Abstract
Background
The aim of this quality improvement (QI) project was to increase Colorectal Cancer (CRC) screening in patients ages 50–75 years from a baseline of 27–40% within 12 months in a primary care clinic in limited resource communities.
Methods
The multidisciplinary QI-team applied the Plan-Do-Study-Act method and developed stakeholder analysis, an Ishikawa fish bone diagram, a process flow map, and a driver diagram. Major barriers to suboptimal CRC screening included limited health literacy, language preferences, absence of stool test options, and knowledge gaps among patients and providers. The outcome measure was CRC screening rates, while stool test and colonoscopy completion rates served as process measures. Major interventions included the use of a patient-navigator, leveraging digital health technology to create a novel CRC screening data dashboard, educating patients and providers, patient centered-shared decision making, and creating messages and educational videos in patient’s preferred languages. We used monthly run charts and statistical process control charts (SPC) for data analysis.
Results
We observed a sustainable, steady increase in CRC screening rates from baseline rates of 27.0–40.0% (n = 1304/3271) during the study period and 45.6% (n = 1493/3,271) six months post-study, with median rates of 34.0% in the run chart and mean rates of 43.0% in the SPC chart. Furthermore, we observed an increase in colonoscopy completion rates during the study and six months post-study to 65.0% (n = 411/631) and 72.9% (n = 461/631) respectively, from a baseline rate of 25.0%, with a median of 63.0% in the monthly run chart.
Conclusion
The increase in CRC screening rates highlights the effectiveness of addressing barriers such as health literacy, language preferences, and knowledge gaps. This underscores the value of a multifaceted approach and the role of a patient navigator in enhancing preventive, patient-centered care. This project focused on population health and addressing social determinants of health to overcome disparities and improve CRC screening in a primary care setting. Continued emphasis on these strategies is likely to further advance colorectal cancer screening efforts.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12913-024-11928-7.
Keywords: Colorectal cancer screening, Primary care, Dashboard
Introduction
Colorectal cancer (CRC) is the third most common cancer diagnosed in both men and women, and the second leading cause of cancer-related deaths in the US [1]. In 2022, an estimated 149,500 new CRC cases and 53,100 deaths occurred in the US, with expected progression of similarly high rates in 2023 [2, 3]. The United States Preventive Services Task Force (USPSTF) recommends routine CRC screening for adults aged 45–75 years. Individuals with a familial history of CRC or other risk factors may require earlier or more frequent screenings [4]. Disparities in screening rates are pronounced in under-resourced communities, notably among African Americans, who face the highest mortality rates from CRC despite having the lowest screening rates [5, 6]. Similar trends are observed among other under-resourced ethnic groups, including Hispanic and Native American populations, also report lower CRC screening rates [7]. Evidence suggests that Social Determinants of Health (SDOH), including low health literacy, language barriers, and lack of access to transportation, contribute to these disparities [8]. CRC screenings play a crucial role in early detection and prevention of CRC [9]. The stool tests and colonoscopy are the most used CRC screening tests. Stool tests, such as the stool DNA test, are non-invasive methods that detect the presence of blood and CRC-associated DNA changes in the stool, offering an accessible screening option [4, 10, 11]. In contrast, colonoscopies are invasive but serve as the gold standard for CRC screening as they enable the detection and removal of precancerous polyps, thus preventing CRC development [4, 10].
The Internal Medicine Clinic (IMC) is the largest community-based academic clinic, located in the urban northern section of the Buffalo, New York. It serves a patient population of about 8,000 individuals in need of resources. The IMC is a part of Kaleida Health, the largest healthcare organization in Buffalo. As of September 2021, the CRC screening rate for the patient population at the IMC was 27%, significantly below the 2021 national average and far from the National Colorectal Cancer Roundtable’s (NCCRT) goal of achieving an 80% CRC screening rate in every community [12]. In 2021, CRC screening based on the most recent guidelines was received by 71.8% of adults aged 50–75 [2, 3]. Our organization supported NCCRT’s goal and provided the highest support to increase CRC screening rates for the IMC patient population. The clinic research team was led by a lead QI physician and resident physician team leaders from the Internal Medicine and preventive Medicine, combined residency program, and Internal Medicine residency program. A multi-disciplinary team including clinic administrative leadership, the clinic medical director and a senior analyst from information technology were highly engaged in these quality improvement initiatives. Furthermore, our health care organization received a grant from American Cancer Society (ACS) to increase CRC screening rates and to mitigate health care disparity in under resourced communities.
We identified significant gaps in colorectal cancer (CRC) screening, including limited access to colonoscopy and stool-based tests, inaccurate data capture for CRC screening, and the inability to track data by race and ethnicity in the existing electronic health records (EHR). The multifaceted strategies implemented focused on patient-centered, shared decision-making and improving colonoscopy access through a patient navigator, all aimed at increasing CRC screening rates.
Methods
The global aim was to mitigate healthcare disparities in evidence-based CRC screening by executing multifaceted strategies, using patient centered shared decision making approach [13] in the target population. The primary focus was to optimize the healthcare delivery system, promote equity, and enhance health outcomes for CRC by addressing SDOH barriers, improving care coordination, and enhancing education within our clinic population. The specific aim of this QI project was to increase the CRC screening rate from 27.0 to 40.0% among patients aged 50–75 years by offering stool-based tests and colonoscopy options to eligible patients at IMC within a 12-month period. The three objectives were to: (1) Increase CRC screening in high-risk patients through completion of colonoscopy and in average-risk patients, increase screening via stool test or colonoscopy completion based on patient’s choice, (2) Enhance digital health technology for accurate medical documentation, (3) Create a population health electronic dashboard to improve timely access and tracking of CRC screening rates by race and ethnicity. High risk patients were defined as patients with a personal or family history of colorectal cancer or pre-cancerous polyps, patients with inflammatory bowel disease (ulcerative colitis or Crohn’s disease) or familial adenomatous polyposis or other hereditary familial cancer syndrome.
Patient population and settings
Our primary care clinic is an academic, community-based IMC in Buffalo, providing care for an under-resourced community. Most patients are black, refugees, immigrants or Hispanic. This population has limited English proficiency, low health literacy and many do not speak English as their first language. Spanish and Arabic are the preferred first languages for some patients. The IMC is the largest primary care clinic in Buffalo, New York, comprised of a diverse, multidisciplinary patient care team of 53 resident physicians, 8 providers, nurses, social workers, referral specialists, gastroenterology (GI) attending, information technology staff and administrative staff. The patient care team speaks over 10 different languages including Spanish and Arabic. The IMC is one of the main sites for resident physicians’ longitudinal, continuity of care experience for the IM residency program at University of Buffalo. This study was reviewed by the human subject institutional review board (IRB) and was classified as exempt. Informed consent was waived by IRB.
Design
The design of this innovative population health QI project was based on a “Safe, Timely, Effective, Efficient, Equitable and Patient-Centered” (STEEEP) model [14] The multidisciplinary QI-team applied Define, Measure, Analyze, Improve and control (DMAIC) approach [15] (Supplementary Table 1). The multidisciplinary QI team was comprised of patients and their families, a patient care team, a patient navigator, the Lead QI physician, two resident team leaders, a liaison from ACS and leadership from the IMC, gastroenterology clinics and information technology department. The multidisciplinary QI team performed a stakeholder analysis to outline stakeholder engagement strategies in weekly group meetings and face to face interviews in the two months. Resident physicians and clinic providers engaged patients during clinic visit encounters. Core QI team members included the lead QI physician, clinic directors, resident team leaders and an ACS liaison. The Core QI team members met weekly to brainstorm various ideas to identify and overcome barriers to suboptimal CRC screening. The lead QI physician, clinic medical director and clinic manager conducted small group feedback sessions with other stakeholders including front line clinic staff, resident physicians, and patients. The QI team developed an Ishikawa fishbone diagram and a driver diagram with engagement of a multidisciplinary team. The QI team used the SQUIRE 2.0 guidelines for this project [16].
Data collection
Our study involved the same group of patients before, during and after the intervention. Therefore, our denominator population remained stable at baseline, during the study and post study period. We identified a target population of 3,271 patients seen in 2021 in the clinic. 40.88% of the whole clinic population (n = 3,271/8,000) was our target population. The QI team used Cerner EHR(s) for CRC screening data during the initial phase of the QI project. Due to inaccuracies and incomplete data in the Cerner EHR(s), additional time was required to collect colonoscopy completion reports from outside the health system, making the process challenging. Colonoscopy orders and completion data were not available from the Cerner EHR. Therefore, We designed a novel, health equity electronic, population health dashboard for CRC screening. Variables comprised of patient’s demographic data including race and ethnicity, and various measures. We designed special reports for the outcome measures, and colonoscopy order and completion rates. This dashboard was created from “HealtheIntent”, a Cerner’s population health platform. HealtheIntent is made up of over 50 data sources including, but not limited to various hospital and health system’s EHRs and claims data of major community health insurances such as Highmark, Independent Health, Univera and Fidelis. The research team was internal to the health care organization and received access for the dashboard and collected data directly from the dashboard. Run charts and SPC charts were created from the data.
We used the DNA stool test, Cologuard (Multitarget (mt)-sDNA), from Exact Sciences laboratories available in the United States. There was a lack of interface between the Cerner EMR and mt-sDNA stool test. The QI team collected data from “EpicCare Link” software by exact sciences laboratories, an electronic patient registry. Resident team leaders accessed this registry and tracked all mt-sDNA stool test orders, status and results. Exact sciences faxed individual patient’s results and these results were scanned in the clinic EHR. Positive results were sent to clinic providers for follow up and notification to patients.
Family of measures
Outcome measure
The outcome measure was to increase the CRC screening rate from 27.0 to 40.0% in patients aged 50–75 by offering mt-sDNA stool-based testing and colonoscopy options for eligible patients at the IMC within a 12-month period. Our study involved the same group of patients before, during and after the intervention. Therefore, our denominator population remained stable at baseline, during the study and post study period. We identified a target population of 3,271 patients seen in 2021 in the clinic. 40.88% of the whole clinic population (n = 3,271/8,000) was our target population. The target population for CRC screening improvements included patients 50–75 years that never had CRC screening or had a normal colonoscopy 10 years ago or had a negative mt-sDNA stool test 3 years ago or had a negative fecal immunochemical test (FIT) one year ago, or those that were due for a repeat surveillance colonoscopy after an initial screening colonoscopy based on history of prior pre-cancerous polys.
Process measures
The QI team used the following process measures of performance: (1) mt-sDNA-Stool test completion rates, (2) Increase in colonoscopy completion rates from the baseline rates of 25.0–50.0%, (3) increase in diagnostic colonoscopy completion rates after a mt-sDNA positive stool test to 30% from the estimated baseline rates of 10% within 12 months, and (4) objective improvement in the resident physicians’ knowledge from education, assessed by pre and post-test multiple choice questions after a PowerPoint Presentation for SDOH, CDC inclusive language and for evidence based guidelines for CRC.
Balance measures
These included an increase in patient and physician satisfaction and increase in colonoscopy wait times due to increase in the demand. We designed 8 questions surveys using a 5-point Likert scale to receive providers’ feedback (Supplementary Fig. 1).
Strategy (interventions)
The QI team implemented a total of 10 interventions based on various secondary drivers (Table 1) with an engagement of 27 members of clinic patient care team and 53 resident physicians. The $20,000 ACS grant was allocated to fund patient navigation services at four hours per week over one year, as well as for printing costs associated with patient mailings, such as postcards, and pocket cards for physicians. The grant also supported patient incentives. Videos and skits were created internally by engaging clinic staff and physicians, incurring no additional costs. Additionally, our healthcare organization’s IT leadership developed a dashboard at no expense. The lead QI physician and resident team leaders contributed time to create postcards, pocket cards, and EHR templates, and facilitated dashboard development, enhancing both patient and provider education. Patient education materials were supplied by ACS at no cost. Although the grant budget included potential costs for transportation and co-pays for diagnostic colonoscopies following positive mt-sDNA stool tests, these funds were ultimately unused due to insurance coverage.
Table 1.
Interventions and “Change Ideas” to Advance Health Equity in CRC screening, S: secondary driver; ACS: American Cancer Society; SDOH: Social Determinants of Health; CDC: Center for Disease Control; NCCRT: National colorectal cancer roundtable
| Interventions and Secondary Drivers | Change Ideas Tested |
|---|---|
|
Intervention 1 (S1, S2) Stakeholder Analysis and Multi disciplinary Team Engagement September 2022-October 2022 |
• Stakeholder Analysis & Partner with Exact Science and ACS liaison • Multi-disciplinary team engagement including front-line staff & patients • Lead QI Physician, resident team leaders and administrative leadership champion • Weekly meeting with core team members, Q5 weekly feedback & data sharing with stakeholders |
|
Intervention 2 (S3, S4, S10, S11, S15) Education to patients & providers November 2021-December 2021 |
• PowerPoint presentation on SDOH & CDC guidelines on principles for inclusive communication education to providers & residents • Structured didactics for residents and clinic staff about USPSTF and ACS CRC guidelines • Offer mt-sDNA-stool test as an option for average risk patients • Transportation vouchers, facilitation by clinic social workers |
|
Intervention 3 (S11, S12, S15) EHR template & Patient Registry January-February 2022 |
• Create Standard algorithm and clinic workflows, create & implement EHR template • Train providers to use EHR reminders & recall for due & overdue patients during clinic visits • Provide access of “Epic care Link” database for stool-DNA test result to registered nurses for tracking & follow up of positive results • Create Process flow map for CRC screening, positive mt-sDNA stool test • Design pocket cards for providers for algorithm and clinic workflows |
|
Intervention 4 (S5, S9, S10) Patient Engagement and Creation of Video March 2022-May 2022 |
• Demonstrate mt-sDNA-stool test kit in patient’s language in examination room • Create customized “Simplified instructions for mt-sDNA stool test in preferred languages to patients • Provide patient education about CRC and screening options by brochures and one on one education in patient’s preferred language by multi-lingual materials • Utilize translator services and use of multilingual residents during clinic visits • Create culturally tailored skits and special messages in video by clinic providers & care team in English, Spanish and Arabic languages |
|
Intervention 5 (S4, S6, S7, S9, S15) Advance Education and Patient Navigator April- May 2022 |
• Provide brochure from Exact Science with QR code for video showing how to perform mt-sDNA stool test at home to patients • Co-pays cost for diagnostic colonoscopy after positive mt-sDNA-stool test • Patient navigation for colonoscopy scheduling and closing the loop • Patient navigator scanning outside mt-sDNA tests and colonoscopy reports as structured data in EHR • Patient education in preferred languages & at patient’s health literacy level including “special messages” from NCCRT |
|
Intervention 6 (S5, S8) Patient Outreach and Leveraging EHR June 2022 |
• Send reminder letter for no-shows and for patients that lost follow up • Create customized multi-lingual postcards for client reminder and recall • Patient navigator for reminder & recall by telephone communication • Reminder calls to return kit and offer education on how to perform the test, scheduling assistance for diagnostic colonoscopy after positive mt-sDNA stool test & screening colonoscopy for high risk patients • Provide client reminders in culturally tailored, patient’s preferred languages • Culturally tailored patient education in patient’s health literacy level |
|
Intervention 7 (S3, S11, S15) Provider Advance Education and Enhancement of Referral Workflow July-August 2022 |
• Refresher education to residents and educating new interns about CRC templates, pocket cards and workflows • Provider group education by PowerPoint Presentation on USPSTF and ACS CRC screening guidelines as structured didactics • Identification of positive mt-sDNA stool test & closing the loop for colonoscopy referrals |
|
Intervention 8 (S15, S8, S10) Patient Incentive September-December 2022 |
• CRC education in weekly huddle with providers & care team • Creation of mass mailing > 2000 copies of reminder cards with information in 3 languages • $25 gift card for completion of mt-sDNA stool test or colonoscopy |
|
Intervention 9 (S1, S10) Stakeholder Reflection & Feedback September-December 2022 |
• Educate providers in shared decision making & motivational interview techniques, offer test of choice based on patient preference for average risk patients • Reinforce use of standard, customized algorithm/clinic Workflow & use of EHR template • Grand rounds to educate about USPSTF & ACS CRC guidelines • Patient feedback for video and CRC projects • Resident feedback on QI tools and workflows created in CRC project |
|
Intervention 10 (S6, S14) Creation of Dashboard & Closing the loop for colonoscopy January-June 2023 |
• Biweekly meeting with Information Technology leadership • Create mt-sDNA stool test scanning folder in EHR to satisfy CRC screening for negative test, activate urgent automated email notification of positive stool test results to registered nurses • Scheduling assistance for urgent diagnostic colonoscopy after positive mt-sDNA stool test • Scheduling assistance for colonoscopy based on patient’s preference for average risk patients & for high risk patients • Created health equity population health CRC dashboard for accurate data capturing & reporting |
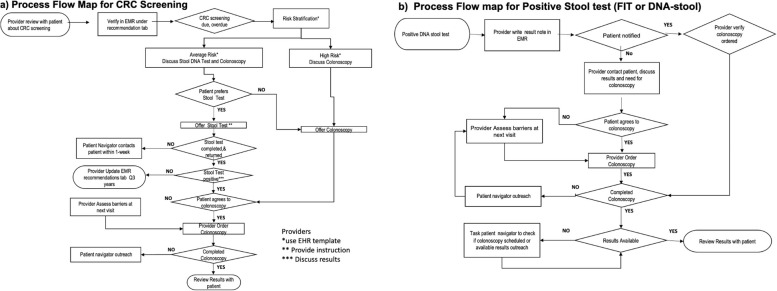
During the pre-study period, we conducted intervention 1 (stakeholder analysis and multidisciplinary team engagement) and intervention 2 (Education to providers, care team and patients). During the study period, we completed 7 interventions (interventions 3–9). We created process flow maps for CRC screening (Fig. 1a) and positive mt-sDNA stool test (Fig. 1b). During the post study period, we implemented intervention 10 (Creation of dashboard and closing colonoscopy referral loop).
Fig. 1.
(a) Process flow map for CRC screening, (b) Process flow map for positive stool test
Table 1.
Data analysis
Data analysis was performed by monthly run charts and a statistical process control chart (SPC), proportion (p) chart by using QI macros software for Excel 2021 (KnowWare International, Denver, CO). We used a run chart and a SPC P chart to plot our data over time to reflect changes related to various interventions. The Run chart and SPC chart are the recommended tools for data analysis for QI projects [17]. We included rules for interpreting run charts and SPC to enhance the robustness of the study findings.
Results
Demographic characteristics of the patient population who completed CRC screening during the pre-study, study and post study period are displayed in Table 2. The demographics of those that completed CRC screening were similar to the general clinic population.
Table 2.
Demographic breakdown of patients who completed colorectal cancer screening during phases of study
| Demographic | Baseline 01/2021–12/2021 (n = 885) |
Study 01/2022–12/2022 (n = 1304) |
Post-Study 01/2023–06/2023 (n = 1493) |
|---|---|---|---|
| Gender | |||
| Female | 506 (57.2%) | 745 (57.1%) | 864 (57.9%) |
| Male | 378 (42.7%) | 557 (42.7%) | 627 (42.0%) |
| Refused/Other | 1 (0.1%) | 2 (0.2%) | 2 (0.1%) |
| Race | |||
| Black | 394 (44.5%) | 587 (45.0%) | 669 (44.8%) |
| White | 365 (41.2%) | 541 (41.5%) | 621 (41.6%) |
| Other | 37 (4.2%) | 51 (3.9%) | 61 (4.1%) |
| Refused/Unknown | 89 (10.1%) | 125 (9.6%) | 142 (9.5%) |
| Ethnicity | |||
| Not Hispanic/Latino | 756 (85.4%) | 1117 (85.7%) | 1279 (85.7%) |
| Hispanic/Latino | 125 (14.1%) | 181 (13.9%) | 208 (13.9%) |
| Refused/Unknown | 4 (0.5%) | 6 (0.5%) | 6 (0.4%) |
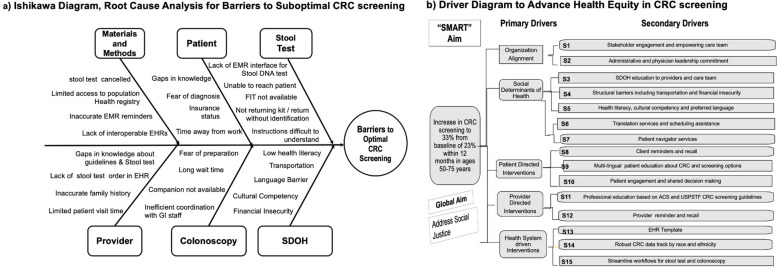
We utilized an Ishikawa fishbone diagram to conduct root cause analysis for barriers to suboptimal CRC screening. The QI team identified SDOH barriers in patients and knowledge gaps among patients and providers as significant factors (Fig. 2a). The team created a driver diagram and identified five primary drivers and fifteen secondary drivers to optimize CRC screening (Fig. 2b: Driver Diagram).
Fig. 2.
(a) Ishikawa Diagram, (b) Diagram Driver
Outcome measures
CRC screening rates
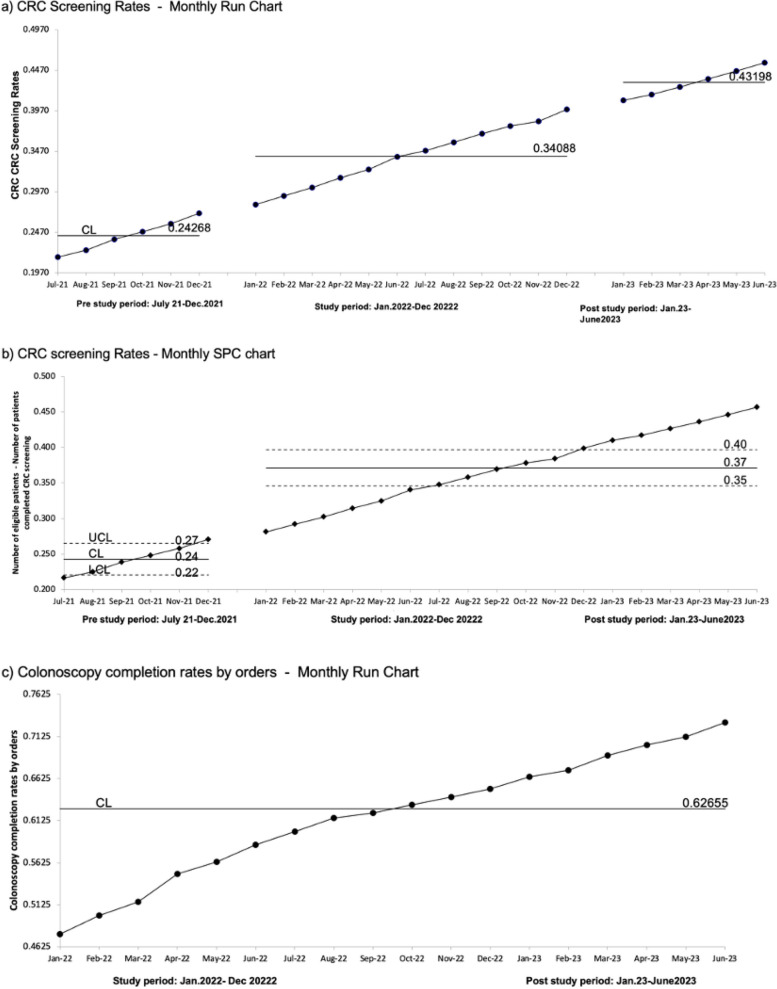
We observed a sustainable, steady increase in CRC screening rates from the baseline rate of 27.0% (n = 885/3,271) to 40.0% (n = 1304/3271) and 45.6% (n = 1493/3,271) during the study and post-study period, respectively. The median and mean were 34.0% and 43.0% during both the study and post-study period respectively, as shown in monthly run chart and SPC chart. We observed the rule of shift, indicated by 6 or more successive points above the baseline which is indicative of variation attributable to nonrandom attributable change in the process based on the run chart rule (Fig. 3a). Our SPC chart demonstrated a shift, 8 successive points on one side of the center line, signaling a special cause variation [18] (Fig. 3b). Rules of run chart and SPC charts confirmed special cause variation, attributable to changes in the process resulting in to improved outcome and demonstrated robustness of this study findings [19]. Our study involved the same group of patients before, during and after the intervention. Therefore, our denominator, population remained stable at baseline, during study and post study period. We identified target population of 3,271 patients seen in 2021 in the clinic. 40.88% of the whole clinic population (n = 3,271/8,000) was our target population.
Fig. 3.
(a) Monthly run chart for CRC screening rates, CL: Control Limit, __: Median, (b) Monthly SPC chart for CRC Screening Rates, UCL: upper control limit: Control Limit, LCL: lower control limit, __: Mean, (c) Colonoscopy Completion Rates by Orders - Monthly Run Chart, CL: Control Limit, __: Median
Process measures
Colonoscopy completion rates
Our baseline colonoscopy completion rate was approximately 25.0% in 2021. We observed a steady increase in colonoscopy completion rates for all ordered colonoscopies during the study period (January 1, 2022 - December 31, 2022) and post-study period (January 1, 2023 - June 30, 2023) to 65.0% (n = 411/631) and 72.9% (n = 461/631) respectively, with a median of 63.0% in the monthly run chart (Fig. 3c).
mt-sDNA stool test completion rates
We observed an increase in mt-sDNA Stool tests completion rates from 73.1% (n = 68/93) at baseline to 81.8% (n = 54/66) and 93.8% (n = 15/16) during the study period and post-study period, respectively.
mt-sDNA stool test positive rates
During the study period, the mt-sDNA Stool test positive rate was 20.4% (n = 11/54).
Diagnostic colonoscopy completion rates after a positive stool test
We followed a total of 30 patients with a positive mt-sDNA stool test during pre-study study and post study period for follow up colonoscopy completions. We observed an increase of follow-up colonoscopy completion rates to 30% within 12 months from the baseline rates of less than 10% for diagnostic colonoscopy among these patients. During the 7-month post-study period, we achieved a 76% diagnostic colonoscopy completion rate (n = 19/25). Out of the 30 patients with positive mt-sDNA stool tests, 4 patients were ineligible for diagnostic colonoscopy due to terminal illness or deceased from other comorbidities. Colonoscopy data could not be obtained for one patient who changed their primary care physician. Five patients declined colonoscopy but are being monitored in the clinic with regular complete blood counts. One patient diagnosed with colon cancer underwent surgery. Most diagnostic colonoscopies revealed precancerous polyps, including tubular adenoma, tubulovillous adenoma, and sessile polyps. A few patients were found to have hemorrhoids, hyperplastic polyps, or a normal colonoscopy.
Qualitative feedback on dashboard
The lead QI physicians collaborated with IT staff and spent about 48 h of total time over a period of 6 months for the design and verification of accuracy of the dashboard. This process was time consuming. Once the dashboard was fully functional, the QI physician leaders started tracking data from the dashboard. The QI physician leaders were able to run reports and collect accurate data more efficiently and share data with stakeholders monthly. Dashboard data is refreshed every 12 h, so we can generate live, up to date data and reports for CRC screening and colonoscopy. The creation and use of a dashboard changed time management by the QI lead physicians. The QI team was able to focus and spend more time in the continuous QI for implemented changes listed in Table 2. Excellent feasibility and acceptability of the dashboard allowed for continuous, dedicated time of QI physicians to guide and foster a positive culture of continuous QI in the clinic. This verbal feedback was received from weekly team meetings.
Improvement in residents’ knowledge
Residents’ average pre- and post-test performance on CRC screening guidelines and SDOH education improved from 74 to 96% and from 85 to 90%, respectively.
Balance measures
Resident and provider feedback survey results
Resident and provider survey results are displayed in supplementary Table 2.
Patient feedback results
Patients reported that they understood stool test and colonoscopy options for CRC screening. Patients also reported that they found education was very useful, so they were able to make decision on CRC screening. The providers received this feedback from the patients during clinic visits.
Discussion
We successfully implemented a QI project to reduce healthcare disparities in CRC screening, which led to an increase in screening rates from 26.0 to 40.0% during the study period, and to 45.6% six months afterward. Our approach involved enhancing screening options, promoting shared decision-making, and addressing SDOH barriers such as language and health literacy. Key strategies included patient education, system navigation by a patient navigator, and engaging both patients and providers in patient-centered care. We also launched a comprehensive CRC population health dashboard in January 2023 to track screening rates by demographics and monitor progress toward health equity goals. Our multifaceted strategies, including educational materials in preferred languages, and face-to-face education, demonstrated significant improvement in screening rates. The dashboard did not directly improve screening rates, but it played a crucial role in facilitating the timely and accurate capture of data, enabling effective sharing with stakeholders. This, in turn, allowed for the development of targeted strategies to improve CRC screening rates. However, challenges such as the lack of interface between multiple EHRs and data integration barriers remain. Employing patient navigators and improving access to colonoscopy are essential for timely completion of diagnostic procedures after a positive stool test.
Previous studies have identified various factors impacting CRC screening rates, including older age, breast cancer survivorship, and urban residence, which correlate with lower participation [20–23]. Socioeconomic factors such as income, education influence and health literacy affect mortality rates [24–27]. To address these, multicomponent strategies are recommended, including patient navigation, reminders, and community engagement [5–7, 24–31]. Offering a range of screening tests and improving access to colonoscopy are also crucial [23, 32]. Patient-provider language concordance is highlighted as essential for culturally competent healthcare delivery [5, 22, 26, 29]. Cost-effectiveness analyses favor organized DNA tests with targeted reminders over universal approaches, emphasizing the importance of inclusive screening program design [33]. Furthermore, advanced dashboards offer decision support tools to analyze data and generate alerts, aiding in identifying high-risk patients. By aggregating data, dashboards facilitate population health management, enabling providers to improve screening rates and address care disparities [34–36]. Our study suggests that the use of a real-time CRC clinical dashboard is an effective tool for healthcare providers to evaluate screening data as it is collected, enabling the identification of gaps in screening coverage. By providing an up-to-date overview of screening rates and individual patient progress, the dashboard allows clinicians to make timely interventions, improve patient care, and optimize screening efforts. Monitoring CRC screening rates are crucial to meet the NCCRT goal of achieving an 80% CRC screening rate in every community [12]. Real-time data enables more targeted, data-driven strategies for improving screening uptake, addressing disparities, and ultimately reducing CRC mortality [37]. As our findings suggest, the implementation of such tools in clinical practice can play a vital role in advancing public health objectives and ensuring that more individuals are screened on schedule, leading to earlier detection and better outcomes.
We learned valuable lessons from this QI project, the first being the importance of a robust population health electronic registry capable of continuous and accurate data capture. The second lesson highlights the importance of identifying barriers to screening, such as SDOH barriers in patients and knowledge gaps among patients and providers as significant factors for CRC screening. Emphasizing the need to overcome these obstacles is crucial to ensure effective preventive care. The third, we recognized the challenges associated with access to follow-up procedures like colonoscopies, including scheduling difficulties and long wait times. Addressing these access issues is vital for timely intervention and management of CRC.
This project has a few limitations. First, we were unable to conduct this study with a control group and were not able to access CRC screening rates data in similar population for comparisons. Second, findings from this study cannot be generalizable to other settings. This study was conducted in an academic, primary care setting with limited resourced, diverse population. Third, we were unable to exclude patients that had a history of CRC cancer or total colectomy, or patients with advanced illness, or receiving palliative care; they were included in the denominator when calculating CRC screening rates. Therefore, we may have under reported our CRC screening rates. Fourth, process measures, specifically DNA stool test completion rates and colonoscopy completion rates are estimated over a very low number of subjects and therefore the results cannot be considered conclusive and should be interpreted with caution. Additionally, our study found a rate of 20% positive results in mt-sDNA stool tests, which is higher than the 12% positive rate reported in a previous study [38] suggesting potential false positives. This supports previous findings that mt-sDNA Cologuard has a false positive rate of approximately 13% [10, 39].
Conclusions
The unwavering commitment of the lead QI physician, organizational administrative leadership, and engagement of a multidisciplinary team and patients were pivotal to the success of this QI project. A novel dashboard provided comprehensive data for CRC screening. Through effective strategies and interventions, we increased CRC screening rates, reduced disparities, and ultimately improved health outcomes for our patients and the broader community.
Supplementary Information
Acknowledgements
We thank Anthony Khoury, a resident team member, Clinic and information technology staff.
Disclaimer
The contents are those of the author(s) and do not necessarily represent the official views of, nor an endorsement by, HRSA, HHS, or the US government.
Authors’ contributions
EB acquisition of data, drafting of manuscript and implementation, EO acquisition of data and implementation, OA acquisition of data, GG data analysis, JR drafting the work or reviewing it critically for important intellectual content and SB responsible for conception, design of the work, data acquisition, data analysis, data interpretation, drafting the work and final approval of work.
Funding
American Cancer Society (ACS) funding but the work was independent.
Social Justice and Equity Award, Graduate Medical Education, University at Buffalo.
Funding is through the Health Resources and Services Administration (HRSA) Primary Care and Enhancement Community Prevention and Maternal Health award T34HP42144-0200.Note. This publication/program was supported by the HRSA of the US Department of Health and Human Services (HHS) as part of an award totaling $2,840,574 with 0% financed with nongovernmental sources.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was reviewed by the University at Buffalo human subject institutional review board and was classified as exempt from human subjects. The University at Buffalo human subject institutional review board determined that the proposed activity is not research involving human subjects (Exempt). It was written and planned as a Quality Improvement project. STUDY00005976. Informed consent was waived by University at Buffalo human subject institutional review board. Survey was developed for this study.
Consent for publication
The University at Buffalo human subject institutional review board determined that the proposed activity is not research involving human subjects. Informed consent was waived by the University at Buffalo human subject institutional review board. No consent for publication necessary.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cancer trends progress report. https://progressreport.cancer.gov.
- 2.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. [DOI] [PubMed] [Google Scholar]
- 3.Key statistics for colorectal cancer. https://www.cancer.org/cancer/types/colon-rectal-cancer/about/key-statistics.html.
- 4.Force USPST, Davidson KW, Barry MJ, Mangione CM, Cabana M, Caughey AB, Davis EM, Donahue KE, Doubeni CA, Krist AH, et al. Screening for colorectal cancer: US preventive services task force recommendation statement. JAMA. 2021;325(19):1965–77. [DOI] [PubMed] [Google Scholar]
- 5.Liss DT, Baker DW. Understanding current racial/ethnic disparities in colorectal cancer screening in the United States: the contribution of socioeconomic status and access to care. Am J Prev Med. 2014;46(3):228–36. [DOI] [PubMed] [Google Scholar]
- 6.Riviere P, Morgan KM, Deshler LN, Demb J, Mehtsun WT, Martinez ME, Gupta S, Banegas M, Murphy JD, Rose BS. Racial disparities in colorectal cancer outcomes and access to care: a multi-cohort analysis. Front Public Health. 2024;12: 1414361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta S, Sussman DA, Doubeni CA, Anderson DS, Day L, Deshpande AR, Elmunzer BJ, Laiyemo AO, Mendez J, Somsouk M, et al. Challenges and possible solutions to colorectal cancer screening for the underserved. J Natl Cancer Inst. 2014;106(4):dju032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Korn AR, Walsh-Bailey C, Correa-Mendez M, DelNero P, Pilar M, Sandler B, Brownson RC, Emmons KM, Oh AY. Social determinants of health and US cancer screening interventions: a systematic review. CA Cancer J Clin. 2023;73(5):461–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeGroff A, Sharma K, Satsangi A, Kenney K, Joseph D, Ross K, Leadbetter S, Helsel W, Kammerer W, Firth R, et al. Increasing colorectal cancer screening in health care systems using evidence-based interventions. Prev Chronic Dis. 2018;15:E100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imperiale TF, Ransohoff DF, Itzkowitz SH, Levin TR, Lavin P, Lidgard GP, Ahlquist DA, Berger BM. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med. 2014;370(14):1287–97. [DOI] [PubMed] [Google Scholar]
- 11.Clebak KT, Nickolich S, Mendez-Miller M. Multitarget Stool DNA Testing (Cologuard) for Colorectal Cancer Screening. Am Fam Physician. 2022;105(2):198–200. [PubMed] [Google Scholar]
- 12.Achieving an 80%. CRC screening rate in every community. https://nccrt.org/.
- 13.Vahdat S, Hamzehgardeshi L, Hessam S, Hamzehgardeshi Z. Patient involvement in health care decision making: a review. Iran Red Crescent Med J. 2014;16(1):e12454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dj B. The guide to achieving STEEEP™ health care Baylor Scott & white health’s quality improvement journey. New York: Routledge, Taylor & Francis Group; 2015. [Google Scholar]
- 15.Monday LM. Define, measure, analyze, improve, Control (DMAIC) Methodology as a Roadmap in Quality Improvement. Glob J Qual Saf Healthc. 2022;5(2):44–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.SQUIRE 2.0 guidelines. https://www.squire-statement.org/index.cfm?fuseaction=Page.ViewPage&PageID=471.
- 17.Brady PW, Tchou MJ, Ambroggio L, Schondelmeyer AC, Shaughnessy EE. Quality Improvement Feature Series Article 2: displaying and analyzing Quality Improvement Data. J Pediatr Infect Dis Soc. 2018;7(2):100–3. [DOI] [PubMed] [Google Scholar]
- 18.Mohammed MA, Worthington P, Woodall WH. Plotting basic control charts: tutorial notes for healthcare practitioners. Qual Saf Health Care. 2008;17(2):137–45. [DOI] [PubMed] [Google Scholar]
- 19.Perla RJ, Provost LP, Murray SK. The run chart: a simple analytical tool for learning from variation in healthcare processes. BMJ Qual Saf. 2011;20(1):46–51. [DOI] [PubMed] [Google Scholar]
- 20.Centra T, Fogg C. Addressing barriers to colorectal cancer screening in a federally qualified health center. J Am Assoc Nurse Pract. 2023;35(7):415–24. [DOI] [PubMed] [Google Scholar]
- 21.Samuel G, Kratzer M, Asagbra O, Kinderwater J, Poola S, Udom J, Lambert K, Mian M, Ali E. Facilitators and barriers to colorectal cancer screening in an outpatient setting. World J Clin Cases. 2021;9(21):5850–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maes-Carballo M, Garcia-Garcia M, Martin-Diaz M, Estrada-Lopez CR, Iglesias-Alvarez A, Filigrana-Valle CM, Khan KS, Bueno-Cavanillas A. A comprehensive systematic review of colorectal cancer screening clinical practices guidelines and consensus statements. Br J Cancer. 2023;128(6):946–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bakhai S, Ahluwalia G, Nallapeta N, Mangat A, Reynolds JL. Faecal immunochemical testing implementation to increase colorectal cancer screening in primary care. BMJ Open Qual. 2018;7(4):e000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salem ME, Puccini A, Trufan SJ, Sha W, Kadakia KC, Hartley ML, Musselwhite LW, Symanowski JT, Hwang JJ, Raghavan D. Impact of Sociodemographic Disparities and Insurance Status on Survival of patients with early-onset Colorectal Cancer. Oncologist. 2021;26(10):e1730–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carethers JM, Doubeni CA. Causes of socioeconomic disparities in colorectal Cancer and intervention Framework and Strategies. Gastroenterology. 2020;158(2):354–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sentell T, Braun KL, Davis J, Davis T. Colorectal cancer screening: low health literacy and limited English proficiency among asians and whites in California. J Health Commun. 2013;18(Suppl 1):242–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yao R, Sykora D, Olson EM, Sanborn D, Himes C, Mohamed AS, Matulis J. Improving colorectal cancer screening disparities among somali-speaking patients in an internal medicine residency clinic. BMJ Open Qual. 2023;12(4):e002391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berkowitz SA, Percac-Lima S, Ashburner JM, Chang Y, Zai AH, He W, Grant RW, Atlas SJ. Building Equity improvement into quality improvement: reducing socioeconomic disparities in colorectal cancer screening as part of population health management. J Gen Intern Med. 2015;30(7):942–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan RJ, Milch VE, Crawford-Williams F, Agbejule OA, Joseph R, Johal J, Dick N, Wallen MP, Ratcliffe J, Agarwal A, et al. Patient navigation across the cancer care continuum: an overview of systematic reviews and emerging literature. CA Cancer J Clin. 2023;73(6):565–89. [DOI] [PubMed] [Google Scholar]
- 30.Kurani SS, McCoy RG, Lampman MA, Doubeni CA, Finney Rutten LJ, Inselman JW, Giblon RE, Bunkers KS, Stroebel RJ, Rushlow D, et al. Association of neighborhood measures of social determinants of health with breast, cervical, and colorectal cancer screening rates in the US Midwest. JAMA Netw Open. 2020;3(3):e200618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soneji S, Iyer SS, Armstrong K, Asch DA. Racial disparities in stage-specific colorectal cancer mortality: 1960–2005. Am J Public Health. 2010;100(10):1912–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Green BB, Fuller S, Anderson ML, Mahoney C, Mendy P, Powell SL. A quality improvement initiative to increase Colorectal Cancer (CRC) screening: collaboration between a Primary Care Clinic and Research Team. J Fam Med. 2017;4(3):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peterse EFP, Meester RGS, de Jonge L, Omidvari AH, Alarid-Escudero F, Knudsen AB, Zauber AG, Lansdorp-Vogelaar I. Comparing the cost-effectiveness of innovative colorectal Cancer screening tests. J Natl Cancer Inst. 2021;113(2):154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ha L, Sikora A. Clinician-designed dashboards. Hosp Pharm. 2023;58(3):225–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Helminski D, Kurlander JE, Renji AD, Sussman JB, Pfeiffer PN, Conte ML, Gadabu OJ, Kokaly AN, Goldberg R, Ranusch A, et al. Dashboards in Health Care settings: protocol for a scoping review. JMIR Res Protoc. 2022;11(3): e34894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gallifant J, Kistler EA, Nakayama LF, Zera C, Kripalani S, Ntatin A, Fernandez L, Bates D, Dankwa-Mullan I, Celi LA. Disparity dashboards: an evaluation of the literature and framework for health equity improvement. Lancet Digit Health. 2023;5(11):e831–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klabunde CN, Lanier D, Breslau ES, Zapka JG, Fletcher RH, Ransohoff DF, Winawer SJ. Improving colorectal cancer screening in primary care practice: innovative strategies and future directions. J Gen Intern Med. 2007;22(8):1195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bering J, Kahn A, Rodriguez E, Ginos B, Ramirez FC, Gurudu SR. Outcomes of cologuard screening at an academic medical center: 1-year results: 230. Official J Am Coll Gastroenterol | ACG. 2017;112:S123–4. [Google Scholar]
- 39.Cotter TG, Burger KN, Devens ME, Simonson JA, Lowrie KL, Heigh RI, Mahoney DW, Johnson DH, Ahlquist DA, Kisiel JB. Long-term follow-up of patients having false-positive multitarget Stool DNA tests after negative screening colonoscopy: the LONG-HAUL cohort study. Cancer Epidemiol Biomarkers Prev. 2017;26(4):614–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.