Abstract
Objectives:
To quantify the proportion of patients receiving high-intensity end-of-life care, identify associated risk factors, and assess how receipt of palliative care impact end-of-life care; as the delivery of such care, and how it relates to palliative care, has not been reported in bladder cancer
Subjects and Methods:
We conducted a retrospective cohort study of patients with bladder cancer who died within 1 year of diagnosis using Surveillance, Epidemiology, and End Results linked Medicare data. The primary outcome was a composite measure of high-intensity end-of-life care (>1 hospital admission, >1 ED visit, or ≥1 ICU admission within the last month of life; receipt of chemotherapy within the last 2 weeks of life; or acute care in-hospital death). Secondary outcomes included the use of such care over time and any association with the use of palliative care. A generalized linear mixed model assessed for independent determinants.
Results:
Overall, 45% of patients received high-intensity end-of-life care. This proportion decreased over time. Patients receiving high-intensity care had higher rates of comorbidities, advanced bladder cancer, and nonbladder cancer cause of death. These patients more often received palliative care but, compared to those not receiving high-intensity care, this occurred farther removed from bladder cancer diagnosis and closer to death.
Conclusions:
Nearly half of Medicare beneficiaries with bladder cancer who die within 1 year of diagnosis receive high-intensity care at the end of life. Palliative care was seldom used and only very near the time of death.
Keywords: Urinary bladder neoplasms, End-of-life care, Palliative care, Palliative medicine, Quality of health care, Health services research, Medicare
1. Introduction
Care provided at the end of life (EoL), particularly the last year of life, places heavy strain on patient quality of life and healthcare systems [1]. This strain can be ameliorated by palliative care; multidisciplinary care aimed at preventing and relieving symptoms and stressors for people with serious illnesses [2]. Palliative care positively impacts patient-focused outcomes and decreases rates of high-intensity EoL care, all without decreasing life expectancy [3,4]. High-intensity EoL care is not always consistent with patients’ preferences [5,6]. It is loosely defined as unplanned healthcare encounters and those that do not comprehensively address a patient’s complex needs or focus on “disease-modifying treatment at the expense of good symptom management” [7].
Delivery of high-intensity EoL care, and how it relates to palliative care services in particular, has not been reported in the bladder cancer population. Indicators of high-intensity EoL care include multiple hospital admissions or emergency department (ED) visits, any intensive care unit (ICU) admission, receipt of chemotherapy in the last 2 weeks of life, and in-hospital death [8]. Administrative datasets have been leveraged to measure these indicators, with studies showing a large burden of high-intensity care in a number of cancer types [9–11]. We recently reported on the very low use of subspecialty palliative care among patients with invasive bladder cancer [12]. However, the rate of palliative care use and receipt of high-intensity EoL care among patients with bladder cancer has yet to be examined.
For these reasons, we conducted a population-based study of high-intensity EoL care among patients with bladder cancer who died within 1 year of diagnosis. We aimed to quantify the proportion of patients receiving high-intensity care, determine changes over time, identify risk factors for receiving high-intensity care, and assess for an association between the delivery of subspecialty palliative care and high-intensity EoL care. We hypothesized that the proportion of patients receiving palliative care would be low, but that these patients would be less likely to receive high-intensity EoL care. Our goal was to establish a baseline rate of high-intensity bladder cancer care at the EoL, so that these outcomes can be used as quality measures in the future.
2. Subjects and Methods
2.1. Data Source and Study Population
We used Surveillance, Epidemiology, and End Results (SEER)–Medicare data to identify patients aged 66 years and older who were diagnosed with bladder cancer between 2008 and 2013. All patients had at least stage II bladder cancer—defined as stage ≥T2 or N+ or M+ disease—derived through the SEER Collaborative Staging algorithm [13]. We restricted the cohort to patients who died within 1 year of diagnosis in order to evaluate trends over time and avoid length time bias. We excluded patients who died within 1 month of diagnosis, since we assessed outcomes over the last month of life.
All patients in the cohort were continuously enrolled in Medicare Parts A and B for 12 months prior to diagnosis to ensure calculation of comorbidity [14]. Patients with gaps in coverage or any Health Maintenance Organization coverage after diagnosis were excluded. We also excluded patients with multiple bladder cancer diagnoses, other malignancies that predated bladder cancer or had missing dates of diagnosis, and bladder cancer diagnosis made at autopsy or death. The final study population consisted of 6066 patients.
2.2. Main outcome measure
Our primary outcome was receipt of high-intensity EoL care received over the last month of life [8]. These indicators were first proposed by Earle and colleagues and are widely adopted in the literature. We defined high-intensity EoL care as a composite measure including 1 or more of the following: >1 hospital admission, >1 ED visit, or ≥1 ICU admission within the last month of life; receipt of chemotherapy within the last 2 weeks of life; and acute care in-hospital death [8].
2.3. Secondary outcomes
Secondary outcomes included individual indicators of high-intensity care, receipt of subspecialty palliative care services, and trends over time. Palliative care receipt was defined as described in previous work; the presence of a claim submitted after bladder cancer diagnosis by a Hospice and Palliative Medicine subspecialty provider identified using Healthcare Finance Administration Specialty codes [12].
2.4. Variable definitions
Patient demographics and pathologic information were obtained using the Patient Entitlement and Diagnosis Summary File. Demographic covariates included age, sex, race/ethnicity, and marital status. Geographic region was determined from SEER region at the time of diagnosis. Local census tract information was used to determine education level (the proportion of a ZIP code population with at least a high school education), population of the county of residence, and median household income. Clinicopathologic covariates included comorbidity [14], tumor/nodal/metastasis stage using the SEER Collaborative Stage algorithm, and extent of disease (advanced or localized bladder cancer). Advanced bladder cancer was defined as TNM stage T4 or N+ or M+ disease; consistent with palliative care guidelines [15].
2.5. Statistical analysis
Patient demographic and clinicopathologic characteristics were presented using frequency/percentages and compared between those who received and did not receive high-intensity EoL care using chi-square tests. We fit a generalized linear mixed model with logit link to assess for determinants of receiving high-intensity EoL care, with health services area as a random effect to account for nesting of patients within regions. The multivariable model contained variables associated with high-intensity EoL care on univariate analysis (P-value <0.1) in addition to marital status (determined to be an important variable a priori). For parsimony, we used linear year as a predictor since adjusted probabilities using year as categorical appeared linear. Results were presented as odds ratios and 95% confidence intervals using a forest plot. From this model, the predicted probability of receiving high-intensity care by year of diagnosis was estimated by averaging subject specific predicted probabilities over the corresponding year. Results were presented as point estimates and 95% bootstrap confidence intervals. The process was repeated for each independent indicator of high-intensity EoL care.
Statistical analysis was performed using SAS v9.4 (Cary, NC), Stata (College Station, TX), and R (version 13.2). Statistical significance was defined as a P-value <0.05. The University of Pittsburgh institutional review board deemed this study exempt from review.
3. Results
We identified 33,367 patients over the age of 65 diagnosed with bladder cancer who met enrollment criteria. Of these, 15,781 (47%) died within 1 year. After excluding patients who died within 1 month of diagnosis or had stage I disease, we arrived at 6,066 patients with stage II or greater bladder cancer who died within 1 year of diagnosis. Overall, 2,728 of 6,066 patients (45%) had at least 1 indicator of high-intensity EoL care.
Patients receiving high-intensity EoL care were more likely to be younger, male, and nonwhite (Table 1). Clinicopathologic factors significantly associated with high-intensity EoL care included comorbidity, advanced bladder cancer, and non-bladder cancer related cause of death (all P < 0.001). The rate of receiving subspecialty palliative care was low at 4.4%. Patients receiving high-intensity care at the EoL were more likely to receive subspecialty palliative care compared to those who did not (5.1% vs. 3.7%, P <0.001). However, patients receiving high-intensity EoL care received palliative care consultation farther out from bladder cancer diagnosis (8.2 vs. 6.4 months, P = 0.1) and closer to death (0.9 vs. 2.3 months, P < 0.001).
Table 1.
Characteristics of study population
| Characteristic | Any high-intensity end-of-life care (n = 2,728) | No high-intensity end-of-life care (n = 3,338) | p-value |
|---|---|---|---|
|
| |||
| Age (%) | <0.001 | ||
| 66–69 | 301 (11.0) | 283 (8.5) | |
| 70–74 | 484 (17.7) | 488 (14.6) | |
| 75–79 | 580 (21.3) | 622 (18.6) | |
| 80 and older | 1,363 (50.0) | 1,945 (58.3) | |
| Sex (%) | 0.004 | ||
| Male | 1,917 (70.3) | 2,230 (66.8) | |
| Female | 811 (29.7) | 1,108 (33.2) | |
| Race/Ethnicity (%) | <0.001 | ||
| White | 2,275 (83.4) | 2,942 (88.1) | |
| Black | 210 (7.7) | 165 (4.9) | |
| Hispanic | 131 (4.8) | 121 (3.6) | |
| Asian | 90 (3.3) | 88 (2.6) | |
| Other | 22 (0.8) | 22 (0.7) | |
| Marital status (%) | 0.2 | ||
| Married | 1,354 (49.6) | 1,606 (48.1) | |
| Not married/Unknown | 1,235 (45.3) | 1,582 (47.4) | |
| Comorbidity (%) | <0.001 | ||
| 0 | 907 (33.2) | 1,248 (37.4) | |
| 1 | 656 (24.0) | 794 (23.8) | |
| 2 | 387 (14.2) | 503 (15.1) | |
| 3 or more | 703 (25.8) | 636 (19.1) | |
| Education in ZIP code of residence (%) | <0.001 | ||
| Low: 75% or fewer with high school education | 404 (14.8) | 387 (11.6) | |
| High: >75% with high school education | 2258 (82.8) | 2890 (86.6) | |
| Missing/Unknown | 66 (2.4) | 61 (1.8) | |
| Population of county of residence (%) | 0.02 | ||
| 249,999 or less | 720 (26.4) | 976 (29.2) | |
| 250,000–999,999 | 477 (17.5) | 612 (18.3) | |
| 1,000,000 or more | 1,531 (56.1) | 1,750 (52.4) | |
| Median income in ZIP code of residence, $ (%) | 0.2 | ||
| 40,000 or less | 523 (19.2) | 585 (17.5) | |
| 40,0001–60,000 | 991 (36.3) | 1,269 (38.0) | |
| 60,001 or more | 1,147 (42.0) | 1,419 (42.5) | |
| Missing/Unknown | 67 (2.5) | 65 (1.9) | |
| Geographic region (%) | 0.001 | ||
| Northeast | 704 (25.8) | 728 (21.8) | |
| South | 643 (23.6) | 784 (23.5) | |
| Central | 347 (12.7) | 489 (14.6) | |
| West | 1,034 (37.9) | 1,337 (40.1) | |
| T stage (%) | |||
| T1 or lower | 85 (3.1) | 90 (2.7) | <0.001 |
| T2 | 1,450 (53.2) | 1,912 (57.3) | <0.001 |
| T3 | 865 (31.7) | 881 (26.4) | |
| T4 | 328 (12.0) | 455 (13.6) | |
| N stage (%) | 0.1 | ||
| N0/NX | 2,311 (74.7) | 2,877 (86.2) | |
| N+ | 417 (15.3) | 461 (13.8) | |
| M stage (%) | 0.1 | ||
| M0/MX | 2,239 (82.2) | 2,792 (83.6) | |
| M+ | 489 (17.9) | 546 (16.4) | |
| Extent of disease ‡ | <0.001 | ||
| Advanced (T4, or N+, or M+) | 1,960 (71.8) | 2,456 (73.6) | |
| Localized (all except T4/N+/M+) | 440 (16.1) | 427 (12.8) | |
| Receipt of palliative care (%) | 139 (5.1) | 125 (3.7%) | <0.001 |
| Diagnosis to palliative care in months, median (IQR) | 8.2 (3.2—16.5) | 6.4 (2.2—14.7) | 0.1 |
| Palliative care to death in months, median (IQR) | 0.9 (0.2—3.3) | 2.3 (1.0—6.3) | <0.001 |
| Cause of death (%) | <0.001 | ||
| Bladder cancer | 1,378 (50.5) | 1,857 (55.6) | |
| Other | 1,350 (49.5) | 1,481 (44.4) | |
| Year of diagnosis (%) | 0.1 | ||
| 2008 | 537 (19.7) | 609 (18.2) | |
| 2009 | 510 (18.7) | 579 (17.3) | |
| 2010 | 498 (18.3) | 589 (17.6) | |
| 2011 | 452 (16.6) | 579 (17.3) | |
| 2012 | 411 (15.1) | 528 (15.8) | |
| 2013 | 320 (11.7) | 454 (13.6) | |
Percentages may not sum to 100 due to rounding.
Advanced stage defined as stage T4 and/or N1 and/or M1.
Of the 2728 patients receiving high-intensity care at the EoL, 50% were admitted to an ICU during the last month of life and 54% died while admitted to an acute care hospital (Table 2). About one third had multiple ED visits or were admitted multiple times over the last month of life; 36% and 30%, respectively. Of all patients receiving high-intensity EoL care, 35% experienced 2 indicators of high-intensity care and 18% experienced 3 or more.
Table 2.
End-of-life care measures
| Characteristic | Total cohort (n = 6,066) | Any high-intensity end-of-life care (n = 2,728) |
|---|---|---|
|
| ||
| Any high-intensity end-of-life care (%) | 2,728 (45.0) | 2,728 (100.0) |
| Multiple admissions (%) | 819 (13.5) | 819 (30.0) |
| Multiple Emergency Department visits (%) | 991 (16.3) | 991 (36.3) |
| Intensive Care Unit admission (%) | 1,367 (22.5) | 1,367 (50.1) |
| Chemotherapy near EoL (%) | 152 (2.5) | 152 (5.6) |
| In-hospital death (%) | 1,463 (24.1) | 1,463 (53.6) |
| Number of high-intensity end of life measures (%) | ||
| 0 | 3,338 (55.0) | 0 (0) |
| 1 | 1,281 (21.1) | 1,281 (47.0) |
| 2 | 952 (15.7) | 952 (34.9) |
| 3 or more | 495 (8.2) | 495 (18.1) |
On multivariable analysis, factors independently associated with increased risk of receiving high-intensity EoL care included receiving subspecialty palliative care (adjusted odds ratio [aOR] 1.40, 95% confidence interval [CI] 1.07–1.84), black race (aOR 1.51, 95% CI 1.17–1.96), Hispanic ethnicity (aOR 1.39, 95% CI 1.03–1.89), comorbidity score of 3 or greater (aOR 1.4, 95% CI 1.28–1.74), and advanced bladder cancer (aOR 1.25, 95% CI 1.07–1.46) (Fig. 1). Factors associated with decreased risk included age of 80 years or more (aOR 0.72, 95% CI 0.59–0.87), female sex (aOR 0.85, 95% CI 0.74–0.96), higher education level (aOR 0.79, 95% CI 0.67–0.94), and later year of diagnosis (aOR 0.95, 95% CI 0.92–0.98).
Fig. 1.
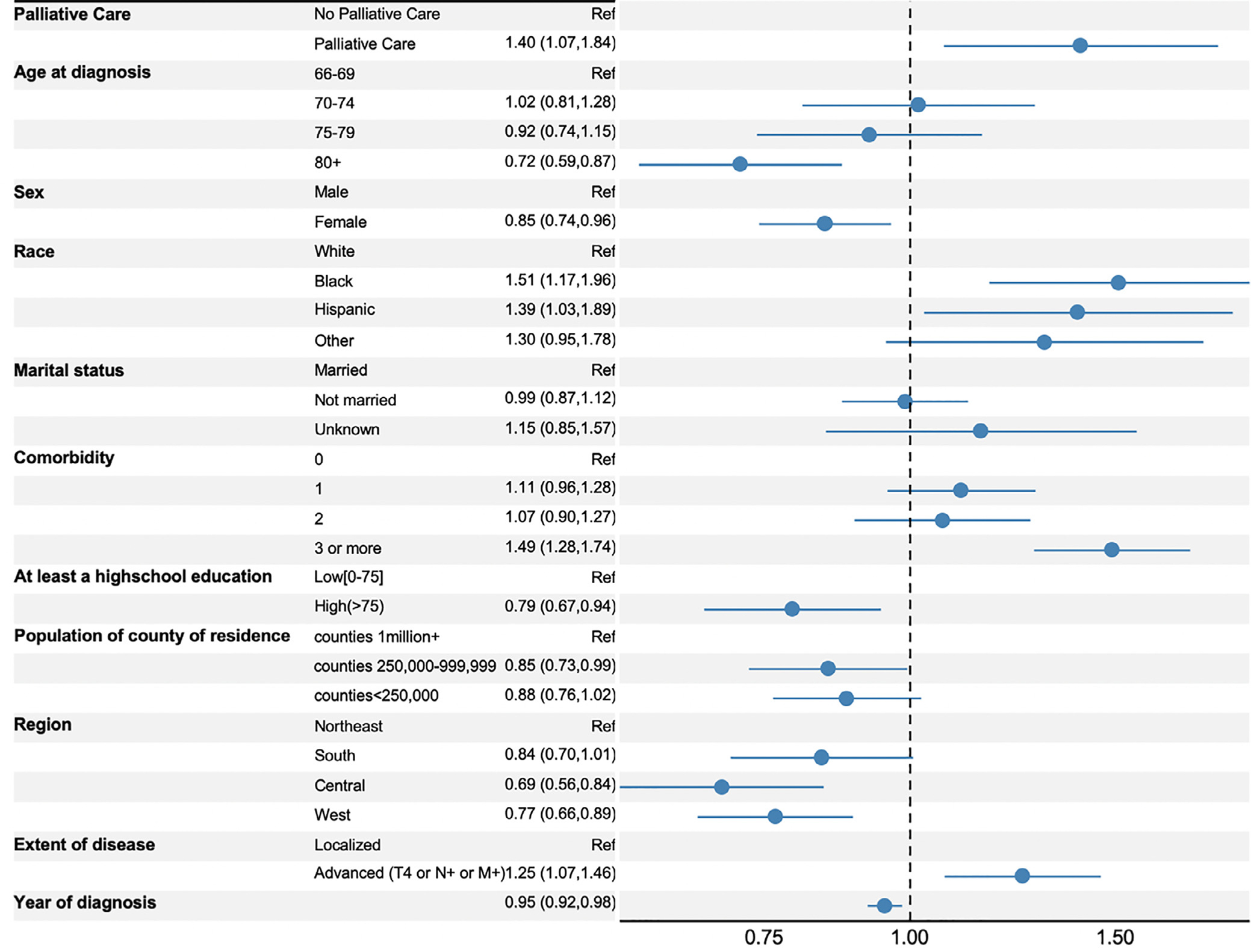
Forrest plot of adjusted odds ratios of receiving any high-intensity end-of-life care. Statistics presented are odds ratios with 95% confidence intervals. Multivariable analysis used a generalized linear mixed model with logit link. Estimates are adjusted for all covariates.
The adjusted probability of receiving high-intensity EoL care significantly decreased from 49% in 2008 to 43% in 2013 (P<0.005) (Fig. 2). The absolute decrease in adjusted probability of multiple hospital admissions (−0.2%) and ICU admission (−0.3%) was negligible, while chemotherapy use (−1.5%) and in hospital death (−6.3%) decreased by larger amounts. Multiple ED admission was the only measure of high-intensity care to increase over time (+0.9%) (Supplementary Fig. 1).
Fig. 2.
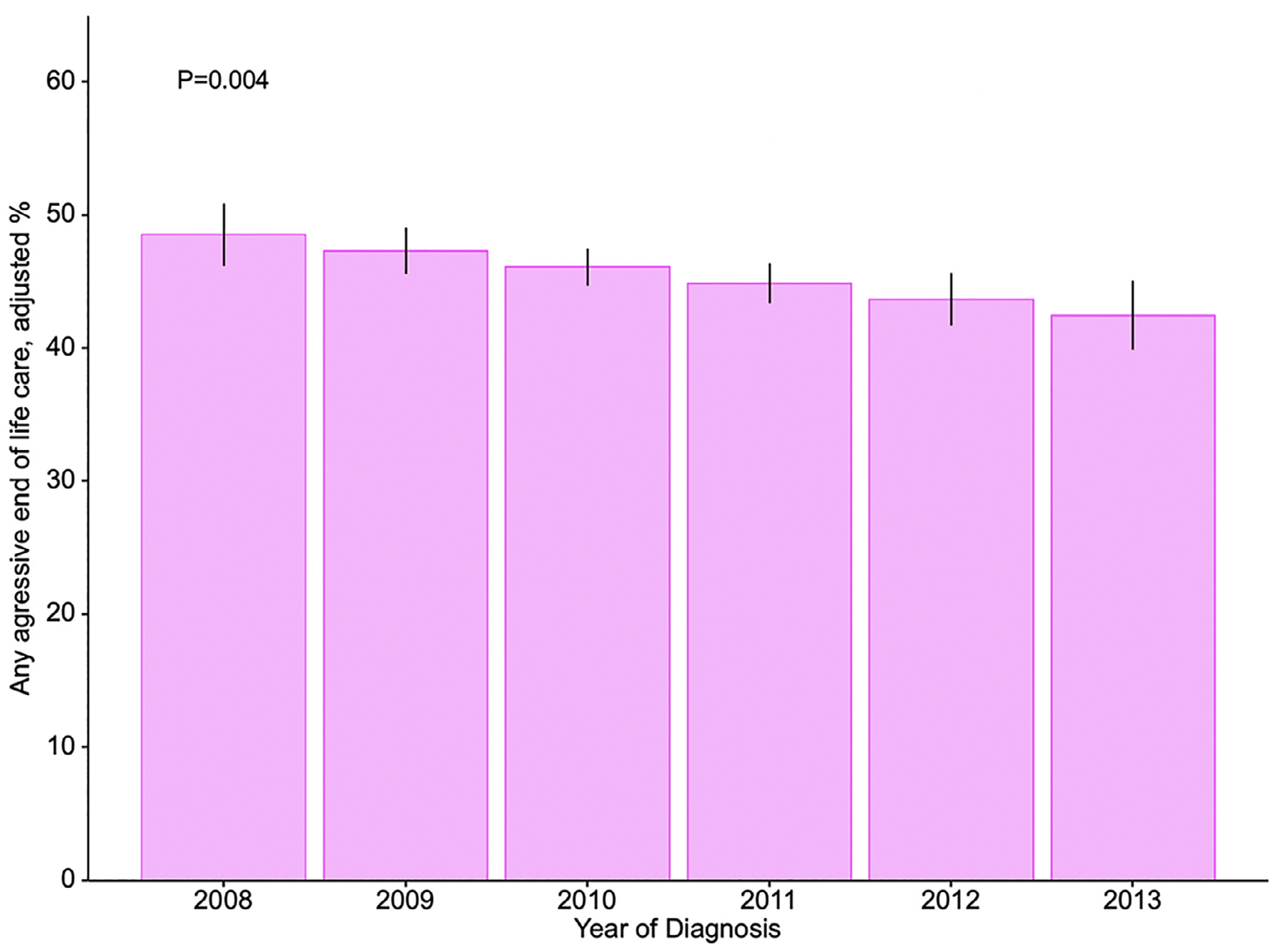
Predicted probability of receiving any high-intensity end-of-life care by year of diagnosis. Estimates obtained with a multivariable generalized linear mixed methods model with logit link and health service area as a random effect to account for patient nesting. Estimates are adjusted for: receipt of palliative care, age, sex, race, marital status, comorbidity (CCI score), education level, population of county of residence, geographical region, extent of disease, and year of diagnosis. Error bars represent 95% bootstrapped confidence intervals.
4. Comment
Nearly half of Medicare beneficiaries with stage II or greater bladder cancer received high-intensity EoL care. These patients were more likely to be younger, non-white men with greater comorbidities and advanced bladder cancer. They were also significantly more likely to receive subspecialty palliative care. Over the study period, the adjusted probability of receiving any high-intensity EoL care decreased by 6%.
A number of impactful findings arise from our study. First, the rate of high-intensity EoL care has not been previously described in the bladder cancer population. Compared to a study of Medicare beneficiaries dying with poor prognosis cancer—mostly lung, pancreatic, colon, and hematologic—we found that patients with bladder cancer were 30% and 60% more likely to have multiple hospital admissions and ED visits, respectively [16]. However, admission to an ICU or in-hospital death were less likely for patients with bladder cancer. The trend towards increased hospital and ED admissions without proportionate increases in ICU treatment and in-hospital death could reflect the poorer survival for patients with lung, pancreatic, colon, and hematologic malignancies [17]. Also, patients with bladder cancer may have local cancer symptoms more amenable to mitigation in the ED or with a short hospital admission. Similar to our study are rates of high-intensity EoL care reported by Barbera et al among women dying of gynecologic malignancies. Over the last month of life, 34% of these women visited the ED, 4% received chemotherapy, and 51% died in an acute care hospital [10].
Secondly, we found that the predicted probability of receiving high-intensity EoL care significantly decreased by 6% over the study period. Between 1993–2004, researchers from Ontario, Canada found that 22% of all patients dying of cancer received high-intensity care; half the rate found in our cohort. However, in this earlier study, aggressive care increased at a rate of 1 percent per year [11]. Increasing rates of high-intensity care reported in studies from the late 1990’s and early 2000’s is thought to result from a “patchwork” palliative care infrastructure at the time [11]. Likewise, the decreasing rate of high-intensity care shown in our study can be attributed to increasing availability of palliative care services and hospice enrollment [16].
Thirdly, and similar to the results of our prior study, the rate of specialty palliative care was low at 4.4% [12]. Interestingly, palliative care was independently associated with an increased odds of receiving high-intensity EoL care. There are several reasons that may explain these findings. First, a small subpopulation receiving palliative care may potentiate any selection bias inherent in SEER-Medicare. Second, there is a known shortage of palliative care providers in the United States, especially in the community setting best equipped to prevent hospitalizations [18,19]. Finally, palliative care consultation could be misused or used in a narrower scope of EoL or “terminal” care. This is the most likely cause of more frequent palliative care use among those receiving high-intensity EoL care. This assessment is substantiated by the timing of subspecialty palliative care consultation relative to bladder cancer diagnosis and death. There was a significant difference in the time from palliative care consultation to death in patients receiving high-intensity care (<1 month) compared to those not receiving high-intensity care (2.3 months); a finding consistent with existing literature that shows decreased high-intensity EoL among patients receiving early palliative care or hospice enrollment [9,20,21]. Goldwasser et al showed that, compared to patients who received “timely” identification of palliative care needs, those with late, very late, or no identification had statistically significant higher rates of chemotherapy (OR range 1.3–2.2), invasive ventilation (1.3–5.2), and death in an ICU (1.3–8.9) [21]. Palliative care consultation among those receiving high-intensity EoL care was also delayed. This reinforces the unfortunate trend of patients receiving subspecialty palliative care either very late in their disease course or not at all [22].
There are a number of implications of this work. First, our findings show the potential impact that the urologic community can have on patients’ EoL care. A study of patients dying of urologic cancer showed that 20% of all outpatient clinic visits during the last year of life are attributed to urology clinics. Patients with newly diagnosed bladder cancer—those most likely to be actively followed by a urologist—have the highest burden of unmet supportive care needs. Overall, 15% of patients across the disease spectrum have more than 10 unmet needs [23]. These data highlight the importance of developing basic primary palliative care skills among urologists and fostering collaborative relationships between palliative care providers and urologists [24]. Providers can quickly screen patients for palliative care needs using the Edmonton Symptom Assessment Scale or the National Comprehensive Cancer Network Distress Thermometer, which can then direct administration of primary palliative care or subspecialty referral. The benefits of early palliative care can be inferred from a study showing that hospice enrollment for patients dying of prostate cancer was associated with lower odds of high-intensity care. This was accomplished while not “rationing” care, as hospice enrollees did not have significantly lower rates of genitourinary procedures (such as foley catheter placement or upper urinary tract decompression), physical therapy, chemotherapy, or outpatient physician visits. The decreased odds of aggressive care strengthened near death, suggesting improved goal-concordant care as a result of hospice enrollment [9]. Other studies have shown that increasing hospice enrollment is associated with declining rates of in-hospital death [25]. Finally, patients, family members, and palliative care providers collaborated with urologists at a Veterans Affairs medical center (Los Angeles, CA) to determine the most efficacious way to deliver comprehensive care to patients with metastatic genitourinary cancer. The investigators found that bringing palliative care consultations to the urologic point of care did not disrupt clinic workflow, improved patient and provider satisfaction, and decreased the need for future clinic visits [26]. Primary palliative care and integrated clinics hold great potential in the improvement of whole-person care, in addition to mitigating goal discordant care as patients near the end of life.
There are a number of policy implications, such as the inclusion of high-intensity EoL care measures in clinical data registries to monitor and improve care for patients with advanced genitourinary cancers. As reimbursement embraces quality-based measures, EoL care interventions will be high-yield for gains in healthcare efficiency and sustainability. Earle et al identified benchmarks for outliers in high-intensity EoL care. The authors proposed that less than 4% of patients should visit the ED, get admitted to an acute care bed, or require ICU care within the last month of life. Additionally, less than 17% of cancer patients should die while admitted to an acute care bed [27]. We hope that the foundational work by Earle et al, in addition to the baseline EoL care rates presented here, will encourage both the measurement of aggressive EoL care and screening for unmet palliative care needs for patients with bladder cancer. While determining causes of variation in EoL care will be crucial in developing interventions or policy, administrative data can only facilitate so much progress. For example, a study of patients with poor prognosis cancers—that is, those with a high likelihood of dying in 1 year—found a very weak association between EoL care and hospital characteristics [28]. Another study found that patient preferences contribute very little to regional variation in EoL care [29]. As such, thought leaders suggest that palliative care clinical trials, communication-based interventions, and qualitative studies will be heavily relied on to improve care of surgical patients in the future [30].
The results of our study must be interpreted in light of some limitations. Our results may slightly overestimate high-intensity EoL care, since exact diagnosis dates are not provided in the SEER-Medicare dataset. Subspecialty palliative care was defined using physician specialty codes rather than ICD-9 codes. We chose this method because the palliative care ICD-9 code lacks sensitivity. Using ICD-9 codes, however, could have potentially improved detection of claims billed for EoL or “terminal” care [31]. The exact cause of death is not reported in SEER-Medicare; only whether death was cancer specific or not. A more rigorous analysis of cause of death may have been able to clarify which patients with localized disease died of rare, unforeseen complications and thus had been receiving appropriate care that unfortunately resulted in demise. Given that half of the cohort was over 80 years of age with comorbidities, this exact scenario is unlikely to have biased our results. Finally, we were not able to ascertain whether hospice enrollment lowered high-intensity EoL care because this data is contained within a separate Medicare file. The work of Bergman et al suggest that this might be the case, however, the cohort of patients qualifying for hospice likely differs significantly from our cohort [9]. We posit that our cohort has wider generalizability, while also recognizing that using the hospice file to further this work should be a future research priority. In fact, ongoing work within our group aims to analyze the Medicare Hospice file to characterize healthcare utilization and EoL care among patients with bladder cancer.
5. Conclusion
Half of Medicare beneficiaries who are diagnosed with bladder cancer and die within 1 year received of high-intensity care during the last month of life. However, palliative care was seldom used and only used very near the time of death. This data is crucial to understanding and improving EoL care for patients with bladder cancer. Our findings support the need for earlier integration of palliative care into standard oncologic care. Conversations across disciplines—such as urology, medical oncology, radiation oncology, palliative care, primary care, and geriatric medicine—should focus on care gaps that contribute to goal-discordant EoL care. Future studies in this area hold the potential to greatly improve quality of life and satisfaction with care.
Supplementary Material
Funding/Disclosures
Bruce Jacobs is supported in part by the University of Pittsburgh Physicians Academic Foundation, P30CA047904 from the National Cancer Institute, the Henry L. Hillman Foundation, and the American Urological Association Data Grant. The remaining authors have no additional funding or disclosures.
Footnotes
Conflicts of Interest
None to disclose (see below for detailed ICMJE statements)
Supplementary materials
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.urolonc.2021.02.008.
References
- [1].IOM (Institute of Medicine). Approaching Death. Washington, D.C.: National Academies Press; 1997. Available at: http://www.nap.edu/catalog/5801. Accessed January 19, 2020. [Google Scholar]
- [2].Center to Advance Palliative Care: About Palliative Care. Available at: https://www.capc.org/about/palliative-care/. Accessed October 6, 2020.
- [3].Davis MP, Temel JS, Balboni T, et al. A review of the trials which examine early integration of outpatient and home palliative care for patients with serious illnesses. Ann. Palliat. Med. 2015;4:99–121. [DOI] [PubMed] [Google Scholar]
- [4].Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non–small-cell lung cancer. N. Engl. J. Med. 2010;363:733–42. [DOI] [PubMed] [Google Scholar]
- [5].Singer PA, Martin DK, Kelner M. Quality End-of-Life Care. J. Am. Med. Assoc. 1999;281:163–8. [DOI] [PubMed] [Google Scholar]
- [6].Bolt EE, Pasman HRW, Willems D, et al. Appropriate and inappropriate care in the last phase of life: an explorative study among patients and relatives. BMC Health Serv. Res. 2016;16:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Henson LA, Gomes B, Koffman J, et al. Factors associated with aggressive end of life cancer care. Support. Care Cancer 2016;24:1079–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Earle CC, Neville BA, Landrum MB, et al. Trends in the aggressiveness of cancer care near the end of life. J. Clin. Oncol. 2004; 22:315–21. [DOI] [PubMed] [Google Scholar]
- [9].Bergman J, Saigal CS, Lorenz KA, et al. Hospice use and high-intensity care in men dying of prostate cancer. Arch. Intern. Med. 2011;171:204–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Barbera L, Elit L, Krzyzanowska M, et al. End of life care for women with gynecologic cancers. Gynecol. Oncol. 2010;118:196–201. [DOI] [PubMed] [Google Scholar]
- [11].Ho TH, Barbera L, Saskin R, et al. Trends in the aggressiveness of end-of-life cancer care in the universal health care system of Ontario. Canada. J. Clin. Oncol. 2011;29:1587–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hugar LA, Lopa S, Yabes J, et al. Palliative care Use Among Patiens with Bladder Cancer. BJU Int 2019:0–3. [DOI] [PubMed] [Google Scholar]
- [13].Page DL, Fleming D, Fritz A. AJCC Cancer Staging Manual. 6th. Philadelphia, PA: Lippincott-Raven; 2002:335–40. [Google Scholar]
- [14].Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J. Clin. Epidemiol. 2000;53:1258–67. [DOI] [PubMed] [Google Scholar]
- [15].Ferrell BR, Temel JS, Temin S, et al. Integration of palliative care into standard oncology care: American society of clinical oncology clinical practice guideline update. J. Clin. Oncol. 2017;35:96–112. [DOI] [PubMed] [Google Scholar]
- [16].Miesfeldt S, Murray K, Lucas L, et al. Association of age, gender, and race with intensity of end-of-life care for medicare beneficiaries with. Cancer. J. Palliat. Med. 2012;15:548–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].U.S. Cancer Statistics Working Group: U.S. Cancer Statistics Data Visualizations Tool, based on 2019 submission data (1999–2017): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute. Available at: www.cdc.gov/cancer/dataviz. Accessed October 9, 2020. [Google Scholar]
- [18].Spetz J, Dudley N, Trupin L, et al. Few hospital palliative care programs meet national staffing recommendations. Health Aff 2016;35:1690–7. [DOI] [PubMed] [Google Scholar]
- [19].Kamal AH, Bull JH, Swetz KM, et al. Future of the Palliative Care Workforce: Preview to an Impending Crisis. Am. J. Med. 2017;130:113–4. [DOI] [PubMed] [Google Scholar]
- [20].Scibetta C, Kerr K, Mcguire J, et al. The costs of waiting: Implications of the timing of palliative care consultation among a cohort of decedents at a comprehensive cancer center. J. Palliat. Med. 2016;19:69–75. [DOI] [PubMed] [Google Scholar]
- [21].Goldwasser F, Vinant P, Aubry R, et al. Timing of palliative care needs reporting and aggressiveness of care near the end of life in metastatic lung cancer: a national registry-based study. Cancer 2018:1–8. [DOI] [PubMed] [Google Scholar]
- [22].Roeland EJ, Triplett DP, Matsuno RK, et al. Patterns of palliative care consultation among elderly patients with cancer. J. Natl. Compr. Cancer Netw. 2016;14:439–45. [DOI] [PubMed] [Google Scholar]
- [23].Chung J, Kulkarni GS, Morash R, et al. Assessment of quality of life, information, and supportive care needs in patients with muscle and non-muscle invasive bladder cancer across the illness trajectory. Support. Care Cancer 2019;27:3877–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hounsome L, Verne J, Woodhams S. End of life care for urological cancer patients. J. Clin. Urol. 2017;10:47–51. [Google Scholar]
- [25].Goodman N, Morden N, Chang C, Fisher E, Wennberg JD. Trends in Cancer Care Near the End of Life. The Dartmouth Altas Project; 2013:1–8. [PubMed] [Google Scholar]
- [26].Bergman J, Ballon-Landa E, Lorenz KA, et al. Community-Partnered Collaboration to Build an Integrated Palliative Care Clinic: The View From Urology. Am. J. Hosp. Palliat. Med. 2016;33:164–70. [DOI] [PubMed] [Google Scholar]
- [27].Earle CC, Neville BA, Landrum MB, et al. Evaluating claims-based indicators of the intensity of end-of-life cancer care. Int. J. Qual. Heal. Care 2005;17:505–9. [DOI] [PubMed] [Google Scholar]
- [28].Morden NE, Chang CH, Jacobson JO, et al. End-of-life care for medicare beneficiaries with cancer is highly intensive overall and varies widely. Health Aff 2012;31:786–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Barnato AE, Herndon MB, Anthony DL, et al. Are regional variations in end-of-life care intensity explained by patient preferences? a study of the US Medicare population. Med. Care 2007;45:386–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lilley EJ, Cooper Z, Schwarze ML, et al. Palliative care in surgery: defining the research priorities. J. Palliat. Med. 2017;20:702–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hua M, Li G, Clancy C, et al. Validation of the V66.7 code for palliative care consultation in a single academic medical center. J. Palliat. Med. 2017;20:372–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


