Abstract
Objective
The clinical evidence on the management for congenital pseudoarthrosis of the tibia (CPT) in adults is limited. The aim of this study is to assess the functional and radiological outcomes of Ilizarov distraction for treating CPT in adults.
Methods
A retrospective analysis was conducted. Between 2013 and 2022, an Ilizarov distraction technique was performed on 14 adults (14 limbs) with CPT in our limb deformity center. There were seven females and seven males with a mean age of 33.7 (range, 18 ~ 53) years. The diagnosis of NF‐1 was confirmed in seven (50.0%) patients. Eight patients had a history of previous surgical failure. The pseudoarthrosis occurred in the middle and lower tibia in all limbs (six left and eight right). The CPT was classified by Crawford classification and Paley classification. The surgical procedures, external fixation time (EFT), and all outcomes and complications were recorded. The Kolmogorov–Smirnov test was performed to test the normality of the data. The American Orthopedic Foot and Ankle Society (AOFAS) ankle‐hindfoot score at the preoperative and final follow‐up was compared by using the Wilcoxon's signed‐rank test. The limb‐length discrepancy (LLD) and a self‐made exercise capacity score at the preoperative and final follow‐up were compared by using the student's t‐test. The clinical and radiological outcomes were assessed by the Inan scale.
Results
The mean EFT of Ilizarov fixator was 19.5 months (range, 7.3 ~ 39.1). At a median follow‐up of 26.8 months (IQR, 20.2 ~ 34.3), bone union of the pseudarthrosis and consolidation of the distraction zone were achieved in all patients. The mean LLD was decreased from 11.3 cm (range, 3.4 ~ 17.3) preoperatively to 1.1 cm (range, 0.3 ~ 3.7) (p < 0.05). The mean or median AOFAS ankle‐hindfoot score was improved from 53.5 (IQR, 26.5 ~ 60.5) preoperatively to 63.9 (range, 53 to 73) at final follow‐up (p < 0.05). The mean score for exercise capacity were improved from 4.9 (range, 1 to 8) preoperatively to 9.6 (range, 7 ~ 12) at final follow‐up (p < 0.05). According to the criteria described by Inan et al., the clinical results were classified as good in 10 and fair in 4, while the radiological results were classified as excellent in three, good in 8, and fair in 2. The success rate was 92.9%, as refracture was defined as treatment failure and occurred in one patient.
Conclusion
Ilizarov distraction provided a suitable treatment option for the CPT in adults, as it could achieve a high rate of bone union, a good correction of secondary deformity, a low risk of refracture, and consequently restore a relatively functional limb.
Keywords: Bone union, Congenital pseudoarthrosis of the tibia, Deformity correction, Ilizarov distraction
Ilizarov distraction could obtain excellent deformity correctio, solid bone union, and low refracture risk for treating the congenital pseudoarthrosis of the tibia in adults.
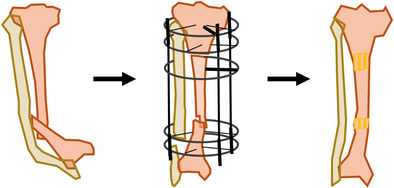
Introduction
Congenital pseudoarthrosis of the tibia (CPT) is a rare disease with an incidence of between 1:140,000 and 1:250,000 live births 1 which is characterized by angulation of the tibia, shortening of the limb, spontaneous fracture, non‐union, and high refracture rate after bone healing. The exact etiology of CPT is not well understood, although approximately 50% to 90% of patients with CPT were reported to be associated with neurofibromatosis‐1 (NF‐1). 2 , 3 Histological analysis of CPT has shown an abnormally thickening periosteum surrounding the pseudoarthrosis, accompanied by the presence of fibrous hamartoma tissue with fibroblasts. 4 , 5 Recent studies have demonstrated that the decreased osteogenic differentiation ability of periosteal mesenchymal stem cells is one of the main pathological mechanisms resulting in recalcitrant non‐union of the tibia, pathological fractures, and pseudarthrosis in CPT. 6 , 7 , 8 Due to this unique and complex pathology, the management of CPT remains one of the most intractable and serious issues for orthopedic surgeons.
It has been reported that four mainstream treatments (rodding, Ilizarov, Ilizarov combined with rodding, and free vascularized fibula graft) of CPT achieved primary union without refracture in only approximately 50.7% (12% to 80%) of children, and thus the orthopedic community has recognized that the treatment of CPT is fraught with complications and therapeutic failure is common. 9 , 10 , 11 , 12 , 13 , 14 However, the vast majority of research and practice have focused on children, and little attention so far has been paid to the management in adults and the evidence concerning optimal treatment strategies for adults with CPT is limited. 15 , 16 , 17 Furthermore, compared to children, the situation may be more complex and even worse in adults with CPT due to the long‐term deformity progression of the tibia. For adults with CPT, beside the presence of pseudoarthrosis, there are more significant limb‐length discrepancy (LLD) and secondary deformities of the tibia and ankle in the affected limb due to the normally developing of the unaffected limb. 18 The correction of these deformities is difficult to accomplish with some treatments alone, such as intramedullary nail fixation or free vascularized fibula graft. In addition, the condition of the surrounding soft tissue is usually poor due to the long‐term presence of asymmetric deformities and a history of failed multiple surgeries, and thus acute deformity correction has a high possibility of soft tissue complications. Some authors even considered amputation as an essential and reasonable treatment option for CPT. 19 , 20 , 21 However, amputation was usually unattractive for patients, especially those in China. For these patients, the aims of treatment for CPT are still as much as possible to achieve bony union of pseudarthrosis and to correct limb malalignment and LLD, and thus to ensure a limb with maximal function and minimal interventions. 2 , 9 , 22 , 23
Ilizarov distraction, as a powerful technique for managing bone loss or limb deformity with serious soft‐tissue contracture, has achieved remarkable success in the treatment of CPT in children. 4 , 24 , 25 , 26 , 27 Thus, we hypothesized that, for adults with CPT, Ilizarov distraction could achieve bony union of pseudarthrosis, correct limb malalignment and length discrepancy, and thus obtain a functional limb. The aim of this study is: (i) to explore the optimal treatment for CPT in adults; (ii) to evaluate the functional and radiological outcomes of Ilizarov distraction for treating CPT in adults; and (iii) to provide Chinese experience of Ilizarov distraction for clinicians around the world in the field of Ilizarov technique.
Patients and Methods
Approved by the Ethics Committee on Human Research of West China hospital (No. 202407), we performed a retrospective study of adult patients with CPT who were treated by Ilizarov distraction in our limb deformity center between 2013 and 2022. Exclusion criteria were age at surgery <18 years, serious neurovascular compromise, less than 1‐year follow‐up, and incomplete clinical information. Finally, as shown in Table 1, a total of 14 patients (14 limbs) with a mean age of 33.7 (range, 18 ~ 53) years were included. There were seven females and seven males. The diagnosis of NF‐1 was confirmed in seven (50.0%) patients. Eight patients had a history of previous surgical failure. The pseudoarthrosis occurred in the middle and lower tibia in all limbs (six left and eight right). The clinical presentation of CPT were classified as Crawford type IV in all patients. 28 According to the Paley classification, there were four with type 3, four with type 4A, five with type 4B, and one with type 4C. 13
TABLE 1.
Descriptive characteristics of patients.
| Case No. | Age at surgery (year) | Sex (F/M) | Side (L/R) | No. previous surgeries | NF‐1 (Y/N) | Crawford classification | Paley classification | Additional procedures | Follow‐up (m) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 39 | F | L | 0 | Y | IV | 4A | Secondary arthrolysis for knee; Secondary bone grafting | 30.4 |
| 2 | 53 | F | L | 2 | N | IV | 4C | Fibular grafting; Secondary bone grafting | 91.8 |
| 3 | 29 | F | R | 2 | N | IV | 4A | Sliding osteotomy; Correction for the equinus deformity | 62.1 |
| 4 | 39 | F | R | 1 | N | IV | 3 | Fibular ORIF | 58.6 |
| 5 | 19 | M | R | 1 | N | IV | 4B | Correction for the femur and foot; Secondary arthrolysis for knee; Secondary ORIF with bone grafting | 31.8 |
| 6 | 23 | F | R | 2 | Y | IV | 4B | Secondary arthrolysis for knee; Secondary ORIF with bone grafting | 21.3 |
| 7 | 30 | M | R | 1 | N | IV | 3 | Secondary correction for the ankle | 20.2 |
| 8 | 18 | M | L | 0 | Y | IV | 4B | / | 23.1 |
| 9 | 36 | M | L | 0 | Y | IV | 3 | Correction for the equinus deformity | 15.4 |
| 10 | 48 | M | L | 2 | N | IV | 3 | Correction for the proximal tibia and foot | 19.8 |
| 11 | 36 | F | R | 0 | N | IV | 4A | Correction for the equinus deformity | 20.8 |
| 12 | 27 | F | R | 1 | Y | IV | 4B | Sliding osteotomy; Secondary correction for the foot | 34.3 |
| 13 | 43 | M | R | 0 | Y | IV | 4B | Correction for the ankle; Secondary ORIF with bone grafting | 17.6 |
| 14 | 32 | M | L | 0 | Y | IV | 4A | Sliding osteotomy; Secondary arthrolysis for knee; Secondary correction for the foot | 33.7 |
| Mean/Median | 33.7 | N/A | N/A | 1.0* | N/A | N/A | N/A | N/A | 26.8* |
Non‐normally distributed data were presented as median.
Surgical Technique
Physical and radiological examinations were carefully performed to develop a personalized surgical plan (Figure 1A–C). All procedures were performed under general anesthesia with tourniquet control. Generally, after exposure, the pseudarthrosis site was resected until cortical bleeding and the medulla was opened by drilling to obtain a normal medullary canal. All the surrounding diseased periosteum and abnormal fibrous tissue were excised. For patients with a small cross‐section of bone at the fracture site, a sliding osteotomy was performed to increase contact area (Figure 1D). Due to prolonged CPT status, compensatory thickening of the fibula may occur in some patients. For those patients, a fibular graft or an acute fibular ORIF was performed to use the fibula as an important support of the limb. An Ilizarov frame, which varied according to the type of deformities in the leg and foot and the length of the proximal tibia, was installed (Figure 1E,F). For patients with a smaller bone defect (usually, <5 cm) at the pseudarthrosis site, an acute shortening was made to obtain bone ends docking under the monitoring of the distal blood perfusion and neural function, and then autogenous cancellous bone with cortical bone graft was harvested from the ipsilateral iliac crest and placed at the docking site. Otherwise, a secondary bone grafting was applied when bone ends docking. Any angulation and rotation deformities at the pseudarthrosis site should be immediately corrected at this time. Then, proximal corticotomy was performed using a drill since all the pseudarthrosis occurred in the distal tibia. During this corticotomy, cool normal saline was used to prevent thermal injury and all surrounding tissues, especially the periosteum, should be carefully protected. For the ankle, spontaneous fusion or combined equinus deformity, supramalleolar osteotomy or progressive correction with a transmalleolar fixator was performed simultaneously to achieve foot compensation in the sagittal plane and effective functional weight‐bearing. For other secondary deformities in the ipsilateral foot, knee, and femur, corrective procedures were usually performed in a second‐stage surgery. Usually, there might be aggravation of equinus deformity or the development of knee contracture after limb lengthening due to the long‐term of LLD, and subsequently, a second‐stage corrective surgery was difficult to avoid. Thus, bone grafting could be performed in the second surgery if necessary. For patients who were unable to tolerate the long‐term external fixation, secondary open reduction and internal fixation (ORIF) was used to replace the Ilizarov fixator.
FIGURE 1.
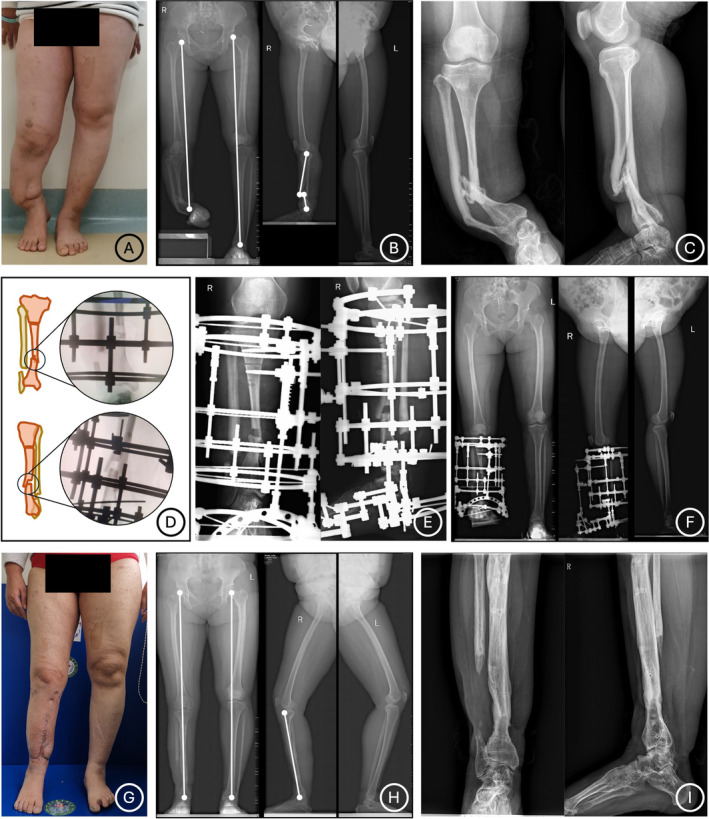
Clinical and radiographical photographs of Ilizarov distraction for a 29‐year‐old female patient (Case No. 3) who has a CPT in right lower limb. (A–C) Preoperative photographs show a CPT characterized with LLD (12.5 cm) and anterolateral bowing and varus deformities. (D‐F) A sliding osteotomy is performed to increase contact area of the CPT site and an Ilizarov distraction technique is applied. (G–I) Solid bone union of the CPT site and excellent correction of limb deformities are obtained as shown in appearance and radiological images at final follow‐up.
Postoperative Management
After a latent period of 5 to 7 days, gradual distraction was initiated at 0.75 to 1 mm/day and adjusted according to the actual osteogenesis of the distraction zone. Plain radiographs, CT scans, and full‐length lower extremity radiographs were used to assess the quality of bone union, consolidation, and the mechanical axis and length of the lower extremity (Figure 1G–I). Pain management, pin‐tract care, joint movement, and weight‐bearing were performed according to clinical routine. Patients were asked to have regular follow‐up visits and unscheduled phone contacts. The adjustment and removal of the Ilizarov frame and any rehabilitation training were supervised by in‐person visits or video calling.
Usually, the length of the affected limb is not required to be identical to that of unaffected limb in these patients due to the long‐term compensatory posture and the presence of secondary stiff deformities in the pelvis and spine. In order to identify an optimal length of the affected limb, patients were encouraged to walk with full weight‐bearing using homemade heightening shoes during the lengthening phase of Ilizarov distraction and to feel their walking function in the situation with a different length of the affected limb. Generally, the frame was removed after full union of the fracture site and solid consolidation of the distraction zone confirmed by CT scans, and a leg or leg‐ankle foot orthosis was used for 3 to 6 months to provide protection during further therapist‐assisted exercises and gait training.
Assessments
Preoperative and postoperative LLD were measured and recorded. The external fixation time (EFT) was recorded from the Ilizarov fixator installation to removal. The American Orthopedic Foot and Ankle Society (AOFAS) ankle‐hindfoot score was used for functional assessment of the affected foot. 29 The exercise capacity of patients was evaluated using a questionnaire of five items (walking, running, jumping, squatting, and going up/down the stairs) and each item was scored from 0 to 3 (“unable” = 0 point, “difficult” = 1 point, “little difficult” = 2 points, and “easy” = 3 points). The clinical and radiological outcomes of CPT were classified as excellent, good, fair, and poor according to a previous study by Inan et al. 30 The satisfaction of patients was investigated according to a five‐point Likert scale, with a score of 1 meaning the lowest degree of satisfaction and a score of 5 meaning the highest degree of satisfaction. Any complications after surgery and during follow‐up were recorded. Pseudoarthrosis recurrence, nonunion, and refracture were defined as treatment failures. The time of bone union of CPT site was recorded started from the time of bone ends docking.
Statistical Analysis
SPSS statistical software package version 24.0 (IBM Inc., Armonk, NY, USA) was used for statistical analysis. To test the normality of the data, the Kolmogorov–Smirnov test was performed. Normally distributed continuous data were recorded as mean with range and compared by using the student's t‐test, while non‐normally distributed data were presented as median with interquartile range (IQR) and compared by using the Wilcoxon's signed‐rank test. Statistical significance was set at p < 0.05.
Results
Treatment Information
Sliding osteotomy was performed in three patients with a small contact cross‐section of bone at the fracture site. For secondary deformities in the ipsilateral foot, knee, and femur, a one‐stage correction was performed in six patients, and a second‐stage correction was performed in six patients. Secondary bone grafting was performed in five patients. Secondary internal fixation was performed to replace the Ilizarov frame in three patients who were unable to tolerate long‐term external fixation and had a strong willingness to undergo surgery.
Radiological Results
Bone union of the pseudarthrosis and consolidation of the distraction zone were achieved in all patients. The mean time of bone union of CPT site was 16.7 weeks (range, 14 ~ 21). The mean EFT was 19.5 months (range, 7.3 ~ 39.1). At a median follow‐up of 26.8 month s(IQR, 20.2 ~ 34.3), a mean tibial lengthening of 10.2 cm (range, 2.5 ~ 14.5) was obtained, and the mean LLD was decreased from 11.3 cm (range, 3.4 ~ 17.3) preoperatively to 1.1 cm (range, 0.3 ~ 3.7) postoperatively (p < 0.05). According to the criteria described by Inan et al., the radiological results were classified as excellent in three patients, good in eight, and fair in two.
Clinical Results
The mean or median AOFAS ankle‐hindfoot score was improved from 53.5 (IQR, 26.5 ~ 60.5) preoperatively to 63.9 (range, 53 to 73) at final follow‐up (p < 0.05). The mean score for exercise capacity were improved from 4.9 (range, 1 to 8) preoperatively to 9.6 (range, 7 ~ 12) at final follow‐up (p < 0.05). According to the criteria described by Inan et al., the clinical results were classified as good in 10 patients and fair in four. The median score of satisfaction in these patients was 4 (IQR, 3 to 5).
Complications
Complications occurred in eight patients. Two patients with delayed wound healing were cured after dressing changes. One patient suffered refracture at the pseudarthrosis site during follow‐up and was successfully healed at 15 weeks after a second ORIF. At final follow‐up, knee or ankle mobility restriction was observed in six patients and residual abduction deformity of the foot was noted in one patient (Figures 2, 3, 4). The success rate was 92.9%, as refracture was defined as treatment failure and occurred in one patient. All outcomes of these patients are shown in Table 2.
FIGURE 2.
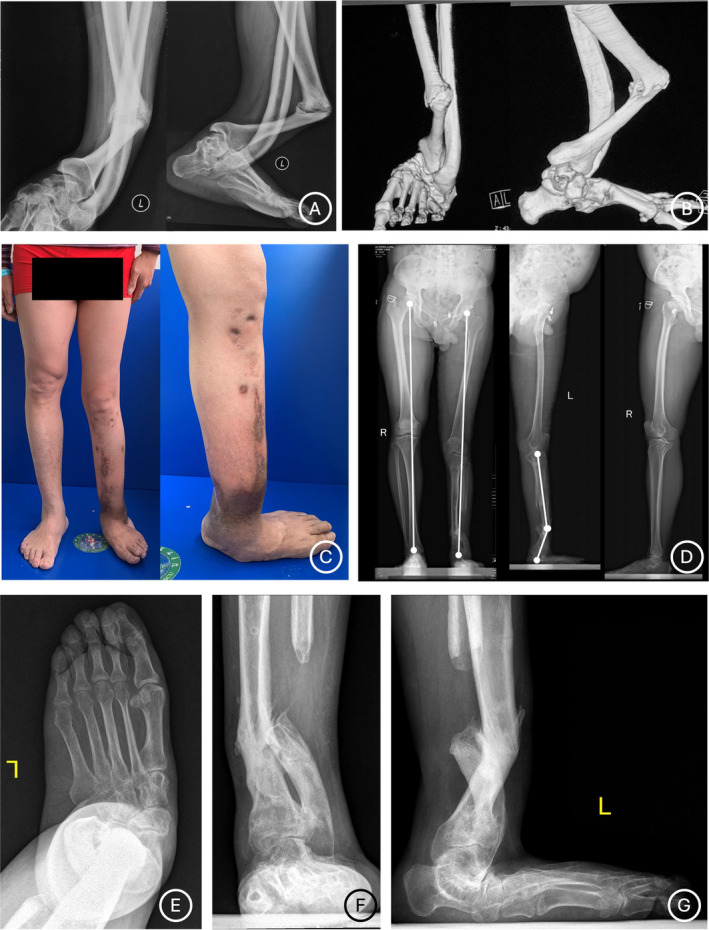
Preoperatively clinical and radiographical photographs of a 58‐year‐old female patient (Case No. 10) who has a CPT in left lower limb and suffered from two previously failed surgeries. (A–B) Radiographical photographs before his previous surgeries show a CPT characterized with significant anterolateral bowing and varus deformities. (C‐G) Appearance and radiological images before our Ilizarov distraction show poor soft‐tissue condition and anterolateral bowing and valgus deformities at the CPT site. Serious secondary deformities of the ipsilateral foot characterized with a collapsed foot arch, valgus, and abduction are noted.
FIGURE 3.
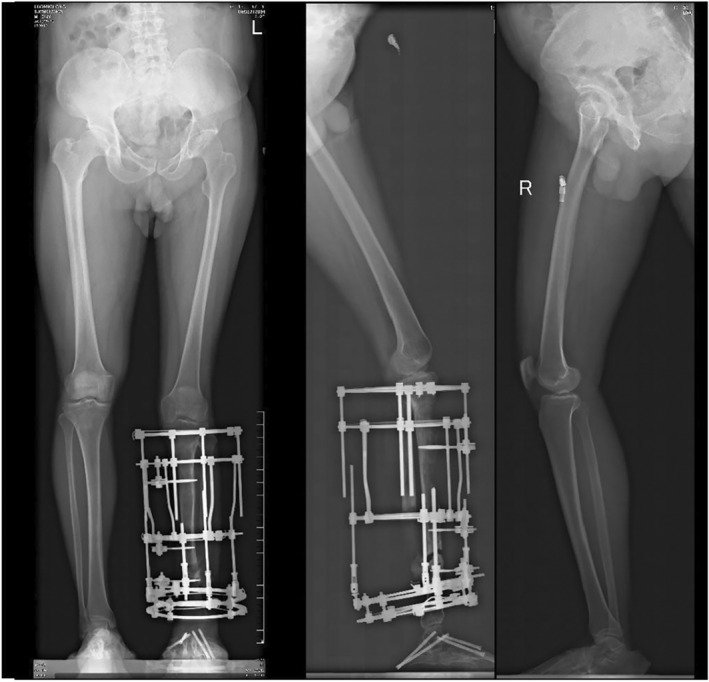
Postoperatively radiographical photographs of the patient (Case No. 10) in Figure 2. The distal fibula was removed due to suspected infection. A corrective surgery for the foot and proximal tibia is performed and a Ilizarov distraction technique is applied.
FIGURE 4.
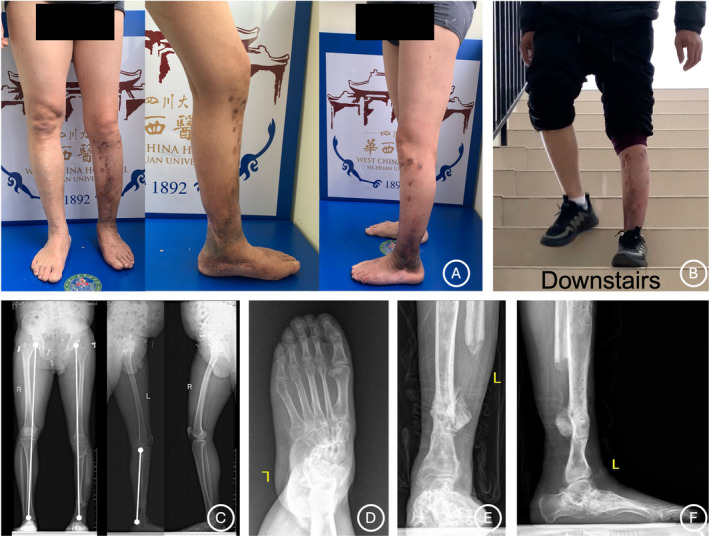
Appearance and radiological images of the patient (Case No. 10) in Figure 2 at final follow‐up. (A‐B) Appearance images of the patient show excellent deformity correction and satisfactory functional reconstruction. (C) Radiological images show solid bone union of the CPT site and excellent correction of limb length. (D‐E) Radiological photographs of the ipsilateral foot show that the significant valgus and flatfoot deformities are corrected but there is still mild abduction deformity remaining.
TABLE 2.
Outcomes of patients.
| Case No. | EFT (m) | Healing time of CPT (w) | Pre. LLD (cm) | Post. LLD (cm) | Pre. AOFAS | Post. AOFAS | Pre. exercise capacity | Post. exercise capacity | Inan clinical results | Inan radiological results | Complications | Satisfaction |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 19.9 | 15 | 7.8 | 2.1 | 64 | 67 | 8 | 12 | Fair | Fair | Knee flexion limited mobility | 4 |
| 2 | 39.1 | 18 | 17.3 | 3.7 | 56 | 67 | 6 | 7 | Good | Good | Delayed wound healing; Ankle joint mobility restriction | 4 |
| 3 | 22.2 | 17 | 12.5 | 0.4 | 56 | 73 | 8 | 12 | Good | Excellent | / | 5 |
| 4 | 17.5 | 14 | 8.6 | 0.5 | 57 | 66 | 5 | 9 | Good | Excellent | / | 4 |
| 5 | 19.9 | 17 | 15.3 | 0.9 | 51 | 53 | 1 | 9 | Fair | Good | Knee flexion limited mobility | 3 |
| 6 | 17.5 | 15 | 15.6 | 1.1 | 68 | 73 | 5 | 8 | Good | Good | Knee flexion limited mobility | 4 |
| 7 | 19.8 | 16 | 10.1 | 0.3 | 22 | 56 | 1 | 9 | Fair | Good | Ankle joint mobility restriction | 4 |
| 8 | 11.7 | 14 | 9.5 | 1.4 | 62 | 73 | 8 | 11 | Good | Excellent | / | 5 |
| 9 | 21.2 | 18 | 11.5 | 0.4 | 60 | 69 | 6 | 11 | Good | Good | / | 4 |
| 10 | 7.3 | 17 | 3.4 | 0.9 | 18 | 64 | 2 | 12 | Good | Good | Residual abduction deformity of the foot | 5 |
| 11 | 21.2 | 19 | 10.6 | 0.7 | 32 | 56 | 3 | 8 | Good | Fair | / | 5 |
| 12 | 19.1 | 16 | 7.8 | 0.4 | 51 | 66 | 5 | 7 | Fair | Good | Delayed wound healing | 4 |
| 13 | 13.2 | 20 | 13.2 | 1.2 | 28 | 73 | 4 | 10 | Good | Good | Refracture; Ankle joint mobility restriction | 4 |
| 14 | 23.6 | 18 | 14.7 | 0.8 | 18 | 66 | 6 | 11 | Good | Good | / | 5 |
| Mean/Median | 19.5 | 16.7 | 11.3 | 1.1 | 53.5* | 63.9 | 4.9 | 9.6 | N/A | N/A | N/A | 4.0* |
Non‐normally distributed data were presented as median.
Discussion
Principal Findings
The Ilizarov technique was first introduced to treat 16 patients with CPT in 1971 and was subsequently widely popularized and applied by many surgeons. 23 , 25 , 27 , 31 Based on the “law of tension‐stress,” the regeneration signal system of living biological tissue could be activated with a continuous tension force by the Ilizarov gradual distraction. 32 , 33 A substantial body of studies for child CPT patients demonstrated that Ilizarov distraction could not only promote bone union of the pseudarthrosis site but also address LLD and the associated multilevel and multidirectional deformities. 4 , 27 , 34 , 35 , 36 However, the clinical evidence on Ilizarov distraction for management of CPT in adults is still limited. Here, we reported the outcomes of the Ilizarov technique for CPT in 14 adults with a median follow up of 26.8 months. At final follow‐up, bone union was achieved in all patients and no amputation was performed.
Ilizarov Technique with or without Intramedullary Rodding
When bone union, deformity correction, and refracture risk are all considered, there is an ongoing debate about whether single Ilizarov distraction or Ilizarov distraction with intramedullary nailing is better for the management of CPT. In 2019, Paley summarized the primary union rate and the refracture rate of the Ilizarov technique with or without intramedullary rodding for treating CPT. 13 A total of 115 children in six studies were treated by the single Ilizarov technique, and the primary union rate and the refracture rate were 93.5% (81% to 100%) and 41% (15% to 68%), respectively. 13 , 27 , 37 , 38 , 39 , 40 , 41 While the Ilizarov technique combined with rodding fixation was applied to a total of 152 children in the other five studies, with primary union achieved in 72% (40% to 100%) of cases and refracture occurring in 17% (0% to 40%) of cases. 13 , 35 , 42 , 43 , 44 , 45 It is obvious that the additional application of intramedullary rodding reduced the risk of refracture but hindered the bone union of pseudarthrosis. For treating CPT in children, the probability of primary union without refracture seems to be at an impasse, or, in other words, it appears to be difficult to achieve higher primary union and lower refracture risk at the same time with whichever technique. 13
Consideration of Bone Union
The bone union of the pseudarthrosis site is an important part of the outcomes in patients with CPT. In our deformity correction center, we preferred to apply the Ilizarov technique without intramedullary nailing for the management of CPT in adults out of concern for economic burden and the damage to the intramedullary microenvironment. There is no doubt that the stability of the periosteal microenvironment is essential for fracture union in healthy people. 46 The application of intramedullary rodding could not only provide stable, strong, and full‐length fixation but also minimize the surgical disturbance to the periosteum. However, the opposite might be true for CPT patients. The pathological periosteum change is a primary mechanism for the pathogenesis of CPT, but the osteogenic microenvironment in bone marrow may not be more affected by the pathology of CPT. 5 , 47 A recent study demonstrated that no significant differences were found in osteogenic ability among bone marrow‐derived mesenchymal stem cells from the fracture site of CPT patients, the iliac crest of CPT patients, and the iliac crest of healthy subjects. 47 Thus, to ensure the bone union of CPT, it appeared particularly critical to maintain the bone marrow microenvironment when the fibrous hamartoma and surrounding diseased periosteum were extensively removed during surgery. Conversely, the use of one‐stage intramedullary rodding might disrupt the intramedullary microenvironment and thus hinder bone healing. This speculation was supported by clinical evidence from a range of studies. A logistic regression analysis by Shah et al. also confirmed the Ilizarov technique combined with intramedullary nailing had a higher risk of unsound union at skeletal maturity for management of CPT in children, which is consistent with Paley. 13 , 48
Consideration of Refracture
With regard to the refracture rate after primary union in CPT, it has been suggested that hamartoma recurrence, a smaller cross‐sectional area of the healed segment, residual ankle valgus, a lack of support by functional bracing or an intramedullary rod, and a younger age at surgery are risk factors. 4 , 27 , 49 Interestingly, we noted a significantly lower risk of refracture (7.14%) in this study, compared with previous studies using similar treatment for CPT in children (41%, 15% to 68%). 13 , 27 , 37 , 38 , 39 , 40 , 41 We guessed that the following reasons might contribute to it. First, in the present study, good‐to‐excellent correction of angulation deformities and secondary ankle deformities was achieved in all patients, which effectively reduced the refracture risk. Second, the use of functional bracing and the supervision for rehabilitation and exercise for 3 to 6 months after primary union could indeed help the patients restore relatively functional abilities and avoid early refracture. Third, good medical compliance in adults might be another reason for the low refracture rate, because adults were prone to consciously protect the affected limb in their daily lives. A final and important point we should highlight is that the age at surgery, as the significant difference between adults and children with CPT, was an influencing factor of refracture that cannot be neglected. 4 Controversy still exists concerning the age at surgery for CPT in children, and some authors suggest performing definitive Ilizarov surgery on children older than 3 years to minimize the refracture risk. 4 And it also remains unclear whether the pathological change of CPT at the adult stage differs from that at the childhood. However, skeletal maturity has been considered to provide a certain degree of protection to the tibia. 50 , 51 , 52 That is why intramedullary nails are suggested to be retained until maturity to minimize the risk of refracture for children with CPT. 18 , 31 And this is also why we believed an additional intramedullary nail in one‐stage surgery might be unnecessary for CPT in adults. Zayda et al. recommended conversion to intramedullary fixation after the complete union of pseudarthrosis to discard the bulky and uncomfortable external fixation and allow joint motion as early as possible. 23 However, whether a second‐stage intramedullary fixation is beneficial needs further confirmation.
The Management of Secondary Deformities in Adults
The severity of secondary deformities is, obviously, another difference between adults and children with CPT. Due to the long‐term compensation, the secondary deformities in adult patients are usually more complex, stiff, and even combined with the presence of arthritis, while these in children are mild and even have not yet occurred. Thus, for adults with CPT, in addition to treatment for CPT itself, focusing on the correction of the secondary deformities is, no doubt, important to obtain a functional limb. Furthermore, the excellent correction of the secondary deformities may, in turn, be beneficial to enhancing bone union as well as consolidation and avoiding refracture at the pseudarthrosis site. However, performing the corrective procedures for secondary deformities in the one‐stage surgery may have a high risk of complications. Moreover, secondary aggravation of these deformities during the limb lengthening was not uncommon. Thus, we believed that for most adult CPT patients, a single surgery usually could not solve all the problems. In our deformity correction center, we preferred to perform a second‐stage correction, which could also provide a suitable chance for bone grafting at the CPT site. We should admit that these secondary deformities in adult patients were not easy to manage compared to those in children. Despite many efforts, it was difficult for patients to fully recover to a normal state after correction. As shown in this study, a mean AOFAS ankle‐hindfoot score of 63.9 (range, 53 to 73) at final follow‐up was relatively poor. And ipsilateral knee or ankle mobility restriction was noted in six patients. Therefore, adequate preoperative communication and appropriate patient selection are crucial.
Limitations
The limitations of this study are the small number of patients with CPT and the short follow‐up. However, given the relative success of management in children, CPT in adults requiring surgical intervention is unusual. To the best of our knowledge, this is already the largest report of adults with CPT in English literature. Another limitation of the study is the retrospective design. Basic research may be needed to elucidate the differences in pathological changes of CPT between the adult stage and childhood. And further clinical studies with a large sample size and long‐term follow‐up results should also be conducted to establish the optimal treatment for CPT in adults.
Prospects of Clinical Application
The management of CPT is challenging to orthopedic surgeons. However, the majority of studies have focused on the treatment in children with CPT and the clinical experience in adults with CPT is still poor. The skeletal maturity, the severity of secondary deformities, and the condition of the surrounding soft tissue in adults with CPT are significantly different from that in child patients. Single Ilizarov distraction could effectively promote bone healing and correct secondary deformities in adult CPT patients though no intramedullary fixation was used. There is also a potential risk concerning refracture despite not as high as that in child patients.
Conclusion
The management of CPT in adults is a challenge for orthopedic surgeons. Ilizarov distraction could provide a suitable treatment option for such patients by achieving a high rate of bone union, a good correction of secondary deformity, a low risk of refracture, and consequently restore a relatively functional limb. Further research is needed to confirm these findings and identify whether an additional intramedullary fixation in one‐stage surgery is necessary in these patients.
Ethics Statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of Interest Statement
The authors declare that they have no conflict of interest.
Author Contributions
Ya‐Xing Li, project administration, formal analysis, writing–original draft, writing–review and editing. Jia Li, project administration, formal analysis, writing–original draft, writing–review and editing. Tingjiang Gan, formal analysis, writing–review and editing. Qirui Geng, writing–review and editing. Xikun Ma, writing–review and editing. Shijiu Yin, formal analysis. Ye Wu, writing–review and editing. Xiang Fang, writing–review and editing. Huiqi Xie, Supervision, writing–review and editing. Hui Zhang, Conceptualization, Project administration, Supervision, writing–review and editing.
Acknowledgments
This research was supported by the 1·3·5 project for disciplines of excellence—Clinical Research Incubation Project, West China Hospital, Sichuan University (Grant No. 22HXFH015), the Natural Science Foundation of Tibet Autonomous Region (Grant No. XZ202201ZR0033G and XZ202201ZY0038G), the Natural Science Foundation of Sichuan Province (Grant No. 2023YFS0014), and the full‐time postdoctoral research and development fund of West China Hospital of Sichuan University (Grant No. 2024HXBH076).
References
- 1. Shah H, Rousset M, Canavese F. Congenital pseudarthrosis of the tibia: management and complications. Indian J Orthop. 2012;46:616–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ari B, Kuyubasi SN. Bilateral congenital pseudarthrosis of the tibia with neurofibromatosis type 1. J Pak Med Assoc. 2021;71:1499–1502. [DOI] [PubMed] [Google Scholar]
- 3. Hefti F, Bollini G, Dungl P, Fixsen J, Grill F, Ippolito E, et al. Congenital pseudarthrosis of the tibia: history, etiology, classification, and epidemiologic data. J Pediatr Orthop B. 2000;9:11–15. [DOI] [PubMed] [Google Scholar]
- 4. Choi IH, Cho TJ, Moon HJ. Ilizarov treatment of congenital pseudarthrosis of the tibia: a multi‐targeted approach using the Ilizarov technique. Clin Orthop Surg. 2011;3:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cho TJ, Seo JB, Lee HR, Yoo WJ, Chung CY, Choi IH. Biologic characteristics of fibrous hamartoma from congenital pseudarthrosis of the tibia associated with neurofibromatosis type 1. J Bone Joint Surg Am. 2008;90:2735–2744. [DOI] [PubMed] [Google Scholar]
- 6. Madhuri V, Mathew SE, Rajagopal K, Ramesh S, Antonisamy B. Does pamidronate enhance the osteogenesis in mesenchymal stem cells derived from fibrous hamartoma in congenital pseudarthrosis of the tibia? Bone Rep. 2016;5:292–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li Z, Liu Y, Huang Y, Tan Q, Mei H, Zhu G, et al. Circ_0000888 regulates osteogenic differentiation of periosteal mesenchymal stem cells in congenital pseudarthrosis of the tibia. iScience. 2023;26:107923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Granchi D, Devescovi V, Baglio SR, Magnani M, Donzelli O, Baldini N. A regenerative approach for bone repair in congenital pseudarthrosis of the tibia associated or not associated with type 1 neurofibromatosis: correlation between laboratory findings and clinical outcome. Cytotherapy. 2012;14:306–314. [DOI] [PubMed] [Google Scholar]
- 9. Shannon CE, Huser AJ, Paley D. Cross‐union surgery for congenital pseudarthrosis of the tibia. Children (Basel). 2021;8:547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu YX, Yang G, Zhu GH, Tan Q, Wu JY, Liu K, et al. Application of the “telescopic rod” in a combined surgical technique for the treatment of congenital pseudarthrosis of the tibia in children. J Orthop Surg Res. 2021;16:532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhong H, Ma S, Cen Y, Ma L, Li D, Liang B, et al. A case report of early unilateral external fixation by 3D printing and computer‐assisted and secondary bone graft internal fixation in pseudarthrosis of the tibia surgery. J Int Med Res. 2020;48:300060520945518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McClure PK, Franzone JM, Herzenberg JE. Challenges with Fassier‐Duval rod exchanges in congenital pseudarthrosis of the tibia: explant roadblock and solution. J Pediatr Orthop B. 2022;31:e95–e100. [DOI] [PubMed] [Google Scholar]
- 13. Paley D. Congenital pseudarthrosis of the tibia: biological and biomechanical considerations to achieve union and prevent refracture. J Child Orthop. 2019;13:120–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vander Have KL, Hensinger RN, Caird M, Johnston C, Farley FA. Congenital pseudarthrosis of the tibia. J Am Acad Orthop Surg. 2008;16:228–236. [DOI] [PubMed] [Google Scholar]
- 15. Kumta SM, Spinner R, Hung LK, Leung PC. Congenital pseudarthrosis of the tibia in adults treated by a free vascularized iliac crest graft. Microsurgery. 1994;15:598–603. [DOI] [PubMed] [Google Scholar]
- 16. Miraj F, Aprilya D. Diagnostic and treatment challenge in adult presentation of congenital pseudoarthrosis of the tibia: a case report. Ann Med Surg (Lond). 2020;58:112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bhowmick K, Varghese VD. Retrograde intramedullary nailing for recurrent fracture in congenital pseudarthrosis of the tibia. J Foot Ankle Surg. 2016;55:1287–1291. [DOI] [PubMed] [Google Scholar]
- 18. El‐Gammal TA, El‐Sayed A, Kotb MM, Saleh WR, Ragheb YF, Refai OA, et al. Crawford type IV congenital pseudarthrosis of the tibia: treatment with vascularized fibular grafting and outcome at skeletal maturity. J Pediatr Orthop. 2021;41:164–170. [DOI] [PubMed] [Google Scholar]
- 19. Westberry DE, Carpenter AM, Tisch J, Wack LI. Amputation outcomes in congenital pseudarthrosis of the tibia. J Pediatr Orthop. 2018;38:e475–e481. [DOI] [PubMed] [Google Scholar]
- 20. O'Donnell C, Foster J, Mooney R, Beebe C, Donaldson N, Heare T. Congenital pseudarthrosis of the tibia. JBJS Rev. 2017;5:e3. [DOI] [PubMed] [Google Scholar]
- 21. McCarthy RE. Amputation for congenital pseudarthrosis of the tibia. Indications and techniques. Clin Orthop Relat Res. 1982;166:58–61. [PubMed] [Google Scholar]
- 22. Khan T, Joseph B. Controversies in the management of congenital pseudarthrosis of the tibia and fibula. Bone Joint J. 2013;95‐B:1027–1034. [DOI] [PubMed] [Google Scholar]
- 23. Zayda AI, Mesregah MK, Zalalo SH, Sakr SA. Functional and radiological outcomes after treatment of congenital pseudarthrosis of the tibia using the Ilizarov technique: a retrospective single‐center study. J Orthop Traumatol. 2022;23:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li Y, Chen Y, Gan T, Qin B, Liu X, Zhang H. An alternative therapeutic strategy for infected large bone defect and massive soft‐tissue loss of leg‐is free flap reconstruction inevitable? Int Orthop. 2021;45:3033–3043. [DOI] [PubMed] [Google Scholar]
- 25. Ilizarov GA, Gracheva VI. Bloodless treatment of congenital pseudarthrosis of the crus with simultaneous elimination of shortening using dosed distraction. Ortop Travmatol Protez. 1971;32:42–46. [PubMed] [Google Scholar]
- 26. Ilizarov GA. Clinical application of the tension‐stress effect for limb lengthening. Clin Orthop Relat Res. 1990;250:8–26. [PubMed] [Google Scholar]
- 27. Paley D, Catagni M, Argnani F, Prevot J, Bell D, Armstrong P. Treatment of congenital pseudoarthrosis of the tibia using the Ilizarov technique. Clin Orthop Relat Res. 1992;280:81–93. [PubMed] [Google Scholar]
- 28. Crawford AH Jr, Bagamery N. Osseous manifestations of neurofibromatosis in childhood. J Pediatr Orthop. 1986;6:72–88. [DOI] [PubMed] [Google Scholar]
- 29. Kitaoka HB, Alexander IJ, Adelaar RS, Nunley JA, Myerson MS, Sanders M. Clinical rating systems for the ankle‐hindfoot, midfoot, hallux, and lesser toes. Foot Ankle Int. 1994;15:349–353. [DOI] [PubMed] [Google Scholar]
- 30. Inan M, El Rassi G, Riddle EC, Kumar SJ. Residual deformities following successful initial bone union in congenital pseudoarthrosis of the tibia. J Pediatr Orthop. 2006;26:393–399. [DOI] [PubMed] [Google Scholar]
- 31. El‐Gammal TA, Ali AE, Kotb MM, Saleh WR, Ragheb YF, Refai OA, et al. Congenital pseudarthrosis of the tibia: long‐term outcome of treatment with intramedullary vascularized fibular graft combined with Ilizarov distraction. J Pediatr Orthop. 2023;43:e487–e492. [DOI] [PubMed] [Google Scholar]
- 32. Ilizarov GA. The tension‐stress effect on the genesis and growth of tissues. Part I. The influence of stability of fixation and soft‐tissue preservation. Clin Orthop Relat Res. 1989;238:249–281. [PubMed] [Google Scholar]
- 33. Ilizarov GA. The tension‐stress effect on the genesis and growth of tissues: part II. The influence of the rate and frequency of distraction. Clin Orthop Relat Res. 1989;239:263–285. [PubMed] [Google Scholar]
- 34. Seo SG, Lee DY, Kim YS, Yoo WJ, Cho TJ, Choi IH. Foot and ankle function at maturity after ilizarov treatment for atrophic‐type congenital pseudarthrosis of the tibia: a comprehensive outcome comparison with normal controls. J Bone Joint Surg Am. 2016;98:490–498. [DOI] [PubMed] [Google Scholar]
- 35. Shabtai L, Ezra E, Wientroub S, Segev E. Congenital tibial pseudarthrosis, changes in treatment protocol. J Pediatr Orthop B. 2015;24:444–449. [DOI] [PubMed] [Google Scholar]
- 36. Ghanem I, Damsin JP, Carlioz H. Ilizarov technique in the treatment of congenital pseudarthrosis of the tibia. J Pediatr Orthop. 1997;17:685–690. [DOI] [PubMed] [Google Scholar]
- 37. El‐Rosasy M. Ilizarov techniques for the management of congenital pseudarthrosis of the tibia. Clin Orthop Surg. 2001;3:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Boero S, Catagni M, Donzelli O, Facchini R, Frediani PV. Congenital pseudarthrosis of the tibia associated with neurofibromatosis‐1: treatment with Ilizarov's device. J Pediatr Orthop. 1997;17:675–684. [DOI] [PubMed] [Google Scholar]
- 39. Borzunov DY, Chevardin AY, Mitrofanov AI. Management of congenital pseudarthrosis of the tibia with the Ilizarov method in a paediatric population: influence of aetiological factors. Int Orthop. 2016;40:331–339. [DOI] [PubMed] [Google Scholar]
- 40. Hissnauer TN, Stiel N, Babin K, Rupprecht M, Hoffmann M, Rueger JM, et al. Bone morphogenetic protein‐2 for the treatment of congenital pseudarthrosis of the tibia or persistent tibial nonunion in children and adolescents: a retrospective study with a minimum 2‐year follow‐up. J Mater Sci Mater Med. 2017;28:60. [DOI] [PubMed] [Google Scholar]
- 41. Ohnishi I, Sato W, Matsuyama J, Yajima H, Haga N, Kamegaya M, et al. Treatment of congenital pseudarthrosis of the tibia: a multicenter study in Japan. J Pediatr Orthop. 2005;25:219–224. [DOI] [PubMed] [Google Scholar]
- 42. Thabet AM, Paley D, Kocaoglu M, Eralp L, Herzenberg JE, Ergin ON. Periosteal grafting for congenital pseudarthrosis of the tibia: a preliminary report. Clin Orthop Relat Res. 2008;466:2981–2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Agashe MV, Song SH, Refai MA, Park KW, Song HR. Congenital pseudarthrosis of the tibia treated with a combination of Ilizarov's technique and intramedullary rodding. Acta Orthop. 2012;83:515–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yan A, Mei HB, Liu K, Wu JY, Tang J, Zhu GH, et al. Wrapping grafting for congenital pseudarthrosis of the tibia: a preliminary report. Medicine (Baltimore). 2017;96:e8835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhu GH, Mei HB, He RG, Liu YX, Liu K, Tang J, et al. Combination of intramedullary rod, wrapping bone grafting and Ilizarov's fixator for the treatment of Crawford type IV congenital pseudarthrosis of the tibia: mid‐term follow up of 56 cases. BMC Musculoskelet Disord. 2016;17:443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lan KC, Wei KT, Lin PW, Lin CC, Won PL, Liu YF, et al. Targeted activation of androgen receptor signaling in the periosteum improves bone fracture repair. Cell Death Dis. 2022;13:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dilogo IH, Mujadid F, Nurhayati RW, Kurniawan A. Evaluation of bone marrow‐derived mesenchymal stem cell quality from patients with congenital pseudoarthrosis of the tibia. J Orthop Surg Res. 2018;13:266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shah H, Joseph B, Nair BVS, Kotian DB, Choi IH, Richards BS, et al. What factors influence union and refracture of congenital pseudarthrosis of the tibia? a multicenter long‐term study. J Pediatr Orthop. 2018;38:e332–e337. [DOI] [PubMed] [Google Scholar]
- 49. Cho T‐J, Choi IH, Lee SM, Chung CY, Yoo WJ, Lee DY, et al. Refracture after Ilizarov osteosynthesis in atrophic‐type congenital pseudarthrosis of the tibia. J Bone Joint Surg Br. 2008;90:488–493. [DOI] [PubMed] [Google Scholar]
- 50. Singer D, Johnston CE. Congenital pseudarthrosis of the tibia: results, at skeletal maturity, of the Charnley‐Williams procedure. JB JS Open Access. 2019;4:e0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tudisco C, Bollini G, Dungl P, Fixen J, Grill F, Hefti F, et al. Functional results at the end of skeletal growth in 30 patients affected by congenital pseudoarthrosis of the tibia. J Pediatr Orthop B. 2000;9:94–102. [DOI] [PubMed] [Google Scholar]
- 52. Crossett LS, Beaty JH, Betz RR, Warner W, Clancy M, Steel HH. Congenital pseudarthrosis of the tibia. Long‐term follow‐up study. Clin Orthop Relat Res. 1989;245:16–18. [PubMed] [Google Scholar]


