Abstract
Background:
High-frequency deep transcranial magnetic stimulation (dTMS) on the anterior cingulate cortex (ACC) and medial prefrontal cortex (mPFC) has been known to be effective in modulating emotional experience but not studied in children and adolescents with externalizing behavior disorders (EBDs). We present a novel protocol for a study that aims to assess the safety and efficacy of adjuvant dTMS in managing emotional dysregulation in EBDs in children and adolescents.
Methods:
The trial is prospectively registered in the Clinical Trial Registry of India (CTRI) at www.ctri.nic.in with registration number: CTRI/2023/03/050701. In total, 40 subjects with age less than 18 years with EBDs would be randomized into two groups (active and sham dTMS); receiving 15 sessions of high-frequency dTMS, each, over 3 weeks. The subjects and rater would remain blind to treatment allocation. Assessments would be done at baseline and immediately after completion of the treatment using the Child Behavior Checklist (CBCL), Difficulty in Emotional Regulation Scale (DERS), Modified Overt Aggression Scale (MOAS), Affective Reactivity Index (ARI), Barratt’s Impulsivity Scale (BIS), Drug Abuse Screening Test (DAST), Children Global Assessment Scale (CGAS), and Clinical Global Impression (CGI). A checklist for side effects will be administered following each session in both groups.
Result:
Data shall be analyzed utilizing the statistical software Statistical Package for Social Sciences for outcome variables as defined for the purpose of the study. Safety of dTMS in young subjects as assessed by TMSens_Q and reduction in scores of DERS would be primary outcome variables. Functional Magnetic Resonance Imaging (fMRI) task-based assessment of the difference in activation of mPFC and ACC at baseline and after application of dTMS and reduction in scores of BIS, ARI, MOAS, CGI, and CGAS would be measured as secondary outcome variables.
Conclusion:
The study’s results are going to provide insight into potential role of dTMS in addressing emotional dysregulation in EBDs in children and adolescents adding one more tool to the armamentarium.
Keywords: Externalizing behavior disorders, children and adolescents, dTMS, anterior cingulate cortex, the medial prefrontal cortex
Key Message:
This protocol aims to investigate the safety and efficacy of high-frequency dTMS for treating emotional dysregulation in children and adolescent having EBDs. Through a randomized trial involving 40 subjects, the research explores dTMS efficacy by targeting the ACC and mPFC. Findings may contribute to expanding treatment options for emotional dysregulation in children and adolescents with EBDs.
Externalizing behaviors are behavioral issues that manifest themselves in children’s outward behavior, such as rule-breaking, aggression, and delinquency. Longitudinal research suggests that an adolescent’s externalizing behaviors are a risk factor for a variety of negative outcomes, including juvenile delinquency, future crime, and violence, 1 and lower educational and occupational attainment in adulthood. 2
A comprehensive model of “externalizing psychopathology” incorporates elements such as impulse dyscontrol, poor attention allocation, heightened emotional reactivity, verbal and physical aggression, rule violations, and substance use/abuse. 3
The externalization construct includes the DSM-5 disorders of attention-deficit/hyperactivity disorder (ADHD), oppositional defiant disorder (ODD) and conduct disorder (CD), disruptive mood dysregulation disorders (DMDD), mood disorders, and substance use disorder. 3
Although externalizing conduct has historically been thought of as a problem with behavior and cognition rather than affect, it is now recognized that these conditions are intimately linked to emotional processes and demand emotional regulation as a primary governing mechanism. 4
Emotion regulation is defined broadly as the capacity to manage one’s own emotional responses and includes strategies to increase, maintain, or decrease the intensity, duration, and trajectory of positive and negative emotions. 5 Emphasizing the role of deficits in emotion regulation, recent findings suggest a connection to the development of disruptive behavior disorders. This highlights the practicality of implementing early intervention programs aimed at children, with a focus on enhancing their emotion regulation skills. 6
Previous anecdotal reports on adult ADHD have shown that emotional experience could be regulated through deep transcranial magnetic stimulation (dTMS). 7 With the advantage of dTMS in stimulating deeper structures like anterior cingulate cortex (ACC) and medial prefrontal cortex (mPFC), the areas mainly involved in emotional regulation, dTMS could be effective as a noninvasive treatment modality in adolescents with externalizing behavior disorders (EBDs).
As per the available literature, no study has envisaged to assess the effects of dTMS on mPFC and ACC in emotional regulation in children and adolescents with EBDs.
Rationale for Study
Untreated externalizing behaviors, especially emotional dysregulation, pose a significant risk for the emergence of negative consequences in subsequent stages of life, like peer rejection, academic underachievement, involvement in criminal activities, and the onset of psychopathological conditions. 8 In recent decades, a multitude of interventions have been devised to address externalizing problems in adolescence. However, the overall impact of these interventions has been observed to be only modest to moderate. 9 Consequently, there arises a need to explore and develop newer interventions to effectively target these problems.
The mPFC, notably the ventromedial-prefrontal cortex (vmPFC), and subgenual anterior cingulate cortex (sgACC), with additional regions in anterior/mid insula, referred as salience network are largely involved in implicit emotional regulation.10, 11 These mPFC regions are known to interact with the amygdala and ventral striatum, which comprise a unique cortical-subcortical network of implicit emotion control, putting regulatory effects, including a decrease in negative affect.12–14
Aberrant mPFC functionality and its functional connectivity to associated limbic structures have been linked to emotional dysregulation, providing additional support for its pivotal role. 15 One transcranial direct current stimulation (tDCS)—fMRI study on modulating emotional experience indicates the potential capacity of tDCS to facilitate brain activation in mPFC regions responsible for implicit regulation of emotion. 16
Transcranial magnetic stimulation (TMS) is a well-known noninvasive diagnostic and therapeutic method that modifies or measures cortical excitability by generating cortical currents in the cerebral parenchyma by depolarizing neural cell membranes using fluctuating extracranial magnetic fields. 17
Preliminary findings indicate that repetitive transcranial magnetic stimulation (rTMS) holds promise in treating treatment-resistant depression among children and adolescents. 18 The positive side effect profile and effectiveness of TMS bolster its potential as a therapeutic approach for this demographic. A study conducted over a 3-year period observed no signs of decline in either depressive symptoms or cognitive capacity, confirming the lasting advantages of this modality for young ones dealing with treatment-resistant depression. 19
In a single open trial, children aged 7 to 12, who received 1 Hz TMS on the lateral dorsolateral prefrontal cortex (L-DLPFC), showed notable improvement in impulsivity/hyperactivity. The study also underscored the safety of TMS in this age group, as only minimal side effects were observed. 20
Given its favorable side effect profile and capacity to stimulate deep brain regions, such as the ACC and mPFC, dTMS may emerge as a promising noninvasive therapeutic option for adolescents grappling with EBDs.
Material and Methods
Aim
To assess safety and efficacy of dTMS in managing emotional dysregulation in children and adolescents with EBD and modulation of functional connectivity between ACC and mPFC.
Study setting: The study would be conducted at the Centre for cognitive neurosciences, which is equipped with the facility of dTMS at Central Institute of Psychiatry, Ranchi, Jharkhand, India. The study obtained approval from the institute’s ethics committee.
Study population: Children and Adolescents of both genders scoring above the cutoff for externalizing behavior as measured by CBCL further diagnosed with DSM 5 for either ADHD, ODD and CD, DMDD, mood disorders (except for those having psychotic symptoms), and substance use.
Sample size was estimated with software G*Power version 3.1.9.7. The sample size calculated (effect size 0.4 [moderate], alpha error 0.05, Power [1-beta] 0.85, number of groups two) was 38, so a sample size of 40 was taken. Total of 40 participants will be taken (20 patients with EBDs in active group G1; 20 patients with EBDs in sham group G2).
Sampling and allocation method: The sample would be recruited using convenient (purposive) sampling. The young subjects would be sequentially randomly assigned to groups with a single random-number sequence (no stratification). The dTMS sessions would be given after the baseline assessments. The subjects and rater would be blind to treatment, but the clinician administering the dTMS would be aware of the treatment group.
Inclusion Criteria for Young Subjects
Children and adolescents scoring above the cutoff for externalizing behavior as measured by CBCL further diagnosed with DSM V for ADHD (CBCL cutoff for boys ≥11, for girls aged 6–11 is 10, 12–18 is 9) ODD (CBCL cutoff for boys aged 6–11 is ≥7, 12–18 is ≥8, for girls aged 6–11 is ≥7, 12–18 is ≥8) and CD (CBCL cutoff for boys aged 6–11 is ≥9, 12–18 is ≥12, for girls aged 6–11 is ≥7, 12–18 is ≥11), DMDD, mood disorders (except for those having psychotic symptoms), and substance use.
Both genders from 8 years to less than 18 years of age.
Children and adolescents providing assent/consent and written informed consent provided by parents/caregivers for the study.
Exclusion Criteria for the Young Subjects
History of chronic or other significant general medical/neurological illness.
History of any psychotic illness.
Children and adolescents who had prior exposure to any mode of brain stimulation in the last 6 months.
Having any metallic implants/parts in the body.
Children and adolescents who are falling below 25 percentiles on Standard Progressive Matrices (SPM).
Description of the Tools
Informed Consent and Patient Instruction Form
Informed consent will be taken from the young subjects and guardians. They will be given information about the whole study procedure and necessary instructions will be given.
Sociodemographic and Clinical Data Sheet
Semi-structured sociodemographic and clinical data sheet: It will include details regarding personal demographics and socioeconomic characteristics, including name, age, sex, education, religion, habitat, family income, family type and structure, diagnosis, duration of illness, past history of illness, and family history of illness. Additional information will be gathered from the institute’s case record file of the participant. The information shall include guardians’ details with regards to age, education and occupation, chief complaints upon presentation, onset and course of illness, presence of any precipitating factor, personal history, premorbid traits, treatment history, investigations, mental status examination, and physical examination findings.
Child Behavior Checklist 6–18 21
As part of the Achenbach System of Empirically Based Assessment (ASEBA21), the Child Behavior Checklist (CBCL)/6–18 is a tool specifically created to evaluate the emotional, behavioral, and social functioning of school-aged children over the preceding 6 months. This paper-and-pencil checklist consists of 112 items and can be efficiently filled out by the child’s parent or caregiver within approximately 15 to 20 minutes. 21 Responses to items are recorded on a 3-point Likert scale as 0 = not true, 1 = somewhat or sometimes true, and 2 = very true or often true. This measurement includes eight empirically derived syndrome scales and six diagnostic scales as per the Diagnostic and Statistical Manual of Mental Disorders.22 Achenbach and Rescorla (2001) mentioned remarkably high values for test-retest item reliability, with 1.00 for the competency items and 0.95 for the problem specific items. The test-retest reliability coefficients for scale scores, including total competence, total adaptive functioning, and total problems, range from 0.91 to 0.95.21 Competence scale Cronbach’s alphas range from 0.63 to 0.79,21 while specific problem scales show alphas between 0.78 and 0.97. Lastly, for DSM-oriented scales, Cronbach’s coefficients range from 0.72 to 0.91. 21
Difficulties in Emotion Regulation Scale 23
The Difficulties in Emotion Regulation Scale (DERS) is a self-report tool consisting of 36 items, created to evaluate clinically significant challenges in emotion regulation. Respondents use a Likert scale for indicating the frequency with which each item applies to them, with responses ranging from 1 (almost never) to 5 (almost always). Elevated scores on the scale denote increased challenges in emotion regulation. The assessment spans six clinically relevant areas of emotion dysregulation, including nonacceptance of emotional responses, limited emotional awareness, constrained access to emotion regulation strategies, challenges in maintaining goal-directed behavior during emotional arousal, impulse control difficulties, and lack of emotional clarity. In the scale’s development, it was observed that DERS exhibited good internal consistency, reliable test–retest measure, and satisfactory validity.23
Modified Overt Aggression Scale 24
The Modified Overt Aggression Scale (MOAS) is a Likert rating scale comprising four components designed to assess and record “frequency and severity” of aggressive episodes. It includes evaluation of verbal aggression, aggression directed toward objects, aggression toward oneself, and aggression toward others. The MOAS exhibits outstanding inter-rater reliability, satisfactory internal consistency, and showcases validity through convergent, divergent, and discriminant measures. 25
Barratt’s Impulsivity Scale2⁶
Barratt’s Impulsivity Scale (BIS) is a commonly used tool for measuring impulsiveness. It is a 30-item self-report measure. Items are scored to yield six first-order factors (attention, motor, self-control, cognitive complexity, perseverance, and cognitive instability impulsiveness) and three second-order factors (attentional, motor, and non-planning impulsiveness). BIS has been established as a reliable and valid tool for measuring impulsivity in adolescents. 27
Drug Abuse Screening Test 28
The Drug Abuse Screening Test (DAST-10) is a concise 10-item screening tool designed for administration by a clinician or for self-administration. Respondents answer each question with a yes or no. This validated and tested tool is utilized to identify problematic drug use, specifically focusing on the previous 12 months. The DAST serves to pinpoint diagnosable drug use problems and has demonstrated high reliability and validity across numerous studies. 29
Children Global Assessment Scale 30
The Children’s Global Assessment Scale (CGAS) is a scale used by mental health professionals to rate the general functioning of youths under 18 years. The scoring scale spans from 1 to 90 or 1 to 100, with higher scores indicating better functioning. Some versions exclude the range from 91 to 100, as scores in this range are seldom observed among individuals seeking health services. The CGAS demonstrated reliability across raters and over time, while also showcasing validity through both discriminant and concurrent measures. 30
Affective Reactivity Index 31
The Affective Reactivity Index (ARI) includes six symptom items and one impairment item focused on irritability. Selection of content of these items was based on a simple definition of irritability, referring to a mood characterized by easy annoyance and touchiness, often accompanied by anger and temper outbursts.31 Each of the individual items is assigned scores of 0, 1, or 2. Total score is derived by summing the scores of the first six items; the seventh item, designated as an impairment item, is analyzed independently. The ARI demonstrates outstanding internal consistency and forms a single factor in both parent- and self-report versions. 31
Clinical Global Impression 32
The Clinical Global Impression—Severity (CGI-S) is evaluated using a 7-point Likert scale, where the severity of illness is expressed with responses from 1 (normal) to 7 (among the most severely ill patients). Higher scores on the Clinical Global Impression of Change (CGI-C) scale indicate degree of change or improvement, from 1 (very much improved) to 7 (very much worse). The ratings for treatment response consider therapeutic efficacy and treatment-related adverse events, from 0 (marked improvement with no side effects) to 4 (unchanged or worse, with side effects outweighing therapeutic effects). The CGI is a valid clinical outcome tool, which is well-suited for regular application in an inpatient setting. 33
The Standard Progressive Matrices 34
Developed by John C. Raven, the SPM is a test administered either individually or in group settings. It assesses intelligence in both children and adults by utilizing nonverbal abstract reasoning tasks. For use in ages from 8 to 65, SPM has 60 problems (five sets of 12), involving completing a pattern or figure with a part missing by choosing the correct missing piece from among six alternatives. Patterns are presented in order of increasing difficulty. It has good reliability and high internal consistency. 35
TMS Adverse Events and Associated Sensations Questionnaire 36
The TMSens_Q, a questionnaire designed to capture secondary effects post TMS application, was developed through a Delphi procedure involving international TMS experts. This collaborative process led to a consensus on questionnaire items, rendering it applicable in clinical and research environments. Employing this structured TMS questionnaire routinely and ensuring consistent reporting of unintended TMS effects is going to play crucial role in monitoring the safety of TMS in our study. This is particularly valuable when implementing new protocols and enhancing the understanding of experimental results.
fMRI Feasibility Checklist
It is a questionnaire which helps us to rule out any contraindications of MRI.
dTMS
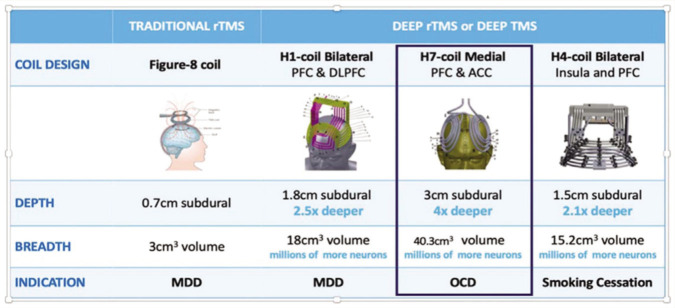
TMS employs using magnetic field to influence the membrane potentials of cortical neurons when applied over scalp. As TMS is capable of directly evoking cortical neural potentials, it is used to modulate cortical excitability and, thus, affect neural networks that are impaired in various psychiatric ailments. Stimulation variables like intensity, frequency, and total number of pulses influence the direction and degree of cortical plasticity. In general, high-frequency stimulation (>5 Hz) is seen to increase cortical excitability (long-term potentiation-like effect) and low-frequency stimulation(<5 Hz) is seen to decrease it (long-term depression-like effect). 37 Unlike typical TMS coils such as the figure-of-8 or round variants, which directly stimulate targets located up to approximately 1 cm beneath the surface of the skull, dTMS can reach depths of around 4 cm beneath the skull’s surface, depending on the specific H-coil utilized. 37 As a result, dTMS could target a variety of deep cortical and adjoining subcortical regions, including the mPFC and the ACC (3 cm3) without significant enhancement of electric fields induced in superficial cortical areas.38, 39
The BrainsWay dTMS system is composed of four main components: an electromagnetic H-coil, TMS stimulator, a cooling system, and a positioning arm. Various H coils have been developed to treat and research different disorders. The H1 dTMS coil has been authorized by the US FDA for treating treatment-resistant depression. The H7 dTMS coil was approved by the US FDA for the treatment of OCD in 2018. The sham stimulation coil and the H7 coil are both located inside a helmet that is attached to the dTMS system. It is possible to deliver both high- and low-frequency pulses through the coil, and many parameters can be changed, including frequency, train length, train duration, and time duration (Figure 1).
Figure 1. Technical Properties of the Various FDA-Cleared TMS Coils. 39 .
Magnetic Resonance Imaging (3T Ingenia, Philips Made, Netherlands)
We will be using a 3T fMRI machine named INGINEA system (Philips Made). Functional magnetic resonance imaging is an approach for defining activity in the healthy and diseased human brain. BOLD fMRI detects local increases in relative blood oxygenation that are most probably a direct consequence of local energy usage. fMRI can be used to produce activation maps showing which parts of the brain area are involved in a particular mental process.
Measurement of Motor Threshold
Measurement of resting motor threshold (RMT) will be done using an electromyography (EMG) system. The active electrode of EMG will be placed on the bilateral tibialis anterior muscle for measuring the motor evoked potentials (MEP). H7 TMS coil will be placed on the scalp and rotated posteriorly along the sagittal plane. Stimuli will be given in an incremental manner till we see a visible twitch in bilateral muscle. Motor threshold is defined as the minimum TMS intensity to produce a predefined MEP in the abductor pollicis brevis muscle in at least 50% of successive trials. 40
Procedure
During the study period, young subjects will be given appropriate medications by the treating team which will be recorded, that is a stable dose of medication. In total, 15 sessions of dTMS will be given once a day for 5 days a week over 3 weeks. TMSens_Q be applied to assess the side effects after dTMS, if any, after each session of dTMS.
dTMS Procedure Active
The helmet with H7 dTMS coil will be placed on the head of the patient. It will be adjusted such that the maximal stimulation is delivered to the ACC and the mPFC. Treatment will be delivered at frequency of 18 Hz at 100% of RMT. An inter-train interval of 20 seconds, a train duration of 2 seconds (for 40 trains per session), and a total of 1440 pulses per session would be delivered.
dTMS Procedure Sham
The helmet of the H7 coil has an inbuilt system to deliver sham stimulation. The helmet will be placed over the head of the young subject and the sham protocol is selected from the dTMS system. The sham H coil is created with a circular winding in the form of a cylindrical tube, positioned above the scalp in such a way that the windings are intentionally kept at a distance to avoid inducing cortical depolarization. The sham coil is designed to produce analogous acoustic artifacts and scalp sensations mimicking the active coil, thereby preserving the blinding of the study.
Assessment
Baseline assessments will be done using CBCL, DERS, ARI, BIS, DAST, MOAS, CAG, and CGI scales. Both groups will receive 15 sessions of dTMS over 3 weeks. TMSens_Q will be applied after every session of dTMS. CBCL, DERS, ARI, BIS, DAST, MOAS, CAG, and CGI scales will be applied again at the end of 3 weeks (Table 1).
Table 1.
Time Schedule of Enrolment, Intervention, and Assessments.
| Parameters | Intervention | ||||||||||||||
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 | Day 10 | Day 11 | Day 12 | Day 13 | Day 14 | Day15 | |
|
Informed
Consent |
× | ||||||||||||||
| Demographic Details | × | ||||||||||||||
| CBCL | × | × | |||||||||||||
| DERS | × | × | |||||||||||||
| MOAS | × | × | |||||||||||||
| ARI | × | × | |||||||||||||
| DAST | × | × | |||||||||||||
| BIS 11 | × | × | |||||||||||||
| CGAS | × | × | |||||||||||||
| CGI | × | × | |||||||||||||
| TMSens_Q | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × |
MRI Procedure
All images will be acquired on a Philips Ingenia 3.0 T whole-body system equipped with a 16-channel head coil, at the fMRI center, Central Institute of Psychiatry.
Structural Data
Three-dimensional T1-weighted structural images will be acquired with the following parameters, echo time (TE) = 2.9 ms, repetition time (TR) = 6.5 ms, flip angle (FA) = 8°, field of view (FOV) = 256 × 256 × 192, matrix = 256 × 256, voxel size = 1 × 1 × 1, slices = 192, scan duration = 5 minutes 39 seconds.
Activation Scans
EPI sequences will be obtained with the following parameters. Scan duration = 600 seconds, TE = 35 ms, TR = 3000 ms, FA = 90°, FOV = 230 × 230 × 144, 36 slices. A total of 200 dynamic scans will be obtained.
MRI Task
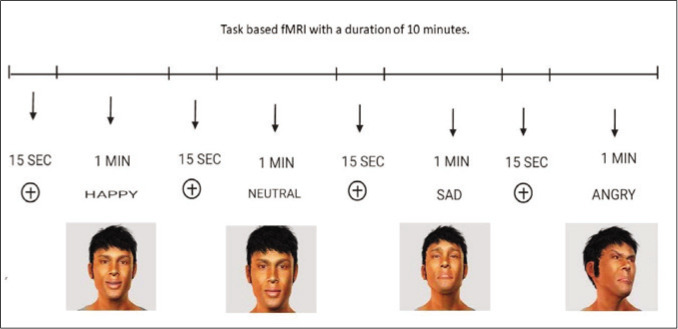
During functional MRI scan, young subjects will perform the Avatar task by using a block design paradigm in which rest conditions and active conditions will be given by turns. The task has been created by E-Prime 3.0 software and delivered through the NORDIK NeuroLabs system. Each trial comprises of 8 one-minute videos of an avatar in happy, angry, sad, and neutral emotions, and a 15-second fixation cross in between. The task will be displayed on a screen at the head end of the patient with viewing through a mirror system placed over the head coil.
Outcomes (Table 2 and Table 3)
Table 2.
Summary of Primary Outcome Variables and Plan of Analysis.
| Variable | Types | Description Test | Baseline Group Diifference | Change In Severity Scores Overtime Between Groups | |
| Primary Outcome Variables | CBCL | Continuous | Mean ± SD | Independent T test | Paired T test |
| DERS | Continuous | Mean ± SD | Independent T test | Paired T test | |
Table 3.
Summary of Secondary Outcome Variables and Plan of Analysis.
| Variable | Types | Description Test | Baseline Group Diifference | Change In Severity Scores Overtime Between Groups | |
| Secondary Outcome Variables |
MOAS | Continuous | Mean ± SD | Independent T test | Paired T test |
| ARI | Continuous | Mean ± SD | Independent T test | Paired T test | |
| DAST | Continuous | Mean ± SD | Independent T test | Paired T test | |
| BIS 11 | Continuous | Mean ± SD | Independent T test | Paired T test | |
| CGAS | Continuous | Mean ± SD | Independent T test | Paired T test | |
| CGI | Continuous | Mean ± SD | Independent T test | Paired T test | |
Primary Outcome Variables
Safety of dTMS in young subjects as assessed by TMSens_Q and reduction in scores of DERS.
Secondary Outcome Variables
fMRI task-based assessment of the difference in activation of mPFC and ACC at baseline and after application of dTMS and reduction in scores of BIS, ARI, MOAS, CGI, and CGAS.
MRI Analysis
In this study, the task-related fMRI data will be processed using CONN v.20.b 41 after proper quality control checking using the preexisting pipelines followed by denoising of the data which would be measured using BOLD activation and the resulting swau (realigned and unwarped + slice-timing corrected + normalized + smoothened) images will be further analyzed using Statistical Parametric Mapping software (SPM) 42 for changes in the network region of the brain using resting state while focusing on default mode network changes.
Statistical Analysis
The results obtained will be analyzed using the computer software program, Statistical Package for Social Sciences-version 25.0 (SPSS-25.0 or the latest available version) for Windows→, with different parametric and nonparametric measures being used, wherever applicable in the following steps:
STEP 1: Sample characteristics would be described with descriptive statistics—percentage, mean, and standard deviation.
STEP 2: Baseline sociodemographic and clinical characteristics would be compared between the groups using independent t-test and chi-square test, as applicable. Continuous variables, such as age, will be compared using an independent t-test, while categorical variables, like gender, will be compared utilizing a chi-square test.
STEP 3: Effect size and statistical power of the test assessment are planned to be done through partial eta squared. A value exceeding 0.5 will be categorized as a large effect size, while a range of 0.2–0.5 will be considered a moderate effect size, and anything below 0.2 will be deemed a mild effect size. The observed power will be determined using a significance level of alpha = 0.05 and tabulated accordingly. We will interpret outcomes with a significance level below 0.05.
STEP 4: The baseline scores of CBCL, DERS, ARI, BIS, DAST, MOAS, CAG, and CGI scales will be compared between patient groups by independent t-test. T-test will be applied to compare the score of CBCL, DERS, ARI, BIS, DAST, MOAS, CAG, and CGI scales at baseline and third week with the active group over time. A t-test will be utilized to compare the scores of CBCL, DERS, ARI, BIS, DAST, MOAS, CAG, and CGI Scales at baseline and third week with the sham group over time.
STEP 5: We plan to employ multivariate repeated measures analysis of variance, implementing the Greenhouse-Geisser test for sphericity correction. The objective will be to evaluate and compare the overall influence of treatment across time for two groups, where treatment serves as the between-group factor and time as the within-subject factor.
Discussion
Disruptive behavior problems represent a prevalent cause for children in elementary school to be referred to psychological services, and they are linked to enduring adverse consequences 43 and can have serious detrimental sequelae such as a higher risk of unemployment, criminality, and mortality in adulthood. 44 Considering the societal and economic burden associated with EBDs, it is hence imperative to search for newer modalities for treatment.
In child neurology and psychiatry, transcranial brain stimulation is being actively researched, primarily with a variety of noninvasive electrical cortical stimulations, especially in conditions where over- or under-activation of focal cortical structures is thought to be a part of the pathophysiology. 45 The positive side effect profile and efficacy observed with TMS support its utilization as a therapeutic intervention for managing depression in children and adolescents. 46 Repetitive stimulation of bilateral dorsolateral prefrontal cortex (DLPFC) through TMS was found to be beneficial in emotional dysregulation in borderline personality disorder and could have therapeutic applications. 47
Therefore, modulating the mPFC and ACC functionality through dTMS shows promise for studying and potentially treating EBDs in children and adolescents. This noninvasive technique could provide an understanding of the neural mechanisms underlying emotional regulation, aiding the development of neuroscience-guided therapies for EBDs.
Conclusion
The outcomes of the study will provide insights into how dTMS could be beneficial in addressing emotional dysregulation within the context of EBDs. This information may assist clinicians in improving the overall management of young individuals with EBDs. There are some inherent limitations to conducting this study though. The first being limited sample size and then not being able to do a redo scan/s on follow up which might preclude a chance of finding network changes in the continuation phase of the treatment. Also, transdiagnostic approach might pose a risk of under or over reporting of a particular diagnostic entity in the limited sample size we are studying.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Zhu J, Yu C, Zhang W, et al. Peer victimization, deviant peer affiliation and impulsivity: predicting adolescent problem behaviors. Child Abuse Negl, 2016; 58: 39–50. [DOI] [PubMed] [Google Scholar]
- 2.Schmengler H, Peeters M, Stevens GWJM, et al. Educational level, attention problems, and externalizing behaviour in adolescence and early adulthood: the role of social causation and health-related selection—the TRAILS study. Eur Child Adolesc Psychiatry, 2023; 32(5): 809–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Psychiatric Association. Disruptive, impulse-control, and conduct disorders. In: Diagnostic and Statistical Manual of Mental Disorders. 5th ed. DSM Library: American Psychiatric Association; 2013 [Google Scholar]
- 4.Eisenberg N, Cumberland A, Spinrad TL, et al. The relations of regulation and emotionality to children’s externalizing and internalizing problem behavior. Child Dev, 2001; 72(4): 1112–1134. [DOI] [PubMed] [Google Scholar]
- 5.Koole SL, and Rothermund K. “I feel better, but I don’t know why”: the psychology of implicit emotion regulation. Cogn Emot, 2011; 25(3): 389–399. [DOI] [PubMed] [Google Scholar]
- 6.Njardvik U, Smaradottir H, and Öst LG. The effects of emotion regulation treatment on disruptive behavior problems in children: a randomized controlled trial. Res Child Adolesc Psychopathol, 2022; 50(7): 895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bleich-Cohen M, Gurevitch G, Carmi N, et al. A functional magnetic resonance imaging investigation of prefrontal cortex deep transcranial magnetic stimulation efficacy in adults with attention deficit/hyperactive disorder: a double-blind, randomized clinical trial. NeuroImage: Clinical, 2021; 30: 102670. DOI: 10.1016/j.nicl.2021.102670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pardini DA, and Fite PJ. Symptoms of conduct disorder, oppositional defiant disorder, attention-deficit/hyperactivity disorder, and callous-unemotional traits as unique predictors of psychosocial maladjustment in boys: advancing an evidence base for DSM-V. J Am Acad Child Adolesc Psychiatry, 2010; 49(11): 1134–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCart MR, Priester PE, Davies WH, et al. Differential effectiveness of behavioral parent-training and cognitive-behavioral therapy for antisocial youth: a meta-analysis. J Abnorm Child Psychol, 2006; 34(4): 527–543. DOI: 10.1007/s10802-006-9031-1 [DOI] [PubMed] [Google Scholar]
- 10.Adamaszek M, D’Agata F, Ferrucci R, et al. Consensus paper: cerebellum and emotion. Cerebellum, 2017; 16(2): 552–576. [DOI] [PubMed] [Google Scholar]
- 11.Bai S, Dokos S, Ho KA, et al. A computational modelling study of transcranial direct current stimulation montages used in depression. Neuroimage, 2014; 87: 332–344. [DOI] [PubMed] [Google Scholar]
- 12.Etkin A, Egner T, and Kalisch R.. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci, 2011; 15(2): 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drevets WC, Savitz J, and Trimble M.. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr, 2008; 13(8): 663–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stein JL, Wiedholz LM, Bassett DS, et al. A validated network of effective amygdala connectivity. Neuroimage, 2007; 36(3): 736–745. [DOI] [PubMed] [Google Scholar]
- 15.Buhle JT, Silvers JA, Wager TD, et al. Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cereb Cortex, 2014; 24(11): 2981–2990. DOI: 10.1093/cercor/bht154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abend R, Sar-El R, Gonen T, et al. Modulating emotional experience using electrical stimulation of the medial-prefrontal cortex: a preliminary tDCS-fMRI study. Neuromodulation, 2019; 22(8): 884–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pell GS, Roth Y, and Zangen A.. Modulation of cortical excitability induced by repetitive transcranial magnetic stimulation: influence of timing and geometrical parameters and underlying mechanisms. Prog Neurobiol, 2011; 93(1): 59–98. [DOI] [PubMed] [Google Scholar]
- 18.Donaldson AE, Gordon MS, Melvin GA, et al. Addressing the needs of adolescents with treatment-resistant depressive disorders: a systematic review of rTMS. Brain Stimul, 2014; 7(1): 7–12. [DOI] [PubMed] [Google Scholar]
- 19.Mayer G, Aviram S, Walter G, et al. Long-term follow-up of adolescents with resistant depression treated with repetitive transcranial magnetic stimulation. J ECT, 2012; 28(2): 84–86. [DOI] [PubMed] [Google Scholar]
- 20.Gómez L, Vidal B, Morales L, et al. Low-frequency repetitive transcranial magnetic stimulation in children with attention deficit/hyperactivity disorder. Preliminary results. Brain Stimul, 2014; 7(5): 760–762. [DOI] [PubMed] [Google Scholar]
- 21.Achenbach TM. International findings with the Achenbach System of Empirically Based Assessment (ASEBA): applications to clinical services, research, and training. Child Adolesc Psychiatry Ment Health, 2019; 13(1). DOI: 10.1186/s13034-019-0291-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hudziak JJ, Copeland W, Stanger C, et al. Screening for DSM-IV externalizing disorders with the Child Behavior Checklist: a receiver-operating characteristic analysis. J Child Psychol Psychiatry, 2004; 45(7): 1299–1307. [DOI] [PubMed] [Google Scholar]
- 23.Gratz KL, and Roemer L.. Multidimensional assessment of emotion regulation and dysregulation: development, factor structure, and initial validation of the Difficulties in Emotion Regulation Scale. J Psychopathol Behav Assess, 2004; 26: 41–54. [Google Scholar]
- 24.Oliver PC, Crawford MJ, Rao B, et al. Modified Overt Aggression Scale (MOAS) for people with intellectual disability and aggressive challenging behaviour: a reliability study. J Appl Res Intellect Disabil, 2007; 20(4): 368–372. [Google Scholar]
- 25.Coccaro EF. The Overt Aggression Scale Modified (OAS-M) for clinical trials targeting impulsive aggression and intermittent explosive disorder: validity, reliability, and correlates. J Psychiatr Res, 2020; 124: 50–57. [DOI] [PubMed] [Google Scholar]
- 26.Patton JH, Stanford MS, and Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol, 1995; 51(6): 768–774. [DOI] [PubMed] [Google Scholar]
- 27.Salisu Rogo I, and Garba A.. Validity and Reliability of Barrat Impulsivity scale (BIS-11), Adolescent conduct disorder scale (ACD-scale) and socioeconomic status scale (SES) for use in adolescence personality research.
- 28.Skinner HA. The drug abuse screening test. Addict Behav, 1982; 7(4): 363–371. [DOI] [PubMed] [Google Scholar]
- 29.Yudko E, Lozhkina O, and Fouts A.. A comprehensive review of the psychometric properties of the Drug Abuse Screening Test. J Subst Abuse Treat, 2007; 32(2):189–198. [DOI] [PubMed] [Google Scholar]
- 30.Shaffer D, Gould MS, Brasic J, et al. A children’s global assessment scale (CGAS). Arch Gen Psychiatry, 1983; 40(11): 1228–1231. [DOI] [PubMed] [Google Scholar]
- 31.Stringaris A, Goodman R, Ferdinando S, et al. The Affective Reactivity Index: a concise irritability scale for clinical and research settings. J Child Psychol Psychiatry, 2012; 53(11): 1109–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guy W. ECDEU assessment manual for psychopharmacology. US Department of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, Psychopharmacology Research Branch, Division of Extramural Research Programs; National Institute ofMental Health; 1976. [Google Scholar]
- 33.Berk M, Ng F, Dodd S, et al. The validity of the CGI severity and improvement scales as measures of clinical effectiveness suitable for routine clinical use. J Eval Clin Pract, 2008; 14(6): 979–983. [DOI] [PubMed] [Google Scholar]
- 34.Leavitt VM. Standard Progressive Matrices. In: Encyclopedia of clinical neuropsychology. Springer, 2011, 2368–2368. [Google Scholar]
- 35.Abdel-Khalek AM. Reliability and factorial validity of the standard progressive matrices among Kuwaiti children ages 8 to 15 years. Percept Mot Skills, 2005; 101(2): 409–412. [DOI] [PubMed] [Google Scholar]
- 36.Giustiniani A, Vallesi A, Oliveri M, et al. A questionnaire to collect unintended effects of transcranial magnetic stimulation: a consensus-based approach. Clin Neurophysiol, 2022; 141: 101–108. DOI: 10.1016/j.clinph.2022.06.008 [DOI] [PubMed] [Google Scholar]
- 37.Tendler A, Barnea Ygael N, Roth Y, et al. Deep transcranial magnetic stimulation (dTMS)—beyond depression. Expert Rev Med Devices, 2016; 13(10): 987–1000. [DOI] [PubMed] [Google Scholar]
- 38.Zangen A, Roth Y, Voller B, et al. Transcranial magnetic stimulation of deep brain regions: evidence for efficacy of the H-coil. Clin Neurophysiol, 2005; 116(4): 775–779. [DOI] [PubMed] [Google Scholar]
- 39.Harmelech T, Roth Y, and Tendler A.. Deep TMS H7 coil: features, applications & future. Expert Rev Med Devices, 2021; 18(12): 1133–1144. [DOI] [PubMed] [Google Scholar]
- 40.Rossini PM, Barker AT, Berardelli A, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol, 1994; 91(2):79–92. [DOI] [PubMed] [Google Scholar]
- 41.Whitfield-Gabrieli S, and Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect, 2012; 2(3): 125–141. [DOI] [PubMed] [Google Scholar]
- 42.Ashburner J. SPM: A history. Neuroimage, 2012; 62(2): 791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kimonis ER, and Frick PJ. Oppositional defiant disorder and conduct disorder grown-up. J Dev Behav Pediatr, 2010; 31(3): 244–254. [DOI] [PubMed] [Google Scholar]
- 44.Scott JG, Giørtz Pedersen M, Erskine HE, et al. Mortality in individuals with disruptive behavior disorders diagnosed by specialist services—a nationwide cohort study. Psychiatry Res, 2017; 251: 255–260. DOI: 10.1016/j.psychres.2017.02.029 [DOI] [PubMed] [Google Scholar]
- 45.Frye RE, Rotenberg A, Ousley M, et al. Transcranial magnetic stimulation in child neurology: current and future directions. J Child Neurol, 2008; 23(1): 79–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Narang P, Madigan K, Sarai S, et al. Is transcranial magnetic stimulation appropriate for treating adolescents with depression?. Innov Clin Neurosci, 2019; 16(9–10): 33–35. [PMC free article] [PubMed] [Google Scholar]
- 47.Molavi P, Aziziaram S, Basharpoor S, et al. Repeated transcranial direct current stimulation of dorsolateral-prefrontal cortex improves executive functions, cognitive reappraisal emotion regulation, and control over emotional processing in borderline personality disorder: a randomized, sham-controlled, parallel-group study. J Affect Disord, 2020; 274: 93–102. [DOI] [PubMed] [Google Scholar]