Abstract
Background
Limited research suggested that liver cirrhosis is an independent risk factor for severe COVID-19, leading to higher hospitalization and mortality rates. This study aimed to identify the prognostic factors and validate scoring systems for predicting mortality in COVID-19 patients with liver cirrhosis.
Methods
This retrospective cohort study extracted electronic health records of patients with COVID-19 who visited the emergency department between April 2021 and September 2022. Adult COVID-19 patients with liver cirrhosis were included, excluding those aged < 18 years and who did not require hospitalization. The primary outcome was in-hospital mortality. The effectiveness of the scoring systems were analyzed for COVID-19 in-house mortality prediction.
Results
A total of 1,368 adult COVID-19 patients with liver cirrhosis were included in this study. Compared with the survival group, the non-survival group had lower vital signs such as systolic blood pressure and blood oxygen saturation, higher levels of white blood cells, creatinine, bilirubin, and C-reactive protein, and longer prothrombin time. Higher rates of intubation, oxygen use, and dexamethasone use were observed in the non-survivor group. The WHO ordinal scale, MELD, and MELD-Na scores showed good predictive ability for in-hospital mortality.
Conclusions
The WHO ordinal scale showed the best performance in predicting mortality in patients with cirrhosis and COVID-19. MELD and MELD-Na scores were also found good performance for mortality prediction. Coagulation function, intubation, and dexamethasone administration were the most significant prognostic factors.
Keywords: COVID-19, Liver cirrhosis, Mortality, MELD score, MELD-Na score, WHO ordinal scale
Background
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), also known as coronavirus 2019 (COVID-19), is highly contagious and has spread worldwide by the end of 2019 [1]. According to the World Health Organization (WHO) data, as of January 7, 2024, over 7 million people died from COVID-19 globally [2]. COVID-19 clinical symptoms range from mild asymptomatic or coughs to respiratory failure caused by severe acute respiratory distress syndrome, which can be fatal [3]. Additionally, older individuals and those with underlying chronic conditions such as cardiovascular disease, diabetes, chronic respiratory diseases, chronic kidney disease, and cancer are more susceptible to severe COVID-19 [4].
Approximately 1 million people worldwide die from cirrhosis each year. Cirrhosis can lead to complications such as ascites, variceal bleeding, hepatic encephalopathy, or non-obstructive jaundice. Superimposed hepatic injury (due to viral, drug-induced, or alcohol-associated hepatitis) or other complications, particularly bacterial infections, may lead to liver and extrahepatic organ failure, which is associated with a high short-term mortality rate [5]. Many studies indicated that COVID-19 can lead to decompensation or worsening of baseline cirrhosis. One possible reason is that SARS-CoV-2 causes direct liver injury and impaired regeneration [6]. However, research on COVID-19 patients with cirrhosis remains limited. Some research reveals that liver cirrhosis is an independent predictive factor for COVID-19 severity, leading to increased hospitalization and mortality rates [7]. An increase in the Child-Pugh and Model for End-Stage Liver Disease (MELD) scores is associated with higher mortality rates in these patients than in their counterparts [7]. Since patients with liver cirrhosis are more susceptible to severe COVID-19, it is imperative to identify high-risk patients and initiate treatment early. Our study aimed to identify the prognostic factors for COVID-19 patients with liver cirrhosis and validate different scoring systems for predicting mortality in these patients.
Methods
Study design and data source
This retrospective cohort study extracted electronic health records (EHR) from the Chang Gung Research Database (CGRD) [8], a de-identified database derived from the EHR of Chang Gung Memorial Hospital (CGMH), currently the largest healthcare system in Taiwan. The CGMH comprises seven medical institutions distributed across Taiwan, provides 10,070 beds, and treats over 280,000 patients annually. The CGRD has collected the EHR of all patients without a specific registration process from the CGMH since 2000 and contains most information, including demographics, laboratory data, inpatient data, outpatient data, emergency patient data, nursing data, disease category data, and treatment data, with hospital medications and procedures.
This study was approved by the Institutional Review Board (IRB no. 202301643B0).
Study setting and population
We retrieved the data of patients who visited the emergency department (ED) and were diagnosed with COVID-19 between April 2021 and September 2022 from the CGRD. From this cohort of COVID-19 patients, we included patients with liver cirrhosis and excluded patients aged < 18 years and those who did not require hospitalization. The patients with a “do not resuscitate (DNR)” order were also excluded since the prognosis could be affected by the patient or family refusing resuscitation or other necessary treatment. Basic demographic data, including age, sex, vital signs, laboratory data, underlying diseases, treatment course, and prognostic data, such as length of stay (LOS), mortality, intubation or not, and intensive care unit (ICU) was collected when these patients visited our ED.
Outcome measures
The primary outcome was in-hospital mortality of COVID-19 patients with liver cirrhosis. The included patients were divided into survival and non-survival groups. The variables and factors were compared and analyzed between the two groups. Different scoring systems, including the model for end-stage liver disease (MELD) score, MELD-Sodium (MELD-Na) score, WHO ordinal scale, and inflammation risk categories, were calculated for the included patients [9–12]. The effectiveness of the scoring systems in predicting in-hospital mortality in COVID-19 patients with liver cirrhosis was further analyzed.
Statistical analysis
Data analysis was performed using IBM SPSS Statistics for Windows (version 24.0; IBM Corp., Armonk, NY, USA). Categorical variables are presented as numbers and percentages, and continuous variables are presented as either mean and standard deviation (SD) or median and interquartile range (IQR), as appropriate. The collected data of the included patients were compared using Student’s t-test or Mann–Whitney U test for continuous variables and Pearson’s chi-square test for categorical variables. Multivariate logistic regression analysis was performed for independent significant factors. Univariate logistic regression analysis was used to analyze different scoring systems and receiver operating characteristic (ROC) curves. A p-value of < 0.05 was considered statistically significant.
Results
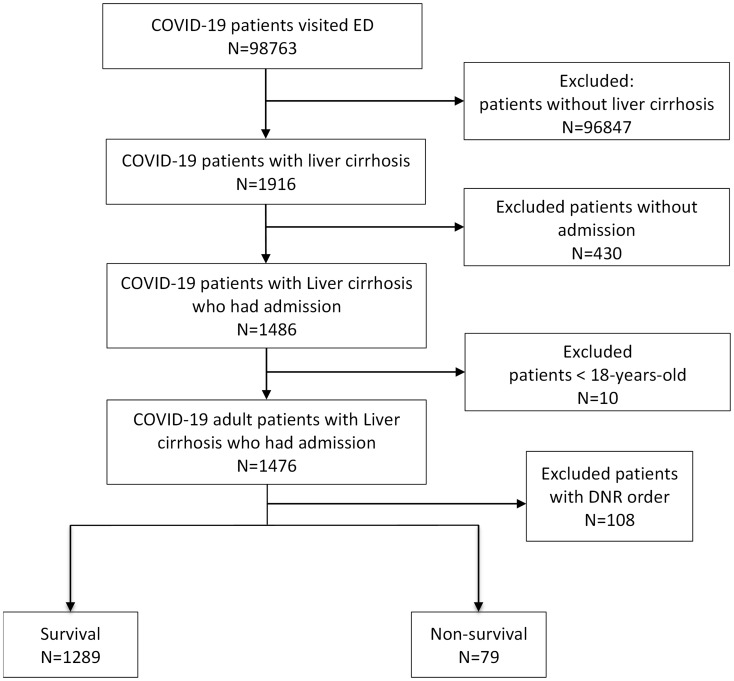
A total of 98,763 patients with COVID-19 confirmed between April 2021 and September 2022 were included in the CGRD database, of which only 1,916 had liver cirrhosis (Fig. 1). After excluding patients aged < 18 years, those who did not require hospitalization, and patients with a “do not resuscitate (DNR)” order, 1,368 COVID-19 adult patients with liver cirrhosis were included. The patients were categorized into survival (N = 1289) and non-survival groups (N = 79).
Fig. 1.
Flow diagram of patient selection
Table 1 presents the patients data in the survival and non-survival groups. Patients in the non-survival group were younger (58.9 vs. 62.82 years, p = 0.014) and had a lower incidence of diabetes (29.11% vs. 46.16%, p = 0.005) than those in the non-survival group. For triage vital signs at ED, compared with the non-survival group, those in the survival group had lower systolic blood pressure (SBP) (119.56 vs. 136.79 mmHg, p < 0.001) and blood oxygen saturation levels (91.23% vs. 95.8%, p = 0.031). Patients of non-survival group had higher white blood cell (WBC) (10.71 vs. 8.15 1000/uL, p = 0.004), higher creatinine levels (2.28 vs. 1.55 mg/dL, p = 0.001), lower hemoglobin levels (9.78 vs. 10.39 g/dL, p < 0.041), higher bilirubin levels (7.03 vs. 2.86 mg/dL, p = 0.002), higher C-reactive protein (CRP) levels (85.65 vs. 48.49 mg/L, p = 0.002), and longer prothrombin time (international normalized ratio, INR, 1.94 vs. 1.38, p < 0.001) than those of survival group. Higher rates of intubation (96.2% vs. 9.39%, p < 0.001), oxygen (56.96% vs. 31.57%, p < 0.001) and dexamethasone (17.72% vs. 5.43%, p < 0.001) use were noted in non-survival group; however, no significant difference in remdesivir (3.8% vs. 3.34%, p = 0.503) use was observed between two groups.
Table 1.
Comparison of characteristics between survival and non-survival groups of COVID-19 patients with liver cirrhosis
| Survival (N = 1289) | Non-survival (N = 79) | p-value | |
|---|---|---|---|
| Demographics | |||
| Age, years, mean (SD) | 62.82(13.8) | 58.9(13.52) | 0.014* |
| Gender, Male, N (%) | 898(69.67) | 58(73.42) | 0.563 |
| Triage vital signs | |||
| BT, ℃ mean (SD) | 36.66(1.1) | 36.35(1.09) | 0.014* |
| SBP, mmHg, mean (SD) | 136.79(29.9) | 119.56(38.33) | < 0.001* |
| DBP, mmHg, mean (SD) | 76.9(16.87) | 70.12(22.83) | 0.013* |
| RR, breaths per minute, mean (SD) | 17.7(3.4) | 18.94(5.53) | 0.052 |
| HR, bpm, mean (SD) | 96.84(19.83) | 97.67(26.96) | 0.791 |
| SpO2, %, mean (SD) | 95.82(4.58) | 91.23(17.76) | 0.031* |
| Initial laboratory data | |||
| WBC, 1000/uL, mean (SD) | 8.15(4.9) | 10.71(7.37) | 0.004* |
| Hb, g/dL, mean (SD) | 10.39(2.52) | 9.78(2.71) | 0.041* |
| Creatinine, mg/dL, mean (SD) | 1.55(1.9) | 2.28(1.83) | 0.001* |
| ALT, U/L, mean (SD) | 57.2(112.96) | 205.91(939.67) | 0.190 |
| Bilirubin, mg/dL, mean (SD) | 2.86(4.01) | 7.03(9.89) | 0.002* |
| CRP, mg/L, mean (SD) | 48.49(62.48) | 85.65(85.83) | 0.002* |
| Sugar, mg/dL, mean (SD) | 164.01(123.49) | 170.71(114.96) | 0.778 |
| Na, mEq/L, mean (SD) | 134.29(7.68) | 134(7.41) | 0.753 |
| K, mEq/L, mean (SD) | 4.04(1.25) | 4.06(0.92) | 0.891 |
| Troponin I, ng/mL, mean (SD) | 0.14(0.77) | 0.18(0.37) | 0.583 |
| D-dimer, ng/mL, mean (SD) | 3717.73(3507.69) | 3575.67(3181.84) | 0.947 |
| Prothrombin Time, INR, mean (SD) | 1.38(0.38) | 1.94(1.08) | < 0.001* |
| Treatment course | |||
| LOS, days, mean (SD) | 15.1(17.13) | 18.84(15.72) | 0.059 |
| ICU admission, N (%) | 78(6.05) | 38(48.10) | < 0.001* |
| ICU LOS, days, mean (SD) | 8.23(11.04) | 12.84(13.93) | 0.005* |
| Treatment | |||
| Oxygen use, N (%) | 407(31.57) | 45(56.96) | < 0.001* |
| Intubation, N (%) | 121(9.39) | 76(96.20) | < 0.001* |
| Remdesivir use, N (%) | 43(3.34) | 3(3.8) | 0.503 |
| Dexamethasone use, N (%) | 70(5.43) | 14(17.72) | < 0.001* |
| Underlying diseases | |||
| Cardiovascular disease, N (%) | 30(2.33) | 3(3.8) | 0.296 |
| Hypertension, N (%) | 644(49.96) | 32(40.51) | 0.130 |
| Congestive heart failure, N (%) | 152(11.79) | 9(11.39) | 1 |
| Cerebrovascular disease, N (%) | 200(15.52) | 8(10.13) | 0.257 |
| Chronic pulmonary disease, N (%) | 204(15.83) | 7(8.86) | 0.133 |
| Diabetes mellitus, N (%) | 595(46.16) | 23(29.11) | 0.005* |
| Malignancy, N (%) | 671(52.06) | 41(51.90) | 1 |
*p < 0.05
BT: body temperature; SBP: systolic blood pressure; DBP: diastolic blood pressure; RR: respiratory rate; HR: heart rate; bpm: beats per minute; WBC white blood cell; Hb: hemoglobin; ALT: alanine transaminase; INR: International Normalized Ratio; LOS: length of stay; ICU: intensive care unit
All the factors were further analyzed by multivariate regression analysis and the results of the variables with significant differences between the survival and non-survival groups are shown in Table 2. Prolonged INR (OR 2.947, p = 0.006), higher rates of dexamethasone use (OR 3.275, p = 0.035), and intubation (OR 191.89, p < 0.001) were observed in the non-survival group.
Table 2.
Multivariate logistic regression analysis between survival and non-survival groups of COVID-19 patients with liver cirrhosis
| Variable | β | OR | 95% CI | p-value |
|---|---|---|---|---|
| Dexamethasone use | 0.5932 | 3.275 | (1.085, 9.889) | 0.035* |
| Intubation | 2.6285 | 191.889 | (25.721, > 999) | < 0.001* |
| INR | 1.0808 | 2.947 | (1.356, 6.405) | 0.006* |
*p < 0.05
OR: Odds Ratio, CI: Confidence Interval; INR: International Normalized Ratio
Table 3 compares the different scoring systems between the survival and non-survival groups. Patients of non-survival group had higher MELD (22.82, IQR 14.82–28.45, p < 0.001) and MELD-Na (24.88, IQR 18.33–29.96, p < 0.001) scores, higher WHO ordinal scale (6, IQR 4–7, p < 0.001) and higher proportion of high inflammation risk category (67.09%, p < 0.001) were also noted in the non-survival group than in the survival group.
Table 3.
Comparison of different scoring systems between survival and non-survival groups
| Survival (N = 1289) | Non-survival (N = 79) | p-value | |
|---|---|---|---|
| MELD score, median (IQR) | 14.19(10.44–19.32) | 22.82(14.82–28.45) | < 0.001* |
| MELD-Na score, median (IQR) | 17.16(13.1-22.54) | 24.88(18.33–29.96) | < 0.001* |
| WHO Ordinal Scale and Inflammation Risk Categories | |||
| WHO Ordinal Scale, median (IQR) | 3(3–4) | 6(4–7) | < 0.001* |
| Inflammation Risk Categories | < 0.001* | ||
| High, N (%) | 460(35.69) | 53(67.09) | |
| Intermediate, N (%) | 298(23.12) | 17(21.52) | |
| Low, N (%) | 531(41.19) | 9(11.39) | |
*p < 0.05
MELD: model for end-stage liver disease; WHO: World Health Organization; IQR: interquartile range;
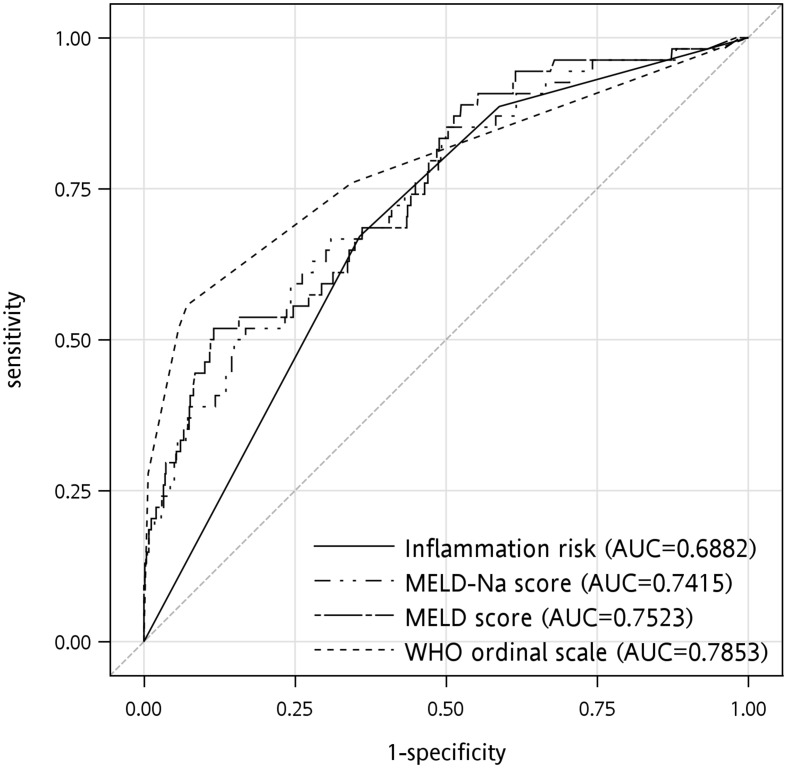
Analysis of the different scoring systems for predicting in-hospital mortality in COVID-19 patients with liver cirrhosis is shown in Table 4. The areas under the curve (AUC) of the MELD and MELD-Na scores were 0.7523 and 0.7415, respectively (Fig. 2). The WHO ordinal scale showed the highest AUC (0.7853), and the inflammation risk category had the lowest AUC (0.6882).
Table 4.
Different scoring systems in predicting mortality of COVID-19 patients with liver cirrhosis
| Variable | β | OR | 95% CI | p-value | |
|---|---|---|---|---|---|
| MELD | 0.1381 | 1.148 | (1.106,1.192) | < 0.001* | |
| MELD-Na | 0.1356 | 1.145 | (1.101,1.191) | < 0.001 | |
| WHO Ordinal Scale | 1.0412 | 2.832 | (2.376,3.377) | < 0.001* | |
| Inflammation Risk Categories | |||||
| High | 0.8732 | 6.798 | (3.317,13.931) | < 0.001* | |
| Intermediate | 0.1702 | 3.366 | (1.482,7.644) | 0.409 | |
| Low | Ref | - | - | - | |
*p < 0.05
OR: Odds Ratio, CI: Confidence Interval: MELD: model for end-stage liver disease; WHO: World Health Organization; Ref: reference
Fig. 2.
The ROC curves and AUCs of different scoring systems including MELD score, MELD-Na score, WHO ordinal severity scale, inflammation risk categories for mortality prediction in COVID-19 patients with liver cirrhosis
Discussion
Patients with liver cirrhosis are more susceptible to COVID-19 owing to their immunocompromised states [13]. A higher mortality rate is indicated among cirrhotic patients infected with COVID-19 than among non-cirrhotic patients [14]. Immune deficiency in cirrhotic patients was considered as one of the reasons, and another possible explanation was that SARS-CoV-2 entered liver cells via angiotensin-converting enzyme-related carboxypeptidase (ACE2) receptors, causing liver damage and impairing regeneration [15]. In patients with decompensated cirrhosis, ACE2 protein expression is higher, making them more susceptible to acute liver injury induced by the virus [16]. Our study categorized COVID-19 patients with liver cirrhosis into survival and non-survival groups to identify the risk factors for death and evaluated different scoring systems for predicting mortality in this high-risk population.
The two risk factors of higher intubation rates and dexamethasone usage, indicating severe respiratory conditions in COVID-19 patients, were observed in the non-survival group. Dexamethasone was recommended for critical cases using oxygen according to global and Taiwan’s COVID-19 pneumonia treatment guidelines [17, 18]. Intubation indicates respiratory failure, the most severe form of COVID-19, and is associated with a poor prognosis [19, 20]. In addition to respiration, our study revealed that prolonged coagulation was a significant risk factor for COVID-19 patients with liver cirrhosis. Coagulation, evaluated by INR, plays a crucial role in predicting liver disease mortality and assessing the cirrhosis severity, as seen in the MELD or Child-Pugh scores [21, 22]. A multicenter study indicated that age and cirrhosis severity, evaluated using the Child-Pugh score, were the most important predictors of mortality in COVID-19 patients with liver cirrhosis [23]. Our study showed that coagulation status was an important risk factor for mortality in cirrhotic patients with COVID-19 compared to other liver function indicators such as bilirubin. A previous study showed similar findings in COVID-19 patients with or without noncirrhotic chronic liver disease [24–26].
In previous studies, individuals with cardiovascular disease, diabetes, chronic respiratory diseases, chronic kidney disease, and cancer were found to be more susceptible to severe COVID-19 [4]. However, in our study, there was no statistically significant difference in the prevalence of these chronic diseases between the survival and non-survival groups. One possible reason was that cirrhotic patients already had poor immune status by liver dysfunction, so the influence on immune system caused by other chronic diseases may be less prominent in these patients. Another explanation was that the prevalence of hepatitis B and C in Taiwan is higher than the global average, resulting in a younger cirrhotic population who had less prevalence of these chronic diseases [27, 28]. Limited sample size may also lead to a lack of statistical significance in our study. Notably, the proportion of diabetes was lower in the non-survival group, which was different from previous research. In addition to the above reasons, this phenomenon may require further research to be fully understood.
According to a previous study, respiration and cirrhotic status were possibly associated with the prognosis of COVID-19 patients with liver cirrhosis; therefore, we analyzed the performance of different scoring systems assessing these two components in predicting mortality in this patient group. COVID-19 is a clinically functional and radiologically dissociated disease and evaluating the respiratory status on ED admission provides the most direct indication of its severity [29]. The WHO ordinal scale is a 9-point scale used to predict the in-hospital mortality of COVID-19 patients according to their respiratory status at the time of hospitalization [9]. Previous studies demonstrated its clinical utility in predicting the prognosis of COVID-19 patients. In our study, the WHO ordinal scale showed the best predictive performance in our patient group compared to the other scoring systems. The results showed that the respiratory status of these patients in the ED was still the essential prognostic factor and that the WHO ordinal scale was an effective tool for predicting mortality in COVID-19 patients with liver cirrhosis.
MELD and MELD-Na scores were used to evaluate cirrhosis status. The MELD score was originally developed to predict three-month mortality in patients with liver cirrhosis following transjugular intrahepatic portosystemic shunt (TIPS) placement [30]. Subsequent studies demonstrated its usefulness in predicting mortality in other groups of patients with liver cirrhosis [11, 21, 31]. The model includes serum bilirubin levels, serum creatinine levels, and prothrombin time. The MELD-Na score is a revised MELD model that adds serum sodium level since the severity of hyponatremia is a marker of the severity of cirrhosis, and the addition of serum sodium concentration improves predictive accuracy [12, 32, 33]. In COVID-19 patients, sodium levels are related to prognosis in previous studies [34, 35]. Hyponatremia is associated with adverse outcomes in COVID-19 patients, and its etiology in COVID-19 patients remain unknown and may be multifactorial. Decreased oral intake, presentation of diarrhea, syndrome of inappropriate antidiuretic hormone (SIADH) due to lung parenchymal injury, disruption of the renin-angiotensin-aldosterone system, or use of medications could contribute to hyponatremia [36–42]. Although the sodium level was probably associated with the outcomes of COVID-19 infection, the MELD-Na score failed to demonstrate better performance in predicting prognosis in our study compared to the MELD score. However, both the MELD and MELD-Na scores showed good performance in predicting mortality in these patients, indicating that the cirrhotic status was also associated with the outcomes of COVID-19 patients with liver cirrhosis. These indicators included in the MELD/MELD-Na score reflected both baseline liver dysfunction and the severity of direct liver injury or cytokine storm caused by COVID-19 infection. Another scoring system for assessing the cirrhotic status is the Child-Pugh score, and some studies have demonstrated the predictive value of the Child-Pugh score in this patient group. However, this was not validated in our study because some data, including the Child-Pugh score and the degree of hepatic encephalopathy and ascites, were not available in our database. In addition, the Child-Pugh score is not convenient to use in the clinic setting of the ED compared to the MELD or MELD-Na scores [7].
Inflammation risk categories are another model applied for in-hospital mortality prediction by dividing three risk categories of COVID-19 patients based on lymphopenia and inflammatory parameters, such as CRP, lactate dehydrogenase (LDH), ferritin, and D-dimer [10]. The performance of the Inflammation Risk Categories in predicting the mortality of COVID-19 patients with liver cirrhosis was not as good as that of the other scoring systems. In a study conducted by Moga et al., the differences in CRP levels between patients with liver cirrhosis and those with COVID-19 infection with or without lung injury were similar [43]. The level of CRP may be a good indicator for bacterial infections but does not significantly increase in viral infections [44]. The inflammatory parameters used in the Inflammation Risk Categories are mostly related to liver metabolism, and liver cirrhosis may cause inaccuracies in these laboratory data [44, 45].
In summary, respiration status was more sensitive than liver dysfunction or inflammation parameters in predicting prognosis of COVID-19 patients with cirrhosis. Since respiratory system was the most affected organ system during COVID-19 infection, the WHO ordinal scale evaluating initial respiration status had the best prediction performance reasonably. However, MELD or MELD-Na scores may still play a role by assessing liver dysfunction, which was different from respiration status in these patients. Evaluating the prognosis of COVID-19 patients with cirrhosis by using WHO ordinal scale and MELD score simultaneously may have the most comprehensive result.
Limitations
Our study had several limitations. First, this was a single-country, multi-center study, which may introduce selection bias and limit the generalizability of the results to other countries. Second, our study relied on database analysis, and some data such as ascites status or the extent of hepatic encephalopathy were unavailable. Some factors were not incorporated into our analysis, and some scoring systems, such as the Child-Pugh score, could not be evaluated. Third, we did not compare patients’ clinical symptoms or pulmonary imaging findings because of the limitations of our database. Fourth, the threshold cycle values for the polymerase chain reaction were not available. Finally, our database does not contain complete baseline liver function data before the patients contracted COVID-19. This may have rendered our analysis incomplete.
Conclusions
Respiration and cirrhosis status were both associated with the prognosis of COVID-19 patients with liver cirrhosis. The WHO ordinal scale evaluated respiration and showed the best performance in predicting the mortality of cirrhotic patients with COVID-19. MELD and MELD-Na scores, which were used to assess the cirrhotic status, were also beneficial tools for the prediction of mortality of these patients. Coagulation function, intubation, and dexamethasone administration were the most significant prognostic factors. Evaluating the prognosis of COVID-19 patients with cirrhosis by using WHO ordinal scale and MELD score simultaneously may have the most comprehensive result.
Acknowledgements
This study was supported by Chang Gung Memorial Hospital. The authors thank all colleagues who provided expertise and substantially assisted with the research, although they may not necessarily agree with all the interpretations presented in this paper.
Abbreviations
- EHR
Electronic health records
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus-2
- COVID-19
coronavirus 2019
- WHO
World Health Organization
- MELD
Model for End-Stage Liver Disease
- CGRD
Chang Gung Research Database
- ED
Emergency department
- LOS
Length of stay
- ICU
Intensive care unit
- DNR
Do not resuscitate
- SBP
Systolic blood pressure
- WBC
White blood cell
- CRP
C-reactive protein
- INR
International normalized ratio
- TIPS
Transjugular intrahepatic portosystemic shunt
Author contributions
SYC and HYL conceived and designed the study. CJN conducted data acquisition, analysis, and interpretation. HYL and YBH provided statistical expertise. SYC drafted the manuscript. CJN and HYL undertook the project administration and supervision. HYL revised the manuscript for intellectual content. All the authors have read and approved the final version of the manuscript.
Funding
This study received no funding.
Data availability
The data that support the findings of this study are available from Linkou Chang Gung Memorial Hospital, but restrictions may apply to the availability of these data, which were approved by individual hospital IRB for the current study, and such they are not publicly available. However, processed datasets can be requested and made available from the authors with the permission of Linkou Chang Gung Memorial Hospital.
Declarations
Human ethics approval
The study was approved by the institutional review board of the Chang-Gung Memorial hospital, Taiwan (IRB no. 202301643B0). This is a retrospective study conducted from de-identified database and informed consent of participation is waived by the Institutional Review Board of CGMH, Taiwan.
Consent to participate
This is a retrospective study conducted from de-identified database and informed consent of participation is waived by the Institutional Review Board of CGMH, Taiwan (IRB no. 202301643B0).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Stokes EK, Zambrano LD, Anderson KN, Marder EP, Raz KM, El Burai Felix S, Tie Y, Fullerton KE. Coronavirus Disease 2019 Case Surveillance - United States, January 22-May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(24):759–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Number of COVID-19 deaths reported to WHO. (cumulative total) [https://data.who.int/dashboards/covid19/deaths?n=c]
- 3.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, et al. Clinical characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18):1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chams N, Chams S, Badran R, Shams A, Araji A, Raad M, Mukhopadhyay S, Stroberg E, Duval EJ, Barton LM, et al. COVID-19: a multidisciplinary review. Front Public Health. 2020;8:383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ginès P, Krag A, Abraldes JG, Solà E, Fabrellas N, Kamath PS. Liver cirrhosis. Lancet. 2021;398(10308):1359–76. [DOI] [PubMed] [Google Scholar]
- 6.Choudhary NS, Dhampalwar S, Saraf N, Soin AS. Outcomes of COVID-19 in patients with cirrhosis or liver transplantation. J Clin Exp Hepatol. 2021;11(6):713–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma P, Kumar A, Anikhindi S, Bansal N, Singla V, Shivam K, Arora A. Effect of COVID-19 on pre-existing liver disease: what hepatologist should know? J Clin Exp Hepatol. 2021;11(4):484–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsai MS, Lin MH, Lee CP, Yang YH, Chen WC, Chang GH, Tsai YT, Chen PC, Tsai YH. Chang Gung Research Database: a multi-institutional database consisting of original medical records. Biomed J. 2017;40(5):263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubio-Rivas M, Mora-Luján JM, Formiga F, Arévalo-Cañas C, Lebrón Ramos JM, Villalba García MV, Fonseca Aizpuru EM, Díez-Manglano J, Arnalich Fernández F, Romero Cabrera JL, et al. WHO Ordinal Scale and inflammation risk categories in COVID-19. Comparative study of the Severity scales. J Gen Intern Med. 2022;37(8):1980–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rubio-Rivas M, Corbella X, Formiga F, Menéndez Fernández E, Martín Escalante MD, Baños Fernández I, Arnalich Fernández F, Del Corral-Beamonte E, Lalueza A, Parra Virto A et al. Risk categories in COVID-19 based on degrees of inflammation: data on more than 17,000 patients from the Spanish SEMI-COVID-19 Registry. J Clin Med 2021, 10(10). [DOI] [PMC free article] [PubMed]
- 11.Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D’Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33(2):464–70. [DOI] [PubMed] [Google Scholar]
- 12.Kim WR, Biggins SW, Kremers WK, Wiesner RH, Kamath PS, Benson JT, Edwards E, Therneau TM. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359(10):1018–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernardi M, Moreau R, Angeli P, Schnabl B, Arroyo V. Mechanisms of decompensation and organ failure in cirrhosis: from peripheral arterial vasodilation to systemic inflammation hypothesis. J Hepatol. 2015;63(5):1272–84. [DOI] [PubMed] [Google Scholar]
- 14.Middleton P, Hsu C, Lythgoe MP. Clinical outcomes in COVID-19 and cirrhosis: a systematic review and meta-analysis of observational studies. BMJ Open Gastroenterol 2021, 8(1). [DOI] [PMC free article] [PubMed]
- 15.Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A, Zhou J, Shi G, Fang N, Fan J. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. biorxiv 2020:2020.2002. 2003.931766.
- 16.Grace JA, Casey S, Burrell LM, Angus PW. Proposed mechanism for increased COVID-19 mortality in patients with decompensated cirrhosis. Hepatol Int. 2020;14(5):884–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.新型冠狀病毒(SARS-CoV-2.)感染臨床處置指引 [https://www.cdc.gov.tw/Category/Page/xCSwc5oznwcqunujPc-qmQ]
- 18.IDSA Guidelines on the Treatment and Management of Patients with COVID-19. [https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/#Recommendations7-9:Glucocorticoids]
- 19.Tobin MJ. Basing Respiratory Management of COVID-19 on physiological principles. Am J Respir Crit Care Med. 2020;201(11):1319–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tobin MJ, Laghi F, Jubran A. Caution about early intubation and mechanical ventilation in COVID-19. Ann Intensive Care. 2020;10(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Said A, Williams J, Holden J, Remington P, Gangnon R, Musat A, Lucey MR. Model for end stage liver disease score predicts mortality across a broad spectrum of liver disease. J Hepatol. 2004;40(6):897–903. [DOI] [PubMed] [Google Scholar]
- 22.Tsoris A, Marlar CA. Use of the child pugh score in Liver Disease. edn. Treasure Island (FL): StatPearls Publishing Copyright © 2023. StatPearls Publishing LLC.; 2023. StatPearls. [PubMed]
- 23.Marjot T, Moon AM, Cook JA, Abd-Elsalam S, Aloman C, Armstrong MJ, Pose E, Brenner EJ, Cargill T, Catana MA, et al. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: an international registry study. J Hepatol. 2021;74(3):567–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu PJ, Feng IC, Lai CC, Ho CH, Kan WC, Sheu MJ, Kuo HT. The mortality of hospitalized patients with COVID-19 and non-cirrhotic chronic liver disease: a retrospective multi-center study. PeerJ. 2023;11:e16582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al Nafea HM, Al-Qahtani MT, Al Gahtani FH, Tabassum H. Blood coagulation, risk factors and associated complications in COVID-19 patients in Saudi Arabia: a retrospective cohort study. Med (Baltim). 2023;102(43):e35621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alizad G, Ayatollahi AA, Shariati Samani A, Samadizadeh S, Aghcheli B, Rajabi A, Nakstad B, Tahamtan A. Hematological and Biochemical Laboratory Parameters in COVID-19 Patients: A Retrospective Modeling Study of Severity and Mortality Predictors. Biomed Res Int 2023, 2023:7753631. [DOI] [PMC free article] [PubMed]
- 27.Yu ML, Chen PJ, Dai CY, Hu TH, Huang CF, Huang YH, Hung CH, Lin CY, Liu CH, Liu CJ, et al. 2020 Taiwan consensus statement on the management of hepatitis C: part (I) general population. J Formos Med Assoc. 2020;119(6):1019–40. [DOI] [PubMed] [Google Scholar]
- 28.Wait S, Chen DS. Towards the eradication of hepatitis B in Taiwan. Kaohsiung J Med Sci. 2012;28(1):1–9. [DOI] [PubMed] [Google Scholar]
- 29.Dhont S, Derom E, Van Braeckel E, Depuydt P, Lambrecht BN. The pathophysiology of ‘happy’ hypoxemia in COVID-19. Respir Res. 2020;21(1):198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31(4):864–71. [DOI] [PubMed] [Google Scholar]
- 31.Wiesner R, Edwards E, Freeman R, Harper A, Kim R, Kamath P, Kremers W, Lake J, Howard T, Merion RM, et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124(1):91–6. [DOI] [PubMed] [Google Scholar]
- 32.Luca A, Angermayr B, Bertolini G, Koenig F, Vizzini G, Ploner M, Peck-Radosavljevic M, Gridelli B, Bosch J. An integrated MELD model including serum sodium and age improves the prediction of early mortality in patients with cirrhosis. Liver Transpl. 2007;13(8):1174–80. [DOI] [PubMed] [Google Scholar]
- 33.Londoño MC, Cárdenas A, Guevara M, Quintó L, de Las Heras D, Navasa M, Rimola A, Garcia-Valdecasas JC, Arroyo V, Ginès P. MELD score and serum sodium in the prediction of survival of patients with cirrhosis awaiting liver transplantation. Gut. 2007;56(9):1283–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Haan L, Ten Wolde M, Beudel M, Olde Engberink RHG, Appelman B, Haspels-Hogervorst EK, Rusch D, van den Gritters NC, Simsek S, Paternotte N, et al. What is the aetiology of dysnatraemia in COVID-19 and how is this related to outcomes in patients admitted during earlier and later COVID-19 waves? A multicentre, retrospective observational study in 11 Dutch hospitals. BMJ Open. 2023;13(11):e075232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rehman FU, Rehan ST, Rind BJ, Valliani K, Asghar MS, Omair F. Hyponatremia causing factors and its association with disease severity and length of stay in COVID-19 patients: a retrospective study from tertiary care hospital. Med (Baltim). 2023;102(45):e35920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gheorghe G, Ilie M, Bungau S, Stoian AMP, Bacalbasa N, Diaconu CC. Is there a relationship between COVID-19 and Hyponatremia? Med (Kaunas) 2021, 57(1). [DOI] [PMC free article] [PubMed]
- 37.Yousaf Z, Al-Shokri SD, Al-Soub H, Mohamed MFH. COVID-19-associated SIADH: a clue in the times of pandemic! Am J Physiol Endocrinol Metab. 2020;318(6):E882–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muaddi L, Ledgerwood C, Sheridan R, Dumont T, Nashar K. Acute renal failure and its complications, indications for Emergent Dialysis, and Dialysis modalities. Crit Care Nurs Q. 2022;45(3):258–65. [DOI] [PubMed] [Google Scholar]
- 40.Valizadeh R, Baradaran A, Mirzazadeh A, Bhaskar L. Coronavirus-nephropathy; renal involvement in COVID-19. J Ren Inj Prev. 2020;9(2):e18. [Google Scholar]
- 41.Hunt RH, East JE, Lanas A, Malfertheiner P, Satsangi J, Scarpignato C, Webb GJ. COVID-19 and gastrointestinal disease: implications for the gastroenterologist. Dig Dis. 2021;39(2):119–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of Coronavirus Disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782–93. [DOI] [PubMed] [Google Scholar]
- 43.Moga TV, Foncea C, Bende R, Popescu A, Burdan A, Heredea D, Danilă M, Miutescu B, Ratiu I, Bizerea-Moga TO et al. Impact of COVID-19 on patients with decompensated liver cirrhosis. Diagnostics (Basel) 2023, 13(4). [DOI] [PMC free article] [PubMed]
- 44.Sproston NR, Ashworth JJ. Role of C-Reactive protein at sites of inflammation and infection. Front Immunol. 2018;9:754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang W, Knovich MA, Coffman LG, Torti FM, Torti SV. Serum ferritin: past, present and future. Biochim Biophys Acta. 2010;1800(8):760–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from Linkou Chang Gung Memorial Hospital, but restrictions may apply to the availability of these data, which were approved by individual hospital IRB for the current study, and such they are not publicly available. However, processed datasets can be requested and made available from the authors with the permission of Linkou Chang Gung Memorial Hospital.