Abstract
Aim: Whether house dust mite (HDM) sublingual immunotherapy (SLIT) is effective for the skin symptoms of adult atopic dermatitis (AD) is unclear.
Methods: HDM SLIT was added to conventional AD treatment for 10 HDM-sensitized AD patients with rhinitis for 2 years.
Results: Seven out of ten enrolled patients completed the study. Eczema Area and Severity Index score was significantly reduced when comparing before treatment and at 24 months follow-up. CD203c ratio in the basophil activation test using HDM extract, skin prick test with HDM extract and Dermatophagoides pteronyssinus/Dermatophagoides farinae specific-IgG4 tended to improve when comparing before treatment and after treatment.
Conclusion: HDM SLIT might be a therapeutic option for AD patients with rhinitis who are sensitized to HDM.
Keywords: : allergy immunotherapy, atopic dermatitis, dermatophagoides farinae, dermatophagoides pteronyssinus, sublingual immunotherapy
Plain Language Summary
What is this article about?
This study examined the effect of house dust mite (HDM) sublingual immunotherapy (SLIT), which is treatment to make the immune system work better to use medicine that is put under the tongue, as an add-on to conventional atopic dermatitis (AD) treatment on the improvement of skin symptoms and immunological response to HDM SLIT in patients with adult AD complicated with rhinitis.
What were the results?
Eczema Area and Severity Index score which is one of the AD assessment indices and represents AD severity, was significantly reduced when comparing before treatment and at 24 months follow-up. The immune response to HDM tended to improve when comparing before treatment and after treatment.
What do the results of the study mean?
HDM SLIT might be a therapeutic option for AD patients with rhinitis who are sensitized to HDM.
1. Introduction
Atopic dermatitis (AD), a multi-pathogenic disease, is characterized by relapsing eczema skin lesions with pruritus. Various causes are involved in a complex mechanism against a background of hypersensitivity of organs including skin related to atopy predisposition and weak skin barrier function [1].
The treatment of AD is based on pharmacotherapy, skin care and avoidance of aggravating factors. AD is exacerbated by a variety of factors including house dust mite (HDM). In fact, most AD patients are sensitized to HDM (Dermatophagoides pteronyssinus (Der p)/Dermatophagoides farinae (Der f)) allergens, which are important factors in exacerbating symptoms [1].
In recent decades, immunoglobulin E (IgE)-mediated allergic diseases have increased worldwide [2]. Allergy immunotherapy (AIT) is advantageous to pharmacotherapy in that it is a disease-modifying causal treatment that induces long-lasting tolerance to the allergen, which prevents additional allergen sensitization [3]. These effects have been demonstrated in allergic rhinitis and asthma [4].
Sublingual immunotherapy (SLIT) and subcutaneous immunotherapy (SCIT) are the two most commonly used treatment forms of AIT. HDM SCIT was recently shown to improve eczema symptoms in AD patients [5]. Additionally, SCIT increased IL-10 production locally and systemically in an AD mouse model, resulting in significant clinical, histological and immunological improvements [6]. SLIT is considered a more convenient and safe treatment alternative to SCIT [7]. However, to date, SLIT is rarely used for the treatment of AD, and therefore, there is little clinical evidence of its use in AD patients [8,9]. Recently, 2-year duration of HDM SLIT was shown to significantly improve clinical symptoms and reduce the amount of ointment required for patients with mild to moderate AD [10]. Langer et al. showed that 18 months of HDM SLIT improved SCORAD (SCORing Atopic dermatitis) [11]. However, there are few reports of the clinical efficacy of HDM SLIT on AD and rhinitis in AD patients. Furthermore, few biomarker studies have investigated the immunological effect of HDM SLIT in patients with AD [8].
Thus, we investigated the effect of HDM SLIT on the improvement of skin symptoms and immunological response in mild to severe adult AD patients complicated with rhinitis.
2. Methods
2.1. Study design
This study was a prospective, open-label, un-blinded and un-controlled study. Patients with mild to severe AD between the age of 20 and 65 years with symptoms of rhinitis and who had positive HDM extract skin prick tests and HDM-specific IgE antibody tests were included in the study. HDM SLIT was added on to the conventional therapy. At the start of treatment and 1, 3, 6, 12, 18 and 24 months after the start of treatment, skin prick tests with HDM extract, blood sampling, thymus and activation-regulated chemokine (TARC), total IgE, Der p Dermatophagoides pteronyssinus-specific IgE (Der p s-IgE), Der p specific immunoglobulin G subclass 4 (Der p s-IgG4), Der f s-IgE, Der f s-IgG4, BAT using HDM extract, Eczema Area and Severity Index (EASI) and total nasal symptom score (TNSS; nasal discharge, sneezing attacks, nasal obstruction and itchy nose), Dermatology Life Quality Index (DLQI) and pruritus visual analog scale (VAS) were evaluated. Detailed data including age, sex, medical history, internal medication history and physical findings were collected from the medical records. Treatment was terminated when SLIT induced severe allergic symptoms occurred. This was a single-arm trial consisting only of the actual drug group.
2.2. Patients
Patients with AD who visited the Dermatological Institute of Kobe University Hospital and agreed to participate in the study were enrolled. The study protocol was approved by the Institutional Review Board (Kobe University; No.290041). Written informed consent was obtained from all participants. AD was diagnosed in accordance with the criteria of the Guidelines for Atopic Dermatitis [1]. Patients who met all of the following criteria were enrolled: symptoms of rhinitis; patients with AD with an EASI score of four or higher; patients positive for skin prick tests with HDM extract and Der p or Der f specific IgE ≧ class 2 (0.7 kU/l); patients who voluntarily consented to the document regarding participation in this study; patients who were 20 to 65 years old; and patients are receiving only conventional AD treatment (antihistamines, topical steroids, topical moisturizers, topical calcineurin inhibitors). Patients who met any of the following criteria were excluded: pregnant and lactating patients; patients with severe bronchial asthma; patients taking oral corticosteroids, JAK inhibitors or cyclosporine; patients receiving biologics such as dupilumab or omalizumab; and patients with prior treatment for AIT.
In Japan, HDM SLIT-tablet is only covered by health insurance for HDM-induced allergic rhinitis and not for patients with atopic dermatitis who are sensitized to HDM. Therefore, AR complication is necessary to properly and safely use HDM-SLIT tablet in the study.
2.3. SLIT
SQ HDM-SLIT tablets (Torii Pharmaceutical, Tokyo, Japan), were used in this study. Patients received 3,300 Japanese Allergen Units (JAU) for the first 7 days and 10,000 JAU daily thereafter [12].
2.4. Skin prick test
HDM allergen extract (mixture of Der p and Der f (1:1), Torii Pharmaceutical, Tokyo, Japan) was used for the skin prick test. The allergen solution was dropped onto a healthy skin surface on the flexion side of the patient's forearm and the allergen solution was gently stabbed into the skin at a right angle to the skin surface using a bifurcated needle. After 15 min, the size of the wheal was measured in mm and the average value of the longest diameter and the diameter perpendicular to its midpoint were taken as the reaction magnitude [13]. Positive (histamine dihydrochloride 10 mg/ml) and negative (50% glycerol – 5% sodium chloride aqueous solution) control solutions (Torii Pharmaceutical, Tokyo, Japan) were used. A reaction with a wheal diameter of 3 mm or more, and more than half of a positive control wheal were judged as positive [14].
2.5. Clinical score
EASI (eczema area and severity index), which is one of the AD assessment indices and represents AD severity, was evaluated for AD disease activity [15]. TNSS (total nasal symptom score) was calculated as the total score (total score: 0 to 16) of 4 nasal symptoms (rhinorrhea, nasal congestion, nasal itching, sneezing) (each nasal symptom score: 0 to 4). DLQI (Dermatology Life Quality Index) is a quality of life measure specific to skin diseases [16]. It consists of 10 questions about skin symptoms and living conditions during the past week. The pruritus VAS shows a 100 mm line, with the left end as “no itch: 0” and the right end as “worst itch imaginable: 100” and marks are placed on the line according to the degree of itching, and the distance (mm) from the left end to the marked area is evaluated as the magnitude of the itch [17].
2.6. Antibody titer & thymus & activation-regulated chemokine
Thymus & activation-regulated chemokine (TARC) was measured by chemiluminescent enzyme immunoassay system (Sysmex, Kobe, Japan). Total IgE, Der p/Der f s-IgE and s-IgG4 were measured by ImmunoCAP assays system (Thermo Fisher Scientific/Phadia, Uppsala, Sweden).
2.7. Basophil activation test
Allergenicity Kit (Beckman Coulter, CA, USA) was used to quantify basophil CD203c expression in accordance with the manufacturer's instructions [18]. We added some reagents to measure additional parameters including CD63 (clone: H5C6; BioLegend, CA, USA). Whole blood samples for the BAT were collected from AD patients into blood collection tubes with ethylenediaminetetraacetic acid (EDTA). The BAT was performed within 24 h of blood sampling. Phosphate-buffered saline (PBS) was used as a negative control. Anti-IgE antibody (clone: E124-2-8D) from the Allergenicity Kit was used as a positive control (1 μg/ml) to stimulate basophils. HDM extract was used as the allergen reagent. HDM extract was diluted in PBS. CD203c and CD63 positive basophils were measured by flow cytometry (FACS Verse; BD Biosciences, NJ, USA) and the flow cytometry data were analyzed with FlowJo software (BD Biosciences, NJ, USA). A previously described gating techinque was used [19]. CD203c and CD63 expressions under anti-IgE antibody stimulation were defined as ‘anti-IgE stimulation Mean fluorescence intensity (MFI)’ and those under HDM extract stimulation were defined as ‘allergen stimulation MFI’. To calculate the responsiveness of basophils, we divided the allergen stimulation MFI by the anti-IgE stimulation MFI and presented it as the ‘CD203c ratio’ or ‘CD63 ratio’.
2.8. Statistical analysis
Data were analyzed and plotted with GraphPad Prism8 software (GraphPad Software Inc., CA, USA). Statistical analyses were performed using the Wilcoxon matched-pairs signed rank test for comparisons between two groups and the Friedman test for comparisons between three groups. To determine correlations among data, Spearman's rank correlation coefficient analysis was performed. Significance was considered for values of p < 0.05.
3. Results
3.1. Study population
Ten AD patients were enrolled in the study, three dropped out. One patient dropped out after 1 month because of an exacerbation of AD, which was considered an adverse event of SLIT; one patient dropped out at 6 months because of the onset of another illness (breast cancer); and one patient refused to come to the hospital and dropped out at 12 months (Figure 1). The characteristics of the AD patients are shown in Table 1. During the 24-month follow-up, antihistamines, topical steroids, topical moisturizer and topical calcineurin inhibitors were administered but the type of internal and external medicines did not change. The EASI, TNSS, DLQI, VAS, TARC, total IgE, Der p/Der f s-IgE and s-IgG4 were examined prior to the time of enrollment. Initially, AD severity was mild to severe according to EASI (Table 1).
Figure 1.
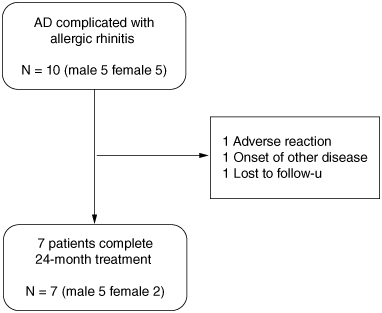
Patient disposition.
AD: Atopic dermatitis.
Table 1.
Clinical baseline characteristics of AD patients with rhinitis.
| Age | Sex | Disease |
Treatment |
Treatment Deatails | EASI | TNSS | DLQI | VAS (mm) | TARC (pg/ml) | Total IgE (kUA/L) | Der p s-IgE (kUA/L) | Der p s-IgG4 (mgA/L) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Duration (year) | Duration (year) | ||||||||||||
| 1 | 25 | Female | 22 | 1.5 | Topical steroid Antihistamines |
16.5 | 11 | 16 | 70 | 361 | 1761.1 | 135.6 | 0.1 |
| 2 | 45 | Male | 16 | 5 | Topical steroid Topical calcineurin inhibitors |
13.2 | 1 | 1 | 0 | 2522 | 23207.3 | 2740 | 0.5 |
| 3 | 47 | Male | 23 | 8 | Topical steroid | 22.8 | 7 | 10 | 60 | 988 | 5137 | 80.3 | 0.2 |
| 4 | 46 | Male | 42 | 5 | Topical steroid Topical calcineurin inhibitors |
27.3 | 5 | 5 | 20 | 3039 | 5467 | 160 | 1.4 |
| 5 | 39 | Female | 36 | 2.5 | Topical steroid Antihistamines |
14.2 | 4 | 8 | 80 | 670 | 1146.4 | 78.6 | 0.2 |
| 6 | 30 | Male | 14 | 1 | Topical steroid Topical calcineurin inhibitors |
5.9 | 9 | 13 | 50 | 310 | 7543.8 | 550 | 0.6 |
| 7 | 45 | Male | 45 | 7 | Topical steroid Topical calcineurin inhibitors |
4.1 | 4 | 4 | 40 | 532 | 348.6 | 11.2 | 0.1 |
Table 1 shows seven registered patients. Patient No. 3 had asthma and patient No. 5 had cholinergic urticaria and asthma. Values of the EASI, TARC, DLQI, TNSS, total IgE, specific-IgE and specific-IgG4 at baseline of this study are shown. The median disease duration was 23 (14–45) (year), The median treatment duration was 5 (1.5–8) (year), The median EASI was 14.2 (4.1–27.3), the median TNSS was 5 (1–11), the median DLQI was 8 (1–6), the median VAS was 50 (0–80) (mm), the median total serum IgE was 5137 (348.6–23207.3) (kUA/L), the median TARC was 670 (310–3039) (pg/ml), the median Der p s-IgE was 135.6 (11.2–2740) (kUA/L), the median Der p s-IgG4 was 0.22 (0.14–1.47) (mgA/L), the median Der f s-IgE was 182.6 (10–1480) (kUA/L) and the median Der f s-IgG4 was 0.31 (0.12–1.78) (mgA/L).
DLQI: Dermatology Life Quality Index; EASI: Eczema area and severity index; TARC: Thymus and activation-regulated chemokine; TNSS: Total nasal symptom score; VAS: Pruritus Visual Analogue Scale.
3.2. Combination treatment efficacy of HDM SLIT based on clinical symptoms
EASI was decreased in all cases when comparing data before treatment (at baseline) with that at 24 months follow-up (at 24 month). Six patients achieved a 50% reduction in the EASI score (EASI-50) and one achieved EASI-75 (Figure 2A). EASI at 24 months showed a significant improvement compared with at baseline (Figure 3A). When comparing data at baseline with at 24 months, the TNSS was improved in three patients, was unchanged in three patients and had worsened in one patient (Figure 2B). No significant difference was observed when comparing data at baseline with at 24 months (Figure 3B). When the DLQI was compared at baseline with at 24 months, two patients showed an improvement of ten points or more, three improved by less than five points, one showed no change and one deteriorated (Figure 2C). There was a tendency to have an improved DLQI at baseline compared with at 24 months (Figure 3C). Regarding pruritus VAS, four patients improved, two showed no change and one had worsened when comparing data at baseline with at 24 months (Figure 2D). Regarding pruritus VAS, no significant difference was found when comparing data at baseline with at 24 months (Figure 3D).
Figure 2.
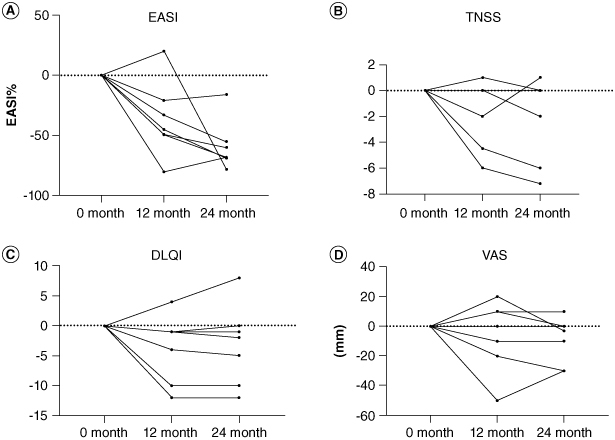
Time-course changes in each clinical parameter regarding the efficacy of HDM SLIT in patients with AD with allergic rhinitis. (A–D) Changes in each clinical parameter from baseline to 12 and 24 months after the start of HDM SLIT.
DLQI: Dermatology Life Quality Index; EASI: Eczema area and severity index; TNSS: Total nasal symptom score; VAS: Pruritus Visual Analogue Scale.
Figure 3.
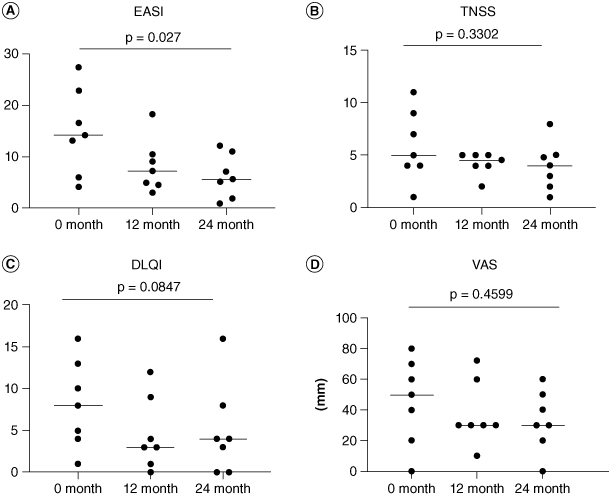
Statistical analysis in each clinical parameter regarding the efficacy of HDM SLIT for patients with AD with allergic rhinitis. (A–D) Significant differences in efficacy before treatment and at 24 months follow-up. The Friedman test was used for comparisons of three groups.
DLQI: Dermatology Life Quality Index; EASI: Eczema area and severity index; TNSS: Total nasal symptom score; VAS: Pruritus Visual Analogue Scale.
3.3. Efficacy evaluation of HDM SLIT based on biomarkers
For TARC, three patients had decreased levels and four had increased levels when comparing data at baseline with at 24 months (Figure 4A). There was no significant difference in TARC levels when comparing data at baseline with at 24 months (Figure 5A). Total IgE was decreased in all seven patients when comparing data at baseline with at 24 months (Figure 4B) but no significant difference was found when comparing data at baseline with at 24 months (Figure 5B). Six patients had a reduced average wheal diameter in the HDM antigen skin prick test at 12 months follow-up after treatment (Figure 4C). There was a tendency of reduced reactions in the skin prick test from baseline to 12 months follow-up (Figure 5C). Because skin prick test with HDM antigen caused localized itching and rash, the skin prick test was discontinued after follow-up until the 12th month in six patients. One patient also refused to follow-up at 12 months. Regarding the change of Der p s-IgE and s-IgG4, Der p s-IgE levels were decreased in four patients when comparing levels at baseline with at 24 months, and Der p s-IgG4 levels were elevated in all patients at 24 months (Figure 4D & E). There was a tendency for reduced Der p s-IgE levels and increased Der p s-IgG4 levels when comparing levels at baseline with at 24 months (Figure 5D & E). Regarding the transition of Der f s-IgE and s-IgG4, Der f s-IgE levels were decreased in three patients when comparing levels at baseline with at 24 months, and Der f s-IgG4 levels were increased in all patients at 24 months (Figure 4F & G). No significant difference was found at 24 months, respectively (Figure 5F & G). Changes in the CD203c ratio and CD63 ratio in the BAT revealed that three patients for CD203c improved prominently after 24 months, and two patients for CD63 improved prominently (Figure 4H & I). At 24 months, the CD203c ratio in the BAT improved significantly and the CD63 ratio in the BAT had a tendency to improve (Figure 5H & I).
Figure 4.
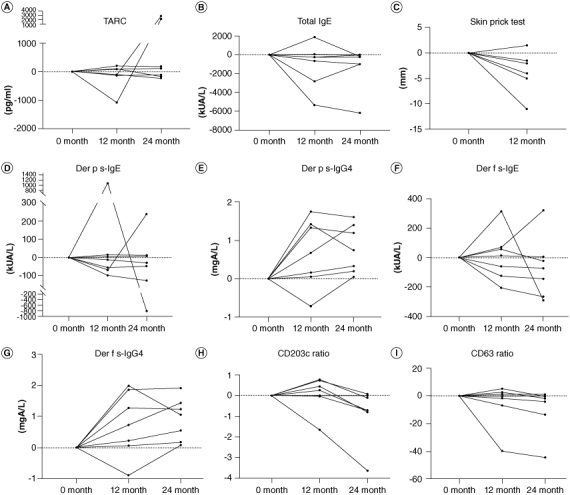
Time-course changes in each biomarker regarding the efficacy of HDM SLIT in patients with AD with allergic rhinitis.
(A, B, D–G) Changes in each biomarker from baseline to 12 and 24 months after the start of HDM SLIT. (C) Changes in each biomarker from baseline to 12 months after the start of HDM SLIT. (H & I) Changes in the surface marker ratio in the basophil activation test from baseline to 12 and 24 months after the start of HDM SLIT. To calculate the responsiveness of basophils, we divided the antigen stimulation MFI by the anti-IgE stimulation MFI and presented it as the ‘CD203c ratio’ or ‘CD63 ratio’.
Der p s-IgE: Dermatophagoides pteronyssinus specific-IgE; Der p s-IgG4: Dermatophagoides pteronyssinus specific-IgG4; Der f s-IgE: Dermatophagoides farinae specific-IgE; Der f s-IgG4: Dermatophagoides farinae specific-IgG4; IgE: Immunoglobulin E; IgG4: Immunoglobulin G subclass 4; TARC: Thymus and activation-regulated chemokine.
Figure 5.
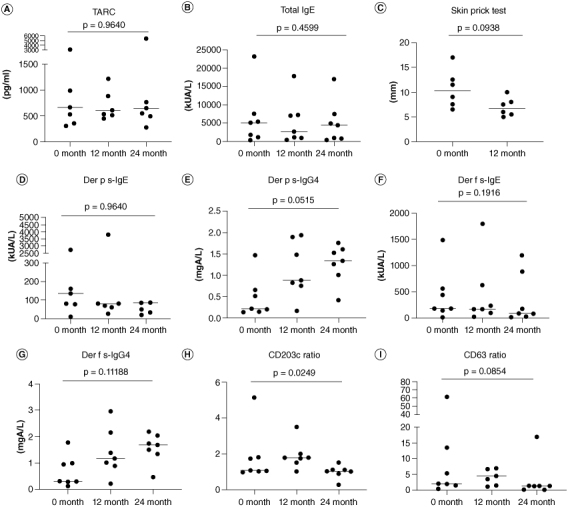
Statistical analysis in each biomarker regarding the efficacy of HDM SLIT for patients with AD with allergic rhinitis.
(A, B, D–I) Significant differences in data before treatment and at 24 months follow-up. The Friedman test was used for comparisons of three groups. (C) Significant differences in data before treatment and at 12months follow-up. The Wilcoxon matched pairs signed rank test was used to compare two groups.
Der p s-IgE: Dermatophagoides pteronyssinus specific-IgE; Der p s-IgG4: Dermatophagoides farinae specific-IgG4; Der f s-IgE: Dermatophagoides farinae specific-IgE; Der f s-IgG4: Dermatophagoides farinae specific-IgG4; IgE: Immunoglobulin E; IgG4: Immunoglobulin G subclass 4; TARC: Thymus and activation-regulated chemokine.
3.4. Correlation between clinical symptoms, the skin prick test & Der p s-IgG4, Der f s-IgG4 values
Finally, we examined the correlation between the reduction of EASI and the increase of Der p s-IgG4, Der f s-IgG4 values, and between the reduction of the average wheal diameter of skin prick test and the increase of Der p s-IgG4, Der f s-IgG4 values. Although not statistically significant, the increase of Der p s-IgG4 and Der f s-IgG4 values were associated with the reduction of EASI (Figure 6A & B). Furthermore, although not statistically significant, the increase of Der p s-IgG4 and Der f s-IgG4 values were associated with the reduction of the average wheal diameter of skin prick test (Figure 6C & D).
Figure 6.
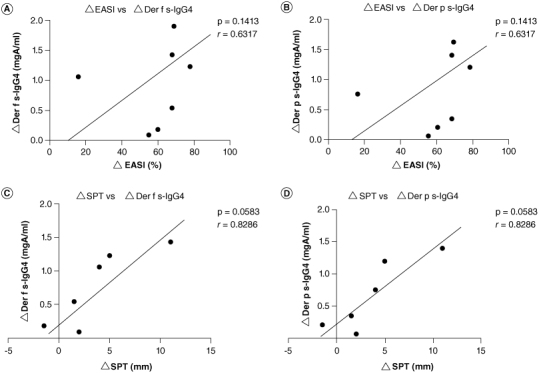
Correlation between EASI and Der p s-IgG4, Der f s-IgG4 values (A & B) and correlation between skin prick test and Der p s-IgG4, Der f s-IgG4 values (C & D). To determine correlations among data, Spearman's rank correlation coefficient analysis was performed.
Der f s-IgG4: Dermatophagoides farinae specific-IgG4; Der p s-IgG4: Dermatophagoides pteronyssinus specific-IgG4; EASI: Eczema area and severity index; SPT: Skin prick test.
4. Discussion
AD is mostly associated with HDM sensitization, but therapeutic approach to HDM allergens have been less highlighted. In this study, we performed HDM SLIT add-on treatment for AD patients with rhinitis, and we focused on the course of improved skin symptoms and immunological response to HDM before and after HDM SLIT add-on treatment.
The present results showed that EASI was significantly improved 24 months after SLIT add-on treatment (Figure 3A). Other previous randomized controlled clinical study of the efficacy of HDM SLIT reported the patients in the SLIT group had significantly decreased ΔSCORAD score from 12 months' treatment compared with the control group [10]. In contrast, other recent randomized, double-blind, placebo-controlled clinical study reported no significant difference in patient SCORAD and EASI improvement compared with the placebo group [11]. However, these two studies and our study can not be compared directly due to the defference that our study is before-and-after comparative study in which AD adult patients with rhinitis were included and also followed them for 6 months longer (24 months) than previous studies. Regarding the DLQI, there was a tendency for improved DLQI when comparing data at baseline with at 24 months (Figures 2C & 3C). However, a previous clinical study reported no significant difference in patient DLQI Improvement compared with the placebo group [11]. This previous study on DLQI was conducted during the Coronavirus Disease 2019 pandemic and the paper stated that it may have affected QoL in the scenario [11]. Regarding the pruritus VAS, there was no significant improvement in pruritus VAS when data were compared at baseline with at 24 months (Figures 2D & 3D) and other previous studies have reported that patients in the SLIT group had no significant changes for VAS scores [11]. Regarding the TNSS, although there were some patients whose TNSS increased or did not change, 3 patients had clear reduction in TNSS and remission of rhinitis symptoms (Figures 2B & 3B). Further research is needed to determine the effect of HDM SLIT as an add-on to conventional AD treatment on patients' pruritic VAS and TNSS.
Regarding biomarkers, an uptrend in Der p/Der f s-IgG4 levels and downtrend of Der p/Der f s-IgE levels was observed (Figure 5D–G). These are similar to biomarker movements in patients with AD and allergic rhinitis without AD in previous studies [4,8]. The CD203c response ratio in the BAT when stimulated with HDM extract was significantly improved (Figures 4H & 5H). Furthermore, the CD63 response ratio in the BAT when stimulated with HDM extract showed a trend toward an improvement (Figures 4I & 5I). These change of CD203c to CD63 response ratio in AD patient's basophil after HDM SLIT as an add-on to conventional AD treatment has not been reported previously. Interestingly, Der p/Der f s-IgG4 tended to be maintained at a high level in the patients where EASI and skin prick tests with HDM antigens were reduced at the time of the 24-month follow-up (Figure 6). These results suggest that HDM SLIT may contribute to an improvement in the clinical symptoms and histamine release with HDM antigen in the skin in AD patients with rhinitis through immunological modulation. SLIT may affect changes in allergen-specific memory T cell and B cell responses to reduce IgE, enhance IgG4 production from B cells and downregulate the activation thresholds of mast cells and basophils in AD patients with allergic rhinitis [20]. Furthermore, It has been noted that basophils are required for acute itching in AD-related inflammation [21]. In other words, a reduced basophil response in the skin upon exposure to HDM may reduce itching, decrease scratching behavior and lead to decreased eczema. The topical and oral medications used to treat AD in patients enrolled in this study were not changed before and after SLIT treatment, only SLIT treatment was added and the EASI and other parameters showed improvement after SLIT treatment. Thus, if appropriate AD patients are selected, the introduction of SLIT for AD patients with allergic rhinitis may contribute to the improvement of clinical symptoms and biomarkers of AD.
One patient dropped out of the study because of exacerbated skin symptoms caused by SLIT. This patient had erythema and itching on her face at the beginning of the study, and the itching worsened one week after HDM SLIT and finally SLIT was discontinued. Thus, SLIT may need to be used with caution when skin symptoms are uncontrolled.
There were some limitations in this small-scale study. Although a control group should have been created and compared, dupilumab or JAK inhibitors became available in Japan during this study and it was difficult for patients to choose to be treated with only conventional AD treatment only during 2 years as the controls to be used in this study. In the future, the number of cases should be increased and the accuracy of statistical analysis of data should be improved. Studies should also be conducted to determine whether the results of this study differ according to the severity of rhinitis and atopic dermatitis.
5. Conclusion
HDM SLIT might be a therapeutic option for AD patients with rhinitis who are sensitized to mites following immunological changes.
Acknowledgments
We thank JL Croxford from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Funding Statement
This work was supported in part by AMED under Grant Number 21ek0410059s0203 and by a Grant-in-Aid for Scientific Research (C) and a Grant-in-Aid for Young Scientists (B) (JSPS KAKENHI Grant Numbers 20K08651 and 19K19722) from the Ministry of Education, Culture, Sports, Science and Technology, Japan (to A Fukunaga and K Washio).
Author contributions
A Fukunaga and A Yoshioka conceived the idea of the study. M Mizuno, Y Oda and S Imamura developed the statistical analysis plan and conducted statistical analyses. M Mizuno and A Fukunaga contributed to the interpretation of the results. H Matsuhara measured specific IgG 4. A Fukunaga, K Washio and K Ohashi-Doi and H Matsuhara supervised the conduct of this study. All authors reviewed the manuscript draft and revised it critically for intellectual content. All authors approved the final version of the manuscript to be published.
Financial disclosure
This work was supported in part by AMED under Grant Number 21ek0410059s0203 and by a Grant-in-Aid for Scientific Research (C) and a Grant-in-Aid for Young Scientists (B) (JSPS KAKENHI Grant Numbers 20K08651 and 19K19722) from the Ministry of Education, Culture, Sports, Science and Technology, Japan (to A Fukunaga and K Washio).
Competing interests disclosure
A Fukunaga has received fees for speaking from Novartis, Sanofi, Takeda, Tanabe-Mitsubishi, Kyowa-Kirin, Kyorin, Kaken and Taiho. A Fukunaga has also received funds for sponsored/joint research from Taiho. H Matsuhara and K Ohashi-Doi are employees of Torii Pharmaceutical Co., Ltd. KW has received fees for speaking from Sanofi.
Ethical conduct of research
The study protocol was approved by the Institutional Review Board of Kobe University (No. 290041).
References
Papers of special note have been highlighted as: •• of considerable interest
- 1.Katoh N, Ohya Y, Ikeda M, et al. Japanese guidelines for atopic dermatitis 2020. Allergol Int. 2020;69(3):356–369. doi: 10.1016/j.alit.2020.02.006 [DOI] [PubMed] [Google Scholar]
- 2.Arbes SJ Jr, Gergen PJ, Elliott L, et al. Prevalences of positive skin test responses to 10 common allergens in the US population: results from the third National Health and Nutrition Examination Survey. J Allergy Clin Immunol. 2005;116(2):377–383. doi: 10.1016/j.jaci.2005.05.017 [DOI] [PubMed] [Google Scholar]
- 3.Durham SR, Leung DY. One hundred years of allergen immunotherapy: time to ring the changes. J Allergy Clin Immunol. 2011;127(1):3–7. doi: 10.1016/j.jaci.2010.11.032 [DOI] [PubMed] [Google Scholar]
- 4.Ohashi-Doi K, Lund K, Mitobe Y, et al. State of the art: development of a sublingual allergy immunotherapy tablet for allergic rhinitis in Japan. Biol. Pharm. Bull. 2020;43(1):41–48. doi: 10.1248/bpb.b19-00093 [DOI] [PubMed] [Google Scholar]
- 5.Bae JM, Choi YY, Park CO, et al. Efficacy of allergen-specific immunotherapy for atopic dermatitis: a systematic review and meta-analysis of randomized controlled trials. J Allergy Clin Immunol. 2013;132(1):110–117. doi: 10.1016/j.jaci.2013.02.044 [DOI] [PubMed] [Google Scholar]; •• Eight randomized controlled trials involving a total of 385 subjects were analyzed and reported that SIT has a significant effect on atopic dermatitis.
- 6.Shin JU, Kim SH, Noh JY, et al. Allergen-specific immunotherapy induces regulatory T cells in an atopic dermatitis mouse model. Allergy. 2018;73(9):1801–1811. doi: 10.1111/all.13465 [DOI] [PubMed] [Google Scholar]
- 7.Canonica GW, Cox L, Pawankar R, et al. Sublingual immunotherapy: World Allergy Organization position paper 2013 update. World Allergy Organ J. 2014;7(1):6. doi: 10.1186/1939-4551-7-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qin YE, Mao JR, Sang YC, et al. Clinical efficacy and compliance of sublingual immunotherapy with Dermatophagoides farinae drops in patients with atopic dermatitis. Int J Dermatol. 2014;53(5):650–655. doi: 10.1111/ijd.12302 [DOI] [PubMed] [Google Scholar]
- 9.You HS, Yang MY, Kim GW, et al. Effectiveness of specific sublingual immunotherapy in Korean patients with atopic dermatitis. Ann Dermatol. 2017;29(1):1–5. doi: 10.5021/ad.2017.29.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu N, Luo H, Liang D, et al. Sublingual immunotherapy in mite-sensitized patients with atopic dermatitis: a randomized controlled study. Postepy Dermatol Alergol. 2021;38(2):69–74. doi: 10.5114/ada.2021.104281 [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Two-year SLIT for HDM was reported to significantly improve clinical symptoms and decrease drug use in patients with mild-moderate AD.
- 11.Langer SS, Cardili RN, Melo JML, et al. Efficacy of house dust mite sublingual immunotherapy in patients with atopic dermatitis: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol Pract. 2022;10(2):539–549.e7. doi: 10.1016/j.jaip.2021.10.060 [DOI] [PubMed] [Google Scholar]; •• Eighteen-month HDM SLIT was reported to improve clinical symptoms and may be effective in HDM-sensitized patients as an add-on treatment for atopic dermatitis.
- 12.Takai T, Okamoto Y, Okubo K, et al. Japanese Society of Allergology task force report on standardization of house dust mite allergen vaccines – secondary publication. Allergol Int. 2015;64(2):181–186. doi: 10.1016/j.alit.2015.01.005 [DOI] [PubMed] [Google Scholar]
- 13.van der Valk JP, Gerth van Wijk R, Hoorn E, et al. Measurement and interpretation of skin prick test results. Clin Transl Allergy. 2015;6:8. doi: 10.1186/s13601-016-0092-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dreborg S. Allergen skin prick test should be adjusted by the histamine reactivity. Int Arch Allergy Immunol. 2015;166(1):77–80. doi: 10.1159/000371848 [DOI] [PubMed] [Google Scholar]
- 15.Hanifin JM, Thurston M, Omoto M, et al. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group. Exp Dermatol. 2001;10(1):11–18. doi: 10.1034/j.1600-0625.2001.100102.x [DOI] [PubMed] [Google Scholar]
- 16.Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)–a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210–216. doi: 10.1111/j.1365-2230.1994.tb01167.x [DOI] [PubMed] [Google Scholar]
- 17.Kido-Nakahara M, Katoh N, Saeki H, et al. Comparative cut-off value setting of pruritus intensity in visual analogue scale and verbal rating scale. Acta Derm Venereol. 2015;95(3):345–346. doi: 10.2340/00015555-1972 [DOI] [PubMed] [Google Scholar]
- 18.Hemmings O, Kwok M, McKendry R, et al. Basophil activation test: old and new applications in allergy. Curr Allergy Asthma Rep. 2018;18(12):77. doi: 10.1007/s11882-018-0831-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deza G, March-Rodríguez A, Sánchez S, et al. Relevance of the Basophil High-Affinity IgE Receptor in Chronic Urticaria: Clinical Experience from a Tertiary Care Institution. J Allergy Clin Immunol Pract. 2019;7(5):1619–1626.e1. doi: 10.1016/j.jaip.2019.01.026 [DOI] [PubMed] [Google Scholar]
- 20.Alvaro-Lozano M, Akdis CA, Akdis M, et al. EAACI allergen immunotherapy user's guide. Pediatr Allergy Immunol. 2020;31(Suppl. 25):1–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang F, Trier AM, Li F, et al. A basophil-neuronal axis promotes itch. Cell. 2021;184(2):422–440.e17. doi: 10.1016/j.cell.2020.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]


