Abstract
Aim: We investigated association between skin adverse events (AEs) and efficacy with dacomitinib in patients with EGFR-positive non-small-cell lung cancer (NSCLC).
Methods: Post hoc analyses from ARCHER 1050 evaluated efficacy in patients who did and did not experience grade ≥2 skin AEs with dacomitinib. Landmark analyses were performed at 3 and 6 months.
Results: In patients who had skin AEs (72.2%) vs. those who did not (27.7%), median progression-free survival was 16.0 vs. 9.2 months, median overall survival (OS) was 37.7 vs. 21.6 months, and objective response rate was 80.2 vs. 61.5%; OS was improved at 3 and 6 months landmark analyses.
Conclusion: Presence of grade ≥2 skin AEs was associated with numerically improved efficacy and represents a valuable biomarker of treatment outcome with dacomitinib in patients with advanced NSCLC.
Clinical Trial Registration: NCT01774721 (ClinicalTrials.gov)
Keywords: : ARCHER 1050, biomarker, dacomitinib, non-small-cell lung cancer, skin disorders
Plain Language Summary
The ARCHER 1050 study assessed how the drugs called dacomitinib and gefitinib affected people with non-small-cell lung cancer (NSCLC) who had mutations in the EGFR gene. In this study, people who were treated with dacomitinib lived longer without their cancer getting worse than people who were treated with gefitinib. Skin adverse reactions were higher in people who were treated with dacomitinib than gefitinib. In this follow-up analysis, researchers wanted to see if the treatment effect of dacomitinib was different between people who had skin adverse reactions and people who did not have skin adverse reactions after treatment with dacomitinib. The results from this analysis showed that after treatment with dacomitinib, half of the people who had skin adverse reactions lived for 16.0 months, and half of the people who did not have skin adverse reactions lived for 9.2 months without their cancer getting worse. This study also showed that half of the people who had skin adverse reactions lived for 37.7 months, and half of the people who did not have skin adverse reactions lived for 21.6 months. In summary, the results from this study showed that the treatment effect of dacomitinib was better in people who had skin adverse reactions after treatment with dacomitinib. Therefore, skin adverse reactions can be a marker of better treatment effect in people with NSCLC who had mutations in the EGFR gene when treated with dacomitinib.
Plain language summary
Article highlights.
Introduction
In the Phase III ARCHER 1050 study, dacomitinib showed improved progression-free survival vs. gefitinib in patients with EGFR-positive non-small-cell lung cancer (NSCLC).
Methods
The association between the presence of skin-related adverse events (AEs) and improved efficacy of EGFR tyrosine kinase inhibitors was assessed.
Results
The presence of grade ≥2 skin AEs was associated with numerically improved efficacy in patients treated with dacomitinib.
Conclusions
Skin AEs may represent a clinically valuable biomarker of treatment outcome in patients with advanced NSCLC treated with dacomitinib.
1. Introduction
Epidermal growth factor receptor (EGFR) mutations are a common subtype and oncogenic driver for non-small-cell lung cancer (NSCLC). Targeted therapy for EGFR mutations in NSCLC includes tyrosine kinase inhibitors (TKIs) such as gefitinib, erlotinib and afatinib. These agents are generally well tolerated and result in improved efficacy responses compared with platinum-based chemotherapy [1].
Dacomitinib is a second-generation, irreversible, EGFR TKI indicated in USA for the first-line treatment of patients with metastatic NSCLC with EGFR exon 19 deletion or exon 21 L858R substitution mutations and in the European Union for the first-line treatment of patients with locally advanced or metastatic NSCLC with EGFR-activating mutations [1–3]. Dacomitinib is associated with more potent EGFR inhibition than first-generation EGFR TKIs [4]. In the Phase III ARCHER 1050 trial (NCT01774721), dacomitinib significantly improved progression-free survival (PFS) and overall survival (OS) vs. gefitinib (by blinded independent radiological central [BIRC] review) as first-line treatment for patients with EGFR mutation-positive NSCLC [1,5,6].
Patients treated with EGFR inhibitors often develop dermatologic toxicities, including acneiform rash, paronychia and pruritus [7,8]. Depending on the specific agent, the [incidence of rash can range from 50 to 100% [8]. The mechanism of these adverse events (AEs) remains unclear. The rash has been hypothesized to result from direct EGFR inhibition in the skin and stimulation of a systemic inflammatory response [9]. Although skin toxicities are rarely fatal, they can lead to treatment disruption or dose modifications [10]. Given that dacomitinib is a potent EGFR inhibitor, these EGFR-related toxicities can be more prevalent with dacomitinib than with first-generation EGFR TKIs [4]. In the ARCHER 1050 study, these skin-related AEs occurred more frequently in patients treated with dacomitinib than with gefitinib [1,5,6]. The most frequently reported AEs of any grade in patients who received dacomitinib (n = 227) were diarrhea (87%), paronychia (62%) and dermatitis acneiform (49%). Grade 3/4 dermatitis acneiform occurred in 14% of patients treated with dacomitinib vs. no patients treated with gefitinib [1]. In addition, these AEs led to dose reductions in 66% of patients receiving dacomitinib [4]. However, the overall safety profile of dacomitinib was similar to that of other EGFR TKIs [4]. In fact, associations between the presence and severity of skin-related AEs and improved treatment efficacy have been reported for the EGFR TKIs afatinib, erlotinib and gefitinib, suggesting that skin-related AEs may be a potential marker of EGFR TKI efficacy [9–12]. In a previous study, patients receiving afatinib who had a grade ≥2 skin rash had longer median PFS than previously seen compared with patients without skin rash, indicating that severe skin rash can be a beneficial marker of efficacy [11]. Similar relationships between skin rash and efficacy were observed with erlotinib [9].
Despite these findings for EGFR TKIs, dacomitinib skin-related AEs have not been evaluated as a favorable-outcome biomarker. We therefore aimed to investigate the association between skin-related AEs and the efficacy of dacomitinib in the ARCHER 1050 trial.
2. Patients & methods
2.1. Study design & patients
The ARCHER 1050 study was an international, multicenter, randomized, open-label, Phase III trial that compared the safety and efficacy of dacomitinib with that of gefitinib in the first-line treatment of patients with advanced EGFR-positive NSCLC. The overall study design of ARCHER 1050 has been published previously [1]. Briefly, patients aged ≥18 years, or ≥20 years in Japan and South Korea, with newly diagnosed stage IIIB/IV or recurrent EGFR-positive NSCLC, ≥1 target lesion that had not been irradiated and was measurable according to Response Evaluation Criteria in Solid Tumors version 1.1 criteria, and an Eastern Cooperative Oncology Group performance status of 0/1 were included in this study. Patients were randomized 1:1 to receive oral dacomitinib 45 mg daily or oral gefitinib 250 mg daily in 28-day cycles. In both treatment arms, patients continued treatment until disease progression, initiation of a new anticancer therapy, unacceptable toxicities, nonadherence, withdrawal of consent, or death. Randomization was stratified by race (Asian vs. non-Asian) and EGFR mutation type (exon 19 deletion vs. exon 21 L858R substitution). The primary end point was PFS by BIRC review. Secondary end points included OS, best overall response, duration of response, overall safety profile and patient-reported outcomes.
Dacomitinib dose reductions of a maximum of two dose levels (30 mg and 15 mg) were permitted for grade ≥3 toxicities or for prolonged grade 2 AEs lasting more than one cycle. For interruption due to grade 3 or intolerable grade 2 toxicity, treatment could be resumed at the same dose level or reduced. For grade 4 toxicities, reduction to the next dose level was mandated. AEs were assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0.
The study was conducted in accordance with Good Clinical Practice (GCP) as required by the International Council for Harmonisation (ICH) guidelines and in accordance with country-specific laws and regulations governing clinical studies. Patients who chose to participate in the study signed an informed consent document. The study protocol, all study protocol amendments, written study patient information, informed consent documentation and any other appropriate study-related information were reviewed and approved by the Institutional Review Board(s) and/or Independent Ethics Committee(s) at each study site.
2.2. Post hoc efficacy analyses according to whether patients experienced grade ≥2 skin-related AEs
The post hoc analyses reported here include subgroups of patients in the dacomitinib arm of ARCHER 1050. Patients who received dacomitinib were stratified according to whether they experienced an all-cause grade ≥2 skin-related AE during the study. Skin-related AEs were defined as any of the following: dermatitis, dermatitis acneiform, dry skin, rash, rash maculopapular, rash pruritic, nail infection, nail toxicity, onycholysis, onychomadesis, palmar-plantar erythrodysesthesia syndrome, paronychia, pruritus, pruritus generalized or xerosis.
Analyses were based on the maximum grade of AE reported (the temporal reduction of grade due to dose reductions was not considered) and included patients who experienced grade ≥2 skin-related AEs, as grade ≥3 skin-related AEs can generally be reduced to grade 2 by dose reduction; the impact of dose reductions on grades is not expected to change which subgroup the patient belongs to. A previous analysis of ARCHER 1050 found that the incidence of grade 3 dermatitis acneiform and paronychia decreased after dose reduction, whereas grade 2 skin-related AEs did not. The observed increase in grade 2 dermatitis acneiform events following dose reduction was hypothesized to be due to grade 3 events converting to grade 2 events [4].
This subgroup analysis examined end points aligned with those in the ARCHER 1050 study. End points included PFS based on BIRC review, OS and best overall response based on BIRC review, according to whether patients had experienced a grade ≥2 skin-related AE at any time during the study. The study also assessed cumulative dacomitinib exposure by subgroup at cycles 3 and 6, defined as the sum of the actual daily dose received by patients from day 1 through the end of the respective cycle. The length of the cycles reflects similar time points in the landmark analysis.
Landmark analyses of PFS and OS were conducted based on first onset of maximum-grade skin-related AE (grade ≥2) from baseline to each specified landmark time point, starting at 1 month; PFS and OS for the patients at risk from each landmark time were assessed from that point forward. This analysis was intended to reduce the possible bias in subgroup analyses. Dose reductions were not considered in these analyses. To evaluate the influence of skin-related AEs on efficacy outcomes (PFS and OS), landmark analyses with landmarks at 3 and 6 months were conducted using the Kaplan-Meier method. In the landmark analysis, patients with PFS time less than or equal to landmark time were excluded from the corresponding analysis. Patients who experienced a skin-related AE before the landmark were assigned to the group with grade ≥2 skin-related AEs, whereas those who did not experience skin-related AEs before the landmark time were assigned to the group without grade ≥2 skin-related AEs. The data cutoff for PFS and OS was 29 July 2016, and 13 May 2019, respectively. The different data cutoff dates led to slight differences in the number of patients from baseline to end results in each group.
2.3. Statistical analyses
Patients were grouped and analyzed according to whether they experienced a grade ≥2 skin-related AE at any time during the study (subgroup analysis) or at specified landmark time points during the study (landmark analysis). PFS and OS were assessed using the Kaplan-Meier method. Kaplan-Meier methods were also used for the landmark analyses at each specified time point. Hazard ratios (HRs) and confidence intervals (CIs) were calculated using unstratified Cox regression. There was no adjustment for multiplicity for the p values presented in these post hoc analyses.
3. Results
3.1. Patients
In the present study, all patients in the dacomitinib arm (n = 227) of ARCHER 1050 were included in both the intention-to-treat and safety populations [1]. Overall, 164 patients (72.2%) reported a grade ≥2 skin-related AE during the study, and 63 patients (27.8%) did not. Sixty-five patients (28.6%) experienced a maximum grade 0/1 skin disorder, 100 patients (44.1%) experienced a maximum grade 2 skin disorder, 62 patients (27.3%) experienced a maximum grade 3 skin disorder and no patients experienced a maximum grade 4 skin disorder. In comparison with patients who did not have grade ≥2 skin-related AEs, a higher proportion of patients who had grade ≥2 skin-related AEs were male (31.1 vs. 47.6%), had an Eastern Cooperative Oncology Group performance status of 1 (62.2 vs. 79.4%) and were smokers or ex-smokers (32.3 vs. 42.9%) (Table 1). The groups had a similar distribution of patients with an Exon19del EGFR mutation (59.8% with grade ≥2 skin-related AE vs. 57.1% without) and a Leu858Arg EGFR mutation (40.2% with grade ≥2 skin-related AE vs. 42.9% without). Median duration of treatment was 15.4 months (range 0.07–60.5 months). At the data cutoff of 13 May 2019, 11 patients (5%) were continuing to receive treatment [5].
Table 1.
Baseline characteristics according to whether patients treated with dacomitinib experienced a grade ≥2 skin-related adverse event.
| Grade ≥2 skin-related AE (n = 164) | No grade ≥2 skin-related AE (n = 63) | |
|---|---|---|
| Age, median (range), years | 62.0 (28–87) | 61.5 (28–81) |
| <65, n (%) | 94 (57.3) | 39 (61.9) |
| ≥65, n (%) | 70 (42.7) | 24 (38.1) |
| Sex, n (%) | ||
| Female | 113 (68.9) | 33 (52.4) |
| Male | 51 (31.1) | 30 (47.6) |
| Race, n (%) | ||
| Asian | 126 (76.8) | 44 (69.8) |
| Chinese | 74 (45.1) | 40 (63.5) |
| Japanese | 38 (23.2) | 2 (3.2) |
| Other East Asian | 14 (8.5) | 2 (3.2) |
| White | 38 (23.2) | 18 (28.6) |
| Black | 0 | 1 (1.6) |
| ECOG performance status, n (%) | ||
| 0 | 62 (37.8) | 13 (20.6) |
| 1 | 102 (62.2) | 50 (79.4) |
| Disease stage at screening, n (%) | ||
| Stage IIIB | 14 (8.5) | 3 (4.8) |
| Stage IV | 145 (88.4) | 58 (92.1) |
| Unknowna | 5 (3.0) | 2 (3.2) |
| Smoking status, n (%) | ||
| Never smoked | 111 (67.7) | 36 (57.1) |
| Former smoker | 44 (26.8) | 21 (33.3) |
| Current smoker | 9 (5.5) | 6 (9.5) |
| Type of EGFR mutationb, n (%) | ||
| Exon19 deletionc | 98 (59.8) | 36 (57.1) |
| Leu858Arg | 66 (40.2) | 27 (42.9) |
Newly diagnosed with stage IV disease at the time of study entry.
EGFR mutations (at randomization) were identified from tumor specimens.
At randomization, no patients in the dacomitinib group had the Thr790Met mutation.
AE: Adverse event; ECOG: Eastern Cooperative Oncology Group; EGFR: Epidermal growth factor receptor.
3.2. Efficacy data for dacomitinib according to whether patients experienced a grade ≥2 skin-related AE
In this subgroup analysis, PFS was significantly longer in patients who experienced a grade ≥2 skin-related AE at any time during the study than in those who did not (HR: 0.639, 95% CI: 0.439–0.928, median 16.0 vs. 9.2 months) (Figure 1A). OS was also substantially longer in patients who experienced a grade ≥2 skin-related AE compared with those who did not (HR: 0.548, 95% CI: 0.381–0.789, median 37.7 vs. 21.6 months) (Figure 1B).
Figure 1.
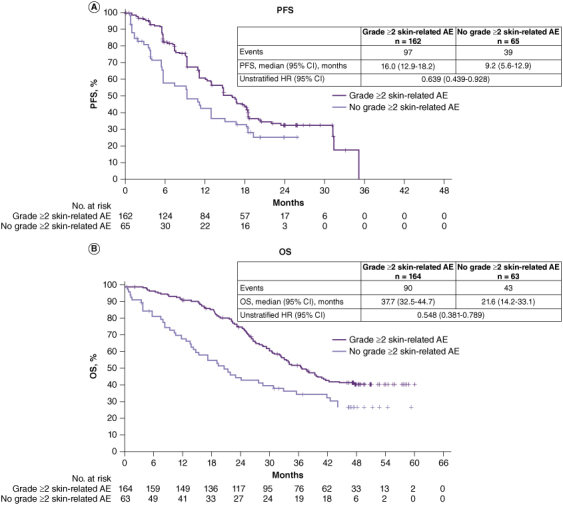
Subgroup analyses of (A) PFS (BIRC review) and (B) OS in patients treated with dacomitinib according to whether they experienced a grade ≥2 skin-related AE.
AE: Adverse event; BIRC: Blinded independent radiological central; CI: Confidence interval; HR: Hazard ratio; OS: Overall survival; PFS: Progression-free survival.
A higher proportion of patients who did versus did not experience a grade ≥2 skin-related AE achieved a complete response (6.8 vs. 1.5%) or partial response (73.5 vs. 60.0%). The proportion of patients who had stable disease or no response was similar across both groups (13.0% in the grade ≥2 skin-related AE group vs. 13.8% in the no grade ≥2 skin-related AE group). The ORR was 80.2% (95% CI: 73.3–86.1%) in patients who experienced a grade ≥2 skin-related AE and 61.5% (95% CI: 48.6–73.3%) in those who did not (Table 2).
Table 2.
Summary of best overall response and clinical benefit response based on BIRC review.
| Grade ≥2 skin-related AE (n = 162) | No grade ≥2 skin-related AE (n = 65) | |
|---|---|---|
| Best overall response, n (%) | ||
| Complete response | 11 (6.8) | 1 (1.5) |
| Partial response | 119 (73.5) | 39 (60.0) |
| Stable/no response | 21 (13.0) | 9 (13.8) |
| Stable/no response and TTF ≥168 days | 11 (6.8) | 1 (1.5) |
| Stable/no response and TTF <168 days | 10 (6.2) | 8 (12.3) |
| Progressive disease | 5 (3.1) | 7 (10.8) |
| Indeterminate | 6 (3.7) | 9 (13.8) |
| Objective response rate (95% exact CI), % a | 80.2 (73.3–86.1) | 61.5 (48.6–73.3) |
| Nominal p valueb | 0.0033 | |
| Clinical benefit response rate (95% exact CI), % a | 87.0 (80.9–91.8) | 63.1 (50.2–74.7) |
| Nominal p valueb | <0.0001 | |
Using the exact method based on binomial distribution.
Two-sided, unstratified; not adjusted for multiplicity.
AE: Adverse event; BIRC: Blinded independent radiological central; CI: Confidence interval; TTF: Time to treatment failure.
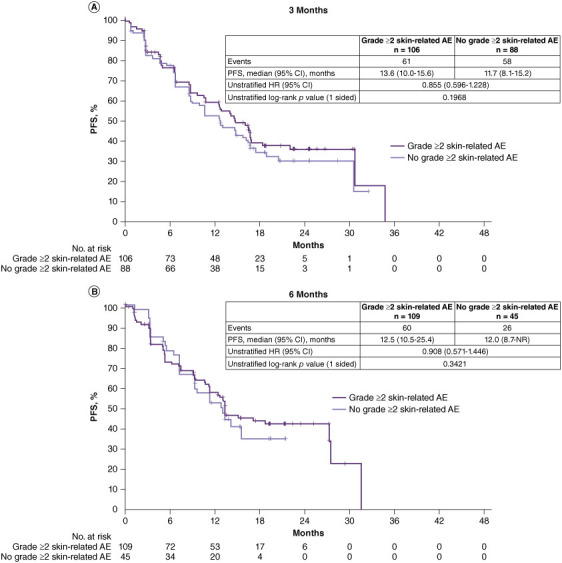
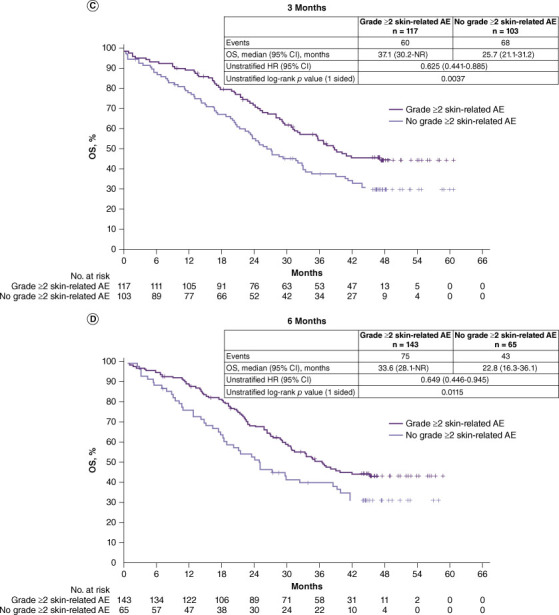
To reduce the possible bias in the subgroup analyses above, landmark analyses were conducted. No significant differences in PFS were observed between patients who did versus did not experience a grade ≥2 skin-related AE at 3 and 6 months. At 3 months, for PFS between patients who experienced a grade ≥2 skin-related AE vs. those who did not, the HR was 0.855 (95% CI: 0.596–1.228, p = 0.1968, median 13.6 vs. 11.7 months) (Figure 2A); at 6 months, for PFS between patients who experienced a grade ≥2 skin-related AE vs. those who did not, HR was 0.908 (95% CI: 0.571–1.446, p = 0.3421, median 12.5 vs. 12.0 months) (Figure 2B). Landmark analyses of PFS at other specified time points are shown in Supplementary Figure S1. There was a trend toward improved OS in the landmark analyses in patients with grade ≥2 skin-related AEs vs. those without grade ≥2 skin-related AEs. At 3 months, OS was significantly longer in patients who experienced a grade ≥2 skin-related AE than those who did not (HR: 0.625, 95% CI: 0.441–0.885, p = 0.0037, median 37.1 vs. 25.7 months) (Figure 2C); at 6 months, OS was significantly longer in patients who experienced a grade ≥2 skin-related AE than those who did not (HR: 0.649, 95% CI: 0.446–0.945, p = 0.01150, median 33.6 vs. 22.8 months) (Figure 2D). Landmark analyses of OS at other specified time points are shown in Supplementary Figure S2.
Figure 2.
Landmark analysis of PFS (BIRC review) and OS in patients treated with dacomitinib according to whether they experienced a grade ≥2 skin-related AE at landmark time points.a (A) PFS at 3 months, (B) PFS at 6 months, (C) OS at 3 months. and (D) OS at 6 months
AE: Adverse event; BIRC: Blinded independent radiological central; CI: Confidence interval; HR: Hazard ratio; NR: Not reached; PFS: Progression-free survival.
aPatients with a PFS time less than or equal to the landmark time were excluded from the corresponding analysis.
3.3. Dacomitinib exposure according to whether patients experienced a grade ≥2 skin-related AE
At cycle 1, cumulative dacomitinib exposure was assessed in 42 patients with and 178 patients without grade ≥2 skin-related AEs. Cumulative exposure was similar in cycle 1; median exposure was 1260 mg in both groups (Table 3). In cycle 3, dacomitinib exposure was assessed in 103 patients with and 94 patients without grade ≥2 skin-related AEs. Cumulative exposure was lower in patients with grade ≥2 skin-related AEs than in those without grade ≥2 skin-related AEs; median exposure was 3330 mg (95% CI: 3074–3311 mg) vs. 3735 mg (95% CI: 3452–3633 mg). In cycle 6, 125 patients with and 56 patients without grade ≥2 skin-related AEs were included in dacomitinib exposure analysis. Median exposure was lower in patients who experienced a grade ≥2 skin-related AE than in those who did not (6054 mg vs. 7515 mg).
Table 3.
Cumulative dacomitinib exposures according to whether patients experienced a grade ≥2 skin-related AE at cycles 1, 3 and 6.
| Grade ≥2 skin-related AEa | No grade ≥2 skin-related AE | |
|---|---|---|
| Cycle 1 b | ||
| n | 42 | 178 |
| Mean (SD), mg | 1171 (179) | 1218 (146) |
| Median (95% CI), mg | 1260 (1117–1226) | 1260 (1197–1240) |
| Cycle 3 c | ||
| n | 103 | 94 |
| Mean (SD), mg | 3193 (613) | 3543 (448) |
| Median (95% CI), mg | 3330 (3074–3311) | 3735 (3452–3633) |
| Cycle 6 d | ||
| n | 125 | 56 |
| Mean (SD), mg | 5901 (1391) | 6930 (959) |
| Median (95% CI), mg | 6045 (5657–6145) | 7515 (6679–7182) |
n refers to the numbers of patients who had the first onset of a grade ≥2 skin-related AE before the end of cycle 1, cycle 3, and cycle 6, respectively.
Sum of actual daily dose received from day 1 through day 28 of cycle 1 (the dose on cycle 2 day 1 is not included).
Sum of actual daily dose received from day 1 through the end of cycle 3 (the dose on cycle 4 day 1 is not included).
Sum of actual daily dose received from day 1 through the end of cycle 6 (the dose on cycle 7 day 1 is not included).
AE: Adverse event; CI: Confidence interval; SD: Standard deviation.
4. Discussion
Although associations between skin-related AEs and improved efficacy have been reported for several EGFR TKIs, skin-related AEs have not been evaluated as a potentially favorable-outcome biomarker in patients with EGFR-mutant NSCLC treated with dacomitinib. In this post hoc subgroup analysis of patients treated with dacomitinib in the Phase III ARCHER 1050 study, patients who experienced a grade ≥2 skin-related AE at any time during the study showed improved PFS, OS and ORR compared with those who did not experience a grade ≥2 skin-related AE. These analyses were based on grade ≥2 skin-related AEs because dose reduction can generally reduce grade 3 disorders to grade 2 while maintaining treatment efficacy. A previous analysis of ARCHER 1050 found that the incidence of grade 3 dermatitis acneiform and paronychia decreased after dose reduction, whereas grade 2 AEs did not [4]. This may be due to grade 3 AEs being converted to grade 2 AEs. Grade 2 skin-related AEs can be managed appropriately without dose reduction in clinical practice. Additionally, using a cutoff of maximum grade ≥2 allowed for the inclusion of enough patients to provide meaningful results.
Although skin-related AEs are commonly observed with dacomitinib, grade ≥2 skin-related AEs may be clinically valuable biomarkers for potentially improved survival and response in patients with advanced NSCLC treated with dacomitinib. In the prior analysis of ARCHER 1050, dose reductions helped manage AEs without the need for permanent discontinuation in the majority of patients, while maintaining efficacy [4]. Our findings are consistent with those of other reports on the association between the presence of skin-related AEs and improved efficacy of EGFR TKIs such as afatinib, erlotinib and gefitinib in patients with advanced NSCLC [9–12].
Previously published literature supports the predictive nature of skin disorders with efficacy. A retrospective study found that grade ≥2 skin rashes were associated with significantly improved PFS (median PFS not reached, n = 5) vs. grade 0/1 skin rashes (median PFS 13.9 months, n = 27, p = 0.0097) in EGFR-positive patients treated with afatinib as a first-line EGFR TKI; however, given the small sample size, these results should be interpreted with caution [11]. Another retrospective study including both EGFR-positive and EGFR-negative patients found that the development of a rash was independently associated with longer time to progression and greater OS in patients with NSCLC receiving erlotinib [9]. An earlier meta-analysis showed that patients who developed a grade 2–4 rash were more likely to respond to treatment with EGFR TKIs than those with no rash (42% vs. 7%) [12]. In 2013, a published systematic review and meta-analysis found skin rash to be predictive of prognosis with regard to disease control rate, ORR, OS and PFS in patients with NSCLC (EGFR mutation status not specified) treated with gefitinib or erlotinib. A subgroup analysis showed that the relationship between rash and efficacy was stronger for gefitinib than erlotinib [10]. These studies provide substantial evidence and data to support the positive relationship between skin-related AEs and the efficacy of EGFR inhibitors.
Although the main analysis revealed improved PFS in patients with grade ≥2 skin-related AEs, landmark analyses showed no substantial differences in PFS between patients who experienced a grade ≥2 skin-related AE and those who did not at each specific time point during the study. This difference in results between the landmark analysis and the subgroup analysis may suggest potential selection bias or survival bias. Patients who remain in the study for a longer duration have more time to develop grade ≥2 skin-related AEs alongside their longer PFS.
The trend of improved OS in patients who did versus did not experience a grade ≥2 skin-related AE was observed at the landmark time points at 3 months and 6 months. The most pronounced differences between the two subgroups were seen during these timeframes. Patient numbers in both subgroups declined at later time points, mainly due to patients experiencing the initial onset of the skin-related AE at an earlier time point. The small sample sizes in the subgroups may have contributed to the overlapping survival curves.
The dacomitinib cumulative exposure data revealed that patients with a grade ≥2 skin-related AE had lower exposures at cycle 3 or later than those without a grade ≥2 skin-related AE. We expect this difference is likely due to a greater number of dose reductions in the skin-related AE group. Furthermore, the efficacy data at landmark times were comparable among patients with and without dose reduction, indicating that dose reductions in patients with grade ≥2 skin-related AEs did not compromise treatment efficacy. Skin-related toxicities, such as rash, have been suggested to be predictive of a better outcome in patients with NSCLC [13]. Some studies reported a correlation between higher drug exposure and rash [14] whereas others suggested a correlation between genetic differences and rash [13]. However, in this post hoc analysis, dacomitinib exposure was lower in patients with a grade ≥2 skin-related AE; therefore, higher drug exposure did not influence the observed efficacy outcome in patients treated with dacomitinib.
Our study included patients with EGFR-positive NSCLC from a large Phase III trial in patients receiving dacomitinib; the large study population and specific inclusion of patients with EGFR mutations contributed to the validity of this study. The study design included a comprehensive definition of skin-related AEs; therefore, this study not only supported previous data on the association between skin rash and EGFR TKI efficacy but also found evidence to support an association for a broader range of skin-related AEs. As dacomitinib is recommended as a treatment option for patients with EGFR-positive NSCLC, the results from this study may be applicable to other EGFR TKIs.
This study had limitations, largely those inherent in post hoc analyses, that should be considered in interpreting the findings. This analysis was not preplanned, which may lead to type I errors and given the potential survival bias and small patient numbers, results should be interpreted carefully. In addition, the open-label study design of ARCHER 1050 could have introduced some bias. Different data cutoff dates were used for PFS and OS analyses, which may have affected the results of this study. In addition, a third of the patient population in the ARCHER 1050 study were Asian, so results of this study may not be applicable to broader populations. Moreover, in the skin-related AE group, a higher proportion of patients were female, had ECOG performance status of 0, had stage IIIB disease and were nonsmokers. These baseline characteristics are predictive of better outcomes, which may have influenced the observed outcomes.
5. Conclusion
In conclusion, our findings support grade ≥2 skin disorders as a potentially clinically valuable biomarker of treatment outcomes in patients with advanced NSCLC treated with dacomitinib. Further large-scale validation studies are warranted.
Supplementary Material
Acknowledgments
The authors would like to thank the study participants and study site personnel. Editorial and medical writing support was provided by C Lavin (on behalf of CMC AFFINITY, McCann Health Medical Communications) and A Dorfstatter of Nucleus Global and funded by Pfizer.
Funding Statement
This study was sponsored by Pfizer, Inc and SFJ Pharmaceuticals. Editorial and medical writing support was funded by Pfizer; however, analysis and interpretation of the data, writing of the report, and decision to submit the article for publication was driven by the authors.
Supplemental material
Supplemental data for this article can be accessed at https://doi.org/10.1080/14796694.2024.2404762
Author contributions
J Li: investigation, writing – review & editing. X Pu: investigation, writing – review & editing. B Zhang: investigation, writing – review & editing. J Zhang: investigation, writing – review & editing. TS Mok: investigation, writing – review & editing. K Nakagawa: investigation, writing – review & editing. R Rosell: investigation, writing – review & editing. Y Cheng: investigation, writing – review & editing. X Zhou: investigation, writing – review & editing. MR Miglorino: investigation, writing – review & editing. S Niho: investigation, writing – review & editing. KH Lee: investigation, writing – review & editing. J Corral: investigation, writing – review & editing. A Pluzanski: investigation, writing – review & editing. J Li: investigation, writing – review & editing. R Linke: writing – review & editing. F Pan: conceptualization, supervision, writing – review & editing. Y Tang: conceptualization, formal analysis, writing – review & editing. W Tan: conceptualization, writing – review & editing. L Wu: writing – review & editing, methodology.
Financial disclosure
This study was sponsored by Pfizer, Inc and SFJ Pharmaceuticals. Editorial and medical writing support was funded by Pfizer; however, analysis and interpretation of the data, writing of the report, and decision to submit the article for publication was driven by the authors.
Competing interests disclosure
TSK Mok reports institutional grants from AstraZeneca, Bristol Myers Squibb, G1 Therapeutics, MSD, Merck Serono, Novartis, Pfizer, Roche, SFJ, Takeda and XCovery; consulting fees or advisory board roles at AbbVie Inc, ACEA Pharma, Adagene, Alpha Biopharma, Amgen, Amoy Diagnostics, AstraZeneca, Bayer, BeiGene, Berry Oncology, Blueprint Medicines Corporation, Boehringer Ingelheim, Bowtie Life Insurance Co, Bristol Myers Squibb, C4 Therapeutics, Cirina, Covidien, CStone Pharmaceuticals, Curio Science, D3 Bio, Da Volterra, Daiichi Sankyo, Daz Group, Eisai, Elevation Oncology, F. Hoffmann-La Roche/Genentech, Fishawack Facilitate, G1 Therapeutics, geneDecode Co, Gilead Sciences, Gritsone Oncology, Guardant Health, Hengrui Therapeutics, HutchMed, Ignyta, Incyte Corporation, Inivata, InMed Medical Communication, IQVIA, Janssen, Janssen Pharmaceutica NV, Jiahui Holdings Co, Lakeshore Biotech, LiangYiHui Healthcare, Lilly, Lucence Health, Lunit USA, Loxo-Oncology, Lucence Health, Medscape LLC/WebMD, MD Health Brazil, Merck Pharmaceuticals HK, Merck Serono, MSD, Mirati Therapeutics, MIRXES, MoreHealth, Novartis, Omega Therapeutics, OrigiMed, OSE Immunotherapeutics, P. Permanyer SL, PeerVoice, Pfizer, Physicians' Education Resource, PriME Oncology, Puma Biotechnology, Qiming Development (HK), Research to Practice, Roche Pharmaceuticals/Diagnostics/Foundation One, Sanofi-Aventis, SFJ Pharmaceuticals, Shanghai BeBirds Translation & Consulting, Simcere of America, Synergy Research, Taiho Pharmaceutical, Takeda Oncology, Takeda Pharmaceuticals HK, Tigermed, Touch Independent Medical Education, Vertex Pharmaceuticals, Virtus Medical Group and Yuban Corporation; support for meeting attendance/travel from AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi Sankyo, MiRXES, MSD, Novartis, Roche and Pfizer; leadership or fiduciary role with ACT Genomics-Sanomics Group, AstraZeneca PLC, Aurora, HutchMed and Lunit USA; and owns stock or stock options at ACT Genomics-Sanomics Group, AstraZeneca, Aurora Tele-Oncology, Biolidics and HutchMed. K Nakagawa reports institutional grants from AbbVie, Amgen, AstraZeneca KK, Bayer Yakuhin, Ltd, Bristol Myers Squibb, Chugai Pharmaceutical, Covance Japan Inc., Daiichi Sankyo, Eisai Co., Ltd., Eli Lilly Japan KK, EP-CRSU Co., Ltd., EPS Corporation, EPS International, GlaxoSmithKline KK, IQVIA Services Japan KK, Janssen Pharmaceutical KK, Japan Clinical Research Operations, Kissei Pharmaceutical Co., Ltd., Mebix Inc., Medical Research Support, Merck Biopharma Co., Ltd., Mochida Pharmaceutical Co., Ltd., MSD KK, Nippon Boehringer Ingelheim Co.,Ltd., Nippon Kayaku Co., Ltd., Novartis Pharma KK, Ono Pharmaceutical, Otsuka Pharmaceutical, PAREXEL International Corp., Pfizer Japan, Pfizer R&D Japan GK, PPD-SNBL KK, PRA Healthsciences, Sanofi KK, SRL, Inc., SymBio Pharmaceuticals Limited., Syneos Health Clinical KK, Sysmex Corporation, Taiho Pharmaceutical and Takeda; lecture fees or honoraria from 3H Clinical Trial Inc., AbbVie, Amgen, AstraZeneca KK, Bayer Yakuhin, Bristol Myers Squibb KK, Care Net, Inc., Chugai Pharmaceutical, CMIC, CMIC ShiftZero KK, Eli Lilly Japan KK, Japan Clinical Research Operations, Kyorin Pharmaceutical, Kyowa Kirin, Life Technologies Japan Ltd., Medical Mobile Communications, Medical Review Co., Ltd., Merck Biopharma, MSD KK, Neo Communication, Nikkei Business Publications, Inc., Nippon Boehringer Ingelheim Co., Ltd., Nippon Kayaku, Novartis Pharma KK, Ono Pharmaceutical, Pfizer Japan, Roche Diagnostics KK, Taiho Pharmaceutical, Taiyo Pharma, Takeda Pharmaceutical and Yodosha; consulting fees from Eli Lilly Japan KK, Kyorin Pharmaceutical, Ono Pharmaceutical and Pfizer Japan; and patents from Daiichi Sankyo. MRM reports consulting fees from AstraZeneca, Novartis, Pfizer, Roche and Takeda; honoraria from AstraZeneca, Novartis, Pfizer, Roche and Sanofi; support for meeting attendance/travel from AstraZeneca, Novartis, Roche, Sanofi and Takeda; and advisory board roles for Pfizer, Roche and Sanofi. S Niho reports funding for the present manuscript from Pfizer; grants paid to his institution from AstraZeneca and Merck Biopharma; advisory board roles for AstraZeneca and Pfizer; and honoraria from AstraZeneca, Boehringer Ingelheim, Chugai, Daiichi Sankyo, Eisai, Eli Lilly, Kyorin, Kyowa Kirin, Merck Biopharma, MSD, Nippon Kayaku, Novartis, Ono Pharmaceuticals, Pfizer, Taiho Pharmaceutical and Takeda. KHL reports advisory board roles for AstraZeneca, Bristol Myers Squibb, Eli Lilly, MSD and Pfizer. J Corral reports grants from AstraZeneca, Bristol Myers Squibb, Janssen, MSD, Pfizer and Takeda; honoraria from AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Janssen, Merck, Pfizer, Roche and Takeda; fees for expert testimony from Roche; support for meeting attendance/travel from AstraZeneca, Bristol Myers Squibb, MSD, Pfizer and Roche; and advisory boards roles for AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Janssen, MSD, Pfizer, Roche and Takeda. A Pluzanski reports honoraria for lectures from AstraZeneca, Bristol Myers Squibb, MSD, Pfizer, Roche and Takeda; advisory board roles for Bristol Myers Squibb, Pfizer and Takeda; and support for meeting attendance/travel from AstraZeneca, Bristol Myers Squibb, MSD and Takeda. R Linke is an employee of SFJ Pharmaceuticals. F Pan was an employee of Pfizer at the time of this study. Y Tang and W Tan are employees of Pfizer and may own company stock. L Wu reports financial support from AstraZeneca, Bajer, BeiGene, Bristol Myers Squibb, CStone, Eli Lilly, Hengrui, Johnson & Johnson, MSD, Novartis, Pfizer, Roche and Sanofi. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Writing disclosure
Editorial and medical writing support was provided by C Lavin (on behalf of CMC AFFINITY, McCann Health Medical Communications) and A Dorfstatter of ClinicalThinking, Inc and funded by Pfizer. However, analysis and interpretation of the data, writing of the report and decision to submit the article for publication was driven by the authors.
Ethical conduct of research
For the original study, the authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved. For these post hoc analyses, no additional approvals were required.
Data availability statement
Upon request and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Wu Y-L, Cheng Y, Zhou X, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18(11):1454–1466. doi: 10.1016/S1470-2045(17)30608-3 [DOI] [PubMed] [Google Scholar]; •• In the randomized Phase III ARCHER 1050 study, dacomitinib significantly improved progression-free survival compared with gefitinib in patients with EGFR mutation-positive non-small-cell lung cancer.
- 2.European Medicines Agency . Vizimpro (dacomitinib) summary of product characteristics [2019; cited 2023 June 26]. Available from: https://www.ema.europa.eu/en/documents/product-information/vizimpro-epar-product-information_en.pdf
- 3.Pfizer . Vizimpro (dacomitinib) prescribing information [December 2020; cited 2023 June 26]. Available from: labeling.pfizer.com/ShowLabeling.aspx?id=11019
- 4.Corral J, Mok TS, Nakagawa K, et al. Effects of dose modifications on the safety and efficacy of dacomitinib for EGFR mutation-positive non-small-cell lung cancer. Future Oncol. 2019;15(24):2795–2805. doi: 10.2217/fon-2019-0299 [DOI] [PubMed] [Google Scholar]; •• Post hoc analyses from the ARCHER 1050 study showed that survival was similar in all dacomitinib-treated patients compared with dacomitinib-treated patients who had dose reductions due to adverse events.
- 5.Mok TS, Cheng Y, Zhou X, et al. Updated overall survival in a randomized study comparing dacomitinib with gefitinib as first-line treatment in patients with advanced non-small-cell lung cancer and EGFR-activating mutations. Drugs. 2021;81(2):257–266. doi: 10.1007/s40265-020-01441-6 [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Overall survival benefit of first-line treatment with dacomitinib vs. gefitinib was maintained after extended follow-up in patients with EGFR mutation-positive non-small-cell lung cancer from the ARCHER 1050 study.
- 6.Cheng Y, Mok TS, Zhou X, et al. Safety and efficacy of first-line dacomitinib in Asian patients with EGFR mutation-positive non-small-cell lung cancer: results from a randomized, open-label, phase III trial (ARCHER 1050). Lung Cancer. 2021;154:176–185. doi: 10.1016/j.lungcan.2021.02.025 [DOI] [PubMed] [Google Scholar]
- 7.Chu CY, Choi J, Eaby-Sandy B, et al. Osimertinib: a novel dermatologic adverse event profile in patients with lung cancer. Oncologist. 2018;23(8):891–899. doi: 10.1634/theoncologist.2017-0582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wacker B, Nagrani T, Weinberg J, et al. Correlation between development of rash and efficacy in patients treated with the epidermal growth factor receptor tyrosine kinase inhibitor erlotinib in two large phase III studies. Clin Cancer Res. 2007;13(13):3913–3921. doi: 10.1158/1078-0432.CCR-06-2610 [DOI] [PubMed] [Google Scholar]
- 9.Kainis I, Syrigos N, Kopitopoulou A, et al. Erlotinib-associated rash in advanced non-small-cell lung cancer: relation to clinicopathological characteristics, treatment response, and survival. Oncol Res. 2018;26(1):59–69. doi: 10.3727/096504017X14913452320194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu HB, Wu Y, Lv TF, et al. Skin rash could predict the response to EGFR tyrosine kinase inhibitor and the prognosis for patients with non-small-cell lung cancer: a systematic review and meta-analysis. PLOS ONE. 2013;8(1):e55128. doi: 10.1371/journal.pone.0055128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nasu S, Suzuki H, Shiroyama T, et al. Skin rash can be a useful marker for afatinib efficacy. Anticancer Res. 2018;38(3):1783–1788. doi: 10.21873/anticanres.12416 [DOI] [PubMed] [Google Scholar]
- 12.Petrelli F, Borgonovo K, Cabiddu M, et al. Relationship between skin rash and outcome in non-small-cell lung cancer patients treated with anti-EGFR tyrosine kinase inhibitors: a literature-based meta-analysis of 24 trials. Lung Cancer. 2012;78(1):8–15. doi: 10.1016/j.lungcan.2012.06.009 [DOI] [PubMed] [Google Scholar]; • A meta-analysis that included 24 publications showed that patients who developed grade 2 to 4 rash were more likely to respond to treatment with EGFR tyrosine kinase inhibitors than those with no rash.
- 13.Dienstmann R, Brana I, Rodon J, et al. Toxicity as a biomarker of efficacy of molecular targeted therapies: focus on EGFR and VEGF inhibiting anticancer drugs. Oncologist. 2011;16(12):1729–1740. doi: 10.1634/theoncologist.2011-0163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rudin CM, Liu W, Desai A, et al. Pharmacogenomic and pharmacokinetic determinants of erlotinib toxicity. J Clin Oncol. 2008;26(7):1119–1127. doi: 10.1200/JCO.2007.13.1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Upon request and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.


