Abstract
Background:
Management of opioid dependence requires titrating medication doses based on withdrawal symptoms, but its clinical assessment presents challenges when it comes to subjective reporting. This study aimed to find out the relationship between heart rate variability (HRV) and opioid withdrawal in patients with opioid dependence.
Methods:
Three groups of adult males were recruited: (a) patients with opioid dependence undergoing inpatient detoxification, (b) patients with opioid dependence stabilized on buprenorphine-based opioid substitution treatment, and (c) healthy controls. Frequency and time-domain parameters of HRV were used in the analysis. The opioid withdrawal was assessed using the Subjective Opiate Withdrawal Scale (SOWS).
Results:
Resting heart rate was found to be significantly different across the three groups (higher in patients stabilized on buprenorphine than the other two groups). In time-domain parameters, the detoxification group had the highest beat-to-beat variability. In frequency-domain parameters, the total power was highest for the detoxification group and lowest for the opioid substitution treatment group. In contrast, the relative power of frequency bands (very low, low, and high) did not vary across the groups at baseline. The SOWS had a weak negative correlation with root mean square of successive differences (RMSSD) in the opioid substitution group and did not have any relationship with HRV parameters in the detoxification group.
Conclusions:
This exploratory study did not find HRV parameters to be robustly associated with subjective withdrawal, except for a negative association with the beat-to-beat variability among patients on opioid substitution treatment. This study adds to information on HRV in patients with opioid dependence.
Keywords: Buprenorphine, heart rate variability, opioid dependence, opioid use disorder, opioid withdrawal
Key Messages:
We explored heart rate variability (HRV) across three groups of individuals: Patients with opioid dependence stabilized on opioid substitution treatment, patients with opioid dependence being detoxified, and healthy controls.
The opioid detoxification group had the highest beat-to-beat variability in time-domain parameters and the highest power in the frequency-domain parameters.
The subjective opioid withdrawal did not correlate with HRV parameters except with the root mean square of successive differences between normal heartbeats (RMSSD) in the detoxification.
Opioid dependence is a chronic and relapsing substance use disorder that impairs the quality of life and often requires medical help to control the illness.1,2 Patients with opioid dependence seek help in the healthcare setting for amelioration of the discomforting withdrawal symptoms.3,4 Assessment of the signs and symptoms of withdrawal is an important component of clinical evaluation of patients with opioid dependence. However, the clinical assessment of opioid withdrawal is constrained by the subjective nature of the assessment methods. Typically, such an assessment is based upon the subjective reporting of the patients combined with the observation of clinicians. Assessment of opioid withdrawal can present clinical conundrums. Thus, clinical judgment, if supplemented with other objective information, can help improve these patients’ decision-making and treatment.
Opioid withdrawal is generally considered a hyper-sympathetic state with an elevation of heart rate, increased sweating, and mydriasis.5,6 Some of these features can be captured with the help of electronic physiological monitoring. Subsequent processing of some of the data can give more meaningful insights. For example, the heart rate can be processed into heart rate variability (HRV). It has been seen that HRV can change with stress, and opioid withdrawal, being a state of relative stress, can potentially result in such changes. A relevant study in this context has found lower high frequency (HF) power values among heroin users as compared to healthy controls. 7 The findings suggested that heroin users have decreased cardiac vagal activity, and methadone maintenance facilitated vagal regulation among the patients. Another study among anesthetized patients during opioid detoxification suggested that opioid withdrawal was associated with sympathetic activation manifested through changes in HRV. 6
Understanding HRV among patients with opioid dependence can have taxonomic and therapeutic implications.8,9 Attempts have been made to classify, categorize, and utilize opioid withdrawal to stratify different groups of patients.10–12 Experts have opined about the necessity of defining, characterizing, and contextualizing opioid withdrawal symptoms. 13 As of now, limited literature exists on the assessment of HRV with opioid withdrawal symptoms, and no study has compared HRV across patients with opioid dependence on opioid substation treatment and opioid detoxification. Hence, in this study, we aimed to use HRV to supplement the assessment of withdrawal symptoms in opioid dependence. We also compared the HRV and autonomic parameters among those undergoing opioid detoxification and those who are maintained on opioid substitution treatment (OST).
Methods
Setting and Participants
This observational study was conducted at a tertiary care addiction treatment setting in India. The treatment facility provides care for patients with substance use disorders. The facility has both inpatient and outpatient services, and patients with opioid use disorders form the majority of the treatment seekers. Treatment is provided in the form of agonist (buprenorphine) or antagonist (naltrexone) medications, supplemented with psychosocial and rehabilitation-based interventions. The facility is affiliated with a publicly funded medical school, and the treatment is largely subsidized.
This study included three groups. Group 1 (detoxification) consisted of adult male patients with opioid dependence (diagnosed as per International Classification of Diseases, 10th revision [ICD-10] criteria) aged less than 65 years, who were planned for inpatient detoxification (i.e., controlled reduction of doses of opioid agonist to make the patient opioid-free). Group 2 (opioid substitution group) consisted of adult patients with opioid dependence aged less than 65 years who were currently on stable doses of buprenorphine for at least four weeks (buprenorphine is given as opioid substitution in patients with long duration of opioid use and who find detoxification extremely challenging). In both these groups, patients were excluded if they had current dependence on any other substance apart from nicotine, a diagnosed additional psychiatric disorder, cardiac or respiratory disease, seizures or other neurological diseases, or recent myocardial infarction. Group 3 (healthy controls) consisted of adult males aged less than 65 years who did not have any psychiatric disorder, any diagnosed cardiac or respiratory disease, any substance use disorder except tobacco dependence, or any seizure or neurological disorders or recent myocardial infarction.
Procedure
The patients of groups 1 (detoxification) and 2 (OST) were assessed in the inpatient and outpatient settings, respectively. They were recruited after obtaining written informed consent. The patients were assessed for demographic and clinical details (duration of opioid use in years, history of injecting drug use in lifetime, history of overdose on opioids, and ever been abstinent more than a month from illicit opioids). The current dose of buprenorphine being administered was recorded. The duration of OST was also recorded in group 2. For both groups, the withdrawal symptoms were assessed using the Subjective Opiate Withdrawal Scale (SOWS). 14 This is a 16-item instrument that is used to assess subjective reporting of opioid withdrawal. Each of the 16 items is rated on a five-point Likert scale from 0 to 4. The total scores can range from 0 to 64, with higher values representing a greater severity and extent of withdrawal symptoms. The instrument takes roughly five to ten minutes to administer. For group 3 (healthy controls), age and gender were recorded.
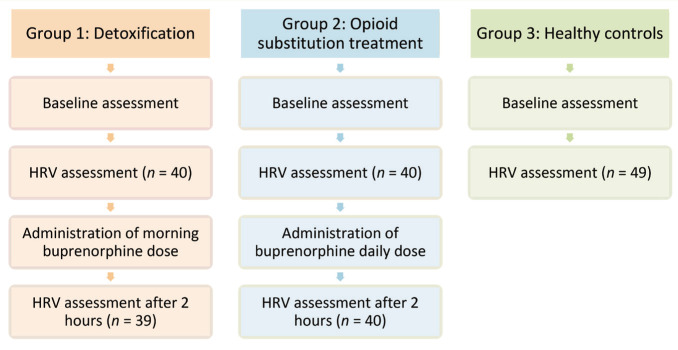
After the baseline assessment in the morning (before administration of buprenorphine), the participant was seated comfortably. Electrodes were placed following lead II placement. A short-term HRV recording was conducted for approximately five minutes. Subjects were instructed to close their eyes and to avoid talking, moving hands, legs, and body, coughing, and sleeping during the recording. Subsequently, the patients (groups 1 and 2) were administered their usual dose of buprenorphine. After two hours, the patients in groups 1 and 2 were again assessed for HRV for five minutes. The acquisition was done using the MP45 Two Channel Data Acquisition System (Biopac Systems Inc). The sample rate of the device was 48000/second. The schematic procedure is presented in Figure 1. The study was done in accordance with the Declaration of Helsinki. The study had institutional ethics committee approval, and the data collection lasted from August 2020 to July 2021.
Figure 1. Schematic Flow of the Study.
The figure describes the study’s flow. HRV, heart rate variability.
Statistical Analysis
Using data from Chang et al., 7 the sample size required with three pairwise comparisons in each group to differentiate based upon HF values was 44. We aimed to have a minimum of 40 participants in each group. The clinical and demographic details were represented by mean, standard deviation, frequency, and percentages. Normality was assessed using the Kolmogorov–Smirnov test. Due to largely non-normal data, scalar data comparisons were made using the Kruskal–Wallis test for independent comparisons of three groups (parametric analysis of variance (ANOVA) for three group comparisons could be done for only one comparison) and the Mann–Whitney U test for comparison of two groups. Nominal data was compared across groups using the chi-squared test. Missing value imputation was not done.
There are three methods of HRV analysis: (a) Time-domain, (b) frequency-domain, and (c) nonlinear methods. We used the frequency and time-domain methods for this study. The frequency-domain method has previously shown a consistent correlation between vagal and sympathetic activity. In this method, the fast Fourier transform (FFT) algorithm was used. Frequency ranges were defined according to the Task Force of The European Society of Cardiology and The North American Society of Pacing and Electrophysiology. These frequencies are classified into very low frequencies (VLF; >25-s cycle length), low frequencies (LF; >6-s cycle length), and high frequencies (HF; 2.5- to 6.0-s cycle length in humans). HRV analysis was done using the Kubios HRV Standard, version 3.5.0. 15
Results
The characteristics of the sample are presented in Table 1. While all the participants were males, the age differed between the three groups, with post-hoc comparisons showing a significant difference between group 1 (detoxification) and group 3 (controls). It was seen that group 1 (detoxification) patients had higher rates of injecting drug use, history of opioid overdose, and expectedly lower current dose of buprenorphine than patients in group 2.
Table 1.
Characteristics of the Sample.
| Variable | Group 1 (Detoxification) (n = 40) |
Group 2 (OST) (n = 40) |
Group 3 (Controls) (n = 49) |
Comparison (P value) |
| Age | 30.0 (10.2) | 33.0 (12.5) | 37.8 (9.6) | KW χ2 = 14.987 (.001)*a |
| Duration of opioid use in years | 9.3 (10.3) | 9.4 (10.1) | NA | U = 759 (.836) |
| History of injecting drug use | 28 (70.0%) | 14 (35.0%) | NA | χ2 = 9.825 (.002)* |
| History of overdose on opioids | 17 (42.5%) | 8 (20.0%) | NA | χ2 = 4.713 (.030)* |
| Ever been abstinent more than a month from illicit opioids | 24 (60%) | 27 (67.5%) | NA | χ2 = 0.487 (.485) |
| Current dose of buprenorphine (mg/day) | 4.2 (4.0) | 13.7 (4.6) | NA | U = 97.5 (<.001)* |
| Duration of OST in years | NA | 3.4 (4.3) | NA | NA |
| SOWS score | 12.9 (13.8) | 7.0 (7.2) | NA | U = 631.0 (.103) |
The table describes the characteristics of the sample in the three groups (detoxification, OST, and control groups). All the individuals in the three groups were males.
*Difference significant at P value <.05.aA post-hoc test reports a significant difference between group 1 and group 3.
KW, Kruskal–Wallis test; NA, not applicable; OST, opioid substitution treatment; SOWS, Subjective Opiate Withdrawal Scale.
Table 2 shows the HRV parameters of the three groups. At baseline, heart rate, standard deviation of all normal RR (NN) intervals (SDNN), RMSSD, and RR tri-index had significant differences. The frequency parameters did not differ between the groups.
Table 2.
Heart Rate Variability (HRV) Parameters Across the Three Groups.
| Variable | Group 1 (Detoxification) (n = 40) |
Group 2 (OST) (n = 40) |
Group 3 (Controls) (n = 49) |
Comparison (P value) |
| Heart rate | 82.3 (16.1) |
92.4 (18.6) |
79.4 (10.6) |
F = 8.575 (<.001)*, G2>G1,G3 |
| SDNN | 134.8 (238.3) |
47.7 (62.0) |
69.8 (77.5) |
KW c2 = 11.350 (.003)* |
| RMSSD | 181.7 (370.9) |
52.5 (89.2) |
68.7 (79.6) |
KW c2 = 12.445 (.002)* |
| RR tri-index | 8.9 (5.3) |
5.0 (2.8) |
8.0 (2.4) |
KW c2 = 32.139 (<.001)* |
| VLF | 0.034 (0.009) |
0.033 (0.013) |
0.033 (0.010) |
KW c2 = 1.021 (.600) |
| LF | 0.087 (0.034) |
0.092 (0.04) |
0.093 (0.032) |
KW c2 = 0.584 (.747) |
| HF | 0.223 (0.071) |
0.208 (0.069) |
0.218 (0.08) |
KW c2 = 2.313 (.315) |
| VLF percentage | 11.8 (17.6) |
13.5 (14.5) |
8.4 (12.4) |
KW c2 = 2.615 (.270) |
| LF percentage | 50 (23.8) |
42.3 (22.8) |
50.7 (19.7) |
KW c2 = 3.129 (.209) |
| HF percentage | 38.1 (24.8) |
44.0 (30.8) |
40.8 (20.9) |
KW c2 = 0.828 (.661) |
| LF/HF ratio | 3.6 (7.6) |
3.6 (5.7) |
3.1 (6.9) |
KW c2 = 0.657 (.720) |
| Total power ms2 | 98334.1 (420350.8) |
2796.1 (6912.4) |
10310.6 (31986.4) |
KW c2 = 15.512 (<.001)* |
The table compares the HRV parameters across the three groups. G1—group 1 (detoxification), G2—group 2 (opioid substitution treatment), G3—group 3 (controls).
HF, high frequency; HRV, heart rate variability; KW, Kruskal–Wallis test; LF, low frequency; OST, opioid substitution treatment; RMSSD, root mean square of successive differences between normal heartbeats; SDNN, standard deviation of all normal RR (NN) intervals; VLF, very low frequency. Post-hoc comparisons were shown for comparisons that had significant differences.
*Significant at P value <.05.
The mean SOWS score in group 1 (detoxification) was 12.9 (±13.8), while that in group 2 (OST) was 7.0 (±7.2). The difference was not significant (Mann–Whitney U test = 631.0, P = .103). The relation of SOWS scores with HRV parameters is presented in Table 3. The SOWS score did not have a significant relationship with any of the parameters in patients undergoing detoxification (group 1). In contrast, they had a weak negative relationship with RMSSD among patients on OST (group 2). The HRV parameters before and after the administration of buprenorphine are shown in Table 4. Apart from a decrease in VLF percentage after administration in the detoxification group, there were no significant differences in HRV parameters before and after the administration of buprenorphine.
Table 3.
Correlation of Heart Rate Variability (HRV) Parameters with Subjective Opioid Withdrawal Scale (SOWS) Score.
| Variable | Group 1 (Detoxification) SOWS Score |
Group 2 (OST) SOWS Score |
| Heart rate | 0.216 (0.181) |
0.166 (0.307) |
| SDNN | 0.102 (0.530) |
–0.267 (0.096) |
| RMSSD | 0.051 (0.755) |
–0.312 (.050)* |
| RR tri-index | 0.077 (0.638) |
–0.191 (0.239) |
| VLF | –0.071 (0.662) |
0.020 (0.900) |
| LF | 0.214 (0.185) |
0.008 (0.959) |
| HF | –0.068 (0.676) |
–0.082 (0.616) |
| VLF percentage | –0.189 (0.244) |
0.035 (0.830) |
| LF percentage | –0.023 (0.889) |
0.171 (0.292) |
| HF percentage | –0.074 (0.651) |
–0.152 (0.351) |
| LF/HF ratio | 0.063 (0.699) |
0.169 (0.296) |
| Total power ms2 | 0.127 (0.435) |
–0.173 (0.286) |
The table presents the correlation of opioid withdrawal with HRV parameters with the p values in the brackets.
HF, high frequency; HRV, heart rate variability; LF, low frequency; RMSSD, root mean square of successive differences between normal heartbeats; OST, opioid substitution treatment; SDNN, standard deviation of all normal RR (NN) intervals; SOWS, Subjective Opiate Withdrawal Scale; VLF, very low frequency.
*Significant at P value <.05.
Table 4.
Heart Rate Variability (HRV) Parameters Before and After Administration of Medication.
| Variable | Group 1 (Detoxification) (n = 39) |
Group 2 (OST) (n = 40) |
||
| Pre | Post | Pre | Post | |
| Heart rate | 82.351 (16.326) |
85.501 (14.944) |
92.374 (18.631) |
90.329 (16.151) |
| SDNN | 136.983 (240.988) |
110.92 (215.122) |
47.67 (62.029) |
50.827 (55.234) |
| RMSSD | 185.172 (375.04) |
148.755 (319.367) |
52.522 (89.19) |
54.665 (74.42) |
| RR tri-index | 8.992 (5.346) |
8.645 (5.405) |
5.025 (2.788) |
5.799 (2.546) |
| VLF | 0.034 (0.009) |
0.035 (0.011) |
0.033 (0.013) |
0.034 (0.012) |
| LF | 0.086 (0.033) |
0.097 (0.033) |
0.092 (0.04) |
0.084 (0.035) |
| HF | 0.225 (0.071) |
0.202 (0.055) |
0.208 (0.069) |
0.203 (0.073) |
| VLF percentage | 12.013 (17.815) |
7.196 (8.56)* |
13.532 (14.512) |
13.672 (14.492) |
| LF percentage | 49.456 (23.866) |
54.827 (21.745) |
42.305 (22.779) |
48.258 (20.911) |
| HF percentage | 38.441 (25.081) |
37.92 (23.825) |
44.024 (30.794) |
37.969 (25.266) |
| LF/HF ratio | 3.593 (7.67) |
2.957 (3.295) |
3.624 (5.728) |
3.529 (4.513) |
| Total power ms2 | 100772.159 (425559.25) |
76528.363 (202185.322) |
2796.056 (6912.399) |
5317.385 (12799.504) |
The table presents the HRV parameters before and after buprenorphine administration in the detoxification and opioid substitution treatment groups.
HF, high frequency; HRV, heart rate variability; LF, low frequency; OST, opioid substitution treatment; RMSSD, root mean square of successive differences between normal heartbeats; SDNN, standard deviation of all normal RR (NN) intervals; VLF, very low frequency,
*Difference between pre- and post-significant at P value <.05
Discussion
This exploratory study aimed to assess the relationship between opioid withdrawal and HRV. HRV parameters being computed through objective automated measurement have the potential of reducing the subjectivity of ascertainment. However, in this study, we did not find a robust relationship between HRV parameters and subjective withdrawal (except a high degree of subjective withdrawal being associated with lower beat-to-beat variability in those who were on opioid substitution). Previous literature has not explored the relationship between the severity and extent of withdrawal with these parameters. The association, even when significant without correction for multiple comparisons, suggests that HRV indices may not be robust markers for subjective withdrawal. There can be several explanations for the same. First, subjective experience of the individual and cognitive appraisal of withdrawal are often not reflected in an absolute manner through HRV, which is rather a marker of physiological and autonomic arousal. Second, the withdrawal symptoms varied considerably across and within the groups. There is research suggesting withdrawal symptoms of opioid dependence may have different specific clusters, suggesting heterogeneity in the experience of opioid withdrawal. 10 Third, the assessment of HRV parameters in the usual clinical scenario may not be able to preclude the effects of varied determinants of HRV applicable to an individual assessment.16,17
A few studies have explored the HRV parameters in patients with opioid dependence in comparison with controls. Lin et al. 9 found a higher SDNN and HF components in patients using heroin while a lower LF/HF ratio as compared to controls. This was not replicated in this study, while another study 7 also did not find a difference in the LF/HF ratio between heroin users and controls. The beat-to-beat variation in heart rate (RMSSD, SDNN, and RR tri-index) was higher in patients undergoing detoxification than controls in this study. The total power is highest in the control group, concurs with Chang et al., 7 who also found higher total power among heroin users than among controls. One possible explanation for the differences in these HRV parameters among the three groups could be the difference in resting heart rate across these groups, as heart rate is inversely associated with the measures of HRV. 18 Interestingly, Levin et al. 19 found the HF and RMSSD to be lower during withdrawal precipitated by naloxone. At the same time, the RMSSD was higher in individuals undergoing detoxification than in opioid substitution in this study. High RMSSD is suggestive of vagal tone. Low vagal tone has been found to be associated with a greater extent of neonatal abstinence syndrome among methadone-exposed infants. 20 Similarly, opioid-treated chronic pain individuals also demonstrated blunted HRV. 21 This suggests that high vagal tone in detoxification enables greater physiological adaptation to opioid withdrawal symptoms. In contrast, lower vagal tone in those undergoing OST may reflect lower cardiac resilience.
No substantial changes were observed in the HRV parameters between predose and two hours after dosing in the detoxification group or the opioid substitution group. This finding is somewhat at variance from Chang et al., 7 who found a change in total power and RMSSD among heroin users after one hour of methadone administration. Change in HF was observed among the noncompliant heroin users but not among the compliant heroin users in the study by Chang et al. 7 Some characteristics of the sample deserve mention. This sample is comprised exclusively of males, which is similar to another set of researchers in Taiwan,8,9 and is representative of the treatment-seeking opioid user population in India. 22 The extent of withdrawal was higher (though not significantly higher) in patients undergoing detoxification (group 1). Additionally, the history of injecting drug use and overdose was higher in the detoxification group. This is contrary to expectations, where OST is the preferred option for those with overdose and those with injecting drug use. This may be ascribed to selection biases, where individuals with less problematic opioid use maintained well on OST were recruited, and inpatient detoxification occurred for those with more problematic opioid use but who were unwilling to daily dispensing for a fair period.
Some of the limitations of this study should be considered while drawing inferences. The sample comprised patients with opioid dependence who sought treatment and consented to partake in the study. Previously, it was found that the ages of patients who underwent detoxification and that of patients who were admitted for opioid substitution at our center were somewhat different. 23 The setting of our assessment was in a routine outpatient and inpatient setting and not that of a noise and ambient temperature-controlled laboratory. Previous researchers have attempted to assess heart rate among drug users in a more ecologically representative location as well. 24 The assessment was conducted at only two-time points, during the morning hours (once before the buprenorphine medication administration and the other 2 hours after administration). Assessment at multiple time points throughout the day may lead to different findings, especially after factoring in meals and diurnal variation. We were unable to control for confounding factors like tobacco use (of patients on OST in the outpatient setting), physical mobility and exertion, and other factors that have been shown to impact HRV. Including participants from only an inpatient setting in the future might allow for better control of these confounders. It is also worth mentioning that, as a usual practice, electrocardiogram (ECG) for HRV is acquired in the supine position. Based on convenience and feasibility, ECG was done in a sitting position in our study. All participants across the three groups underwent ECG recording in the sitting position. Therefore, posture is not a factor that could influence the differences in the outcome variables in this study. Another limitation was the measurement of SOWS only at baseline. Measurement post-administration of buprenorphine would have provided means to assess the correlation between HRV and subjective withdrawal after medication and the effect of buprenorphine administration on subjective withdrawal in these patients. The inclusion of only male participants also limits the generalizability of our study. The inability to control for potential confounders like age, body mass index, tobacco use, medication use and dosing, temperatures, and dietary patterns is another limitation. Longitudinal repeated assessments could also have enriched the study findings, and this was not pursued in this study.
Conclusions
This preliminary study hence offers some observations about HRV in patients with opioid dependence, either undergoing detoxification or on OST. The study gives some insight into the differences in HRV parameters between these patients with opioid dependence and healthy controls in both the frequency and time domains. The withdrawal scores may not have a robust relationship with HRV parameters, though positive associations would have conferred substantive clinical relevance. Moreover, HRV parameters do not change substantially over two hours of buprenorphine dosing. This study essentially has negative findings. Nevertheless, negative findings can be instructive and inform further research pursuits. Future research may explore whether HRV parameters can be useful in determining subgroups of patients with opioid withdrawal, assessing cardiac functioning / vagal tone over a longitudinal horizon of several months to years, and exploring the contribution of various individual-related and assessment-related parameters in the interpretation of HRV in patients with opioid dependence.
Acknowledgments
The study was funded by intramural grant of the All India Institute of Medical Sciences, New Delhi (A758) to Siddharth Sarkar.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Declaration regarding the use of generative AI: None used.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Mitchell SG, Gryczynski J, Schwartz RP, et al. Changes in quality of life following buprenorphine treatment: Relationship with treatment retention and illicit opioid use. J Psychoactive Drugs, 2015; 47(2): 149–157. DOI: 10.1080/02791072.2015.1014948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strada L, Vanderplasschen W, Buchholz A, et al. Measuring quality of life in opioid-dependent people: A systematic review of assessment instruments. Qual Life Res Int J Qual Life Asp Treat Care Rehabil, 2017; 26(12): 3187–3200. DOI: 10.1007/s11136-017-1674-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mannelli P, Peindl KS, Lee T, et al. Buprenorphine-mediated transition from opioid agonist to antagonist treatment: State of the art and new perspectives. Curr Drug Abuse Rev, 2012; 5(1): 52–63. DOI: 10.2174/1874473711205010052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stotts AL, Dodrill CL and Kosten TR. Opioid dependence treatment: Options in pharmacotherapy. Expert Opin Pharmacother, 2009; 10(11): 1727–1740. DOI: 10.1517/14656560903037168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kienbaum P, Thürauf N, Michel MC, et al. Profound increase in epinephrine concentration in plasma and cardiovascular stimulation after mu-opioid receptor blockade in opioid-addicted patients during barbiturate-induced anesthesia for acute detoxification. Anesthesiology, 1998; 88(5): 1154–1161. DOI: 10.1097/00000542-199805000-00004 [DOI] [PubMed] [Google Scholar]
- 6.McDonald T, Hoffman WE, Berkowitz R, et al. Heart rate variability and plasma catecholamines in patients during opioid detoxification. J Neurosurg Anesthesiol, 1999; 11(3): 195–199. [DOI] [PubMed] [Google Scholar]
- 7.Chang LR, Lin YH, Kuo TBJ, et al. Cardiac autonomic modulation during methadone therapy among heroin users: A pilot study. Prog Neuropsychopharmacol Biol Psychiatry, 2012; 37(1): 188–193. DOI: 10.1016/j.pnpbp.2012.01.006 [DOI] [PubMed] [Google Scholar]
- 8.Huang WL, Lin YH, Kuo TBJ, et al. Methadone-mediated autonomic functioning of male patients with heroin dependence: The influence of borderline personality pattern. PloS One, 2012; 7(5): e37464. DOI: 10.1371/journal.pone.0037464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin IM, Ko JM, Fan SY, et al. Heart rate variability and the efficacy of biofeedback in heroin users with depressive symptoms. Clin Psychopharmacol Neurosci, 2016; 14(2): 168–176. DOI: 10.9758/cpn.2016.14.2.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunn KE, Weerts EM, Huhn AS, et al. Preliminary evidence of different and clinically meaningful opioid withdrawal phenotypes. Addict Biol, 2020; 25(1): e12680. DOI: 10.1111/adb.12680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Northrup TF, Stotts AL, Green C, et al. Opioid withdrawal, craving, and use during and after outpatient buprenorphine stabilization and taper: A discrete survival and growth mixture model. Addict Behav, 2015; 41: 20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ware OD and Dunn KE. Clinically meaningful individual differences in opioid withdrawal expression. Exp Clin Psychopharmacol, 2023; 31(6): 1005–1009. DOI: 10.1037/pha0000654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunn KE and Strain EC. Establishing a research agenda for the study and assessment of opioid withdrawal. Lancet Psychiatry, 2024; S2215–0366 (24) 00068-3. DOI: 10.1016/S2215-0366(24)00068-3 [DOI] [PubMed] [Google Scholar]
- 14.Handelsman L, Cochrane KJ, Aronson MJ, et al. Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse, 1987; 13(3): 293–308. DOI: 10.3109/00952998709001515 [DOI] [PubMed] [Google Scholar]
- 15.Tarvainen MP, Niskanen JP, Lipponen JA, et al. Kubios HRV--heart rate variability analysis software. Comput Methods Programs Biomed, 2014; 113(1): 210–220. DOI: 10.1016/j.cmpb.2013.07.024 [DOI] [PubMed] [Google Scholar]
- 16.Fatisson J, Oswald V and Lalonde F.. Influence diagram of physiological and environmental factors affecting heart rate variability: An extended literature overview. Heart Int, 2016; 11(1): heartint.5000232. DOI: 10.5301/heartint.5000232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tiwari R, Kumar R, Malik S, et al. Analysis of heart rate variability and implication of different factors on heart rate variability. Curr Cardiol Rev, 2021; 17(5): 74–83. DOI: 10. 2174/1573403X16999201231203854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsuji H, Larson MG, Venditti FJ, et al. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation, 1996; 94(11): 2850–2855. DOI: 10.1161/01.cir.94.11.2850 [DOI] [PubMed] [Google Scholar]
- 19.Levin CJ, Wai JM, Jones JD, et al. Changes in cardiac vagal tone as measured by heart rate variability during naloxone-induced opioid withdrawal. Drug Alcohol Depend, 2019; 204: 107538. DOI: 10.1016/j.drugalcdep.2019.06.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jansson LM, DiPietro JA, Elko A, et al. Infant autonomic functioning and neonatal abstinence syndrome. Drug Alcohol Depend, 2010; 109(1): 198–204. DOI: 10.1016/j.drugalcdep.2010.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts RL and Garland EL. Association between opioid use disorder and blunted heart rate variability among opioid-treated chronic pain patients. Addict Biol, 2022; 27(6): e13230. DOI: 10.1111/adb.13230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarkar S, Balhara YPS, Gautam N, et al. A retrospective chart review of treatment completers versus non-completers among in-patients at a tertiary care drug dependence treatment center in India. Indian J Psychol Med, 2016; 38(4): 296–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh VV, Dhawan A, Chadda RK, et al. A prospective three-month naturalistic follow-up study of outcomes of patients with opioid dependence discharged on buprenorphine or oral naltrexone. Indian J Psychol Med, 2023; 45(1): 26–32. DOI: https://doi.org/10.1177/02537176211066739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kennedy AP, Epstein DH, Jobes ML, et al. Continuous in-the-field measurement of heart rate: Correlates of drug use, craving, stress, and mood in polydrug users. Drug Alcohol Depend, 2015; 151: 159–166. DOI: 10.1016/j.drugalcdep.2015.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]