Abstract
BACKGROUND:
Patients with primary cutaneous melanoma are at increased risk for subsequent new primary melanomas. Indoor tanning is a recognized risk factor for melanoma. This study was aimed at determining the association between indoor tanning and the occurrence of multiple primary melanoma.
METHODS:
This was a retrospective case-control study of cases with multiple primary melanoma and sex-matched controls with single primary melanoma retrieved at a 1:2 ratio from the Biological Sample and Nevus Bank of the Melanoma Center of the University of Pittsburgh Cancer Institute. Logistic regression models were used to examine the association between multiple primary melanoma and risk factors.
RESULTS:
In total, 330 patients (39.1% men) with a median age of 51 years were enrolled. Compared with patients who had a single primary melanoma, patients with multiple melanomas were younger at the diagnosis of their first primary melanoma and were more likely to be discovered at stage 0 or I and to have had indoor tanning exposure, a family history of melanoma, atypical moles, dysplastic nevi, and a Breslow thickness less than 1 mm. Compared with patients’ first melanomas, subsequent melanomas were more likely to be thinner or in situ. The estimated probability of the locus for the second primary being the same as that for the first primary melanoma was 34%. In a multivariate analysis after adjustments for age, a family history of melanoma, the presence of atypical and dysplastic nevi, and recreational sun exposure, indoor tanning remained significantly associated with the occurrence of multiple primary melanoma (odds ratio, 2.75; 95% confidence interval, 1.07–7.08; P = .0356).
CONCLUSIONS:
Indoor tanning is associated with an increased risk of second primary melanoma. Subsequent melanomas are more likely to be thin or in situ and to occur in different anatomic locations.
Keywords: exposure, indoor tanning, melanoma, multiple primary
INTRODUCTION
Cutaneous melanoma is the fifth most common cancer in the United States and accounts for 5.6% of all new cancer cases. The incidence of melanoma has increased over the last 10 years, with an estimated 100,350 new cases in 2020 in the United States.1 Patients with melanoma are at increased risk of developing subsequent melanoma, and the risk is reported to be approximately 1% to 8% and highest within the first 2 years after the diagnosis of the first primary.2 The risk factors for cutaneous melanoma include, but are not limited to, phenotypic characteristics of skin such as a high density of nevi, red hair color, atypical nevi, and an inability to tan; ultraviolet radiation exposure; a family history of melanoma; and immunosuppression. The factors associated with the development of subsequent melanoma include dysplastic nevi, a family history of melanoma, mutations in CDKN2A, a high number of benign nevi, and an age older than 60 years at the diagnosis of the primary melanoma.3–7
Ultraviolet radiation from indoor tanning facilities is a risk factor for cutaneous melanoma and nonmelanoma skin cancers. Tanning bed use is more common among young White females.8 Although the percentage of indoor tanning adults has decreased in the United States, there is still a unique population of adolescents who have increased risk of exposure.9 Indoor tanners have a 20% to 75% increased risk of developing melanoma in comparison with never tanners.10 The risk increases even more with exposure at a younger age and with the use of tanning facilities for more than 10 years, for 50 or more hours, and for more than 100 sessions in a lifetime.11,12 Whether indoor tanning exposure increases the risk of subsequent melanoma as well has not been evaluated previously.
We conducted a case-control study to determine the association between indoor tanning exposure and multiple primary melanoma. We hypothesized that tanning bed exposure is associated with an increased risk of multiple primary melanoma. The secondary objectives of our study were to assess the clinical and pathological characteristics of primary and subsequent melanomas and the characteristics of melanoma in tanners.
MATERIALS AND METHODS
Data Source and Study Population
This study was approved by University of Pittsburgh Institutional Review Board. We conducted a retrospective case-control study in which cases with multiple primary melanoma and sex-matched controls with single primary melanoma were recruited from the Melanoma Center of the University of Pittsburgh Cancer Institute at a 1:2 ratio as part of the Human Biological Sample and Nevus Image Banking and Analysis Protocol within the time window of January 1996 to October 2019. This biospecimen and data banking study provides an organized resource of appropriately preserved and retrievable biological samples and digital images of nevi, melanoma, and other lesions for use in research. Patients who provided informed consent to be entered into this database were included in this study. Patient-reported information about a family history of melanoma, recreational sunbathing, indoor tanning exposure, and sunscreen use, clinical characteristics such as the stages and locations of primary and subsequent melanomas, and skin phenotype characteristics such as the skin type, number of benign nevi, and presence of atypical nevi were obtained from electronic medical records. The location of each melanoma was classified as head and neck, upper extremity, lower extremity, or trunk. To determine the dose-response effect, the indoor tanning exposure was recorded in 3 categories based on tanning bed sessions over a lifetime: never tanners, 10 sessions or fewer, and more than 10 sessions. The 10-session threshold was determined as a cutoff on the basis of the Australian Familial Melanoma Study, which showed that the risk of first primary melanoma was higher in patients with more than 10 sessions of sunbed exposure with odds ratios (ORs) of 1.41 and 2.01 for ever tanners and tanners with more than 10 sessions in comparison with nontanners, respectively.13
A family history of melanoma, recreational sunbathing, and sunscreen use were recorded as binary variables. Pathological characteristics were obtained from pathology reports. Clinical atypia was used to define an atypical mole, and architectural and cytological atypia was used to define a dysplastic nevus. Demographic data included the age at diagnosis of first melanoma and sex. Patients with multiple primary melanoma were defined as those with 2 or more primary melanomas according to the pathology reports. The diagnosis time for first and subsequent melanomas was recorded, and the time to second melanoma was defined as the timeline between the diagnoses of first and second melanomas. Melanomas that were diagnosed within 30 days of each other were described as synchronous. The follow-up time for patients with a single primary was calculated from the diagnosis time of the melanoma to either the time of death or last follow-up.
Statistical Analysis
Continuous variables were summarized as medians and interquartile ranges (IQRs), and categorical variables were summarized as frequencies and percentages at each level. These variables were compared between single primary melanoma and multiple primary melanoma with the chi-square test for categorical data and with the Wilcoxon rank sum test for age. For comparisons between the first primary and the second primary, the sign test was used for stage, which was an ordinal variable, and the 2-sample t test was used for continuous variables. For comparison between tanners and nontanners, the chi-square test (or Fisher exact test if cell counts were small) was used for categorical data; the Wilcoxon rank sum test was used for continuous data such as age. Univariate logistic regression models were used to examine the association between multiple primary melanoma and risk factors. Multivariate logistic regression models were used to study the effect of indoor tanning on multiple primary melanoma after adjustments for age, a family history of melanoma, the presence of atypical and dysplastic nevi, and recreational sun exposure. The effect of tanning bed exposure on the time to the second primary melanoma was tested with Cox proportional hazards models. ORs and 95% confidence intervals (CIs) were reported. All tests were 2-sided with α = .05. Data were analyzed with SAS 9.4 (SAS Institute, Inc, Cary, North Carolina).
RESULTS
Patient Characteristics
A total of 330 patients were enrolled: 110 patients with multiple primary melanoma and 220 patients with single primary melanoma who were sex-matched. The baseline characteristics of the patients are summarized in Table 1. The median age at the diagnosis of melanoma was 51 years (IQR, 40–61 years). Patients with multiple melanomas were younger at the diagnosis of their first melanoma. The median age at the time of diagnosis was 52 years (IQR, 43–62 years) for the single melanoma group and 46 years (IQR, 34–58 years) for the multiple melanoma group; 129 of the patients (39%) were male. Male patients were older than female patients at the time of the diagnosis of their first melanoma. The majority of the patients had Fitzpatrick skin type I or II and were discovered at stage I. Twenty-five (21%) had at least 1 exposure to indoor tanning, with 17% reporting more than 10 sessions; 37 (11%) reported a family history of melanoma.
TABLE 1.
Baseline Characteristics of the Patients (n = 330)
| Variable | Value |
|---|---|
|
| |
| Age, median (IQR), y | 51 (40–61) |
| Stage, No. (%) | |
| 0 | 14 (5) |
| I | 158 (51) |
| II | 85 (27) |
| III | 51 (16) |
| IV | 3 (1) |
| Skin type, No. (%) | |
| I | 87 (26) |
| II | 203 (62) |
| III | 38 (12) |
| IV | 2 (1) |
| Indoor tanning, No. (%) | |
| >10 sessions | 57 (17) |
| No | 258 (79) |
| ≤10 sessions | 13 (4) |
| Location, No. (%) | |
| UE | 78 (24) |
| LE | 89 (27) |
| Trunk | 124 (38) |
| HN | 37 (11) |
| FH of melanoma, No. (%) | |
| Yes | 37 (11) |
| No | 293 (89) |
| Atypical moles, No. (%) | |
| Yes | 70 (21) |
| No | 260 (79) |
| Breslow thickness, No. (%) | |
| <1 mm | 136 (42) |
| 1 to <2 mm | 93 (29) |
| 2 to <4 mm | 54 (17) |
| ≥4 mm | 41 (13) |
| Dysplastic nevi, No. (%) | |
| Yes | 35 (11) |
| No | 295 (89) |
| Benign nevi, No. (%) | |
| >15 | 144 (44) |
| <15 | 186 (56) |
| Recreational sunbathing, No. (%) | |
| Yes | 245 (74) |
| No | 85 (26) |
| Sunscreen use, No. (%) | |
| Yes | 131 (40) |
| No | 30 (9) |
| Irregular | 169 (51) |
| BRAF, No. (%) | |
| Wild | 45 (65) |
| Mutated | 24 (35) |
Abbreviations: FH, family history; HN, head and neck; IQR, interquartile range; LE, lower extremity; UE, upper extremity.
Melanoma Characteristics
The median time between the diagnoses of first and second primary melanomas was 13 months. The number of subsequent melanomas ranged from 1 to 6. Twenty-five patients with multiple primary melanoma (22.7%) had 3 or more primary melanomas. Fifty-three of the subsequent melanomas (48.2%) occurred within the first year after the diagnosis of the first primary. Nineteen patients (5.8%) had synchronous melanoma. The most common location for a second primary melanoma was the trunk, which was followed by a lower extremity.
Comparison of Patients With Single Primary Melanoma and Patients With Multiple Primary Melanoma
In comparison with patients who had a single primary melanoma, patients with multiple melanomas were more likely to be discovered at stage 0 or I (68% vs 49%; P < .0001) and to have had a family history of melanoma (18% vs 8%; P = .0045), indoor tanning exposure (34% vs 10%; P < .0001), atypical moles (37% vs 13%; P < .0001), dysplastic nevi (21% vs 5%; P < .0001), and a Breslow thickness less than 1 mm (61% vs 33%; P < .0001; Table 2). We further stratified patients into 2 cohorts (age < 60 years and age ≥ 60 years). Among patients younger than 60 years, a comparison of patients who had a single primary melanoma and patients who had multiple melanomas showed that these second primary melanomas were more likely to be discovered at stage 0 or I (72% vs 50%; P < .0001) and to have occurred among patients who had indoor tanning exposure (41% vs 21%; P < .0001), a family history of melanoma (19% vs 8%; P = .0199), atypical moles (41% vs 16%; P < .0001), dysplastic nevi (22% vs 8%; P = .0024), and a Breslow thickness less than 1 mm (65% vs 37%; P = .0003). Among patients who were 60 years old or older, a comparison of patients who had a single primary melanoma and patients with multiple melanomas showed that the patients with multiple primaries were more likely to have had indoor tanning exposure (16% vs 0%; P < .0041) and dysplastic nevi (16% vs 1%; P < .0041; see Supporting Table 1). Because of the small sample size, these findings will need to be confirmed in larger studies. A family history of melanoma, atypical moles, and melanoma with a Breslow thickness less than 1 mm were more common in patients with multiple primary ones but did not reach statistical significance, perhaps because of the low sample size. The median follow-up times for patients with single primary melanoma and patients with multiple primary melanoma were 65 and 60 months, respectively.
TABLE 2.
Comparisons of Single Primary Melanoma and Multiple Primary Melanoma
| Variable | Single Primary Melanoma (n = 220) | Multiple Primary Melanoma (n = 110) | P |
|---|---|---|---|
|
| |||
| Age, y | .0060 | ||
| Mean (SD) | 51 (15) | 47 (15) | |
| Median (IQR) | 52 (43–62) | 46 (34–58) | |
| Sex, No. (%) | |||
| Male | 86 (39) | 43 (39) | |
| Female | 134 (61) | 67 (61) | |
| Stage, No. (%) | <.0001 | ||
| 0 | 0 (0) | 14 (13) | |
| I | 101 (49) | 57 (55) | |
| II | 63 (31) | 22 (21) | |
| III | 40 (19) | 11 (11) | |
| IV | 2 (1) | 1 (1) | |
| Skin type, No. | .2564 | ||
| (%) | |||
| I | 57 (26) | 30 (28) | |
| II | 131 (60) | 72 (66) | |
| III | 30 (14) | 8 (7) | |
| IV | 2 (1) | 0 (0) | |
| Indoor tanning, No. (%) | <.0001 | ||
| >10 sessions | 21 (10) | 36 (34) | |
| No | 188 (85) | 70 (64) | |
| ≤10 sessions | 11 (5) | 2 (2) | |
| Location, No. (%) | .5906 | ||
| UE | 55 (25) | 23 (21) | |
| LE | 55 (25) | 34 (31) | |
| Trunk | 86 (39) | 38 (36) | |
| HN | 24 (11) | 13 (12) | |
| FH of mela-noma, No. (%) | .0045 | ||
| Yes | 17 (8) | 20 (18) | |
| No | 203 (92) | 90 (82) | |
| Atypical moles, No. (%) | <.0001 | ||
| Yes | 29 (13) | 41 (37) | |
| No | 191 (87) | 69 (63) | |
| Breslow thickness, No. (%) | <.0001 | ||
| <1 mm | 73 (33) | 63 (61) | |
| 1 to <2 mm | 69 (31) | 24 (23) | |
| 2 to <4 mm | 43 (20) | 11 (11) | |
| ≥4 mm | 35 (16) | 6 (6) | |
| Dysplastic nevi, No. (%) | <.0001 | ||
| Yes | 12 (5) | 23 (21) | |
| No | 208 (95) | 87 (79) | |
| Benign nevi, No. (%) | <.0001 | ||
| >15 | 117 (53) | 27 (25) | |
| <15 | 103 (47) | 83 (75) | |
| Recreational sunbathing, No. (%) | .0018 | ||
| Yes | 175 (80) | 70 (64) | |
| No | 45 (20) | 40 (36) | |
| Sunscreen use, No. (%) | .0011 | ||
| Yes | 77 (35) | 54 (50) | |
| No | 15 (7) | 15 (14) | |
| Irregular | 128 (58) | 41 (37) | |
| BRAF, No. (%) | .5918 | ||
| Wild | 29 (63) | 16 (68) | |
| Mutated | 17 (37) | 7 (32) | |
Abbreviations: FH, family history; HN, head and neck; IQR, interquartile range; LE, lower extremity; UE, upper extremity.
Association Between Indoor Tanning Exposure and Multiple Primary Melanoma
In the univariate analysis, tanning bed use was associated with an increased risk of a second primary melanoma (OR, 3.19; 95% CI, 1.85–5.50; P < .0001; Table 3). Other significant factors included the presence of atypical and dysplastic nevi, a family history of melanoma, and more than 15 benign nevi (Table 4). In the multivariate analysis, after adjustments for age, a family history of melanoma, the presence of atypical and dysplastic nevi, and recreational sun exposure, indoor tanning remained significantly associated with multiple primary melanoma (OR, 2.75; 95% CI, 1.07–7.08; P = .0356). Lifetime tanning bed exposure of more than 10 sessions was associated with an increased risk of a second primary melanoma (OR in univariate analysis, 4.60; 95% CI, 2.52–8.42; P < .0001; OR in multivariate analysis, 4.32; 95% CI, 1.54–12.15; P = .0026).
TABLE 3.
Indoor Tanning Association With Multiple Primary Melanoma
| Indoor Tanning | OR (95% CI) | P |
|---|---|---|
|
| ||
| Yes/no | ||
| Univariate analysis | 3.19 (1.85–5.50) | <.0001 |
| Multivariate analysis | 2.75 (1.07–7.08) | .0356 |
| >10 sessions/no | ||
| Univariate analysis | 4.60 (2.52–8.42) | <.0001 |
| Multivariate analysis | 4.32 (1.54–12.15) | .0026 |
Abbreviations: CI, confidence interval; OR, odds ratio.
TABLE 4.
Risk Factors Associated With Multiple Primary Melanoma
| Factor | OR (95% CI) | P |
|---|---|---|
|
| ||
| Age | 0.99 (0.97–1.02) | .6783 |
| Indoor tanning | 4.32 (1.54–12.15) | .0026 |
| FH of melanoma | 2.71 (1.36–5.43) | .0048 |
| Dysplastic nevus | 4.43 (2.10–9.36) | .0001 |
| Atypical nevi | 4.03 (2.32–6.99) | <.0001 |
| Benign nevi | 2.75 (1.19–6.33) | .0179 |
| Recreational sunbathing | 0.37 (0.09–1.53) | .1705 |
| Sunscreen use | 0.05 (0.01–0.38) | .0227 |
| Blistering sunburn | 13.95 (7.69–25.32) | <.0001 |
Abbreviations: CI, confidence interval; FH, family history; OR, odds ratio.
Primary Melanoma Versus Subsequent Melanoma
Compared with patients’ first melanomas, subsequent melanomas were more likely to be thinner (0.6 vs 1.2 mm; P = .0007) or in situ (24% vs 13%; P = .0004; Table 5). The most common sites for a second primary melanoma were an upper extremity (34%) and then the trunk (29%). The estimated probability of a melanoma locus that was the same for the first and second primary melanomas was 34% with an exact 95% CI (25%–44%).
TABLE 5.
Comparison of First and Subsequent Primaries
| Variable | First Primary | Second Primary | P |
|---|---|---|---|
|
| |||
| Stage, No. (%) | |||
| 0 | 14 (13) | 25 (24) | .0004 |
| I | 57 (54) | 67 (64) | |
| II | 22 (21) | 9 (9) | |
| III | 11 (10) | 3 (3) | |
| IV | 1 (1) | 1 (1) | |
| Breslow thickness, mean (SD), mm | 1.2 (1.5) | 0.6 (0.8) | .0007 |
| Location, No. (%) | |||
| UE | 23 (21) | 36 (33) | The probability of the same location is 34% with an exact 95% CI (25%-44%). |
| LE | 34 (31) | 25 (23) | |
| Trunk | 38 (35) | 31 (29) | |
| HN | 13 (12) | 15 (14) | |
| Vulva | 0 (0) | 1 (1) | |
Abbreviations: CI, confidence interval; HN, head and neck; LE, lower extremity; UE, upper extremity.
Tanners Versus Nontanners
The prevalence of tanning bed exposure was highest in patients younger than 30 years, and indoor tanners were younger at the time of the diagnosis of their first primary melanoma (Fig. 1). Females were more likely to have indoor tanning exposure than males (86% vs 14%; P < .0001). In the whole cohort, tanners were more likely to be diagnosed at stage 0 or I, have atypical moles, have a melanoma with a Breslow thickness less than 1 mm, and have a melanoma located on the trunk (not statistically significant). Synchronous melanoma and more than 1 second primary melanoma were more prevalent among tanners (Table 6). The median time for the development of subsequent melanoma was 14 months (IQR, 5–84 months) for nontanners and 11 months (IQR, 1–34 months) for tanners. Tanning bed exposure was associated with decreased time to the development of subsequent melanoma (hazard ratio, 1.511; 95% CI, 1.003–2.276; P = .0485; Fig. 2).
Figure 1.
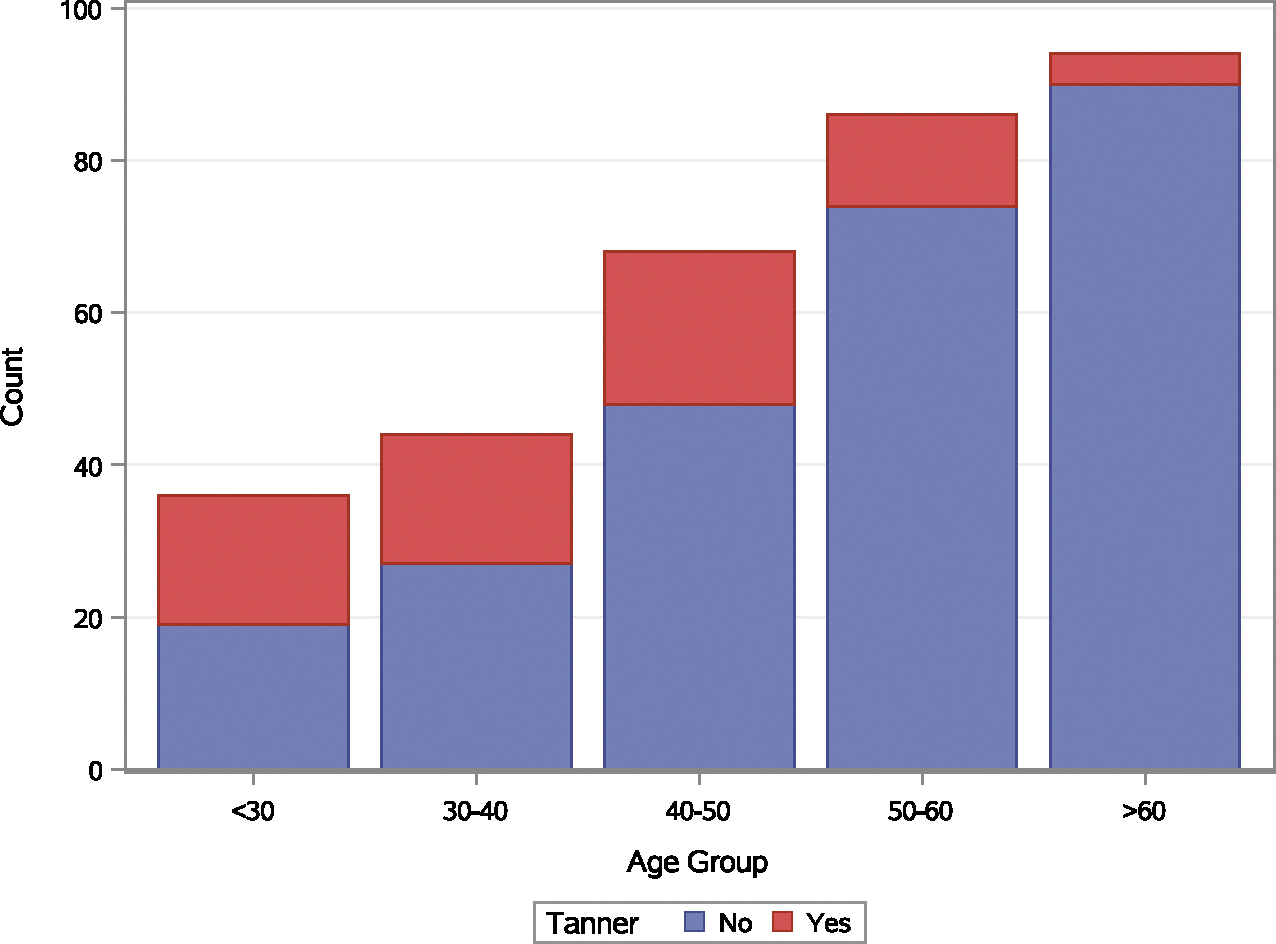
Age of primary melanoma by age group and tanning bed exposure.
TABLE 6.
Demographic, Clinical, and Pathological Characteristics of Melanoma Based on Tanning Bed Exposure
| Variable, No. (%) | Tanners (n = 70) | Nontanners (n = 258) | P |
|---|---|---|---|
|
| |||
| Age, median (IQR), y | 40 (30–49) | 53 (44–63) | <.0001 |
| Stage, No. (%) | .0040 | ||
| 0 | 5 (7) | 7 (3) | |
| I | 44 (64) | 114 (48) | |
| II | 7 (10) | 78 (33) | |
| III | 12 (17) | 39 (16) | |
| IV | 1 (1) | 2 (1) | |
| Sex, No. (%) | <.0001 | ||
| Female | 60 (86) | 139 (54) | |
| Male | 10 (14) | 119 (46) | |
| Location, No. (%) | .0664 | ||
| UE | 8 (12) | 70 (27) | |
| LE | 24 (35) | 65 (25) | |
| Trunk | 29 (42) | 93 (36) | |
| HN | 8 (12) | 29 (11) | |
| Atypical moles, No. (%) | <.0001 | ||
| Yes | 30 (43) | 40 (16) | |
| No | 40 (57) | 218 (84) | |
| Breslow thickness, No. (%) | <.0001 | ||
| <1 mm | 47 (67) | 87 (35) | |
| 1 to <2 mm | 14 (20) | 79 (31) | |
| 2 to <4 mm | 7 (10) | 47 (19) | |
| ≥4 mm | 2 (3) | 39 (15) | |
| Dysplastic nevi, No. (%) | .2251 | ||
| Yes | 10 (14) | 24 (9) | |
| No | 60 (86) | 234 (91) | |
| Benign nevi, No. (%) | <.0001 | ||
| >15 | 16 (23) | 128 (50) | |
| <15 | 54 (77) | 130 (50) | |
| Mutated (yes) | 4 (6) | 20 (8) | |
| Ulceration, No. (%) | <.0001 | ||
| Yes | 6 (9) | 53 (21) | |
| No | 64 (91) | 199 (79) | |
| Synchronous MPM, No. (%) | .0020 | ||
| Yes | 8 (20) | 11 (6) | |
| No | 32 (80) | 188 (94) | |
| >1 second primary, No. (%) | <.0001 | ||
| Yes | 13 (29) | 13 (6) | |
| No | 32 (71) | 188 (94) | |
| Breslow thickness of second primary, No. (%) | .6666 | ||
| <1 mm | 29 (76) | 52 (78) | |
| 1 to <2 mm | 8 (21) | 10 (15) | |
| 2 to <4 mm | 1 (2) | 3 (4) | |
| ≥4 mm | 0 (0) | 2 (3) | |
| Stage of second primary, No. (%) | .1516 | ||
| 0 | 13 (34) | 13 (19) | |
| I | 19 (50) | 49 (70) | |
| II | 4 (11) | 6 (9) | |
| III | 2 (5) | 1 (1) | |
| IV | 0 (0) | 1 (1) | |
Abbreviations: HN, head and neck; IQR, interquartile range; LE, lower extremity; MPM, multiple primary melanoma; UE, upper extremity.
Figure 2.
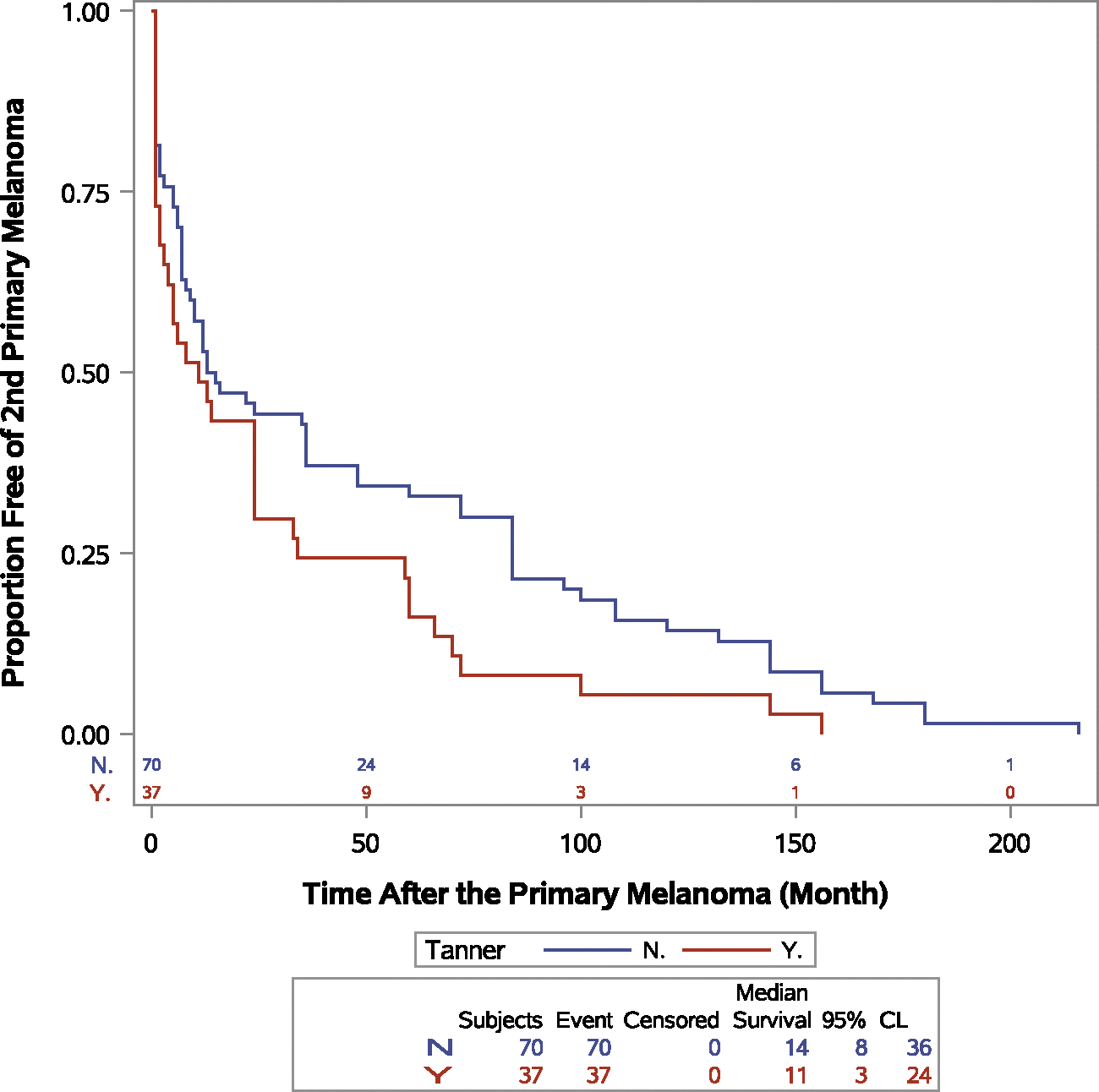
Event-free probability for the second primary melanoma by tanning bed exposure (P = .0485). CL indicates confidence level; N, no; Y, yes.
DISCUSSION
Patients with melanoma have an increased risk of developing a second primary during their lifetime. With the increasing incidence of cutaneous melanoma and the improved overall survival of patients, the risk of a second primary is likely to further increase.14,15 Indoor tanning is extensively used in the United States, especially among young adults, despite being a well-established risk factor for primary cutaneous melanoma. The Food and Drug Administration classifies indoor tanning devices as class II medical devices.16
In this study, we aimed to determine the association between indoor tanning and the occurrence of multiple primary melanoma. We included cases with multiple primary melanoma and controls who had a single primary melanoma. Patients with multiple melanoma, compared with patients with a single primary melanoma, were younger at the diagnosis of their first melanoma and were more likely to be discovered at stage 0 or I and to have had indoor tanning exposure, atypical moles, dysplastic nevi, and a Breslow thickness less than 1 mm. The prevalence of a positive family history was higher in patients with multiple primary melanoma. With further stratification by age, the difference remained statistically significant in patients younger than 60 years at the diagnosis of first melanoma. Two patients at the ages of 23 and 25 years with a family history of melanoma had a CDKN2A mutation. The risk for subsequent melanomas was higher in younger patients with a family history of melanoma, and this raises the consideration of genetic counseling referral for these patients.17
Indoor tanning is associated with an increased risk of second primary melanoma, and the risk is higher with a lifetime tanning bed exposure of more than 10 sessions. Atypical and dysplastic nevi, a family history of melanoma, and a high number of benign nevi were other significant risk factors as described in previous studies.5,18 The previous studies evaluating risk factors for multiple primary melanoma report conflicting results regarding tanning bed use. Solarium indoor tanning use was reported not to be associated with multiple primary melanoma in a retrospective case-control study conducted in Austria. Notably, patients in that study reported external risk factors related to sun exposure such as outdoor occupation and time spent outside at leisure that were also not significant.19 In contrast, Li et al20 reported a short median duration of second primary development in tanning bed users (7.5 vs 42 months; P = .027) and a higher percentage with a second primary within the first year after the diagnosis of early melanoma (67% vs 28%; P = .011). In our study, 48.2% of the patients developed subsequent melanoma within the first year of the diagnosis. One patient had 4 primary melanomas, with the last one diagnosed 28 years after the first primary; this highlights the importance of continuous follow-up examinations. The Surveillance, Epidemiology, and End Results database review by Bradford et al21 showed that the relative risk of subsequent melanoma continues to remain high even more than 20 years after the first primary melanoma diagnosis. The Melanoma Institute of Australia established a risk prediction model with a Harrell C statistic of 0.73 for the development of subsequent melanoma. The model includes sex, age at first primary melanoma, previous keratinocyte cancer, family history of melanoma, outdoor recreational activities, skin color, nevus density, ability to tan, polygenic risk score, CDKN2A mutation, site, and histological subtype.22 The model has not been validated yet in other populations. The study highlights the difference in the risk of developing subsequent melanoma based on individuals’ cumulative risk factors. However, this study did not include information about tanning bed exposure in the questionnaire. One explanation for this could be that since 2015 there has been a complete ban on indoor tanning use in Australia.
Second primary melanomas were thinner, as reported in previous studies.4,21,23 This is likely due to increased patient awareness about the disease, risk factors, and protective strategies and adherence to strict follow-up visit schedules at the Melanoma Center of the University of Pittsburgh Cancer Institute. The latter may not be a consistent pattern at all outpatient cancer clinics. Subsequent primaries were more likely to be diagnosed in different anatomical locations, and this highlights the importance of a total body skin examination and the utility of total body photography. Second primaries were more likely to occur in upper extremities, which were followed by the trunk. Other studies have reported lower extremities and the head and neck area as being the most common sites for second primary melanoma.24,25
The tanning bed exposure frequency was higher among females and younger adults, and this was consistent with previously reported data.26,27 The compliance with indoor tanning legislation remains an important issue. The mean compliance with age restrictions and warning labels was 65% and 44%, respectively.28 Our study demonstrated a decreased median time to the development of the second primary in tanners.
The strengths of our study include the robust clinical data set collected as part of the Human Biological Sample and Nevus Image Banking and Analysis Study and the long follow-up of the control group, which captured both melanoma in situ and invasive melanoma in patients. The limitations of our study include its retrospective design, its derivation from a single melanoma center, and a possible recall bias due to the accuracy of indoor tanning usage reporting by patients. Details regarding the use of protective strategies were not collected in this study.
In conclusion, indoor tanning is strongly associated with an increased risk of second primary melanoma. Subsequent melanomas are more likely to be thin or in situ and to occur in different anatomic locations. Our findings highlight the importance of avoiding indoor tanning use and regular follow-up visits, especially for patients who have other established risk factors for subsequent melanoma. Current guidelines for the follow-up of patients with invasive melanoma are tailored to discover recurrent local-regional and distant metastatic disease without discussion about the development of subsequent melanoma. Furthermore, there are no established follow-up guidelines for patients with melanoma in situ. Indeed, Pomerantz et al29 showed that after 10 years of follow-up, patients with melanoma in situ were more likely to develop subsequent invasive melanomas than those with initial invasive melanoma. The question remains to be answered whether follow-up visits should be modified among these patients with increased numbers of visits and with closer surveillance for new lesions as well as a lower threshold for performing biopsies of new lesions.
Supplementary Material
Footnotes
CONFLICT OF INTEREST DISCLOSURES
John M. Kirkwood reports consultancy for Bristol-Myers Squibb, Checkmate, Novartis, and Amgen and research support from Bristol-Myers Squibb, Amgen, Checkmate, Castle Biosciences, Immunocore LLC, Iovance, and Novartis. The other authors made no disclosures.
Additional supporting information may be found in the online version of this article.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Ferrone CR, Ben Porat L, Panageas KS, et al. Clinicopathological features of and risk factors for multiple primary melanomas. JAMA. 2005;294:1647–1654. [DOI] [PubMed] [Google Scholar]
- 3.Berwick M, Orlow I, Hummer AJ, et al. The prevalence of CDKN2A germ-line mutations and relative risk for cutaneous malignant melanoma: an international population-based study. Cancer Epidemiol Biomarkers Prev. 2006;15:1520–1525. [DOI] [PubMed] [Google Scholar]
- 4.Hwa C, Price LS, Belitskaya-Levy I, et al. Single versus multiple pri-mary melanomas. Cancer. 2012;118:4184–4192. [DOI] [PubMed] [Google Scholar]
- 5.Titus-Ernstoff L, Duray PH, Ernstoff MS, Barnhill RL, Horn PL, Kirkwood JM. Dysplastic nevi in association with multiple primary melanoma. Cancer Res. 1988;48:1016–1018. [PubMed] [Google Scholar]
- 6.Titus-Ernstoff L, Perry AE, Spencer SK, et al. Multiple primary mela-noma: two-year results from a population-based study. Arch Dermatol. 2006;142:433–438. [DOI] [PubMed] [Google Scholar]
- 7.Olsen CM, Pandeya N, Thompson BS, et al. Association between phenotypic characteristics and melanoma in a large prospective cohort study. J Invest Dermatol. 2019;139:665–672. [DOI] [PubMed] [Google Scholar]
- 8.Le Clair MZ, Cockburn MG. Tanning bed use and melanoma: estab-lishing risk and improving prevention interventions. Prev Med Rep. 2016;3:139–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guy GP Jr, Watson M, Seidenberg AB, Hartman AM, Holman DM, Perna F. Trends in indoor tanning and its association with sunburn among US adults. J Am Acad Dermatol. 2017;76:1191–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boniol M, Autier P, Boyle P, Gandini S. Cutaneous melanoma attributable to sunbed use: systematic review and meta-analysis. BMJ. 2012;345:e4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lazovich D, Vogel RI, Berwick M, Weinstock MA, Anderson KE, Warshaw EM. Indoor tanning and risk of melanoma: a case-control study in a highly exposed population. Cancer Epidemiol Biomarkers Prev. 2010;19:1557–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallagher RP, Spinelli JJ, Lee TK. Tanning beds, sunlamps, and risk of cutaneous malignant melanoma. Cancer Epidemiol Biomarkers Prev. 2005;14:562–566. [DOI] [PubMed] [Google Scholar]
- 13.Cust AE, Armstrong BK, Goumas C, et al. Sunbed use during adolescence and early adulthood is associated with increased risk of early-on-set melanoma. Int J Cancer. 2011;128:2425–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381:1535–1546. [DOI] [PubMed] [Google Scholar]
- 15.Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:472–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Food and Drug Administration. Indoor tanning raises risk of melanoma: FDA strengthens warnings for sunlamp products [press release]. Published May 2014. Accessed September 01, 2020. https://www.fda.gov/radiation-emittingproducts/radiation-emitting-products-and-procedures/tanning
- 17.Chen T, Fallah M, Försti A, Kharazmi E, Sundquist K, Hemminki K. Risk of next melanoma in patients with familial and sporadic melanoma by number of previous melanomas. JAMA Dermatol. 2015;151:607–615. [DOI] [PubMed] [Google Scholar]
- 18.Siskind V, Hughes MCB, Palmer JM, et al. Nevi, family history, and fair skin increase the risk of second primary melanoma. J Invest Dermatol. 2011;131:461–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Müller C, Wendt J, Rauscher S, et al. Risk factors of subsequent primary melanomas in Austria. JAMA Dermatol. 2019;155:188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Kulkarni M, Trinkaus K, Cornelius LA. Second primary melanomas: increased risk and decreased time to presentation in patients exposed to tanning beds. J Am Acad Dermatol. 2018;79:1101–1108. [DOI] [PubMed] [Google Scholar]
- 21.Bradford PT, Freedman DM, Goldstein AM, Tucker MA. Increased risk of second primary cancers after a diagnosis of melanoma. Arch Dermatol. 2010;146:265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cust AE, Badcock C, Smith J, et al. A risk prediction model for the development of subsequent primary melanoma in a population-based cohort. Br J Dermatol. 2020;182:1148–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Savoia P, Quaglino P, Verrone A, Bernengo MG. Multiple primary melanomas: analysis of 49 cases. Melanoma Res. 1998;8:361–366. [DOI] [PubMed] [Google Scholar]
- 24.Moore MM, Geller AC, Warton EM, Schwalbe J, Asgari MM. Multiple primary melanomas among 16,570 patients with melanoma diagnosed at Kaiser Permanente Northern California, 1996 to 2011. J Am Acad Dermatol. 2015;73:630–636. [DOI] [PubMed] [Google Scholar]
- 25.Menzies S, Barry R, Ormond P. Multiple primary melanoma: a single centre retrospective review. Melanoma Res. 2017;27:638–640. [DOI] [PubMed] [Google Scholar]
- 26.Falzone AE, Brindis CD, Chren MM, et al. Teens, tweets, and tanning beds: rethinking the use of social media for skin cancer prevention. Am J Prev Med. 2017;53(3 suppl 1):S86–S94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noar SM, Myrick JG, Morales-Pico B, Thomas NE. Development and validation of the Comprehensive Indoor Tanning Expectations Scale. JAMA Dermatol. 2014;150:512–521. [DOI] [PubMed] [Google Scholar]
- 28.Reimann J, McWhirter JE, Papadopoulos A, Dewey C. A systematic review of compliance with indoor tanning legislation. BMC Public Health. 2018;18:1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pomerantz H, Huang D, Weinstock MA. Risk of subsequent melanoma after melanoma in situ and invasive melanoma: a population-based study from 1973 to 2011. J Am Acad Dermatol. 2015;72:794–800. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


