Introduction
Massive irreparable rotator cuff tears present a significant challenge for the orthopedic surgeon. Multiple treatment options have been described including partial repair with and without graft augmentation, interposition grafts, debridement, tendon transfer, superior capsule reconstruction, and reverse total shoulder arthroplasty (TSA) [7,10,28]. More recently, the InSpace subacromial balloon spacer (originally OrthoSpace, now Stryker) was developed for temporary and possibly definitive treatment of massive irreparable rotator cuff tears. The product has been in use in Europe for over a decade, receiving US Food and Drug Administration (FDA) clearance in July 2021 [23,24].
The biomechanical principle employed by the subacromial balloon spacer is depression of the humeral head and restoration of the native glenohumeral alignment in the coronal plane, resulting in frictionless gliding and reduced subacromial shear forces [3,14,17,20,21]. The InSpace balloon is made from poly(l-lactide-co-ε-caprolactone) (PLCL), a polymer that utilizes the strength of poly(l-lactide) and the flexibility of ε-caprolactone in a 70:30 ratio. It degrades over 12 months via a foreign body reaction, leading to elimination of the material [26]. The ideal candidate for this procedure is the low-demand, older patient with pain secondary to supraspinatus tendon tear, minimal glenohumeral arthritis, and an intact subscapularis who has failed non-operative treatment [3,17,25 –27]. Complications associated with the use of this balloon include persistent pain requiring additional surgery, infection, nerve injury and dysesthesia, synovitis, foreign body reaction, and migration of the implant [2,4 –6,9,15,16,18,19].
We present the case of a patient who underwent arthroscopic placement of the subacromial balloon spacer with subsequent early rupture and deflation of the device associated with acute-onset atraumatic shoulder pain and functional limitation at 3.5 weeks postoperatively. This complication has not previously been described in the literature.
Case Report
The patient described in this case report provided informed consent to describe the details of her case. An 82-year-old woman presented with a 1-month history of left shoulder pain following a ground-level fall onto her left side. The history, physical examination, and imaging studies including magnetic resonance imaging (MRI) and plain radiographs revealed complete tears of the supraspinatus and infraspinatus tendons with retraction to the glenoid, Goutallier grade 2 fatty atrophy of the supraspinatus and infraspinatus and grade 0 fatty atrophy of the teres minor and subscapularis, mild superior migration of the humeral head relative to the glenoid with a subacromial space measuring 2.2 mm, a Bigliani type I acromion without a spur, and no significant glenohumeral joint arthritis (Fig. 1). After 3 months of conservative treatment including oral non–steroidal anti-inflammatory medications and steroid injections into the glenohumeral joint, her pain persisted, primarily with overhead function. Active range of motion (ROM) was measured at 30° of forward flexion, 30° of abduction, 20° of external rotation, and internal rotation to the level of L1. She was noted to have full passive ROM. Given her advanced age and low demand, she elected to undergo arthroscopic debridement of the glenohumeral joint and subacromial space, with insertion of the InSpace subacromial balloon spacer.
Fig. 1.
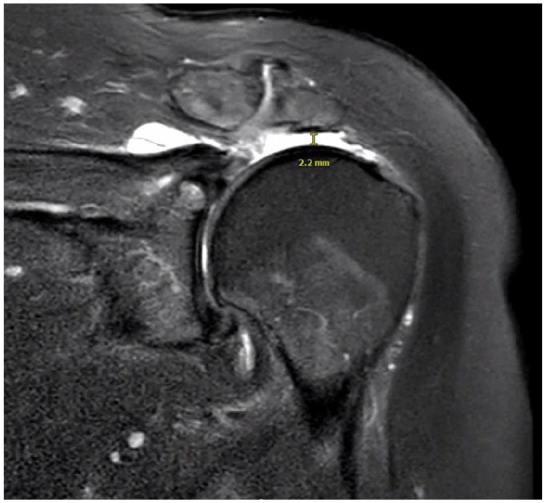
Preoperative coronal T1-weighted magnetic resonance image of left shoulder. The humeral head is superiorly elevated with a subacromial space measurement of 2.2 mm.
The patient’s medical history was notable for obstructive sleep apnea, asthma, and atrial fibrillation. Her height was 62’’, weight was 202 lbs, and body mass index (BMI) was 37. Preoperatively, she was medically cleared for surgery by her cardiologist.
After the administration of regional and general anesthesia, she underwent left shoulder arthroscopy in the beach-chair position. Initial diagnostic arthroscopy revealed age-appropriate minimal arthritic changes, complete tears of the supraspinatus and infraspinatus tendons, and intact teres minor and subscapularis tendons; there was no evidence of instability or adhesive capsulitis. Passive ROM was noted to be 170° of forward elevation, 160° of abduction, 60° external rotation with the arm at 0° of abduction; with the arm at 90° of abduction, there was 70° of internal rotation before the scapula began to lift off the posterior chest wall.
Extensive arthroscopic debridement and total synovectomy were performed with the use of standard arthroscopic techniques. She then underwent subacromial decompression without acromioplasty. Excess tendon was debrided from the greater tuberosity, and the subacromial space scar tissue was removed and debrided for placement of the balloon spacer. A small (40 mm × 50 mm) balloon was selected and placed according to the manufacturer’s protocol. The balloon was filled with 16 cc of physiologic saline and was noted to be stable and in the appropriate position. No complications were noted during or immediately after the procedure.
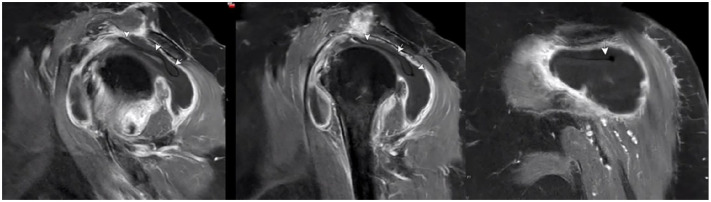
At routine follow-up 2 weeks after surgery, the patient reported satisfaction with the procedure and pain control and had started physical therapy. Three-and-a-half weeks after surgery, the patient experienced acute-onset atraumatic left shoulder pain with associated ROM limitations, primarily abduction and forward flexion. Evaluation in the emergency department revealed the absence of systemic symptoms including fever and a normal white blood cell count (6.29 k/cmm), although her C-reactive protein level and erythrocyte sedimentation rate were elevated (129.8 mg/L and 38 mm/h, respectively). She was hospitalized because of severe pain. Given concern for possible infection, MRI of the shoulder was ordered and demonstrated deflation of the subacromial balloon spacer with mild posterior migration of the balloon under the acromion process and surrounding hemarthrosis (Fig. 2). In addition, the humeral head was superiorly elevated with a subacromial space measurement of 2.1 mm (Fig. 3). Pain was controlled with the use of opioids, and infection was ruled out given her absence of systemic symptoms and reassuring white blood cell count. The patient was discharged home in stable condition.
Fig. 2.
Sagittal and coronal T1-weighted magnetic resonance images at 4 weeks postoperatively, 6 days after the onset of acute left shoulder pain. White arrows indicate deflated subacromial balloon spacer. There is a large glenohumeral joint effusion communicating with subacromial and subdeltoid bursal space likely due to a combination of postsurgical inflammation, synovitis, and saline that was previously confined to the balloon.
Fig. 3.
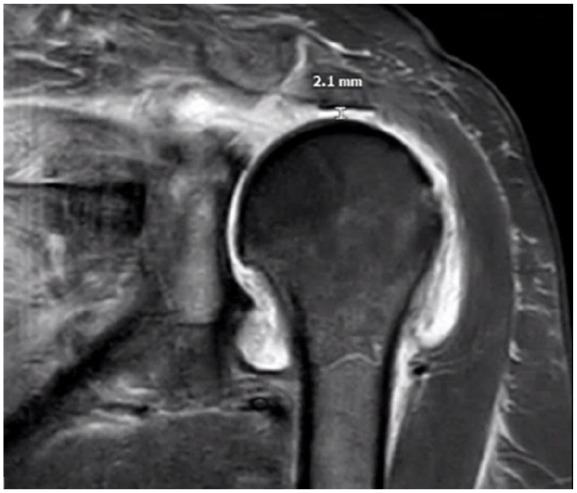
Coronal T1-weighted magnetic resonance image at 4 weeks postoperatively, 6 days after the onset of acute left shoulder pain. The humeral head is superiorly elevated with a subacromial space measurement of 2.1 mm.
During evaluation in clinic 1 week after hospital admission, the patient reported continued pain and functional limitation. C-reactive protein level and erythrocyte sedimentation rate had trended down (22.1 mg/L and 33 mm/h, respectively). Further treatment options were discussed including watchful waiting, steroid injection, repeat arthroscopy with removal of the deflated balloon and replacement with a new balloon, or reverse TSA. She elected to undergo reverse TSA.
One week later, she underwent uncomplicated reverse TSA, during which a large hemarthrosis within the subacromial space and communicating with the glenohumeral space was encountered. The subacromial balloon spacer was removed atraumatically, and it was noted to be completely deflated. Inspection revealed a tear at the seam of the balloon at the 8:00 position relative to the glenoid (12:00 position) and the balloon neck from the lateral side (6:00 position) (Fig. 4). The balloon was returned to the manufacturer for further evaluation.
Fig. 4.
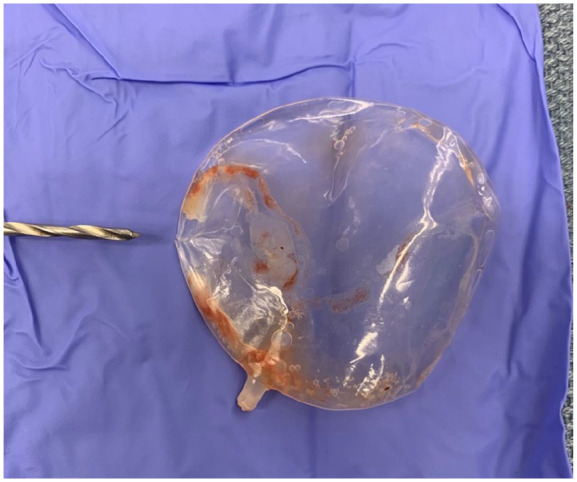
Ruptured subacromial balloon spacer following removal during reverse total shoulder arthroplasty procedure. Drill bit points to tear at the 8:00 position relative to the glenoid being at the 12:00 position.
At the most recent follow-up visit 6 months after reverse TSA, the patient reported no pain and increased mobility; ROM was measured at 150° of forward flexion, 140° of abduction, 50° of external rotation, and internal rotation to the level of L5.
Discussion
This case illustrates a previously unreported complication, atraumatic early balloon rupture associated with acute-onset severe pain, arising from the use of the InSpace subacromial balloon spacer, which received FDA clearance in 2021 for the treatment of massive irreparable rotator cuff tears. These tears can pose a significant challenge to orthopedic surgeons [7,10,23,24,28]. Given the many surgical options available, along with the short period that the InSpace device has been in use in the United States, limited data are available on the clinical utility and complications of the implant [1,8,9,11 –13,22,26,27]. Previously reported complications of the subacromial balloon spacer include persistent pain requiring additional or revision surgery, superficial or deep infection, nerve injury or dysesthesia, synovitis, foreign body reaction, and implant migration [2,4 –6,9,15,16,18,19].
To our knowledge, no similar case of subacromial balloon spacer rupture has been described. Senekovic et al [19] reported early deflation of a subacromial balloon spacer at 6 weeks in 1 of 24 patients, but this patient’s symptoms and the radiographic findings were not described. Ruiz Iban et al [16] reported unsatisfactory outcomes in 3 patients at 6, 6, and 7 weeks, respectively; all 3 patients were converted to reverse TSA, and no remnant of the balloon spacer could be appreciated. The authors postulated that these findings may be due to early rupture and subsequent reabsorption of the implant. Neither study reported acute-onset symptomatic loss of function or pain associated with early implant failure. In addition, deflation at 6 or more weeks was reported for all patients in both studies.
Fury et al [5] describe a case of subacromial balloon spacer–augmented rotator cuff repair failure associated with rice-body synovitis in a 66-year-old patient. The patient’s postoperative course was notable for persistent pain and difficulty progressing with mobility exercises. At 4 months postoperatively, MRI demonstrated a large effusion with rice-body synovitis, axillary lymphadenopathy, and failure of the cuff repair. Arthroscopic debridement revealed marked synovial fluid, innumerable rice bodies with a diffusely hyperemic synovium, and balloon fragmentation. The authors theorized that the diffuse inflammatory reaction negated the possibility of tissue healing and that the medial row knots may have caused an abrasive process and altered the typical breakdown process of the balloon.
Our case differs from that of Fury et al in that it was not confounded by an additional procedure (rotator cuff repair). In addition, we observed a tear along the seam of the balloon with failure observed at 3.5 weeks postoperatively, rather than fragmentation observed at 4 months postoperatively. Furthermore, we did not observe any signs of foreign body reaction or rice-body synovitis in our case. Finally, the patient reported by Fury et al suffered from severe pain and mobility limitations immediately following surgery, whereas our patient reported well-controlled pain and had begun progressing with physical therapy by 2 weeks after surgery.
Why the subacromial balloon spacer in our patient failed is unclear. A detailed history describing the onset of her acute pain revealed no precipitating trauma that may have caused inadvertent rupture. Bloodwork and vital signs during hospitalization ruled out infection. Given thorough preoperative inspection of the balloon in association with the patient’s overall reassuring status postoperatively, a preoperative or intraoperative tear of the balloon is unlikely. An MRI performed after the onset of acute pain revealed the collapse of the balloon with a superiorly migrated humeral head and a subacromial space measuring 2.1 mm. This is nearly identical to the subacromial space measured on MRI preoperatively (2.2 mm). With an inflated balloon in place, the humeral head is expected to be depressed. A biomechanical study by Lobao et al [14] of massive irreparable rotator cuff tears demonstrated an average humeral head depression of 6.2 mm after insertion of a subacromial balloon when compared to the acromiohumeral interval before balloon insertion.
Our case demonstrates Hamada 3 changes on plain radiographs, suggesting that the balloon may have been subjected to pressure overload during inflation. Because the goal is to depress the humeral head approximately 6 mm, the inferior capsule may have restricted this translocation and acted to increase the pressure on the wall of the balloon to a critical level, even though the recommended volume of saline was used to inflate the device. This pressure could have led to the premature failure. Because this patient demonstrated normal passive ROM in the clinic and on the operating room table, a capsular release was not performed. Had there been evidence of adhesive capsulitis, the balloon spacer procedure would have been aborted.
Indeed, removal and inspection of the balloon during the patient’s reverse TSA demonstrated a tear in the seam of the balloon. Based on the balloon positioning within the subacromial space, there was no bony contact that could have caused the split in the seam, as it was anterior to the glenoid and medial in the position of the sub-coracoid recess. In addition, an anterior portal was not used in the insertion process, and so no surgical tools contacted the balloon anteriorly. It is also possible that early failure of this device may have occurred secondary to a defect in the seam of the balloon leading to atraumatic rupture.
The major strength of this case report is the description of a previously unreported complication related to a recently cleared medical device. In addition, the balloon spacer was recovered during the subsequent surgery, allowing for visual inspection of the failed product. As with all case reports, this case may not be generalizable to all patients undergoing subacromial balloon spacer placement.
In conclusion, given the lack of robust evidence demonstrating benefits from the use of a subacromial balloon spacer, and in accordance with the present case, long-term studies investigating objective and patient-reported outcomes, including complications, of the subacromial balloon spacer are warranted.
Supplemental Material
Supplemental material, sj-pdf-1-hss-10.1177_15563316241257233 for Symptomatic Early Rupture of the InSpace Subacromial Balloon Spacer: A Case Report by Jack Mangan and Adam Shafritz in HSS Journal®
Supplemental material, sj-pdf-2-hss-10.1177_15563316241257233 for Symptomatic Early Rupture of the InSpace Subacromial Balloon Spacer: A Case Report by Jack Mangan and Adam Shafritz in HSS Journal®
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Human/Animal Rights: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2013.
Informed Consent: Informed consent was obtained from the patient included in this case report.
Level of Evidence: Level V: Case Report.
Required Author Forms: Disclosure forms provided by the authors are available with the online version of this article as supplemental material.
ORCID iD: Jack Mangan  https://orcid.org/0000-0002-9773-0717
https://orcid.org/0000-0002-9773-0717
References
- 1. Berk AN, Cregar WM, Gachigi KK, et al. Outcomes of subacromial balloon spacer implantation for irreparable rotator cuff tears: a systematic review and meta-analysis. J Shoulder Elbow Surg. 2023;32(10):2180–2191. 10.1016/j.jse.2023.04.016. [DOI] [PubMed] [Google Scholar]
- 2. Calvo E, Valencia M, Merino-Garcia JA, Luengo-Alonso G. Symptomatic foreign body reaction secondary to subacromial balloon spacer placement: a case report. J Shoulder Elbow Surg. 2020;29(8):e313–e316. 10.1016/j.jse.2020.02.029. [DOI] [PubMed] [Google Scholar]
- 3. Dasari SP, Khan ZA, Swindell HW, Mehta N, Kerzner B, Verma NN. Subacromial balloon spacer: indications, rationale, and technique. JBJS Essent Surg Tech. 2022;12(2):e21.00069. 10.2106/JBJS.ST.21.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Deranlot J, Herisson O, Nourissat G, et al. Arthroscopic subacromial spacer implantation in patients with massive irreparable rotator cuff tears: clinical and radiographic results of 39 retrospectives cases. Arthroscopy. 2017;33(9):1639–1644. 10.1016/j.arthro.2017.03.029. [DOI] [PubMed] [Google Scholar]
- 5. Fury MS, Cirino CM, White AE, Bauer TW, Taylor SA. Rice-body synovitis, foreign body reaction, and rotator cuff failure after subacromial balloon spacer augmentation of a rotator cuff repair: a case report. JBJS Case Connect. 2023;13(2):e23.00009. 10.2106/JBJS.CC.23.00009. [DOI] [PubMed] [Google Scholar]
- 6. Gervasi E, Maman E, Dekel A, Cautero E. Fluoroscopy-guided biodegradable spacer implantation using local anesthesia: safety and efficacy study in patients with massive rotator cuff tears. Musculoskelet Surg. 2016;100(suppl 1):19–24. 10.1007/s12306-016-0433-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hughes JD, Davis B, Whicker E, et al. Nonarthroplasty options for massive, irreparable rotator cuff tears have improvement in range of motion and patient-reported outcomes at short-term follow-up: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2023;31(5):1883–1902. 10.1007/s00167-022-07099-9. [DOI] [PubMed] [Google Scholar]
- 8. Johns WL, Ailaney N, Lacy K, Golladay GJ, Vanderbeck J, Kalore NV. Implantable subacromial balloon spacers in patients with massive irreparable rotator cuff tears: a systematic review of clinical, biomechanical, and financial implications. Arthrosc Sports Med Rehabil. 2020;2(6):e855–e872. 10.1016/j.asmr.2020.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Knapik DM, Williams BT, Verma NN. Balloon spacers in the management of massive rotator cuff tears: a focus on clinical outcomes. Ann Joint. 2020;6:19. 10.21037/AOJ-20-35. [DOI] [Google Scholar]
- 10. Kucirek NK, Hung NJ, Wong SE. Treatment options for massive irreparable rotator cuff tears. Curr Rev Musculoskelet Med. 2021;14(5):304–315. 10.1007/s12178-021-09714-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kunze KN, Moran J, Cecere R, et al. High rate of clinically meaningful achievement in outcomes after subacromial balloon spacer implantation for massive irreparable rotator cuff tears: a systematic review and meta-analysis. Am J Sports Med. 2024;52(1):286–294. 10.1177/03635465231155916. [DOI] [PubMed] [Google Scholar]
- 12. Kunze KN, Moran J, Taylor SA, et al. Subacromial balloon spacer implantation for massive irreparable rotator cuff tears is associated with restoration of the acromiohumeral interval and glenohumeral center of pressure: a systematic review and meta-analysis of controlled laboratory studies. Am J Sports Med. 2023;51(14):3870–3879. 10.1177/03635465221150652. [DOI] [PubMed] [Google Scholar]
- 13. Liu F, Dong J, Kang Q, Zhou D, Xiong F. Subacromial balloon spacer implantation for patients with massive irreparable rotator cuff tears achieves satisfactory clinical outcomes in the short and middle of follow-up period: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2021;29(1):143–153. 10.1007/s00167-019-05834-3. [DOI] [PubMed] [Google Scholar]
- 14. Lobao MH, Canham RB, Melvani RT, Abboud JA, Parks BG, Murthi AM. Biomechanics of biodegradable subacromial balloon spacer for irreparable superior rotator cuff tears: study of a cadaveric Model. J Bone Joint Surg Am. 2019;101(11):e49. 10.2106/JBJS.18.00850. [DOI] [PubMed] [Google Scholar]
- 15. Prat D, Tenenbaum S, Pritsch M, Oran A, Vogel G. Sub-acromial balloon spacer for irreparable rotator cuff tears: is it an appropriate salvage procedure? J Orthop Surg (Hong Kong). 2018;26(2):2309499018770887. 10.1177/2309499018770887. [DOI] [PubMed] [Google Scholar]
- 16. Ruiz Iban MA, Lorente Moreno R, Ruiz Diaz R, et al. The absorbable subacromial spacer for irreparable posterosuperior cuff tears has inconsistent results. Knee Surg Sports Traumatol Arthrosc. 2018;26(12):3848–3854. 10.1007/s00167-018-5083-3. [DOI] [PubMed] [Google Scholar]
- 17. Savarese E, Romeo R. New solution for massive, irreparable rotator cuff tears: the subacromial “biodegradable spacer.” Arthrosc Tech. 2012;1(1):e69–e74. 10.1016/j.eats.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Senekovic V, Poberaj B, Kovacic L, Mikek M, Adar E, Dekel A. Prospective clinical study of a novel biodegradable sub-acromial spacer in treatment of massive irreparable rotator cuff tears. Eur J Orthop Surg Traumatol. 2013;23(3):311–316. 10.1007/s00590-012-0981-4. [DOI] [PubMed] [Google Scholar]
- 19. Senekovic V, Poberaj B, Kovacic L, et al. The biodegradable spacer as a novel treatment modality for massive rotator cuff tears: a prospective study with 5-year follow-up. Arch Orthop Trauma Surg. 2017;137(1):95–103. 10.1007/s00402-016-2603-9. [DOI] [PubMed] [Google Scholar]
- 20. Singh S, Reeves J, Langohr GDG, Johnson JA, Athwal GS. The effect of the subacromial balloon spacer on humeral head translation in the treatment of massive, irreparable rotator cuff tears: a biomechanical assessment. J Shoulder Elbow Surg. 2019;28(10):1841–1847. 10.1016/j.jse.2019.03.036. [DOI] [PubMed] [Google Scholar]
- 21. Singh S, Reeves J, Langohr GDG, Johnson JA, Athwal GS. The subacromial balloon spacer versus superior capsular reconstruction in the treatment of irreparable rotator cuff tears: a biomechanical assessment. Arthroscopy. 2019;35(2):382–389. 10.1016/j.arthro.2018.09.016. [DOI] [PubMed] [Google Scholar]
- 22. Stewart RK, Kaplin L, Parada SA, Graves BR, Verma NN, Waterman BR. Outcomes of subacromial balloon spacer implantation for massive and irreparable rotator cuff tears: a systematic review. Orthop J Sports Med. 2019;7(10):2325967119875717. 10.1177/2325967119875717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stryker. InSpace subacromial balloon spacer. Available at: https://www.stryker.com/us/en/sports-medicine/products/inspace.html. Published 2023. Accessed May 17, 2023.
- 24. US Food and Drug Administration. Medical device databases. Device classification under section 513(f)(2)(De Novo). Available at: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/denovo.cfm?id=DEN200039. Published 2021. Accessed May 17, 2023.
- 25. Verma N, Srikumaran U, Roden CM, et al. InSpace implant compared with partial repair for the treatment of full-thickness massive rotator cuff tears: a multicenter, single-blinded, randomized controlled trial. J Bone Joint Surg Am. 2022;104(14):1250–1262. 10.2106/JBJS.21.00667. [DOI] [PubMed] [Google Scholar]
- 26. Viswanath A, Drew S. Subacromial balloon spacer—where are we now? J Clin Orthop Trauma. 2021;17:223–232. 10.1016/j.jcot.2021.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wright MA, Abboud JA, Murthi AM. Subacromial balloon spacer implantation. Curr Rev Musculoskelet Med. 2020;13(5):584–591. 10.1007/s12178-020-09661-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhou X, Zhang X, Jin X, Deng J, Zhang Z, Yu Y. Multiple surgical treatment comparisons for irreparable rotator cuff tears: a network meta-analysis. Medicine (Baltimore). 2023;102(22):e33832. 10.1097/MD.0000000000033832. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-hss-10.1177_15563316241257233 for Symptomatic Early Rupture of the InSpace Subacromial Balloon Spacer: A Case Report by Jack Mangan and Adam Shafritz in HSS Journal®
Supplemental material, sj-pdf-2-hss-10.1177_15563316241257233 for Symptomatic Early Rupture of the InSpace Subacromial Balloon Spacer: A Case Report by Jack Mangan and Adam Shafritz in HSS Journal®