Abstract
Continuous glucose monitors (CGMs) improve glycemic outcomes and quality of life for many people with diabetes. Research and clinical practice efforts have focused on CGM initiation and uptake. There is limited understanding of how to sustain CGM use to realize these benefits and limited consideration for different reasons/goals for CGM use. Therefore, we apply the Information-Motivation-Behavioral Skills (IMB) model as an organizing framework to advance understanding of CGM use as a complex, ongoing self-management behavior. We present a person-centered, dynamic perspective with the central thesis that IMB predictors of optimal CGM use vary based on the CGM use goal of the person with diabetes. This reframe emphasizes the importance of identifying and articulating each person’s goal for CGM use to inform education and support.
Keywords: behavior, continuous glucose monitoring, diabetes mellitus, hemoglobin A1c, quality of life
Introduction
Continuous glucose monitors (CGMs) have transformed diabetes self-management, with access to real-time glucose levels, viewable patterns over time, alerts to cue behavioral responses to prevent/manage hypo/hyperglycemia, and ability to share data with others including clinicians, family, and friends. 1 In the United States, CGM is standard of care for type 1 diabetes,2,3 and uptake is rapidly increasing for type 2 diabetes across medication regimens. 4 Consistent CGM use is required to realize the benefits of automated insulin delivery (AID) systems, 5 and CGM use without AID can improve adults’ hemoglobin A1c (HbA1c), time in range (TIR), reduce hypoglycemia, and improve quality of life.6-11
However, outcomes do not improve universally and substantial interindividual variability in CGM wearing and viewing CGM data likely drive variability in benefits.11-14 Complex ongoing behaviors have different predictors than one-time actions like filling the first CGM prescription. The Information-Motivation-Behavioral Skills (IMB) model 15 has been successfully applied to explain and promote ongoing health behaviors in diabetes16-23 and other contexts.15,20,24 Herein, we apply the IMB model to understand CGM use as a complex ongoing self-management behavior and propose meaningful additions to include CGM use goals (i.e., why an individual is using CGM and what they hope to gain from it). These goals vary across individuals and time and can help define education/behavioral supports and operationalize CGM use. Centering CGM use goals creates a framework that is applicable across diabetes types.
IMB Model for CGM Use
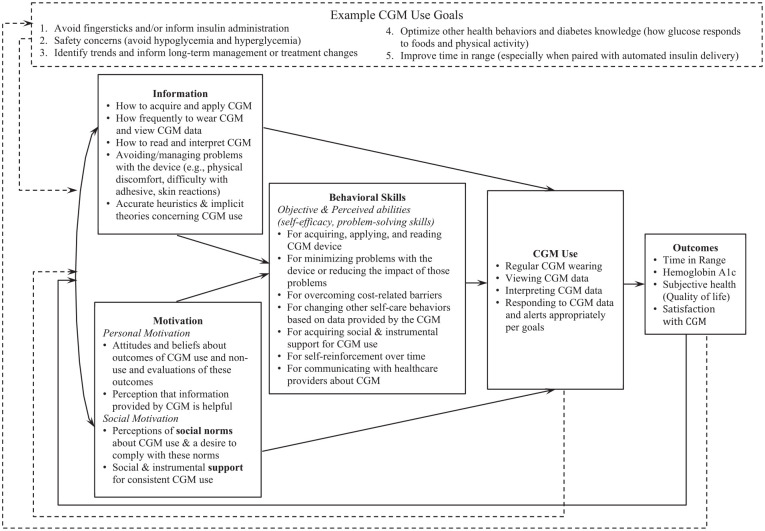
The IMB model 15 stipulates that to consistently perform a health behavior, an individual needs behavior-specific information, personal and social motivation, and behavioral skills.15,19,24-27 The primary outcome for the IMB model is the behavior. Here, that behavior is CGM use conceptualized as a continuous variable, least to most optimal (in contrast to a dichotomous variable, using vs. not). For instance, wearing a CGM for a higher proportion of time is associated with better diabetes outcomes, although exactly how much wear time is necessary for benefits is undetermined.28-30 Sustained CGM wearing requires behaviors to navigate barriers related to adhesive, skin issues, insurance coverage, and ensuring supplies are available when needed. Particularly among CGM users not using an AID system, additional behaviors are a necessary part of beneficial CGM use including checking CGM data throughout the day, viewing data trends, and responding to data and alerts.
In addition to defining “CGM use” as a complex ongoing behavior, our application of the IMB model categorizes different determinants of sustained CGM use and highlights their interrelatedness. Table 1 includes specific examples of barriers and facilitators of CGM use, organized by IMB domain. The model stipulates that more optimal CGM use should be associated with favorable health outcomes (Figure 1) which, in turn, contribute to increased information, motivation, and, by extension, behavioral skills over time via a feedback loop.
Table 1.
Factors Relevant to CGM Use Organized by Information, Motivation, and Behavioral Skills.
| Information | Knowing how often to change sensor and/or transmitter |
| Knowing how to order more supplies when running low | |
| Knowing where is best to apply the device | |
| Understanding basic knowledge about in-range and out-of-range glucose values | |
| Understanding the meaning of glucose trend arrows and how to respond to them | |
| Understanding how to access and interpret trend data | |
| Knowing how often and when to check CGM data | |
| Knowing how to calibrate and when it is necessary | |
| Knowing when fingersticks are needed | |
| Knowing there is a glucose CGM lag time (especially when glucose levels are rising or falling or during exercise) | |
| Knowing what to do in response to an out-of-range value | |
| Personal motivation | Expectations of CGM and its effects on glucose variability and quality of life |
| Perceived helpfulness of information from CGM | |
| Level of ease with wearing and changing CGM (e.g., adhesive, skin irritation, pain) | |
| Level of distress or meaning ascribed when glucose numbers fluctuate | |
| Feelings about having something on your body or “needing” a technological tool | |
| Experience of fears/worries about short- and long-term impacts of diabetes | |
| Perception of the cost-benefit ratio of CGM (e.g., if benefits are worth the cost, time, frustration, and bodily space) | |
| Level of concern about the visibility of CGM (e.g., drawing attention to having diabetes) | |
| Level of concern about false alarms (particularly when sleeping, about waking bed or room partners) | |
| Feelings about needing to explain diabetes or CGM to others | |
| Social motivation | Feelings of connection with others with diabetes (e.g., noticing that someone else also has a CGM and possibly starting a conversation) |
| Level of comfort with checking CGM and managing diabetes (e.g., administering insulin) around others (e.g., in public, at work) | |
| Social network’s values around health information (e.g., are others using data/CGM to inform health decisions outside of diabetes management?) | |
| Messaging from health care providers about utility of CGM data | |
| Emotional support from family/friends for navigating barriers or stressors related to CGM use | |
| Level of ease with experiencing/managing alerts/alarms around others | |
| Family/friends knowledge on how to respond to alerts/alarms | |
| CGM data-sharing relationships with friends/family | |
| Instrumental support for obtaining, wearing, and changing CGM | |
| Instrumental support for navigating insurance logistics pertaining to supplies and coverage | |
| Behavioral skills | Organizing and replenishing supplies |
| Managing side effects (e.g., pain, discomfort, adhesive problems, skin irritation) | |
| Setting, adjusting, and responding to alerts (e.g., adjusting to avoid alert fatigue) | |
| Self-cueing CGM checks regularly and at important times during the day | |
| Dosing/adjusting medications in response to CGM data | |
| Communicating with health care providers about problems/questions about CGM | |
| Retrieving, viewing, and interpreting CGM trend data | |
| Adjusting activities/lifestyle based on CGM data | |
| Planning and executing fingersticks when taking an intended break from CGM or between refills/during gaps in insurance coverage | |
| Contacting the CGM company and/or insurance if problems arise with refilling or using CGM (e.g., sensor failure) |
Figure 1.
Information-Motivation-Behavioral Skills (IMB) Model of CGM Use.
Curved lines indicate associations/correlations; straight lines indicate causal paths; dashed lines indicate our additions to the IMB model of CGM use. Additions to the IMB model which are new and specific to CGM use include (1) a feedback loop from CGM use to information, motivation, and, by extension, behavioral skills; (2) CGM use goals as an overarching frame informing relevant IMB domains, CGM use, and outcomes; (3) a feedback loop from outcomes to CGM use goals indicating goals change over time depending on outcomes of CGM use.
Given that CGM use itself provides frequent feedback, we propose a new second feedback loop from CGM use back to information and motivation capturing the learning and increased motivation that comes from seeing changes in glucose levels or TIR in response to behaviors or medication changes. We also propose a new overarching frame for the model which acknowledges each person’s goal for CGM use and its influence on IMB components and the definition of “optimal” use (Table 2). Relationships between the IMB model components (solid lines; Figure 1) are expected to remain as hypothesized regardless of CGM use goal. The IMB model already allows for each component of the model to vary—we add CGM use goal to organize that variation and suggest optimal CGM use and intended health outcome(s) may be different based on one’s goal. A new third feedback loop from outcomes to CGM use goal allows for goals to change over time.
Table 2.
Example CGM Use Goals With Associated Features of Optimal Use and Example Characteristics of Persons With Diabetes.
| CGM Use Goal | Features of Optimal CGM Use | Common Among Persons Who |
|---|---|---|
| Avoid fingersticks and/or inform insulin administration | • Consistent wear and/or a plan to use fingersticks during CGM breaks • Remembering to check before meals/consistent with insulin dosing schedule • Responding effectively and quickly to checks and/or alerts (including dosing insulin appropriately) |
• Check glucose values frequently • Use prandial insulin • Experience pain from or difficulty/distress related to fingersticks |
| Safety concerns (avoid hypoglycemia and hyperglycemia) | • Consistent wear and/or a plan to use fingersticks during CGM breaks • Remembering to check at important times in routine (before work or before driving) • Setting appropriate alerts • Responding quickly and effectively to checks and/or alerts • Ensuring important others receive alerts for out-of-range values and know how to respond |
• Have hyper/hypoglycemia unawareness • Prefer their glucose values to run higher or lower than recommended • Worry about or have experienced severe hypo/hyperglycemia events |
| Identify trends and inform long-term management or treatment changes | • Wear time sufficient to inform treatment changes (e.g., 2 weeks – 3 months) • Viewing and interpreting trend data • Sharing data with health care provider(s) to inform changes to medication regimen • Sharing with health care provider(s) to advise on/confirm plans for patient-directed changes (timing of medications, meals or snacks) |
• Have lower quality of life or experience distress associated with their treatment regimen or glucose excursions • Have started a new medication and want to evaluate its effectiveness • Have persistently high HbA1c values |
| Optimize other health behaviors and diabetes knowledge (how glucose responds to foods and physical activity) | • Wear could be short-term or sporadic or long-term and consistent • Reviewing data at end of the day and/or checking before/after meals/snacks and physical activity • Engaging educated others in reviewing CGM data • Changing diet (composition or timing) and planning snacks/meals around physical activity in the future • Using predictive numbers to adjust/plan for activities |
• Are newly diagnosed • Are participating in Diabetes Self-Management Education and Support • Do not use prandial insulin • Experienced recent unexplained increases in HbA1c |
Information
CGM use information includes accurate knowledge about wearing a CGM, viewing and interpreting data, and responding when challenges arise. It also includes having accurate heuristics and theories that support CGM use as opposed to inaccurate heuristics (e.g., any glucose variation is harmful). Knowledge of how and when to read CGM data and respond to alerts requires general diabetes education and knowledge about in-range and out-of-range glucose values, the meaning of trend arrows, and how to access and interpret trend data. Certain information and heuristics are essential for enhancing trust in the CGM glucose values: knowing about false lows due to compression of the device, understanding discrepancies between CGM values and fingerstick values, and knowing how long it takes for insulin administration to affect values. Knowledge that uncontrollable factors (hormonal changes, weather, illness, stress) affect glucose may be necessary when accessing continuous glucose data. Per the IMB model, levels of information are associated with levels of motivation. Information and motivation directly impact behavioral skills and indirectly impact CGM use.
Motivation
Personal motivation reflects individuals’ attitudes about CGM use which are based on beliefs or expectations that CGM can benefit them, and perceptions of the information provided by the CGM as helpful. A person’s overall perception of knowledge about their diabetes-related numbers can range from useful and informative to distressing, anxiety-inducing, or shame-inducing. Continuous glucose monitors can exacerbate or challenge these existing perceptions, impacting motivation for ongoing use. Other relevant beliefs are the meaning of “needing” a medical device or technology and of wearing a device on the body. Relevant expectations require balancing positive expectations of how CGM can provide benefit with realistic expectations about potential burdens of CGM use. Maintaining CGM use may require realistic expectations that include experiences of distress and hassles as well as benefits of CGM use.
Social motivation for CGM use rests on perceived social norms endorsing CGM use and social and instrumental support for CGM use. Relevant social norms include concerns about the visibility of CGM and diabetes stigma. In some groups, CGM use may be normative, whereas in other groups, a person wearing CGM may have to address repeated inquiries about the device. Continuous glucose monitor alarms in social, work, or public settings may increase stigma and negative feedback about diabetes. Social and instrumental support for CGM use includes reinforcement from clinicians that CGM is useful and informative. This may involve clinicians reviewing CGM data to inform clinical care decisions and expressing the importance of these data. In addition, family/friends may offer emotional support (listening and expressing empathy for hassles and feelings about addressing others’ inquiries) and instrumental support including help navigating insurance, family/friends knowing how to respond to CGM alerts or alarms. Others’ response to seeing CGM on the body or hearing alerts may be harmful or conflictual and nighttime alarms may strain relationships with bed or room partners.
Sharing CGM data with friends/family may be a social motivation factor unique to CGM. The literature on CGM data sharing demonstrates benefits for both the person with diabetes and the share partner. 31 However, to ensure a successful data-sharing relationship, it is critical to establish ground rules.32,33 Ground rules are intended to honor the person with diabetes’s preferences and autonomy by setting forth expectations of how the friend/family should respond to the data. Knowing and articulating CGM use goals may inform ground rules and maximize benefits of sharing.
Personal and social motivation influences one another. For instance, personal beliefs may affect interest in CGM data sharing. CGM may be viewed as increasing autonomy and reducing concerns of family members, or as impeding autonomous diabetes management. The degree to which sharing CGM data supports or interferes with those perceptions likely informs comfort with data sharing. Similarly, social norms, personal beliefs, or values around privacy may reduce motivation to seek social support through CGM data sharing.
Behavioral Skills
Behavioral skills include objective and perceived abilities to maintain CGM use across different situations including obtaining and applying the device; setting, adjusting, and responding to alerts; dosing/adjusting insulin in response to CGM data; and/or adjusting activities/lifestyle based on CGM data. Moreover, behavioral skills include problem-solving skills to overcome challenges with CGM use, such as managing and overcoming technical difficulties, and communicating with health care providers or insurance companies if problems/questions arise.
Information and motivation work primarily through behavioral skills to affect CGM use. For instance, having accurate knowledge of how to respond to an out-of-range CGM alert (information), the belief that addressing an out-of-range CGM alert is beneficial (personal motivation), and comfort addressing an out-of-range value around others (social motivation) all affect whether a person will have self-efficacy and adequate behavioral skills to respond to CGM alerts consistent with their broader goals to manage diabetes.
Model Application
Optimal education and support for CGM use will require identification of each person’s goal to guide which aspects of IMB skills to emphasize. For example, a person using prandial insulin whose goal is to avoid hypoglycemia may prefer to avoid gaps in CGM use, develop habits to view CGM data at key times, and respond to alerts and arrows in a timely fashion. In this case, CGM-related education and support may emphasize information about the meaning of arrows and alerts and associated required changes in insulin administration. Social motivation may include sharing alerts for hypoglycemia with others with whom they spend time (partners, coworkers). Desired benefits may include increasing TIR and improving quality of life.
In contrast, a person not using prandial insulin may use CGM with the goal of optimizing other health behaviors, allowing for more time-limited use (wearing when making behavior changes or undergoing changes in their medications) and viewing trends at the end of each day or week. Education may focus on how CGM informs dietary, physical activity, or medication adherence changes. CGM education and support may focus on how to view and interpret trend data and emphasize the feedback loop from glucose data to information and motivation. They may choose to share and/or review data with someone who understands how behaviors affect glucose for people with diabetes (e.g., a health care professional, family/friend with diabetes). Desired benefits may be lower HbA1c or more TIR.
Conclusion
CGM use is a complex ongoing self-management behavior, conceptualized with the IMB model to guide efforts to realize long-term glycemic and quality-of-life benefits for people with diabetes. This framework can be applied to understand heterogeneous experiences and outcomes of CGM use, and to guide education, support, and interventions for CGM use. This model emphasizes that CGM is a tool to support a person with diabetes and highlights how diabetes education can be tailored to identify what information is needed in accordance with an individual’s goals and desired outcomes. Certain goals may be more prevalent based on diabetes characteristics like diabetes duration, medication regimen, HbA1c level, and frequency of hyper/hypoglycemia. CGM goals may evolve depending on life demands and time/experience with diabetes and with CGM, thus, ongoing evolving support may be needed for sustained and effective CGM use.
The IMB model is designed to explain, predict, and guide interventions on an individual level, with acknowledgment of the role of social motivation and its drivers. The model does not incorporate health care system-level factors known to be critical (CGM-related costs, insurance coverage, billing codes, and expertise of clinicians)34-36 or aspects of the CGM technology/device. However, a strength of the IMB model is the dynamic consideration of changes in the individual’s knowledge, motivation, and behavioral skills which are affected by these factors.
At this point, the IMB model of CGM use is proposed as a useful organizing framework based on existing literature and our experiences with clinical care and intervention research. Important next steps include identifying/developing necessary measures and applying them toward validation of the IMB model of CGM use. Further research should elucidate which specific aspects of each IMB component are most relevant for different CGM use goals. We also suggest operationalization of sustained CGM use should allow different degrees of ongoing use to be considered “optimal” for different use cases and goals. Our framework can inform the types and frequency of clinician support required, which is essential as use of CGM in primary care settings increases.4,36 IMB model interventions should tailor content to individuals’ goals and barriers, dynamically over time, and evaluate intervention effects on CGM use and anticipated outcomes.15,16,22
Footnotes
Abbreviations: AID, automated insulin delivery; CGM, continuous glucose monitor; HbA1c, hemoglobin A1c; IMB, information-motivation-behavioral skills; TIR, time in range
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: All authors’ contributions to this work were supported by the Leona M. and Harry B. Helmsley Charitable Trust (Author LSM is PI). Author NAA received consulting fees from Diathrive Health and has a grant from Dexcom for equipment being used in an NIH-funded research project. KH received consulting fees from Cecelia Health and Sanofi.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This collaboration was supported by the Leona M. and Harry B. Helmsley Charitable Trust (grant no. R-2203-05822).
ORCID iDs: Lindsay S. Mayberry  https://orcid.org/0000-0002-0654-4151
https://orcid.org/0000-0002-0654-4151
Jennifer K. Raymond  https://orcid.org/0000-0003-1866-4932
https://orcid.org/0000-0003-1866-4932
Molly L. Tanenbaum  https://orcid.org/0000-0003-4222-4224
https://orcid.org/0000-0003-4222-4224
Sarah S. Jaser  https://orcid.org/0000-0002-7958-7662
https://orcid.org/0000-0002-7958-7662
Nancy Allen  https://orcid.org/0000-0001-7358-2265
https://orcid.org/0000-0001-7358-2265
Diana Naranjo  https://orcid.org/0000-0001-8039-0616
https://orcid.org/0000-0001-8039-0616
Michelle Litchman  https://orcid.org/0000-0002-8928-5748
https://orcid.org/0000-0002-8928-5748
Korey Hood  https://orcid.org/0000-0001-5730-7749
https://orcid.org/0000-0001-5730-7749
References
- 1. Continuous Glucose Monitoring. National Institute of Diabetes and Digestive and Kidney Diseases. Published 2017. Accessed July 24, 2024. https://www.niddk.nih.gov/health-information/diabetes/overview/managing-diabetes/continuous-glucose-monitoring#:~:text=an%20artificial%20pancreas%3F-,What%20is%20continuous%20glucose%20monitoring%3F,or%20days%20to%20see%20trends.
- 2. Peters AL, Ahmann AJ, Battelino T, et al. Diabetes technology—continuous subcutaneous insulin infusion therapy and continuous glucose monitoring in adults: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(11):3922-3937. [DOI] [PubMed] [Google Scholar]
- 3. American Diabetes Association. Diabetes technology: standards of medical care in diabetes-2019. Diabetes Care. 2019;42(suppl 1):S71-S80. [DOI] [PubMed] [Google Scholar]
- 4. Mayberry LS, Guy C, Hendrickson CD, McCoy AB, Elasy T. Rates and correlates of uptake of continuous glucose monitors among adults with type 2 diabetes in primary care and endocrinology settings. J Gen Intern Med. 2023;38(11):2546-2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Breton MD, Kovatchev BP. One year real-world use of the control-IQ advanced hybrid closed-loop technology. Diabetes Technol Ther. 2021;23(9):601-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Seidu S, Kunutsor SK, Ajjan RA, Choudhary P. Efficacy and safety of continuous glucose monitoring and intermittently scanned continuous glucose monitoring in patients with type 2 diabetes: a systematic review and meta-analysis of interventional evidence. Diabetes Care. 2024;47(1):169-179. [DOI] [PubMed] [Google Scholar]
- 7. Champakanath A, Akturk HK, Alonso GT, Snell- Bergeon JK, Shah VN. Continuous glucose monitoring initiation within first year of type 1 diabetes diagnosis is associated with improved glycemic outcomes: 7-year follow-up study. Diabetes Care. 2022;45(3):750-753. doi: 10.2337/dc21-2004. [DOI] [PubMed] [Google Scholar]
- 8. Sanderson EE, Abraham MB, Smith GJ, Mountain JA, Jones TW, Davis EA. Continuous glucose monitoring improves glycemic outcomes in children with type 1 diabetes: real-world data from a population-based clinic. Diabetes Care. 2021;44(9):e171-e172. doi: 10.2337/dc21-0304. [DOI] [PubMed] [Google Scholar]
- 9. Maiorino MI, Signoriello S, Maio A, et al. Effects of continuous glucose monitoring on metrics of glycemic control in diabetes: a systematic review with meta-analysis of randomized controlled trials. Diabetes Care. 2020;43(5):1146-1156. doi: 10.2337/dc19-1459. [DOI] [PubMed] [Google Scholar]
- 10. Lin TM, Jacquelyn A, Illesca N, et al. Improving continuous glucose monitoring uptake in underserved youth with type 1 diabetes: the impact study. Diabetes Technol Ther. 2023;25(1):13-19. doi: 10.1089/dia.2022.0347. [DOI] [PubMed] [Google Scholar]
- 11. Tanenbaum ML, Hanes SJ, Miller KM, Naranjo D, Bensen R, Hood KK. Diabetes device use in adults with type 1 diabetes: barriers to uptake and potential intervention targets. Diabetes Care. 2017;40(2):181-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kubiak T, Mann CG, Barnard KC, Heinemann L. Psychosocial aspects of continuous glucose monitoring: connecting to the patients’ experience. J Diabetes Sci Technol. 2016;10(4):859-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Naranjo D, Tanenbaum ML, Iturralde E, Hood KK. Diabetes technology: uptake, outcomes, barriers, and the intersection with distress. J Diabetes Sci Technol. 2016;10(4):852-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Akturk HK, Dowd R, Shankar K, Derdzinski M. Real-world evidence and glycemic improvement using Dexcom G6 features. Diabetes Technol Ther. 2021;23(suppl 1):S21-S26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fisher JD, Fisher WA, Amico KR, Harman JJ. An Information-Motivation-Behavioral skills model of adherence to antiretroviral therapy. Health Psychol. 2006;25(4):462-473. doi: 10.1037/0278-6133.25.4.462. [DOI] [PubMed] [Google Scholar]
- 16. Mayberry LS, Osborn CY. Empirical validation of the Information-Motivation-Behavioral skills model of diabetes medication adherence: a framework for intervention. Diabetes Care. 2014;37(5):1246-1253. doi: 10.2337/dc13-1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nelson LA, Wallston KA, Kripalani S, LeStourgeon LM, Williamson SE, Mayberry LS. Assessing barriers to diabetes medication adherence using the Information-Motivation-Behavioral skills model. Diabetes Res Clin Pract. 2018;142:374-384. doi: 10.1016/j.diabres.2018.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ferrari M, Speight J, Beath A, Browne JL, Mosely K. The Information-Motivation-Behavioral skills model explains physical activity levels for adults with type 2 diabetes across all weight classes. Psychol Health Med. 2021;26(3):381-394. doi: 10.1080/13548506.2020.1749292. [DOI] [PubMed] [Google Scholar]
- 19. Meunier S, Coulombe S, Beaulieu MD, et al. Longitudinal testing of the Information-Motivation-Behavioral skills model of self-care among adults with type 2 diabetes. Patient Educ Couns. 2016;99(11):1830-1836. doi: 10.1016/j.pec.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 20. Zarani F, Besharat MA, Sadeghian S, Sarami G. The effectiveness of the Information-Motivation-Behavioral skills model in promoting adherence in CABG patients. J Health Psychol. 2010;15(6):828-837. doi: 10.1177/1359105309357092. [DOI] [PubMed] [Google Scholar]
- 21. Osborn CY, Amico KR, Cruz N, et al. A brief culturally tailored intervention for Puerto Ricans with type 2 diabetes. Health Educ Behav. 2010;37(6):849-862. doi: 10.1177/1090198110366004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nelson LA, Greevy RA, Spieker A, et al. Effects of a tailored text messaging intervention among diverse adults with type 2 diabetes: evidence from the 15-month REACH randomized controlled trial. Diabetes Care. 2021;44(1):26-34. doi: 10.2337/dc20-0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gavgani RM, Poursharifi H, Aliasgarzadeh A. Effectiveness of Information-Motivation and Behavioral skill (IMB) model in improving self-care behaviors & Hba1c measure in adults with type 2 diabetes in Iran-Tabriz. Procedia Soc. Behav Sci. 2010;5:1868-1873. [Google Scholar]
- 24. Amico KR, Toro-Alfonso J, Fisher JD. An empirical test of the Information, Motivation and Behavioral skills model of antiretroviral therapy adherence. AIDS Care. 2005;17(6):661-673. doi: 10.1080/09540120500038058. [DOI] [PubMed] [Google Scholar]
- 25. Starace F, Massa A, Amico KR, Fisher JD. Adherence to antiretroviral therapy: an empirical test of the Information- Motivation-Behavioral skills model. Health Psychol. 2006;25(2):153-162. [DOI] [PubMed] [Google Scholar]
- 26. Osborn CY, Egede LE. Validation of an Information–Motivation–Behavioral skills model of diabetes self-care (IMB-DSC). Patient Educ Couns. 2010;79(1):49-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gao J, Wang J, Zhu Y, Yu J. Validation of an Information–Motivation–Behavioral skills model of self-care among Chinese adults with type 2 diabetes. BMC Public Health. 2013;13(1):1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tanenbaum ML, Adams RN, Hanes SJ, et al. Optimal use of diabetes devices: clinician perspectives on barriers and adherence to device use. J Diabetes Sci Technol. 2017;11(3):484-492. doi: 10.1177/1932296816688010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chase HP, Beck RW, Xing D, et al. Continuous glucose monitoring in youth with type 1 diabetes: 12-month follow-up of the Juvenile Diabetes Research Foundation continuous glucose monitoring randomized trial. Diabetes Technol Ther. 2010;12(7):507-515. [DOI] [PubMed] [Google Scholar]
- 30. Tansey M, Laffel L, Cheng J, et al. Satisfaction with continuous glucose monitoring in adults and youths with type 1 diabetes. Diabet Med. 2011;28(9):1118-1122. [DOI] [PubMed] [Google Scholar]
- 31. Polonsky WH, Fortmann AL. Impact of real-time continuous glucose monitoring data sharing on quality of life and health outcomes in adults with type 1 diabetes. Diabetes Technol Ther. 2021;23(3):195-202. doi: 10.1089/dia.2020.0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Litchman ML, Allen NA, Colicchio VD, et al. A qualitative analysis of real-time continuous glucose monitoring data sharing with care partners: to share or not to share? Diabetes Technol Ther. 2018;20(1):25-31. doi: 10.1089/dia.2017.0285. [DOI] [PubMed] [Google Scholar]
- 33. Allen NA, Litchman ML, Chamberlain J, Grigorian EG, Iacob E, Berg CA. Continuous glucose monitoring data sharing in older adults with type 1 diabetes: pilot intervention study. JMIR Diabetes. 2022;7(1):e35687. doi: 10.2196/35687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Been RA, Lameijer A, Gans ROB, et al. The impact of socioeconomic factors, social determinants, and ethnicity on the utilization of glucose sensor technology among persons with diabetes mellitus: a narrative review. Ther Adv Endocrinol Metab. 2024;15:20420188241236289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ni K, Tampe CA, Sol K, Richardson DB, Pereira RI. Effect of CGM access expansion on uptake among patients on Medicaid with diabetes. Diabetes Care. 2023;46(2):391-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Oser TK, Hall TL, Dickinson LM, et al. Continuous glucose monitoring in primary care: understanding and supporting clinicians’ use to enhance diabetes care. Ann Fam Med. 2022;20(6):541-547. [DOI] [PMC free article] [PubMed] [Google Scholar]