with a type I error rate (α) of 5% and a type II error rate (β) of 10%, with one-sided tests. Previous trials have shown short median PFS in patients with platinum-sensitive relapse who responded to re-challenge with platinum doublet: 5.5 months for BRCA-mutated patients and less than 4 months for non-BRCA-mutated patients. We anticipate a slightly shorter PFS (<4 months) in our population, as most patients will have received a PARP inhibitor and are required to have non-progression on platinum chemotherapy rather than an objective response per RECIST criteria. The combination of OSE2101 and pembrolizumab is hypothesized to outperform both OSE2101 alone and best supportive care (BSC). If the combination does not outperform BSC, no difference is expected between OSE2101 alone and BSC.
Hierarchical testing (fixed sequence procedure) will be employed to compare the treatment arms, using three one-sided log-rank tests in a predefined order:
-
1.
**H1**: Arm C (OSE2101 + pembrolizumab) vs Arm A (BSC)
- If H1 is statistically significant, proceed to:
-
2.
**H2**: Arm C (OSE2101 + pembrolizumab) vs Arm B (OSE2101)
- If both H1 and H2 are statistically significant, proceed to:
-
3.
**H3**: Arm B (OSE2101) vs Arm A (BSC)
All one-sided tests in this sequence will use a nominal α value of 0.05. If H1 is not rejected, further tests will be exploratory.
The sample size is calculated to ensure 90% power to detect an improvement in PFS for the combination therapy compared with BSC, with a hazard ratio (HR) of 0.57 (assuming median PFS increases from 4 to 7 months under the exponential distribution of PFS), and an imbalanced randomization of 2:1. This design requires 121 events (progression or deaths) to test H1. Assuming a 2-year accrual period and a minimum 1-year follow-up per patient, 180 patients need to be randomized across Arm A (n = 45), Arm B (n = 45) and Arm C (n = 90) to observe the required events.
With 45 patients in the OSE2101 monotherapy arm, the trial will have 78% power to detect an HR of 0.57 compared with the BSC arm.
3.8. Statistical methods
3.8.1. Primary end point analysis
PFS will be defined from the date of randomization to the date of first documented disease progression (according to RECIST v1.1) as reported by the investigator, or death from any cause. PFS will be estimated using the Kaplan–Meier method. Patients who are alive and free of progression at the cut-off dates will be censored at the date of their last tumor assessment.
For each treatment group, the median PFS and the probability of PFS at 4, 6 and 12 months will be reported along with two-sided 95% confidence intervals (CIs). The comparison of PFS between Arm C (combination therapy) and Arm A (BSC) will be conducted using a one-sided log-rank test, stratified by the randomization stratification factor. HRs and their CIs will be estimated using a Cox model, also stratified by the randomization stratification factor. If any stratum has no events, it will be combined with another stratum. The proportional hazards assumption will be visually checked with Schoenfeld residuals and tested for a linear time trend using the Therneau test at a 5% significance level. Potential interactions between treatment effect and the stratification factor will be explored, although the small sample sizes may limit the power of these analyses.
3.8.2. Secondary end points analyses
3.8.2.1. Best Overall Response Rate (ORR)
Response in OC will be assessed according to RECIST 1.1 in the Objective Response analysis set. ORR is defined as the proportion of subjects achieving a CR or PR among patients with measurable disease. Patients without radiological assessments will be considered treatment failures (included in the denominator). Patients who receive no treatment after randomization will be excluded from the analysis (not included in the denominator).
The ORR for each treatment arm, along with the exact two-sided 95% CIs, will be estimated. Additionally, the difference in response rates and the stratified odds ratio, with corresponding 95% CIs, will be calculated. The Cochran–Mantel–Haenszel test will be used to compare the overall treatment arms, controlling for stratification factors. If significant, pairwise comparisons between arms will be conducted without adjustments for multiple testing.
A sensitivity analysis excluding non-evaluable patients will also be performed.
3.8.2.2. Safety analysis
In the Safety analysis set, safety and tolerability will be assessed in terms of AEs, serious AE, AE of special interest, immune related AE, toxic deaths, laboratory data; AE will be described according to Medical Dictionary for Regulatory Activities (MedDRA) terms (version 23.0) and graded according to NCI Common Terminology Criteria for Adverse Events (CTC-AE) version 5.0 [32].
3.8.2.3. Time to subsequent treatment (TTST-1 & TTST-2), overall survival
Time to start of subsequent therapy or death (TTST) is measured from the date of randomization to the date of anti-cancer therapy following the discontinuation of the study treatment (TTST-1) or the second treatment (TTST-2), or until death. Patients without such events will be censored at the date of their last tumor assessment.
Both TTST end points will be estimated using the Kaplan–Meier method and compared with log-rank tests, stratified by the best response to chemotherapy in the full analysis set population. If the initial comparison is rejected, pairwise comparisons between treatment arms will be performed. Stratified HRs and their CIs will be estimated using a stratified Cox semi-parametric model. The proportional hazards assumption will be visually checked with Schoenfeld residuals and tested for a linear time trend with the Therneau test at a 5% significance level.
Details of subsequent therapies aimed at controlling OC will be reported, as well as the cause of death by treatment arms.
3.9. Safety monitoring
The Data Safety Monitoring Board will be constituted by the sponsor and will be composed of statisticians and clinical experts. Severe toxicity (Grade 3 or 4 non hematological or Grade 4 hematological) at least possibly related to the study treatments, will be closely monitored. An interim safety analyses has been performed after 40 patients being treated at least during 3 weeks (30 in the combined investigational arms). This analysis performed in June 2023 was reassuring and led to the implantation of a mitigation plan for CRS with adjunction of ketoprofen and an antihistamine as premedication before OSE2101 injection.
3.10. Ethical conduct
An Institutional Review Board/Ethics Committee from each study site approved the final study protocol, including the final version of the Informed Consent Form. Each patient is given full and adequate oral and written information about the nature, purpose, possible risk and benefit of the study, with signed written and dated informed consent. Investigators of each center are in charge of enrolment of each participant.
Appropriate coded identification (i.e., patient number) will be used to protect confidentiality. The investigator will make a separate confidential record of these details (patient identification code list) to permit identification of all patients enrolled in a clinical trial in case follow-up is required.
3.11. Current status
To date 316 patients have been screened: 152 HLA-A2+ and 155 HLA-A2- (5 with results pending). Overall, 107 patients have been randomized, eight due for randomization and 37 screen-failed. Among 155 HLA-A2- patients, 22 are screen failed and 110 have been included in the observational cohort. Four patients have withdrawn consent before being tested. Screen-failures are largely attributable to progressive disease after HLA-A2 testing.
4. Conclusion
Patients with OC presenting with platinum sensitive relapse post-PARP inhibition and bevacizumab represent a crucial unmet medical need. They have no available maintenance strategies and recent data suggest that progression after prior PARP inhibitor exposure is associated with particularly poor outcomes [33,34]. Novel effective maintenance therapies are thus needed like proteomics technologies (mass spectrometry and protein array analysis) which have advanced the dissection of the underlying molecular signaling events. Proteomics analysis of OC, as well as their adaptive responses to therapy, can uncover new therapeutic choices, which can reduce the emergence of drug resistance and potentially improve patient outcomes [35].
Anti-PD1/PDL1 alone is clearly not sufficient to stimulate an effective antitumor immune response in OC. Whether the combination with a therapeutic cancer vaccine targeting several tumor-associated antigens is worthy of investigation.
This multicentre international Phase II proof of concept study will determine whether maintenance with vaccine OSE2101 and pembrolizumab can improve PFS in women with no progression after platinum-based CT and previous exposure to PARP inhibition and bevacizumab.
Supplementary Material
Funding Statement
This study was supported by ARCAGY-GINECO; OSE Immunotherapeutics and Merck Sharp & Dohme Corp. (MSD), a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, known as MSD outside of the US and Canada.
Supplemental material
Supplemental data for this article can be accessed at https://doi.org/10.1080/14796694.2024.2386922
Financial disclosure
This study was supported by ARCAGY-GINECO; OSE Immunotherapeutics and Merck Sharp & Dohme Corp. (MSD), a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, known as MSD outside of the US and Canada.
Frederik Marmé: Coordinating PI (institutional, no financial interest) for AGO Research GmbH, AstraZeneca, German Breast Group, Gilead/Immunomedics, Roche; Local PI (institutional, no financial interest) for Eisai, GSK, MSD, Novartis, Roche, Vaccibody; funding (institutional, no financial interest) from AstraZeneca, Lilly, Seagen. Thibault De La Motte Rouge: Grants or contracts from MSD, Novartis, Pfizer, Seagen (institutional). Renaud Sabatier: Research grants from Astra-Zeneca; nonfinancial support from MSD and GSK. Jérôme Alexandre: Research fund from Janssen, GSK, MSD; travel and accommodations from Eisai and Astra Zeneca. Jean-Sébastien Frenel: Support for attending meetings and/or travel from Pfizer, Lilly, Novartis, Astra Zeneca, Clovis Oncology, GSK, Gilead, Daiichi Sankyo, Seagen, MSD. Alexandre Leary: Research fund from Astra Zeneca, Clovis, GSK, MSD, Ability, Zentalis, Agenus, Iovance, Sanofi, Roche, OSEimmuno, BMS. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Competing interests disclosure
O Tredan: Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Roche, Pfizer, Novartis-Sandoz, Lilly, MSD, Astra-Zeneca, Pierre Fabre, Seagen, Daiichi-Sankyo, Gilead, Eisai, Menarini-Stemline, Veracyte; support for attending meetings and/or travel from Roche, Pfizer, Novartis-Sandoz, Lilly, MSD, Astra-Zeneca, Seagen, Daiichi-Sankyo, Gilead; participation on a Data Safety Monitoring Board or Advisory Board from Roche, Pfizer, Novartis-Sandoz, Lilly, MSD, Astra-Zeneca, Pierre Fabre, Seagen, Daiichi-Sankyo, Gilead, Eisai. F Marmé: Participation on an Advisory Board from AstraZeneca, EISAI, GenomicHealth, Gilead/Immunomedics, Immunicom, MSD, Myriad, Novartis, PharmaMar, Roche, Seagen; invited speaker by AstraZeneca, Clovis, Daiichi Sankyo, GSK, GSK/Tesaro, Lilly, Pfizer, Seagen. X Paoletti: Consulting or advisory role for MSD, Ipsen (personal). C Lebreton: Honoraria from Eisai, Clovis Oncology, MSD Oncology, GlaxoSmithKline; travel and accommodations from GlaxoSmithKline and MSD Oncology. Thibault De La Motte Rouge: Consulting fees for AstraZeneca, GSK, Clovis Oncology, Pfizer, Gilead, Seagen, Sanofi; honoraria for lectures, presentations, speakers from GSK, MSD; support for attending meetings and/or travel from Pfizer, Gilead, Eisai; participation on a data safety board or advisory board from MSD. R Sabatier: Consulting fees from GSK and EISAI. TV Gorp: Consulting fees (via institution) from AstraZeneca, BioNTech, Eisai, GSK, ImmunoGen, Incyte, Karyopharm, MSD, OncXerna, Seagen, Tubulis, Zentalis. Sponsored research (via institution): Amgen, AstraZeneca, Roche. Honoraria for lectures (via institution): GSK, ImmunoGen, MSD. L Gladieff: Institutional honoraria for symposium participation and support for attending meetings from MSD. J Alexandre: Honoraria from GSK, Astra Zeneca, Eisai, MSD. J-S Frenel: Consulting fees from Pfizer, Lilly, Novartis, Astra Zeneca, Clovis Oncology, GSK, Gilead, Daiichi Sankyo, Seagen, Exact Science, MSD; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Lilly, Novartis, Astra Zeneca, Gilead, Daiichi Sankyo, Seagen, MSD. Al Leary: Honoraria/expenses from Astra Zeneca, Clovis, GDK; participation at a consulting/advisory board for Astra Zeneca, Clovis, GSK, MSD, Merck Serono, Ability, Zentalis, Agenus, Blueprint. R Kabirian: No disclosure. L Eberst: No disclosure. A Angelergues: No disclosure. M Fabbro: No disclosure. L Mansi: No disclosure. E Kaczmarek: No disclosure. T Grellety: No disclosure. L Favier: No disclosure. J Welz: No disclosure. The authors have no other competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript apart from those disclosed.
Ethical conduct of research
An Institutional Review Board/Ethics Committee approved the final study protocol, including the final version of the Informed Consent Form. Each patient is given full and adequate oral and written information about the nature, purpose, possible risk and benefit of the study, with signed written and dated informed consent. The study will be conducted in accordance with the principles outlined in the Declaration of Helsinki and the International Conference on Harmonisation Guidelines for Good Clinical Practice.
Data availability statement
The datasets generated and/or analyzed during the current study will be available from the corresponding author upon reasonable request. Data will be shared in accordance with institutional guidelines and will require a data use agreement to ensure the confidentiality of participants.
Previous presentation
Presented at ASCO, May 31, 2023 - Abstract TPS5618 [36].
References
- 1.Pavlidis N, Rassy E, Vermorken JB, Assi T, Kattan J, Boussios S, et al. . The outcome of patients with serous papillary peritoneal cancer, fallopian tube cancer, and epithelial ovarian cancer by treatment eras: 27 years data from the SEER registry. Cancer Epidemiol. 2021;75:102045. doi: 10.1016/j.canep.2021.102045 [DOI] [PubMed] [Google Scholar]
- 2.Webb PM, Jordan SJ. Epidemiology of epithelial ovarian cancer. Best Pract Res Clin Obstet Gynaecol. 2017;41:3–14. doi: 10.1016/j.bpobgyn.2016.08.006 [DOI] [PubMed] [Google Scholar]
- 3.González-Martín A, Harter P, Leary A, Lorusso D, Miller RE, Pothuri B, et al. . Newly diagnosed and relapsed epithelial ovarian cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34:833–848. doi: 10.1016/j.annonc.2023.07.011 [DOI] [PubMed] [Google Scholar]
- 4.Shah S, Cheung A, Kutka M, Sheriff M, Boussios S. Epithelial ovarian cancer: providing evidence of predisposition genes. Int J Environ Res Public Health. 2022;19:8113. doi: 10.3390/ijerph19138113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vergote I, Gonzalez-Martin A, Lorusso D, Gourley C, Mirza MR, Kurtz J-E, et al. . Clinical research in ovarian cancer: consensus recommendations from the Gynecologic Cancer InterGroup. Lancet Oncol. 2022;23:e374–e384. doi: 10.1016/S1470-2045(22)00139-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G, et al. . A Phase III trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365:2484–2496. doi: 10.1056/NEJMoa1103799 [DOI] [PubMed] [Google Scholar]
- 7.Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, et al. . Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365:2473–2483. doi: 10.1056/NEJMoa1104390 [DOI] [PubMed] [Google Scholar]
- 8.Ray-Coquard I, Pautier P, Pignata S, Pérol D, González-Martín A, Berger R, et al. . Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med. 2019;381:2416–2428. doi: 10.1056/NEJMoa1911361 [DOI] [PubMed] [Google Scholar]
- 9.González-Martín A, Pothuri B, Vergote I, DePont Christensen R, Graybill W, Mirza MR, et al. . Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2019;381:2391–2402. doi: 10.1056/NEJMoa1910962 [DOI] [PubMed] [Google Scholar]
- 10.Selle F, Joly F, Gladieff L, Prulhière K, Leary A, Kalbacher E, et al. . Prise en charge des carcinomes ovariens de haut grade séreux et/ou endométrioïdes de stades avancés (III-IV) et testing HRD-BRCA en 2023: actualisation selon les données publiées et/ou présentées en 2022. Bulletin du Cancer. 2023;110:6S5–6S9. doi: 10.1016/S0007-4551(23)00329-6 [DOI] [PubMed] [Google Scholar]
- 11.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamanishi J, Mandai M, Ikeda T, Minami M, Kawaguchi A, Murayama T, et al. . Safety and antitumor activity of anti–PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2015;33:4015–4022. doi: 10.1200/JCO.2015.62.3397 [DOI] [PubMed] [Google Scholar]
- 13.Disis ML, Taylor MH, Kelly K, Beck JT, Gordon M, Moore KM, et al. . Efficacy and safety of avelumab for patients with recurrent or refractory ovarian cancer: Phase Ib results from the JAVELIN solid tumor trial. JAMA Oncol. 2019;5:393. doi: 10.1001/jamaoncol.2018.6258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matulonis UA, Shapira-Frommer R, Santin AD, Lisyanskaya AS, Pignata S, Vergote I, et al. . Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: results from the Phase II KEYNOTE-100 study. Ann Oncol. 2019;30:1080–1087. doi: 10.1093/annonc/mdz135 [DOI] [PubMed] [Google Scholar]
- 15.Sha D, Jin Z, Budczies J, Kluck K, Stenzinger A, Sinicrope FA. Tumor mutational burden as a predictive biomarker in solid tumors. Cancer Discov. 2020;10:1808–1825. doi: 10.1158/2159-8290.CD-20-0522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwok G, Yau TCC, Chiu JW, Tse E, Kwong Y-L. Pembrolizumab (Keytruda). Hum Vaccin Immunother. 2016;12:2777–2789. doi: 10.1080/21645515.2016.1199310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X, Schwartz J-CD, Guo X, Bhatia S, Cao E, Chen L, et al. . Structural and functional analysis of the costimulatory receptor programmed death-1. Immunity. 2004;20:337–347. doi: 10.1016/S1074-7613(04)00051-2 [DOI] [PubMed] [Google Scholar]
- 18.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, et al. . CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fikes JD, Sette A. Design of multi-epitope, analogue-based cancer vaccines. Expert Opinion Biol Ther. 2003;3:985–993. doi: 10.1517/eobt.3.6.985.21255 [DOI] [PubMed] [Google Scholar]
- 21.Kandalaft LE, Powell DJ, Singh N, Coukos G. Immunotherapy for ovarian cancer: what's next? J Clin Oncol. 2011;29:925–933. doi: 10.1200/JCO.2009.27.2369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keogh E, Fikes J, Southwood S, Celis E, Chesnut R, Sette A. Identification of new epitopes from four different tumor-associated antigens: recognition of naturally processed epitopes correlates with HLA-A-0201-binding affinity. J Immunol. 2001;167:787–796. doi: 10.4049/jimmunol.167.2.787 [DOI] [PubMed] [Google Scholar]
- 23.Barve M, Bender J, Senzer N, Cunningham C, Greco FA, McCune D, et al. . Induction of immune responses and clinical efficacy in a Phase II trial of IDM-2101, a 10-epitope cytotoxic t-lymphocyte vaccine, in metastatic non–small-cell lung cancer. J Clin Oncol. 2008;26:4418–4425. doi: 10.1200/JCO.2008.16.6462 [DOI] [PubMed] [Google Scholar]
- 24.Besse B, Felip E, Garcia Campelo R, Cobo M, Mascaux C, Madroszyk A, et al. . Randomized open-label controlled study of cancer vaccine OSE2101 versus chemotherapy in HLA-A2-positive patients with advanced non-small-cell lung cancer with resistance to immunotherapy: ATALANTE-1. Ann Oncol. 2023;34:920–933. doi: 10.1016/j.annonc.2023.07.006 [DOI] [PubMed] [Google Scholar]
- 25.Soares KC, Rucki AA, Wu AA, Olino K, Xiao Q, Chai Y, et al. . PD-1/PD-L1 blockade together with vaccine therapy facilitates effector T-Cell infiltration into pancreatic tumors. J Immunother. 2015;38:1–11. doi: 10.1097/CJI.0000000000000062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuai R, Ochyl LJ, Bahjat KS, Schwendeman A, Moon JJ. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nature Mater. 2017;16:489–496. doi: 10.1038/nmat4822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X, Wen H, Xiao X, Ren Z, Tan C, Fu C. Design of a novel multi-epitope vaccine candidate against endometrial cancer using immunoinformatics and bioinformatics approaches. J Biomol Struct Dyn. 2023;1–17. doi: 10.1080/07391102.2023.2263213 [DOI] [PubMed] [Google Scholar]
- 28.De Groot AS, Moise L, Terry F, Gutierrez AH, Hindocha P, Richard G, et al. . Better epitope discovery, precision immune engineering, and accelerated vaccine design using immunoinformatics tools. Front Immunol. 2020;11:442. doi: 10.3389/fimmu.2020.00442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sufyan M, Shahid F, Irshad F, Javaid A, Qasim M, Ashfaq UA. Implementation of vaccinomics and in-silico approaches to construct multimeric based vaccine against ovarian cancer. Int J Pept Res Ther. 2021;27:2845–2859. doi: 10.1007/s10989-021-10294-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dobhal S, Chauhan K, Kumar S, Shikha S, Jogi M, Kumar D, et al. . In silico identification of MHC displayed tumor associated peptides inovarian cancer for multi-epitope vaccine construct. Endocr Metab Immune Disord Drug Targets. 2024;24(12):1401–1413. doi: 10.21203/rs.3.rs-2577222/v1 [DOI] [PubMed] [Google Scholar]
- 31.Schlom J, Gulley JL. Vaccines as an integral component of cancer immunotherapy. J Am Med Assoc. 2018;320:2195. doi: 10.1001/jama.2018.9511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National Institutes of Health National Cancer Institute, USA. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf
- 33.Frenel JS, Kim JW, Aryal N, Asher R, Berton D, Vidal L, et al. . Efficacy of subsequent chemotherapy for patients with BRCA1/2-mutated recurrent epithelial ovarian cancer progressing on olaparib versus placebo maintenance: post-hoc analyses of the SOLO2/ENGOT Ov-21 trial. Ann Oncol. 2022;33:1021–1028. doi: 10.1016/j.annonc.2022.06.011 [DOI] [PubMed] [Google Scholar]
- 34.Harter P, Mouret-Reynier M-A, Lorusso D, Cropet C, Guerra EM, Wolfrum-Ristau P, et al. . Efficacy of subsequent therapies in patients (pts) with advanced ovarian cancer (AOC) in the Phase III PAOLA-1/ENGOT-ov25 trial according to whether disease progression occurred during or after the end of olaparib (ola) maintenance. J Clin Oncol. 2023;41:5550–5550. doi: 10.1200/JCO.2023.41.16_suppl.555037801674 [DOI] [Google Scholar]
- 35.Ghose A, Gullapalli SVN, Chohan N, Bolina A, Moschetta M, Rassy E, et al. . Applications of proteomics in ovarian cancer: Dawn of a new era. Proteomes. 2022;10:16. doi: 10.3390/proteomes10020016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leary A, Gladieff L, Sabatier R, Paoletti X, Marmé F, Van Gorp T, et al. . TEDOVA/GINECO-OV244b/ENGOT-ov58 trial: neo-epitope based vaccine OSE2101 alone or in combination with pembrolizumab vs best supportive care (BSC) as maintenance in platinum-sensitive recurrent ovarian cancer with disease control after platinum. J Clin Oncol. 2023;41(Suppl. 16):TPS5618–TPS5618. doi: 10.1200/JCO.2023.41.16_suppl.TPS5618 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study will be available from the corresponding author upon reasonable request. Data will be shared in accordance with institutional guidelines and will require a data use agreement to ensure the confidentiality of participants.


