Abstract
Digital twin is a new concept that is rapidly gaining recognition especially in the medical field. Indeed, being a virtual representation of real-world entities and processes, a digital twin can be used to accurately represent the patients’ disease, clarify the treatment target, and realize personalized and precise therapies. However, despite being a revolutionary concept, the diffusion of digital twins in type 1 diabetes (T1D) is still limited. In this systematic review, we analyzed structure, operating conditions, and characteristics of digital twins being developed for T1D. Our search covered published documents until March 2024: 220 publications were identified, 37 of which were duplicated entries; in addition, 173 publications were removed after inspection of titles, abstracts, and keywords; and finally, 11 publications were fully reviewed, of which 8 were deemed eligible for inclusion. We found that all eight methodologies are not comprehensive multi-scale virtual replicas of the individual with T1D, but they all focus on describing glucose-insulin metabolism, aiming to simulate glucose concentration resultant from therapeutic interventions. In this review, we will compare and analyze different factors characterizing these digital twins, such as operating principles (mathematical model, twinning procedure, validation and assessment) and the key aspects for practical adoption (inclusion of physical activity, data required for twinning, open-source availability). We will conclude the paper listing which, in our opinion, are the current limitations and future directives of digital twins in T1D, hoping that this article can be helpful to researchers working on diabetes technologies to further develop the use of such an important instrument.
Keywords: digital twins, diabetes technology, virtual replica, replay, simulation
Introduction
Digital Twin: Definition and Characteristics
Digital twin is a new concept that nowadays is widely used, especially in the medical field. However, despite its occasional adoption since 2002 under other names, eg, “virtual twin,” the first official appearance was in 2010 in documents released by the National Aeronautics and Space Administration (NASA), in which a digital twin was described as “an integrated multi-physics, multi-scale, probabilistic ultra-realistic simulation of a vehicle or system that uses the best available physical models, sensor updates, fleet history, etc, to mirror the life of its flying twin.”1,2
Probably, one of the most effective definitions of digital twin is the one released by the Digital Twin Consortium: “a digital twin is a virtual representation of real-world entities and processes, synchronized at a specified frequency and fidelity.” 3 Therefore, a digital twin is more than just model, as it has to be very precise and accurate in describing the physical object being twinned, it should be based on past and real-time data related to the entity to be described, and it should be able to adjust its behavior as new data become available. 4 As correctly highlighted by Wang and colleagues, “a digital twin is not simply a digital clone of the physical system. Instead, it’s an intelligent counterpart.” 5
The previous definition can be completed by the key properties of digital twins:6-8
Connected: it requires a network of sensors to get data and integrate and communicate these data through various integration technologies;
Homogeneous: requiring data, it is both consequence and enabler of data homogenization and the decoupling of the information from its physical artifact (anonymization);
Reprogrammable and smart: through sensors, artificial intelligence, and predictive analytics;
Maker of digital traces: it leaves traces that can be used by engineers, eg, to diagnose where the problem occurred;
Modular: it should be possible to identify poorly performant modules and replace them with better fitting ones.
Digital Twin in Medicine and Type 1 Diabetes
Starting from the description provided above and the main features/characteristics, it is straightforward to acknowledge that the digital twin represents a potential revolution in the field of medicine, as a digital twin of the human body or of a part of it can provide robust, precise, and effective medical services at limited cost, without any intervention on the individuals, except the need of collecting data. 9 Even more important in the field of medicine, a digital twin can be used to accurately represent the patients’ disease, clarify the treatment target, and realize personalized and precise therapies. However, despite being a revolutionary concept, the diffusion of digital twins in medicine is still limited. According to Botin-Sanabria et al, 10 only 1% of the industrial application digital twin–based are in the medicine/health care field. According to the authors, the main reasons for such a limited diffusion are that gathering and/or using clinical-related data is more critical than in other engineering fields because of data privacy problems, complexity in creating reliable and detailed human models, medical liability, timely feedback, and vocabulary ambiguity in electronic medical records.
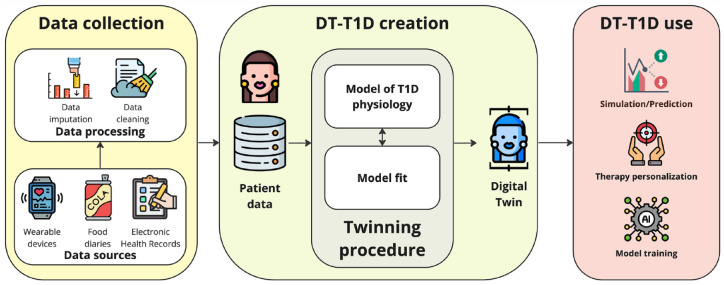
How does a digital twin in medicine work? Without any loss of generality, we can describe it directly by declining the digital twin concept into type 1 diabetes (T1D), as presented at the Diabetes Technology Meeting 2023, 11 which is schematized in Figure 1. Digital twins in T1D require a computer model that serves as a “blueprint” to represent a patient with T1D. Then, by combining data from various sources, such as electronic medical records, genetic information, wearable devices (eg, continuous glucose monitoring—CGM—sensors, insulin smart pens, insulin pumps, closed-loop control systems, etc), and food diaries (properly processed to comply to the peculiar model input), it is possible to generate the digital twin of the T1D individual resorting to a suitable twinning procedure that allows personalizing such a blueprint to the patient. Then, the T1D digital twin can be used to simulate/predict different scenarios, such as how a particular drug/lifestyle or changes in the treatment regimen might affect glucose control, personalize T1D therapy, and train artificial intelligence models.
Figure 1.
DT-T1D general schema.
To better understand the diffusion of the development and use of digital twins in type 1 diabetes (DT-T1D), we conducted a systematic review to map research in this area.
Methods
Study Design
The primary objective of this review was to identify and evaluate currently available methodologies for DT-T1D, aiming to understand their operational mechanisms and delineate their main characteristics along with their strengths and limitations. To achieve this goal, we conducted an extensive literature search encompassing various scholarly databases, including PubMed, Web of Science, IEEE Xplore, and Scopus. These databases were selected for their comprehensive coverage of peer-reviewed journal articles and conference proceedings in the fields of medicine, engineering, and computer science, which are pertinent to DT-T1D research.
Search Strategy
The search strategy adopted the following query: (“digital twin” OR “replay”) AND “type 1 diabetes.” This query was designed to capture studies that specifically address DT-T1D by including also the “replay” keyword to ensure coverage of relevant literature exploring simulation-based approaches for T1D close to the digital twin concept and rationale. The selected timeframe for the search spanned the last 20 years, from April 1, 2004, to March 31, 2024, to encompass a substantial body of literature while focusing on recent advancements and developments in DT-T1D methodologies. Finally, we decided to exclude papers written in languages other than English, non–peer-reviewed papers, conference abstracts, and papers presenting decision support systems for T1D that use a digital twin framework as a core component.
Results
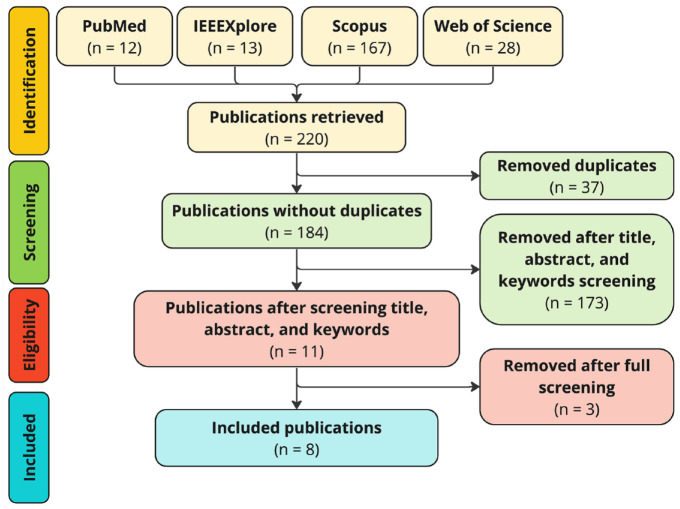
The screening process is summarized in the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) 12 flow diagram shown in Figure 2.
Figure 2.
PRISMA flow diagram of the article selection process.
Comprehensively, 220 publications were identified, 37 of which were duplicated entries. In addition, 173 publications were removed after inspection of titles, abstracts, and keywords. Consequently, 11 publications were fully reviewed, of which 8 were deemed eligible for inclusion. Particularly, we excluded the following three works: (1) Patek et al, 13 (2) Diaz et al, 14 and (3) Silfvergren et al. 15 Briefly, we excluded (1) because it represents an old version of an already included work (ie, the method of Hughes et al 16 ), (2) because it presented a decision support system for T1D that uses the digital twin framework as one of its core components, and (3) because it involved healthy people and people with type 2 diabetes rather than people with T1D.
The eight DT-T1D methodologies selected through the procedure are not comprehensive multi-scale, from cellular to whole organism complexity, virtual replicas of the individual with T1D. Instead, these methodologies are the tools that focus on describing the dynamics of glucose-insulin metabolism, aiming to simulate plasma glucose concentration resultant from therapeutic interventions, such as alterations in carbohydrate intake during meals, insulin dosage administered throughout the day, and physical activity.
Below, we compared and analyzed different factors which, following our expertise, condense both the operating principles and the key aspects for practical adoption of the included studies.
Comparison of Considered Digital Twins in Type 1 Diabetes Operating Principles
As summarized in Table 1 and analyzed below in details, we described each work in terms of four factors that condense their operating principles:
Table 1.
Summary of Factors That Describe the Operating Principles of Included DT-T1D Methodologies.
| DT-T1D method | Model | Twinning procedure | Validation process | Comparison with other methodologies |
|---|---|---|---|---|
| Cappon et al 17 | Composite model of previous works18-21 | Identification of a subset of most relevant model parameters via Markov Chain Monte Carlo | Tested the ability to replay ground-truth data generated using the UVa/Padova T1D Simulator (version 2018 22 ) when the meal/insulin regimen used for twinning is altered | Compared with Hughes et al 16 |
| Colmegna et al 23 | Model of UVa/Padova Simulator (version 2014 24 ) | Identification of a subset of most relevant model parameters along with a variability component that captures daily variations in insulin sensitivity via Maximum A Posteriori regression | Tested the ability to replay ground-truth data generated using the UVa/Padova T1D Simulator (version 2014 24 ) when the meal/insulin regimen used for twinning is altered | No |
| Deichmann et al 25 | Composite model of previous works,18,26,27 and a custom model of physical activity effect | Identification of a subset of most relevant model parameters via Maximum Likelihood (least squares) regression | Tested the ability to replay ground-truth data generated using the UVa/Padova T1D Simulator (version 2007 28 ) when the meal/insulin regimen used for twinning is altered | No |
| Goodwin et al 29 | Transfer functions of meal and insulin to glucose summed to an envelope of low-order-model responses capturing model uncertainty | Identification of transfer function poles and gains as those minimizing a custom cost function | Tested the ability to predict single-meal scenarios in a controlled clinical trial setup | No |
| Haidar et al 30 | Haidar et al 30 | Model parameters identified via Markov Chain Monte Carlo | No. Just tested the ability to fit a set of real glucose traces | No |
| Hughes et al 16 | Composite model of previous works,18,19 and a custom model of meal absorption | Two steps: (1) Identification of a subset of most relevant model parameters via Maximum Likelihood (least squares) regression and (2) model residual error is fit by deconvolution to explain and represent unmodeled phenomena | Tested the ability to replay ground-truth data generated using the UVa/Padova T1D Simulator (version 2018 22 ) when the meal/insulin regimen used for twinning is altered | No |
| Visentin et al 31 | UVa/Padova T1D Simulator (version 2014 24 ) | Model parameters identified via Maximum A Posteriori regression | No. Just tested the ability to fit a set of real glucose traces | No |
| Young et al 16 | Resalat et al 32 | Before exercising, the patient is matched to a virtual patient of an existing virtual population 32 . The digital twin is the virtual patient with the closest simulated glucose trace in the 4 hours preceding each exercise session | No | No |
DT-T1D methods are reported in an alphabetical order.
the mathematical model used to represent the subject’s digital twin;
the adopted twinning procedure;
the procedure used to validate the proposed DT-T1D;
the presence of results aimed to compare the proposed DT-T1D vs other state-of-the-art methods.
Model
Trivially, a fundamental aspect that characterizes a specific DT-T1D framework is the mathematical model employed to represent the glucose-insulin dynamics of people with T1D and obtain their digital twin. We found that three out of eight (37.5%) DT-T1D methods16,17,25 used a composite model of glucose-insulin kinetics with the Bergman’s minimal model 18 at its core, two out eight methodologies (25.0%)23,31 used the model of Dalla Man et al, 24 one out of eight works (12.5%) 33 used the Resalat et al’s 32 model, and two out of eight works (25.0%)29,30 used a custom description of T1D physiology.
Twinning procedure
Regarding the employed approach to run the twinning procedure, this aspect is tied to the model used to create the digital twin. Indeed, the chosen model identification technique is not universal and it is selected to be compatible with the peculiar model structure and deal with potential model identifiability issues. We found that two out of eight (25.0%) DT-T1D used Markov Chain Monte Carlo–based techniques,17,30 two out of eight (25.0%) opted for a Maximum A Posteriori regression algorithm,23,31 three out of eight (37.5%) used a Maximum Likelihood (least squares) regression approach,16,25,29 whereas the method of Young et al 33 twinned each patient by simply matching him/her to the virtual patient of an existing virtual population 32 who exhibits the most similar glucose trace in a specific before-exercise time window.
Validation process
The validation procedure of a DT-T1D is far from trivial. The most common and accepted approach, which is adopted in four out of eight (50%) DT-T1D methodologies,16,17,25,23 relies on the use on synthetic data sets generated using sophisticated simulators which integrate maximal models of T1D glucose-insulin physiology.24,26,34,35 At first, these simulators are used to establish a starting point scenario, against which the fitting performance of the DT-T1D can be evaluated. Afterwards, the simulators are used to produce different scenarios, which serve as new reference points to test the DT-T1D’s ability to accurately replicate them. This solution is the only one that allows obtaining the glycemic ground truth resulting from an alteration of the original treatment regime. Following a different direction, we found that only one out of eight (12.5%) DT-T1D validated their results using real data. 29 Particularly, they used data collected in a two-week at-home study where subjects were asked to have standardized breakfasts but with different insulin therapy regimes. However, given the many factors that can perturbate glucose levels over these two weeks, 29 limited to qualitatively evaluate whether the replayed glucose traces were “biologically consistent” with those observed during the trial. Finally, two out of eight (25.0%) methodologies31,30 just compared the quality of data fit and model parameter distributions obtained after twinning without actually challenging the system to replicate different therapy regimes, whereas Young et al 33 did not assessed the DT-T1D per se, but they only evaluated the performance of a decision support system for exercise management having the proposed DT-T1D as core component.
Comparison with other digital twins in type 1 diabetes methodologies
Finally, a fundamental aspect in analyzing the performance of a DT-T1D framework is comparing the reported results with those obtained from other similar approaches to demonstrate the novelty of the proposed methodology, its strength and weaknesses, and, ultimately, its potential impact. Of note, as summarized in Table 1, we found that only Cappon et al (ie, one out of eight [12.5%]) compared their DT-T1D framework against other state-of-the-art approaches, ie, the method of Hughes et al. 16
Comparison of Key Factors for Practical Adoption of Digital Twins in Type 1 Diabetes
When reviewing new technologies for health care as DT-T1D, it is important to consider factors able to qualitatively evaluate their practical use. Indeed, useless to say, if a new methodology is hard to be replicated and/or it requires data that are difficult to be collected, such as plasma samples, to function, its potential for adoption within the health care system may be severely limited.
To this aim, as reported in Table 2 and discussed in detail below, for each included work, we identified four key aspects that, based on our experience, are desirable when using DT-T1D as tools to both evaluate and/or design new therapies and methodologies for T1D management:
Table 2.
Summary of Key Factors to Facilitate the Practical Adoption of the Included DT-T1D Methodologies to Evaluate and/or Design Therapies for T1D Management.
| DT-T1D method | Possibility to replay physical activity | Possibility to replay psychological/social factors | Required data for twinning | Open-source availability |
|---|---|---|---|---|
| Cappon et al 17 | No | No | Glucose, Meal, Insulin | Yes. MATLAB implementation: https://github.com/gcappon/replay-bg Python implementation: https://github.com/gcappon/py_replay_bg |
| Colmegna et al 23 | No | No | Glucose, Meal, Insulin | No |
| Deichmann et al 25 | Yes | No | Glucose, Meal, Insulin, Accelerometry | Yes. Python implementation: https://gitlab.com/csb.ethz/t1d-exercise-model |
| Goodwin et al 29 | No | No | Glucose, Meal, Insulin | No |
| Haidar et al 30 | No | No | Plasma glucose, Meal, Plasma insulin | No |
| Hughes et al 16 | No | No | Glucose, Meal, Insulin | No |
| Visentin et al 31 | No | No | Plasma glucose, Meal, Plasma insulin | No |
| Young et al 33 | Yes | No | Glucose, Meal, Insulin, Heart Rate | No |
DT-T1D methods are reported in an alphabetical order.
the possibility to represent and replay physical activity;
the possibility to represent and replay psychological/social factors;
the data that are required to create the digital twin, ie, to run the twinning procedure;
the availability of the proposed work as an open-sourced software package.
Possibility to represent and replay physical activity
Physical activity has a major impact on glucose concentration thus requiring people with T1D extra attention in order to keep it in the normal euglycemic range before, during, and after activities. As such, adopting a DT-T1D that incorporates a model of physical activity capable of capturing and replicating its impact on glucose levels certainly enhances its practical utility. However, we found that only two out of eight (25.0%)25,33 methodologies integrate this functionality. Specifically, drawing from the research of Roy et al, 36 Breton et al, 37 and Lenart et al 25 , Lenart and Parker 38 used a physical activity model driven by accelerometer data counts to detect movements, correlate them with physical activity intensity, and trigger an increase in insulin sensitivity, glucose uptake, and glucose production. In contrast, Young et al 33 employed the model proposed by Resalat et al, 32 leveraging changes in heart rate relative to a baseline value to induce similar effects.
Possibility to represent and replay psychological/social factors
Psychological and social factors are well-known to influence glucose control, playing a significant role in the overall management of T1D. Unfortunately, our review revealed that none of the eight identified DT-T1D models incorporate these critical aspects. This gap highlights an important area for future research, suggesting that integrating psychological and social dimensions into DT-T1D could significantly enhance their effectiveness. Addressing this deficiency opens new perspectives and opportunities for improving the holistic management of T1D through more comprehensive and inclusive modeling approaches.
Required data for twinning
As anticipated, the type of data necessary to run the twinning procedure and create the digital twin plays a fundamental role for the practical use of DT-T1D frameworks. Indeed, such data should be easily obtainable and as much as possible reliable in order to avoid generating wrong replicas of the individuals with T1D. Driven by this rationale, we found that most of the included methodologies—ie, six out of eight (75.0%)16,17,25,23,29,33—operate using easily accessible information, ie, glucose data collected from CGM, meal logs, insulin recordings, and accelerometer/heart rate signals measured with a physical activity tracker, whereas the remaining two methodologies31,30 require to invasively collect plasma glucose and plasma insulin samples, limiting their broad practical adoption.
Open-source availability
Finally, in our evaluation, we assessed if the DT-T1D systems under consideration were available as open-source software, as this aspect promotes transparency, accessibility for health care organizations with limited budgets, and customizability for tailoring them to specific needs and workflows. However, only two out of eight (25.0%) DT-T1D systems17,16 were found to be open-source. This limitation underscores the need for greater availability of open-source solutions in this domain.
Current use of Digital Twins in Type 1 Diabetes Frameworks
The DT-T1D frameworks clearly represent powerful simulation environments that can be used to leverage retrospective data to first obtain a cohort of digital twins that mimic the physiological variability of the observed population with T1D, and then set up in silico clinical trials (ISCTs) aimed to test “what would have potentially happened” if other alternative therapies “would have been adopted” instead of those originally reported. Following this direction, the DT-T1D of Cappon et al 17 has been adopted in the literature to validate new insulin dosing strategies targeting hyperglycemic episodes, 39 new meal insulin bolus calculators, 40 and to assess the use of an interpretable glucose prediction algorithm based on LSTM (Long Short-Term Memory). 41
Another possible application of DT-T1D is to use them as an active component of decision support systems for T1D management. For example, in Young et al, 33 they proposed a new decision support system leveraging their DT-T1D for personalizing the management of glucose during and after physical exercise showing, in a dedicated ISCT, the potential improvement of glucose outcomes thanks to its use. Finally, one great possibility provided by DT-T1D is using it to tune the insulin therapy for specific individuals. This is facilitated by the increasing ease of gathering real-life data, making it ideal to exploit such large data sets for further assessment or even personalization of new therapies. This perspective was explored by Diaz et al, 14 El Fathi et al, 42 and Fabris et al, 43 where the authors proposed and validated, through simulation, the adoption of the DT-T1D of Hughes et al, 16 as the core element to automatically adapt the insulin treatment regimen of individuals with T1D using a closed-loop system.
Conclusion
Digital twins in type 1 diabetes are emerging methodologies with the potential to revolutionize T1D management. By harnessing the power of digital replicas to simulate and predict individualized metabolic responses, these methodologies offer unprecedented insights into the complex interplay of factors influencing blood glucose dynamics. Through continuous monitoring, personalized modeling, and data-driven interventions, DT-T1D holds promise in optimizing treatment strategies, enhancing patient outcomes, and advancing our understanding of diabetes pathophysiology.
In this systematic review, we analyzed eight DT-T1D frameworks, highlighting their main characteristics and limitations. Particularly, alongside discussing their clear outstanding contributions in the domain of diabetes technology, we identified four common challenges that future work must address.
First, most of the considered methodologies lack proper validation on real-world data, which is crucial for any technology, especially those tied to the health care domain where accuracy, reliability, and safety must be guaranteed. As mentioned above, in the context of DT-T1D, this is far from trivial as it would be necessary to set up clinical trials where data are collected under the same initial and surrounding physiological conditions, which is not feasible in the real-world scenario. However, even if not possible, researchers must put their effort into envisioning new validation protocols and set up studies aimed at comparing, with a fair degree of error, the predictions or outcomes generated by DT-T1D with real-world data or clinical observations.
Second, there is a need for studies aimed at comparing the prediction accuracy of different DT-T1D and understanding their strengths, weaknesses, and unique contributions. Indeed, by benchmarking DT-T1D approaches against existing methods or similar models, researchers can demonstrate their novelty and effectiveness and help identify areas for improvement and innovation within the field.
In addition, physical exercise and psychological/social models are missing in most of the reviewed DT-T1D, despite their significant on glucose levels. Fostered by the recent proliferation of wearable devices like smartwatches that can track physical activity and stress, there is indeed a unique opportunity to leverage these data to incorporate such an important factor into DT-T1D.
Third, we found that code implementations of most of the considered methodologies are not available. The aim being promoting transparency, collaboration, and accessibility within the scientific community, making DT-T1D open-source would allow researchers to scrutinize and validate the underlying algorithms, encourages continuous improvement, and ensures broader accessibility for health care practitioners and patients by also enabling customization and adaptation to diverse clinical settings, ultimately enhancing cost-effectiveness and scalability.
As a final note, it is important to remark that, as summarized in Table 3, creating accurate DT-T1D requires addressing several critical patient factors. Indeed, pharmacoadherence, social determinants of health, and disease heterogeneity all introduce potential barriers that can impact the reliability and effectiveness of the twinning procedure of DT-T1D. Overcoming these challenges involves integrating comprehensive and precise data, along with developing adaptable models that can account for individual variations.
Table 3.
Summary of Patient Factors That Might be Barriers to the Use of DT-T1D.
| Patient factor | Description | Potential barrier |
|---|---|---|
| Pharmacoadherence | Consistency with which patients follow their prescribed medication regimens | Non-adherence can lead to inaccurate twinning and, by product, wrong predictions |
| Social determinants of health | Conditions in which people are born, grow, live, work, and age (eg, socioeconomic status) | These factors can influence health outcomes and access to health care |
| Heterogeneity of the disease | Variation in disease manifestation and progression among different patients | This variability can complicate the creation of accurate digital twins |
Footnotes
Abbreviations: CGM, continuous glucose monitoring; DT-T1D, digital twin in type 1 diabetes; ISCT, in silico clinical trials; LSTM, Long Short-Term Memory; NASA, National Aeronautics and Space Administration; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; T1D, type 1 diabetes.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was partially supported by MIUR under the initiative “PRIN: Programmi di Ricerca Scientifica di Rilevante Interesse Nazionale (2020),” project ID: 2020X7XX2P, project title: “A noninvasive tattoo-based continuous GLUCOse Monitoring electronic system FOR Type-1 diabetes individuals (GLUCOMFORT).”
ORCID iDs: Giacomo Cappon  https://orcid.org/0000-0003-4358-9268
https://orcid.org/0000-0003-4358-9268
Andrea Facchinetti  https://orcid.org/0000-0001-8041-2280
https://orcid.org/0000-0001-8041-2280
References
- 1. Glaessgen E, Stargel D. The digital twin paradigm for future NASA and U.S. Air force vehicles. In: 53rd AIAA/ASME/ASCE/AHS/ASC structures, structural dynamics and materials conference; 2012 April 23-26; Honolulu, HI, USA. [Google Scholar]
- 2. Negri E, Fumagalli L, Macchi M. A review of the roles of digital twin in CPS-based production systems. In: Crespo Márquez A, Macchi M, Parlikad A, eds. Value Based and Intelligent Asset Management. Cham: Springer; 2020:291-307. [Google Scholar]
- 3. What is a digital twin? [Internet]. The digital twin consortium; c2024. https://www.digitaltwinconsortium.org/initiatives/the-definition-of-a-digital-twin/. Accessed April 1, 2024.
- 4. Wright L, Davidson S. How to tell the difference between a model and a digital twin. Adv Model Simul. 2020;7(1):13. [Google Scholar]
- 5. Joshi S, Shamanna P, Dharmalingam M, et al. Digital twin enabled personalized nutrition. Endocr Pract. 2023;29(12):960-970. [DOI] [PubMed] [Google Scholar]
- 6. Yoo Y, Boland R, Lyytinen K, Majchrzak A. Organizing for innovation in the digitized world. Organ Sci. 2012;23(5):1398-1408. [Google Scholar]
- 7. Tilson D, Lyytinen K, Sørensen C. Digital infrastructures: the missing is research agenda. Inf Syst Res. 2010;21(4):748-759. [Google Scholar]
- 8. Rosen R, von Wichert G, Lo G, Bettenhausen KD. About the importance of autonomy and digital twins for the future of manufacturing. IFAC: PapersOnline. 2015;48(3):567-572. [Google Scholar]
- 9. Xames D Md, Topcu TG. A systematic review of digital twin research for healthcare systems: research trends, gaps, and realization challenges. IEEE Access. 2024;12:4099-4126. [Google Scholar]
- 10. Botin-Sanabria DM, Mihaita AS, Peimbert-Garcia RE, Ramirez-Moreno MA, Ramizer-Mendoza RA, Lozoya-Santos JdJ. Digital twin technology challenges and applications: a comprehensive review. Remote Sens. 2022;14(6):1335. [Google Scholar]
- 11. Tian T, Aaron RE, DuNova AY, et al. Diabetes technology meeting 2023 [published online ahead of print March 25, 2024]. J Diabetes Sci Technol. doi: 10.1177/19322968241235205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moher D, Liberati A, Tetzlaff J, Altmann DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Int Med. 2009;151(4):264-269. [DOI] [PubMed] [Google Scholar]
- 13. Patek S, Lv D, Ortiz EA, et al. Empirical representation of blood glucose variability in a compartmental model. In: Kirchsteiger H, Jørgensen J, Renard E, del Re L, eds. Prediction Methods for Blood Glucose Concentration. Lecture Notes in Bioengineering. Cham: Springer; 2015:133-157. [Google Scholar]
- 14. Diaz C JL, Villa-Tamayo MF, Moscoso-Vasquez M, Colmegna P. Simulation-driven optimization of insulin therapy profiles in a commercial hybrid closed-loop system. Comput Meth Prog Bio. 2023;242:107830. [DOI] [PubMed] [Google Scholar]
- 15. Silfvergren O, Simonsson C, Ekstedt M, Lundberg P, Gennemark P, Cedersund G. Digital twin predicting diet response before and after long-term fasting. PLoS Comput Biol. 2022;18(9):e1010469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hughes J, Gautier T, Colmegna P, Fabris C, Breton MD. Replay simulations with personalized metabolic model for treatment design and evaluation in type 1 diabetes. J Diabetes Sci Technol. 2021;15(6):1326-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cappon G, Vettoretti M, Sparacino G, Favero SD, Facchinetti A. ReplayBG: a digital twin-based methodology to identify a personalized model from type 1 diabetes data and simulate glucose concentrations to assess alternative therapies. IEEE Trans Biomed Eng. 2023;70(11):3227-3238. [DOI] [PubMed] [Google Scholar]
- 18. Bergman RN, Ider YZ, Bowden CR, Cobelli C. Quantitative estimation of insulin sensitivity. Am J Physiol. 1979;236(6):E667-E677. [DOI] [PubMed] [Google Scholar]
- 19. Schiavon M, Dalla Man C, Cobelli C. Modeling subcutaneous absorption of fast-acting insulin in type 1 diabetes. IEEE Trans Biomed Eng. 2018;65(9):2079-2086. [DOI] [PubMed] [Google Scholar]
- 20. Dalla Man C, Camilleri M, Cobelli C. A system model of oral glucose absorption: validation on gold standard data. IEEE Trans Biomed Eng. 2006;53(12, pt 1):2472-2478. [DOI] [PubMed] [Google Scholar]
- 21. Vettoretti M, Favero SD, Sparacino G, Facchinetti A. Modeling the error of factory-calibrated continuous glucose monitoring sensors: application to Dexcom G6 sensor data. Annu Int Conf IEEE Eng Med Biol Soc. 2019;2019:750-753. [DOI] [PubMed] [Google Scholar]
- 22. Visentin R, Campos-Náñez E, Schiavon M, et al. The UVA/Padova type 1 diabetes simulator goes from single meal to single day. J Diabetes Sci Technol. 2018;12(2):273-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Colmegna P, Wang K, Garcia-Tirado J, Breton MD. Mapping data to type 1 diabetes. Control Eng Pract. 2020;103:104605. [Google Scholar]
- 24. Man CD, Micheletto F, Lv D, Breton M, Kovatchev B, Cobelli C. The UVA/PADOVA type 1 diabetes simulator: new features. J Diabetes Sci Technol. 2014;8(1):26-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Deichmann J, Bachmann S, Burckhardt MA, Pfister M, Szinnai G, Kaltenbach HM. New model of glucose-insulin regulation characterizes effects of physical activity and facilitates personalized treatment evaluation in children and adults with type 1 diabetes. PLoS Comput Biol. 2023;19(2):e1010289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hovorka R, Canonico V, Chassin LJ, et al. Nonlinear model predictive control of glucose concentration in subjects with type 1 diabetes. Physiol Meas. 2004;25(4):905-920. [DOI] [PubMed] [Google Scholar]
- 27. Nucci G, Cobelli C. Models of subcutaneous insulin kinetics. Comput Meth Progr Bio. 2000;62(3):249-257. [DOI] [PubMed] [Google Scholar]
- 28. Dalla Man C, Rizza RA, Cobelli C. Meal simulation model of the glucose-insulin system. IEEE Trans Biomed Eng. 2007;54(10):1740-1749. [DOI] [PubMed] [Google Scholar]
- 29. Goodwin GC, Seron MM, Medioli AM, Smith T, King BR, Smart CE. A systematic stochastic design strategy achieving an optimal tradeoff between peak BGL and probability of hypoglycaemic events for individuals having type 1 diabetes mellitus. Bio Sig Proc Con. 2020;57:101813. [Google Scholar]
- 30. Haidar A, Wilinska ME, Graveston JA, Hovorka R. Stochastic virtual population of subjects with type 1 diabetes for the assessment of closed-loop glucose controllers. IEEE Trans Biomed Eng. 2013;60(12):3524-3533. [DOI] [PubMed] [Google Scholar]
- 31. Visentin R, Man CD, Cobelli C. One-day Bayesian cloning of type 1 diabetes subjects: toward a single-day UVA/Padova type 1 diabetes simulator. IEEE Trans Bio Eng. 2016;63(11):2416-2424. [DOI] [PubMed] [Google Scholar]
- 32. Resalat N, El Youssef J, Tyler N, Castle J, Jacobs PG. A statistical virtual patient population for the glucoregulatory system in type 1 diabetes with integrated exercise model. PLoS ONE. 2019;14(7):e0217301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Young G, Dodier R, Youssef JE, et al. Design and in silico evaluation of an exercise decision support system using digital twin models. J Diabetes Sci Technol. 2024;18(2):324-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wilinska ME, Chassin LJ, Acerini CL, Allen JM, Dunger DB, Hovorka R. Simulation environment to evaluate closed-loop insulin delivery systems in type 1 diabetes. J Diabetes Sci Technol. 2010;4(1):132-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kanderian SS, Weinzimer S, Voskanyan G, Steil GM. Identification of intraday metabolic profiles during closed-loop glucose control in individuals with type 1 diabetes. J Diabetes Sci Technol. 2009;3(5):1047-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Roy A, Parker RS. Dynamic modeling of exercise effects on plasma glucose and insulin levels. J Diabetes Sci Technol. 2007;1(3):338-347. doi: 10.1177/193229680700100305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Breton MD. Physical activity-the Major unaccounted impediment to closed loop control. J Diabetes Sci Technol. 2008;2(1):169-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lenart PJ, Parker RS. Modeling exercise effects in type I diabetic patients. IFAC Proceed Vol. 2002;35(1):247-252. [Google Scholar]
- 39. Pellizzari E, Prendin F, Cappon G, Sparacino G, Facchinetti A. drCORRECT: an algorithm for the preventive administration of postprandial corrective insulin boluses in type 1 diabetes management [published online ahead of print December 29, 2023]. J Diabetes Sci Technol. doi: 10.1177/19322968231221768. [DOI] [PubMed] [Google Scholar]
- 40. Noaro G, Cappon G, Vettoretti M, Sparacino G, Favero SD, Facchinetti A. Machine-learning based model to improve insulin bolus calculation in type 1 diabetes therapy. IEEE Trans Biomed Eng. 2021;68(1):247-255. [DOI] [PubMed] [Google Scholar]
- 41. Prendin F, Pavan J, Cappon G, Del Favero S, Sparacino G, Facchinetti A. The importance of interpreting machine learning models for blood glucose prediction in diabetes: an analysis using SHAP. Sci Rep. 2023;13(1):16865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. El Fathi A, Fabris C, Breton MD. Titration of long-acting insulin using continuous glucose monitoring and smart insulin pens in type 1 diabetes: a model-based carbohydrate-free approach. Front Endocrinol (Lausanne). 2022;12:795895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fabris C, Gautier T, Breton M. Automated adaptation of insulin treatment in type 1 diabetes. Annu Int Conf IEEE Eng Med Biol Soc. 2021;2021:5039-5042. [DOI] [PubMed] [Google Scholar]