Abstract
BACKGROUND
The frequent suboptimal efficacy of endoscopic ultrasound-guided fine-needle biopsy (EUS-FNB) to culture pancreatic cancer (PC) organoids (PCOs) poses a major challenge in the advancement of personalized medicine for advanced PC.
AIM
To explore how to obtain appropriate puncture tissues from EUS-FNB and optimize the strategy for efficiently constructing PCOs, providing an efficient tool for the advancement of personalized medicine.
METHODS
Patients who underwent EUS-FNB for the diagnosis of PC tissue were prospectively enrolled. We refined the endoscopic biopsy procedures and organoid cultivation techniques. All tissue specimens verified by on-site pathological assessment were cultured in a semi-suspended medium in a microfluidic environment. We assessed differences in PCOs cultured beyond and below five generations examining patient demographics, specimen and organoid attributes, and the sensitivity of organoids to a panel of clinical drugs through cell viability assays.
RESULTS
In this study, 16 patients with PC were recruited, one sample was excluded because onsite cytopathology showed no tumor cells. Successful organoid generation occurred in 93.3% (14 of 15) of the EUS-FNB specimens, with 60% (9 of 15) sustaining over five generations. Among these patients, those with a history of diabetes, familial cancer, or larger tumors exhibited enhanced PCO expandability. The key factors influencing long-term PCOs expansion included initial needle sample quality (P = 0.005), rapid initiation of organoid culture post-isolation (P ≤ 0.001), and high organoid activity (P = 0.031). Drug sensitivity analysis revealed a partial response in two patients following therapeutic intervention and surgery and stable disease in four patients, indicating a moderate correlation between organoid response and clinical outcomes.
CONCLUSION
Optimal initial needle sampling, rapid and precise biopsy sample processing, process isolated samples as soon as possible, and sufficient cellular material are crucial for successful cultivating PCOs. High organoid activity is an important factor in maintaining their long-term expansion, which is essential for shortening the time of drug sensitivity analysis and is the basis of PC research.
Keywords: Endoscopic ultrasound-guided fine-needle biopsy, Pancreatic cancer organoid, Puncture technique, Cultivation optimization strategy, Drug screening
Core Tip: We optimized the endoscopic ultrasound puncture process to obtain pancreatic cancer tissue and cultivate pancreatic cancer organoids. Adequate material from the first puncture, rapid and accurate puncture operations, and rapid entry of puncture samples into the culture stage are important factors for successfully cultivating pancreatic cancer organoids. Organoid activity is the key to long-term expansion.
INTRODUCTION
Pancreatic cancer (PC) is characterized by an insidious onset and often has ambiguous clinical manifestations; it predominantly presents in advanced stages, either locally advanced (30%-35%) or metastatic (50%)[1]. However, surgical intervention, a critical treatment modality, is feasible for only a limited subset (15%-20%) of patients with PC[2]. This scenario underscores the pivotal role played by diagnostic biopsies and tailored neoadjuvant therapies in potentially achieving tumor remission to facilitate surgical resection, thereby attaining universality in managing borderline resectable and locally advanced PC. Endoscopic ultrasound-guided tissue acquisition (EUS-TA) has emerged as an integral diagnostic tool, and the diagnostic accuracy of solid pancreatic tumors is commendably high at 91.5%[3,4]. Moreover, the advent of EUS-guided fine-needle biopsy (EUS-FNB), particularly with the latest end-cutting designs, has proven effective in preserving cellular architecture, thereby establishing definitive diagnoses of malignancies across various solid lesions[5].
The burgeoning field of organoid technology, particularly in the context of PC, offers promising avenues for research and therapeutic applications. Since 2018, the breakthrough generation of PC organoids (PCOs) from samples using EUS-FNB with a 22-gauge needle has been enabling the rapid creation of organoids at initial diagnosis[6]. This advanced sampling technique ensures the procurement of adequate specimens from patients at all stages of PC, facilitating developing preclinical models closely mirroring the histological and biological characteristics of the original tumor tissue. These models retain the genetic diversity and heterogeneity of the tumor, allowing the establishment of gene polymorphism profiles, high-throughput drug testing, longitudinal chemosensitivity assessments, PCOs xenograft models, and analyses of the tumor immune microenvironment[7,8].
Although most PCOs are derived from surgical specimens, post-biopsy chemotherapy is the sole treatment option for many patients with advanced PC who are ineligible for surgery. The limited responsiveness of PC to anticancer drugs and its tendency towards refractoriness necessitates advancements in personalized medicine for PC. The slow progress in the development of PCOs technology is primarily due to the lack of standardized and repeatable techniques, particularly in cases with limited biopsy samples. Research has indicated that the efficiency of organoid cultivation from EUS-TA sourced PCOs, with success rates ranging between 36.4% to 87% across various models, is typically lower than that of those derived from surgical specimens[9]. Additionally, the frequency of these cultures extending beyond five generations was also comparatively less[10]. In general, a stable culture and sufficient organoids can be successfully produced for high-throughput drug screening with successive passage of organoid lines for more than five times.
Our study aimed to optimize the puncture procedures, sample handling, and culture methodologies to enhance the success rates of PCO generation. This endeavor should bolster basic and preclinical research on advanced PC, thereby contributing significantly to personalized medicine.
MATERIALS AND METHODS
This work has been reported in accordance with the strengthening the reporting of prospective cohort studies in surgery guideline[11].
Patient selection and study design
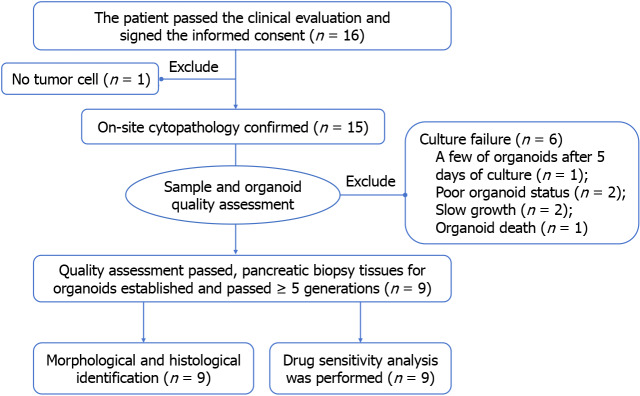
Patients who underwent EUS-FNB for suspected borderline resectable, locally advanced, or unresectable PC were evaluated based on radiological findings from December 2022 to July 2023 (Figure 1). Informed consent was obtained from each participant or their legal representative. This study included all those that fulfilled the inclusion criteria except for those with inadequate tumor tissue from EUS-FNB or concurrent malignancies. The study aimed to investigate a rapid culture technique for PCOs, drug sensitivity testing, and establish a correlation between in vitro drug responses and clinical disease control. The primary endpoint was obtaining a successful culture of organoids, and the secondary endpoint were drug sensitivity analysis and clinical relevance of the organoids. The study protocol was approved by the Ethics Committee of Chongqing General Hospital (No. KY S2022-045-01) and registered at Chinese Clinical Trial Registry (No. ChiCTR2200064388).
Figure 1.
Study scheme. Finally, 9 patients successfully constructed organoids and completed identification and drug screening.
EUS procedure details
Two skilled endosonographer performed all the EUS-FNB procedures using a curvilinear echoendoscope (Olympus Medical Systems, Tokyo, Japan). Tissue samples were acquired using a 22-gauge AcquireTM EUS fine needle biopsy device (Boston Scientific, United States). Previous research has indicated that the DNA yield from a 22-gauge EUS-TA needle is notably higher in tumor cells than that from other needle types[12]. We employed one or two additional sampling using a 22-gauge FNB needle, focusing on the active tumor periphery. A sector puncture approach was used, continuously adjusting the needle angle to optimize sample collection from the most suitable site. Necrotic cores and blood vessels were avoided by immediate on-site cytopathological evaluation. The remaining tissue was preserved in a freezing solution and transported to the laboratory via a cold chain within 10 minutes.
Organoid generation and maintenance
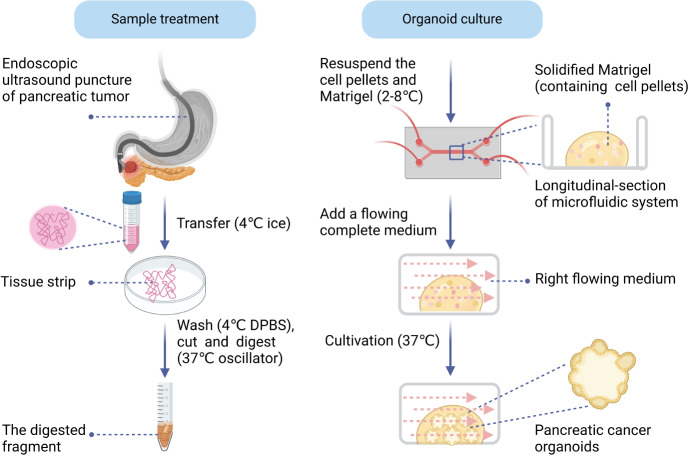
In this study, we used a semi-suspended organoid culture method combined with a microfluidic system (Figure 2). Specifically, tissue samples obtained via EUS-FNB were initially rinsed in ice-cold Dulbecco’s phosphate-buffered saline (without calcium and magnesium) (Thermo Fisher Scientific, catalog: 14190144) and then finely minced. Subsequently, a pre-warmed digestion solution was added, and this mixture was incubated in a 37 °C shaker at 140 rpm for 30 minutes following the gradient method. After digestion, the supernatant was centrifuged at 1000 rpm for 5 minutes. The supernatant was discarded, preserving the cell pellets, which were then resuspended in an appropriate volume of Matrigel (Corning, catalog: 356231), and placed onto a 48-well microfluidic culture plate. This setup was transferred to a 37 °C incubator to solidify the dome. Subsequently, a suitable volume of pre-warmed KingcultureTM organoid complete growth medium, with an approximate flow rate of 10-20 μL/minute, was added to the microfluidic system for cultivation. The complete medium was refreshed every 2-3 days. Additionally, each sample was assessed using a sample score scale and an organoid score scale, which provided the success rate and duration of the culture (Supplementary Tables 1 and 2).
Figure 2.
Schematic diagram of sample treatment from endoscopic ultrasound-guided fine-needle biopsy and organoid culture process. DPBS: Dulbecco’s phosphate-buffered saline.
During passage, the old medium was removed and pre-warmed Dispase II (Thermo Fisher Scientific, catalog: 17105041) was added. The dome structure in the well was gently disrupted with up-and-down motions of a pipette tip. After digestion according to the above method, the precipitation was retained and an appropriate amount of TrypLE was added (Thermo Fisher, catalog: 12605010). The digestion steps were repeated until the mixture showed small clusters of cells (ranging from 2-10 cells). The subsequent addition of Matrigel to this mixture complete the medium into a new generation culture. When performed the drug sensitivity analysis after the organoids have grown in sufficient numbers. Generally, the morphological changes, size and quantity of organoids were observed every 12-24 hours after the addition of drugs to provide sufficient data support for accurately drawing the rea under curve.
Histopathological assessment of PCOs
Daily photographic documentation was employed to monitor morphological diversity and quantitative alterations from the initiation of organoid culture. After attaining a substantial organoid count, histological characterization and drug sensitivity assessments were conducted. Tissue samples and corresponding organoids were processed into formalin-fixed paraffin-embedded blocks, followed by routine hematoxylin-eosin staining. Additionally, Ki-67 staining, a marker of cell proliferation, was performed on deparaffinized sections following standard diagnostic protocols.
Chemotherapy drug sensitivity analysis
Chemotherapeutic agents were introduced approximately 72 hours after organoid seeding for the organoids that expanded to the fifth generation. The study utilized the following standard first-line therapies: Gemcitabine plus albumin-paclitaxel (AG regimen) and FOLFIRINOX (oxaliplatin, fluorouracil, irinotecan, leucovorin). Moreover, Food and Drug Administration-approved targeted therapies for PC, including poly ADP-ribose polymerase-1/2/3 inhibitors (olaparib) and epidermal growth factor receptor (EGFR) inhibitors (erlotinib and nituzumab), were incorporated into the PCOs susceptibility analysis. Drug reactivity was quantified using the area under curve and cell inhibition rate. The clinical efficacy of sensitive drugs was primarily gauged using progression-free survival and overall survival metrics, with a data cutoff date of October 15, 2023.
Statistical analysis
Normally distributed continuous variables in this study were presented as mean ± SD and analyzed via a two-tailed Student’s t test. Categorical variables underwent analysis using Fisher’s exact test in conjunction with χ2 test and Mann-Whitney U test (the variable has some degree of increase or decrease). Statistical analyses were performed using statistical product and service solutions (SPSS) software (version 25.0; SPSS Inc, Chicago, IL, United States), and flow charts were generated using R version 4.2.0 software (https://cran.r-project.org/).
RESULTS
Demographic and clinical profile of study participants
In this study, 16 patients with PC were recruited with 15 EUS pancreatic biopsy specimens were processed for PCO culture. Successful establishment of PCOs was achieved in 14 cases (93.3%); however, 5 of these did not progress beyond the fifth generation, as detailed in Table 1. The cohort consisted predominantly of man (10 patients, 66.7%), and the mean age was 60.6 ± 8.6 years. The average maximal diameter of the pancreatic tumors was 4.1 ± 1.5 cm. Most patients were diagnosed with locally advanced and unresectable PC, frequently exhibiting metastases to the liver, lungs, or peritoneum. According to the American Joint Committee on Cancer 8th edition, clinical tumor node metastasis stages were predominantly stage III-IV (12 patients, 80%). All participants were classified as pancreatic ductal adenocarcinoma except for one patient, identified as patient No.2, who was pathologically diagnosed with adenocarcinoma featuring sarcomatoid carcinoma. Analysis of the clinical data from these 15 samples indicated that patients with successful PCO cultures tended to have larger tumors and a history of diabetes and familial malignancies. However, these observations were not statistically significant and warrant further investigation with a larger sample size.
Table 1.
Baseline characteristics of the patients, n (%)
|
Characteristics
|
Organoid culture successful (n = 9)
|
Organoid culture unsuccessful (n = 6)
|
P value
|
| Sex (male) | 6 (66.7) | 3 (50.0) | 0.622 |
| Age (year), mean ± SD | 60.3 ± 3.5 | 69.6 ± 5.4 | 0.088 |
| BMI, mean ± SD | 22.7 ± 1.3 | 21.6 ± 1.3 | 0.361 |
| Smoking | 3 (33.3) | 1 (17.7) | 0.604 |
| Drinking | 3 (33.3) | 1 (17.7) | 0.604 |
| Tumor size (cm), mean ± SD | 4.2 ± 0.6 | 3.4 ± 0.3 | 0.159 |
| Tumor volume (cm3), mean ± SD | 72.4 ± 24.1 | 29.4 ± 8.8 | 0.085 |
| Hypertension | 1 (11.1) | 2 (33.3) | 0.525 |
| Diabetes | 4 (66.7) | 1 (16.7) | 0.580 |
| Family of history malignancy | 3 (33.3) | 0 (0) | 0.229 |
| Personal of history malignancy | 1 (11.1) | 0 (0) | 1.000 |
| History of abdominal operation | 1 (11.1) | 1 (16.7) | 1.000 |
| Location, head-neck | 5 (55.6) | 4 (66.7) | 0.608 |
| Clinical TNM staging, III/IV | 7 (77.8) | 6 (100.0) | 0.622 |
| T stage, T3-T4 | 6 (66.7) | 5 (83.3) | 0.245 |
| N stage, N1 | 7 (87.5) | 4 (66.7) | 0.538 |
| M stage, M1 | 4 (44.4) | 3 (50.5) | 1.000 |
| CA19-9 (U/mL), mean ± SD | 774.4 ± 299.4 | 1098.6 ± 382.7 | 0.349 |
| CA125, mean ± SD | 34.9 ± 11.5 | 55.3 ± 16.6 | 0.514 |
| CEA, mean ± SD | 4.7 ± 1.1 | 4.1 ± 0.7 | 0.372 |
| Time from initial diagnosis to puncture biopsy (month), mean ± SD | 2.5 ± 0.8 | 1.0 ± 0.1 | 0.526 |
| Ki-67 (%), mean ± SD | 28.9 ± 7.2 | 18.0 ± 3.7 | 0.264 |
| IHC, CA19-9 (+) | 7 (100.0) | 5 (83.3) | |
| IHC, CEA (+) | 5 (83.3) | 4 (100.0) | 1.000 |
| IHC, P53 (+) | 5 (71.4) | 3 (60.0) | 1.000 |
BMI: Body mass index; TNM: Tumor node metastasis; CA: Carbohydrate antigen; CEA: Carcinoembryonic antigen; IHC: Immunohistochemistry.
Compared with patients in the conventional group who did not undergo organoid culture during the same period, those in the culture group under similar circumstances (3 patients, 18.8%) had a small amount of mucosal bleeding at the puncture point during the operation (which could be relieved after depotassic renal adrenal saliner). Simultaneously, 10 patients in the conventional group (15.9%) showed no statistical difference between the two groups (P = 0.782). All patients did not develop pancreatitis requiring surgical treatment, infection and perforation, and their lives returned to normal 24 hours after EUS-FNB.
Influential factors in successful organoid cultivation from EUS-FNB PC samples
Our study further explored organoid cultures from PC samples obtained via EUS-FNB using a 22-gauge needle (Table 2). Based on findings from other studies[13], FNB typically yielded adequate diagnostic material with an initial needle puncture. Secondary needle biopsies from six patients often resulted in hemorrhagic samples, leading to tissue entrapment in erythrocytes and potentially reduced cell viability (15%-50%, 16.7% vs 55.6%, Table 2). This observation aligns with the findings of Lacomb et al[14], in which only one of these secondary samples successfully yielded an organoid culture (P = 0.005, Table 2). We obtained an organoid culture from a biopsy sample weighing 0.008 g. The earliest organoid emerged on the fourth day post-culture, exhibiting rapid growth and activity, and drug sensitivity testing was completed within 26 days. Only one sample was excluded from laboratory analysis because of negative onsite cytopathology results.
Table 2.
Pancreatic puncture sample information and organoid culture information, n (%)
|
Characteristics
|
Organoid culture successful (n = 9)
|
Organoid culture unsuccessful (n = 6)
|
P value
|
| Sample information | |||
| Puncture, 1st needle | 8 (89.0) | 1 (16.7) | 0.005 |
| Sample weight (g), mean ± SD | 0.0463 ± 0.0100 | 0.0387 ± 0.0041 | 0.494 |
| The time from isolation to organoid culture (hour), mean ± SD | 6.2 ± 1.5 | 18.7 ± 1.5 | 0.001 |
| Proportion of tumor cells in the sample | 0.785 | ||
| > 70% | 0 (0) | 0 (0) | |
| 20%-70% | 3 (33.3) | 2 (33.3) | |
| 5%-20% | 5 (55.6) | 4 (66.7) | |
| < 5% | 1 (11.1) | 0 (0) | |
| Cell activity | 0.288 | ||
| > 50% | 0 (0) | 0 (0) | |
| 15%-50% | 5 (55.6) | 1 (16.7) | |
| 5%-15% | 3 (33.3) | 5 (83.3) | |
| < 5% | 1 (11.1) | 0 (0) | |
| Sample quality score, mean ± SD | 55.9 ± 2.5 | 50.2 ± 1.9 | 0.120 |
| Organoid information | |||
| Organoids grow in the first day after processing | 8 (88.9) | 3 (50.0) | 0.107 |
| Organoid proliferation | 0.414 | ||
| Moderate | 1 (11.1) | 0 (0) | |
| Slow | 8 (88.9) | 6 (100.0) | |
| Organoid activity | 0.031 | ||
| > 50% | 0 (0) | 0 (0) | |
| 15%-50% | 5 (55.6) | 0 (0) | |
| 5%-15% | 4 (44.4) | 6 (100.0) | |
| < 5% | 0 (0) | 0 (0) | |
| Organoid quality score, mean ± SD | 64.2 ± 1.6 |
Our data suggest that the interval from sample collection to organoid culture initiation should ideally be < 24 hours. However, shorter processing times significantly enhanced PCO culture success (P ≤ 0.001, Table 2). The three dimensional microfluidic system potentially influences the general slow proliferation rate of organoids. Samples with less than 5% tissue cellularity still demonstrated high organoid activity post-initial formation, expanding to the fifth generation and completing drug sensitivity testing within 34 days. This indicates that organoid vitality (P = 0.031) is a critical factor for their rapid expansion (Table 2).
Morphological and histological analysis of PCOs
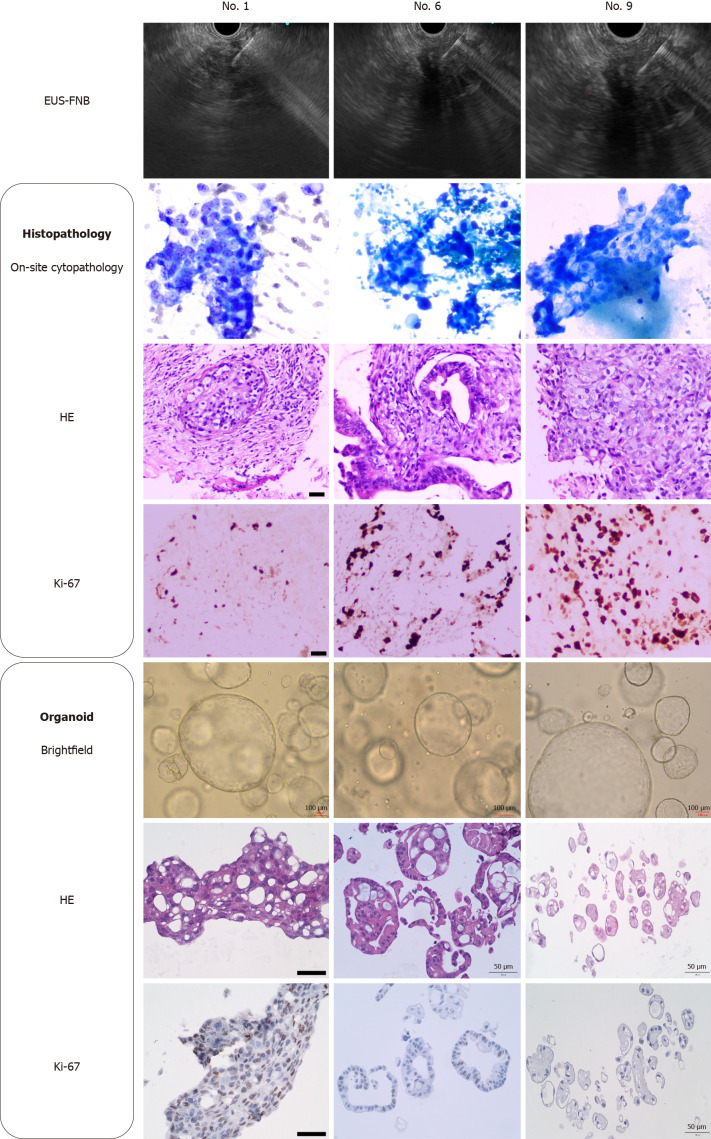
In this study, 60% (9 out of 15) of the PC specimens successfully expanded beyond the fifth passage for drug sensitivity assays, while the remaining six failed organoid cultivation owing to various factors, in agreement with previous studies[10,15]. Predominantly, the unsuccessful cases (4 out of 6, 66.7%) were halted because suboptimal organoid conditions or sluggish growth rates. Additionally, two specimens failed to initiate cultures, which was attributed to either low tumor cellularity or insufficient organoid formation, as observed under bright-field microscopy by day 5. Histological examinations were conducted on the nine successfully cultured samples. These analyses revealed morphological congruence between the catheter-like structures observed in the original PC biopsy specimens and their respective organoids (Figure 3).
Figure 3.
Histological identification of biopsy tissue and organoids from three samples (patient No. 1, No. 6 and No. 9). Bright-field images of pancreatic cancer organoids taken at 4 days of culture. Scale bars of organoid bright-field: 100 µm, scale bars of hematoxylin-eosin staining and Ki-67: 50 µm. EUS-FNB: Endoscopic ultrasound-guided fine-needle biopsy; HE: Hematoxylin-eosin staining.
Correlation of organoid drug sensitivity with clinical outcomes in PC
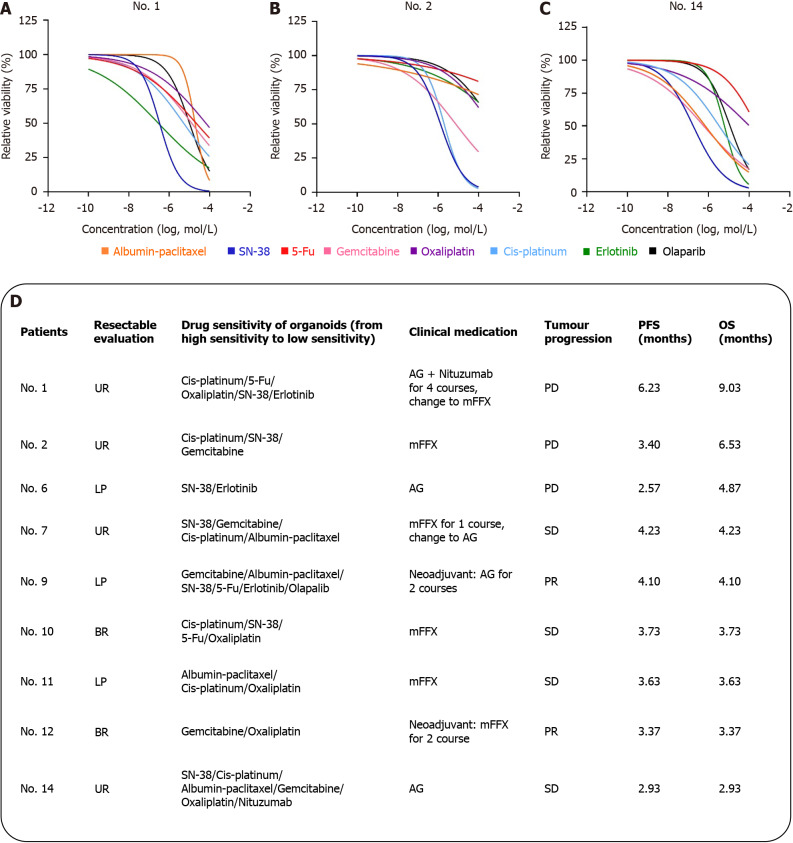
Figure 4 illustrates the 9 PCOs were successfully developed for drug sensitivity assays from the 15 patient specimens in our study. The average duration that covers all periods of culture, identification, drug sensitivity and freezing (2 tubes of organoids were frozen in each patient for subsequent resuscitation culture and molecular experiments) to complete these tests was 30 ± 3.1 days. Sensitivity analysis revealed varied responses: 33.3% of the patients (3 out of 9) showed the highest sensitivity to SN-38 or Cis-platinum, 22.2% (2 out of 9) to gemcitabine, and 11.1% (1 out of 9) to albumin-paclitaxel. Despite these findings, it is important to note that these drugs are only beneficial for a limited subset of PC patients[16,17]. Remarkably, two patients (No. 9 and No. 12) exhibited a partial tumor response, transitioning from a locally progressive or borderline resectable to a surgically resectable state, leading to successful tumor resection. Postoperative pathological assessment of neoadjuvant therapy using the college of American pathologists grading system (modified Ryan) indicated a grade 3 response (minimal tumor cell retreatment with predominant cell presence) and negative pathological resection margins.
Figure 4.
Drug sensitivity analysis and clinical relevance of organoids. A: Curve drug sensitivity curves of patients No. 1; B: Curve drug sensitivity curves of patients No. 2; C: Curve drug sensitivity curves of patients No. 14; D: Listed the sensitive drugs and clinical protocols for No. 9 patients. Assessment of resectable patients according to National Comprehensive Cancer Network guidelines: Unresectable, local progression, borderline resectable. Patient tumor response was measured using RESIST 1.1 criteria and categorized as partial response, stable disease, and progressive disease. AG: Gemcitabine combined with albumin-paclitaxel; mFFX: Modified FOLFIRINOX, oxaliplatin, irinotecan, fluorouracil and calcium leucovorin; UR: Unresectable; LP: Local progression; BR: Borderline resectable; 5-Fu: Fluorouracil; PD: Progressive disease; SD: Stable disease; PR: Partial response; OS: Overall survival; PFS: Progression-free survival.
However, the median follow-up duration in this study was relatively short, at 4.1 months (interquartile range 3.5-5.7 months), with no recorded mortality during this period. Further longitudinal studies are required to comprehensively evaluate the long-term clinical outcomes associated with drug responses.
DISCUSSION
In this study, we used a semi-suspended medium in conjunction with a microfluidic system to cultivate PCOs. This approach, coupled with efficient EUS-FNB techniques, enabled the acquisition of ample samples with minimal needle usage, thus forming the cornerstone of successful organoid culture. Rapid sample processing and refined culture methodologies have significantly enhanced the success rate of PCOs culture.
PC is known for its high malignancy, low resection rates, and limited drug sensitivity, which contributes to its persistently high mortality rate. The lethality of PC is largely attributed to the tumor microenvironment, characterized by complex cell-cell and paracrine interactions, particularly between cancer-associated fibroblasts and cancer cells, which contribute to resistance against multi-agent chemotherapy[18]. The National Comprehensive Cancer Network 2019 guidelines advocate universal germline testing for patients with PC[19], highlighting prevalent mutations in oncogenes like KRAS (90%-95%) and tumor suppressor genes such as CDKN2A, TP53, and SMAD4[20]. However, challenges in targeting these mutations, particularly KRAS, owing to their high affinity for guanosine triphosphate/guanosine diphosphate and escape mechanisms limit the clinical impact of small molecule inhibitors[21]. Current precision medicine models focus on maintaining mutation fidelity but often overlook RNA states and expression heterogeneity[22]. Recent findings indicate a novel intermediate PC transcription cell state, underscoring the influence of tumor microenvironments on cancer cell transcriptional phenotypes and drug responses[23]. This plasticity of PC cells and their microenvironment presents exploitable vulnerabilities. As pioneered by Boj et al[24], organoid technology for PC was pioneered in 2015, which offers a comprehensive model encompassing tumor and microenvironmental aspects. The unparalleled advantages in stability, congruence with human PC cells, and drug sensitivity testing[25,26] provided by organoids play a pivotal role in disease mechanism exploration, drug efficacy, multi-omics sequencing, personalized medicine, and tissue regeneration compared to those by traditional models, such as 2 dimensional cell cultures, patient-derived xenografts, and genetically engineered mouse models[27,28].
The successful culture of PCOs from EUS-FNB has opened new avenues in precision medicine for advanced PC, where in most patients (80%) are relegated to pharmacotherapy[6]. However, challenges such as low success rates, difficulty in long-term expansion, and prolonged culture periods (approximately 2 to 3 months) of EUS-FNB samples impede personalized treatment strategies[9,10]. Our study addressed these issues through strategic optimizations in needle selection, puncture site, sample transportation, medium selection, and culture conditions. The choice of a 22-gauge FNB and the precise selection of the biopsy site were pivotal in our study for acquiring adequate tumor cellular material. The limited tumor cell yield in PC biopsies resulting from the fibrotic microenvironment surrounding the PC cells[29,30] poses a significant challenge for PCOs cultivation. Our technique involved a fan-shaped puncture approach that targets the periphery of the lesion and continuously adjusting the needle angle to optimize sample collection. Necrotic regions that can detrimentally affect organoid production should be avoided[31]. Prompt delivery of the biopsy specimen to the laboratory for processing and following the on-site pathological confirmation was crucial. This rapid transfer, facilitated by a temperature-controlled transport box, ensured optimal preservation of the organoid culture medium at 4 °C, a factor influencing the quality of tumor cells, diverging from the 24 hours time frame cited in previous studies[6].
The KingMed system employed in our study is a specialized organoid culture medium for PC. Organoid culture media have been shown to significantly influence the response to standard chemotherapies and the morphological and transcriptomic profiles within the same organoid culture[32]. This underscores the critical role of the culture medium. The KingMed system, which utilizes the properties of Matrigel, enables a semi-suspended culture technique for early stage pancreatic tumor organoids, effectively reducing early cell apoptosis. This approach facilitated the growth of the first organoid within four days in most of our samples (93.3%), surpassing the growth rates observed in organoids derived from surgical specimens[33].
Pancreatic intraepithelial lesion increases extracellular matrix deposition and the stroma amount up to 90% of the whole tumor volume. Moreover, PC is characterized by high interstitial pressure and occurs in a hypoxic conditions[34]. Most in vitro PC models have limited translational relevance, as these fail to recapitulate relevant aspects of PC complexity. Microfluidic systems can provide a biomechanical microenvironment for growing cells. Compared to traditional well plate cultures, organs-on-a-chip culture require only a few cells for experimental studies. Hence, it was feasible to use these for patient stratification and for individualization of therapies[35]. Additionally, the incorporation of a microfluidic system simulated the in vivo fluid dynamics, thereby more accurately replicating the original tissue microenvironment. Despite these advancements, a subset of specimens (33.3%) failed to progress to the fifth generation, precluding drug sensitivity analysis, a phenomenon referred to as “lack of expansion” in the literature[28]. Clinically, only a minority of EGFR-inhibitors-responsive patients proceeded with inhibitor therapy because of the factors such as uncertain efficacy, patient preference, drug availability, side effects, and cost considerations.
Although our study was limited by the small number of cases, the two groups (successful and unsuccessful) could not overcome the bias of properties of each tumor. In the future, it will be successful PCOs cultivation through univariate and multivariate analysis. Furthermore, drug sensitivity analysis can be conducted immediately without having to grow for five generations to enhance the efficiency of drug sensitivity analysis in organoids and effectively meet the demands of rapid durg utilization. Optimization of drug concentration and combination proportions are also pivotal aspects that require attention in future endeavors. Our study provides valuable insights into the culture of organoids from endoscopic needle biopsies in PC. This methodology potentially supports high-throughput drug screening[36,37], co-culture technologies[38], and advancements in regenerative medicine for PC[39].
CONCLUSION
The quality of a biopsy sample is fundamental for successful organoid culture. Our study delineates a viable process for culturing organoids from PC needle biopsies, facilitating the evaluation of drug sensitivities and enabling a multidimensional approach to personalized medicine. The next critical step is to harness this promising tool to provide more detailed insights into the mechanisms of early detection, progression, and drug resistance in PC.
Footnotes
Institutional review board statement: This study was reviewed and approved by the Ethics Committee of Chongqing General Hospital. The ethics review number: No. KY S2022-045-01.
Clinical trial registration statement: The protocol registered at Chinese Clinical Trial Registry. The registration identification number: No. ChiCTR2200064388.
Informed consent statement: All participants or their legal guardian signed informed consent forms allowing their puncture samples and medical data to be used for scientific research.
Conflict-of-interest statement: The authors declare that there is no conflict of interest.
CONSORT 2010 statement: The authors have read the CONSORT 2010 Statement, and the manuscript was prepared and revised according to the CONSORT 2010 Statement.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade C, Grade C
Novelty: Grade B, Grade C
Creativity or Innovation: Grade B, Grade C
Scientific Significance: Grade B, Grade B
P-Reviewer: Zhang HJ S-Editor: Fan M L-Editor: A P-Editor: Yuan YY
Contributor Information
Jia-Li Yang, Institute of Hepatopancreatobiliary Surgery, Chongqing General Hospital, Chongqing University, Chongqing 401147, China.
Jun-Feng Zhang, Institute of Hepatopancreatobiliary Surgery, Chongqing General Hospital, Chongqing University, Chongqing 401147, China.
Jian-You Gu, Institute of Hepatopancreatobiliary Surgery, Chongqing General Hospital, Chongqing University, Chongqing 401147, China.
Mei Gao, Institute of Hepatopancreatobiliary Surgery, Chongqing General Hospital, Chongqing University, Chongqing 401147, China.
Ming-You Zheng, Institute of Hepatopancreatobiliary Surgery, Chongqing General Hospital, Chongqing University, Chongqing 401147, China.
Shi-Xiang Guo, Institute of Hepatopancreatobiliary Surgery, Chongqing General Hospital, Chongqing University, Chongqing 401147, China.
Tao Zhang, Institute of Hepatopancreatobiliary Surgery, Chongqing General Hospital, Chongqing University, Chongqing 401147, China. tzhang04@126.com.
Data sharing statement
Datasets generated during the current study are available upon reasonable request.
References
- 1.Park W, Chawla A, O'Reilly EM. Pancreatic Cancer: A Review. JAMA. 2021;326:851–862. doi: 10.1001/jama.2021.13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halbrook CJ, Lyssiotis CA, Pasca di Magliano M, Maitra A. Pancreatic cancer: Advances and challenges. Cell. 2023;186:1729–1754. doi: 10.1016/j.cell.2023.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kandel P, Wallace MB. Advanced EUS Guided Tissue Acquisition Methods for Pancreatic Cancer. Cancers (Basel) 2018;10 doi: 10.3390/cancers10020054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Archibugi L, Testoni SGG, Redegalli M, Petrone MC, Reni M, Falconi M, Doglioni C, Capurso G, Arcidiacono PG. New era for pancreatic endoscopic ultrasound: From imaging to molecular pathology of pancreatic cancer. World J Gastrointest Oncol. 2019;11:933–945. doi: 10.4251/wjgo.v11.i11.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakai Y, Chang KJ. EUS-guided fine-needle injection for pancreatic cancer: back to the future. Gastrointest Endosc. 2020;92:1053–1054. doi: 10.1016/j.gie.2020.06.025. [DOI] [PubMed] [Google Scholar]
- 6.Tiriac H, Bucobo JC, Tzimas D, Grewel S, Lacomb JF, Rowehl LM, Nagula S, Wu M, Kim J, Sasson A, Vignesh S, Martello L, Munoz-Sagastibelza M, Somma J, Tuveson DA, Li E, Buscaglia JM. Successful creation of pancreatic cancer organoids by means of EUS-guided fine-needle biopsy sampling for personalized cancer treatment. Gastrointest Endosc. 2018;87:1474–1480. doi: 10.1016/j.gie.2017.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vilgelm AE, Bergdorf K, Wolf M, Bharti V, Shattuck-Brandt R, Blevins A, Jones C, Phifer C, Lee M, Lowe C, Hongo R, Boyd K, Netterville J, Rohde S, Idrees K, Bauer JA, Westover D, Reinfeld B, Baregamian N, Richmond A, Rathmell WK, Lee E, McDonald OG, Weiss VL. Fine-Needle Aspiration-Based Patient-Derived Cancer Organoids. iScience. 2020;23:101408. doi: 10.1016/j.isci.2020.101408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y, Li N, Zhu Y. Pancreatic Organoids: A Frontier Method for Investigating Pancreatic-Related Diseases. Int J Mol Sci. 2023;24 doi: 10.3390/ijms24044027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tong T, Zhang C, Li J, Deng M, Wang X. Preclinical models derived from endoscopic ultrasound-guided tissue acquisition for individualized treatment of pancreatic ductal adenocarcinoma. Front Med (Lausanne) 2022;9:934974. doi: 10.3389/fmed.2022.934974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JH, Kim H, Lee SH, Ku JL, Chun JW, Seo HY, Kim SC, Paik WH, Ryu JK, Lee SK, Lowy AM, Kim YT. Establishment of Patient-Derived Pancreatic Cancer Organoids from Endoscopic Ultrasound-Guided Fine-Needle Aspiration Biopsies. Gut Liver. 2022;16:625–636. doi: 10.5009/gnl210166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mathew G, Agha R, Albrecht J, Goel P, Mukherjee I, Pai P, D'Cruz AK, Nixon IJ, Roberto K, Enam SA, Basu S, Muensterer OJ, Giordano S, Pagano D, Machado-Aranda D, Bradley PJ, Bashashati M, Thoma A, Afifi RY, Johnston M, Challacombe B, Ngu JC, Chalkoo M, Raveendran K, Hoffman JR, Kirshtein B, Lau WY, Thorat MA, Miguel D, Beamish AJ, Roy G, Healy D, Ather HM, Raja SG, Mei Z, Manning TG, Kasivisvanathan V, Rivas JG, Coppola R, Ekser B, Karanth VL, Kadioglu H, Valmasoni M, Noureldin A STROCSS Group. STROCSS 2021: Strengthening the reporting of cohort, cross-sectional and case-control studies in surgery. Int J Surg. 2021;96:106165. doi: 10.1016/j.ijsu.2021.106165. [DOI] [PubMed] [Google Scholar]
- 12.Park JK, Lee JH, Noh DH, Park JK, Lee KT, Lee JK, Lee KH, Jang KT, Cho J. Factors of Endoscopic Ultrasound-Guided Tissue Acquisition for Successful Next-Generation Sequencing in Pancreatic Ductal Adenocarcinoma. Gut Liver. 2020;14:387–394. doi: 10.5009/gnl19011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bang JY, Hawes R, Varadarajulu S. A meta-analysis comparing ProCore and standard fine-needle aspiration needles for endoscopic ultrasound-guided tissue acquisition. Endoscopy. 2016;48:339–349. doi: 10.1055/s-0034-1393354. [DOI] [PubMed] [Google Scholar]
- 14.Lacomb JF, Plenker D, Tiriac H, Bucobo JC, D'souza LS, Khokhar AS, Patel H, Channer B, Joseph D, Wu M, Tuveson DA, Li E, Buscaglia JM. Single-Pass vs 2-Pass Endoscopic Ultrasound-Guided Fine-Needle Biopsy Sample Collection for Creation of Pancreatic Adenocarcinoma Organoids. Clin Gastroenterol Hepatol. 2021;19:845–847. doi: 10.1016/j.cgh.2020.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hermans E, Van der Merwe SW, Depreeuw J, Dekervel J, Radaelli E, Roskams T, van Pelt Jos J, Topal B, Verslype C, Prenen H, Van Steenbergen W, Nevens F, Lambrechts D, Amant F. Successful application of endoscopic ultrasound-guided fine needle biopsy to establish pancreatic patient-derived tumor xenografts: a pilot study. Endoscopy. 2016;48:1016–1022. doi: 10.1055/s-0042-113597. [DOI] [PubMed] [Google Scholar]
- 16.Zhu H, Wei M, Xu J, Hua J, Liang C, Meng Q, Zhang Y, Liu J, Zhang B, Yu X, Shi S. PARP inhibitors in pancreatic cancer: molecular mechanisms and clinical applications. Mol Cancer. 2020;19:49. doi: 10.1186/s12943-020-01167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med. 2008;358:1160–1174. doi: 10.1056/NEJMra0707704. [DOI] [PubMed] [Google Scholar]
- 18.Ho WJ, Jaffee EM, Zheng L. The tumour microenvironment in pancreatic cancer - clinical challenges and opportunities. Nat Rev Clin Oncol. 2020;17:527–540. doi: 10.1038/s41571-020-0363-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crowley F, Gandhi S, Rudshteyn M, Sehmbhi M, Cohen DJ. Adherence to NCCN Genetic Testing Guidelines in Pancreatic Cancer and Impact on Treatment. Oncologist. 2023;28:486–493. doi: 10.1093/oncolo/oyad044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wood LD, Canto MI, Jaffee EM, Simeone DM. Pancreatic Cancer: Pathogenesis, Screening, Diagnosis, and Treatment. Gastroenterology. 2022;163:386–402.e1. doi: 10.1053/j.gastro.2022.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buscail L, Bournet B, Cordelier P. Role of oncogenic KRAS in the diagnosis, prognosis and treatment of pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2020;17:153–168. doi: 10.1038/s41575-019-0245-4. [DOI] [PubMed] [Google Scholar]
- 22.Whitcomb DC, Shelton CA, Brand RE. Genetics and Genetic Testing in Pancreatic Cancer. Gastroenterology. 2015;149:1252–1264.e4. doi: 10.1053/j.gastro.2015.07.057. [DOI] [PubMed] [Google Scholar]
- 23.Raghavan S, Winter PS, Navia AW, Williams HL, DenAdel A, Lowder KE, Galvez-Reyes J, Kalekar RL, Mulugeta N, Kapner KS, Raghavan MS, Borah AA, Liu N, Väyrynen SA, Costa AD, Ng RWS, Wang J, Hill EK, Ragon DY, Brais LK, Jaeger AM, Spurr LF, Li YY, Cherniack AD, Booker MA, Cohen EF, Tolstorukov MY, Wakiro I, Rotem A, Johnson BE, McFarland JM, Sicinska ET, Jacks TE, Sullivan RJ, Shapiro GI, Clancy TE, Perez K, Rubinson DA, Ng K, Cleary JM, Crawford L, Manalis SR, Nowak JA, Wolpin BM, Hahn WC, Aguirre AJ, Shalek AK. Microenvironment drives cell state, plasticity, and drug response in pancreatic cancer. Cell. 2021;184:6119–6137.e26. doi: 10.1016/j.cell.2021.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boj SF, Hwang CI, Baker LA, Chio II, Engle DD, Corbo V, Jager M, Ponz-Sarvise M, Tiriac H, Spector MS, Gracanin A, Oni T, Yu KH, van Boxtel R, Huch M, Rivera KD, Wilson JP, Feigin ME, Öhlund D, Handly-Santana A, Ardito-Abraham CM, Ludwig M, Elyada E, Alagesan B, Biffi G, Yordanov GN, Delcuze B, Creighton B, Wright K, Park Y, Morsink FH, Molenaar IQ, Borel Rinkes IH, Cuppen E, Hao Y, Jin Y, Nijman IJ, Iacobuzio-Donahue C, Leach SD, Pappin DJ, Hammell M, Klimstra DS, Basturk O, Hruban RH, Offerhaus GJ, Vries RG, Clevers H, Tuveson DA. Organoid models of human and mouse ductal pancreatic cancer. Cell. 2015;160:324–338. doi: 10.1016/j.cell.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen H, Zhuo Q, Ye Z, Xu X, Ji S. Organoid model: A new hope for pancreatic cancer treatment? Biochim Biophys Acta Rev Cancer. 2021;1875:188466. doi: 10.1016/j.bbcan.2020.188466. [DOI] [PubMed] [Google Scholar]
- 26.Tuveson D, Clevers H. Cancer modeling meets human organoid technology. Science. 2019;364:952–955. doi: 10.1126/science.aaw6985. [DOI] [PubMed] [Google Scholar]
- 27.Tiriac H, Plenker D, Baker LA, Tuveson DA. Organoid models for translational pancreatic cancer research. Curr Opin Genet Dev. 2019;54:7–11. doi: 10.1016/j.gde.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grossman JE, Muthuswamy L, Huang L, Akshinthala D, Perea S, Gonzalez RS, Tsai LL, Cohen J, Bockorny B, Bullock AJ, Schlechter B, Peters MLB, Conahan C, Narasimhan S, Lim C, Davis RB, Besaw R, Sawhney MS, Pleskow D, Berzin TM, Smith M, Kent TS, Callery M, Muthuswamy SK, Hidalgo M. Organoid Sensitivity Correlates with Therapeutic Response in Patients with Pancreatic Cancer. Clin Cancer Res. 2022;28:708–718. doi: 10.1158/1078-0432.CCR-20-4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allaway RJ, Fischer DA, de Abreu FB, Gardner TB, Gordon SR, Barth RJ, Colacchio TA, Wood M, Kacsoh BZ, Bouley SJ, Cui J, Hamilton J, Choi JA, Lange JT, Peterson JD, Padmanabhan V, Tomlinson CR, Tsongalis GJ, Suriawinata AA, Greene CS, Sanchez Y, Smith KD. Genomic characterization of patient-derived xenograft models established from fine needle aspirate biopsies of a primary pancreatic ductal adenocarcinoma and from patient-matched metastatic sites. Oncotarget. 2016;7:17087–17102. doi: 10.18632/oncotarget.7718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Puleo F, Nicolle R, Blum Y, Cros J, Marisa L, Demetter P, Quertinmont E, Svrcek M, Elarouci N, Iovanna J, Franchimont D, Verset L, Galdon MG, Devière J, de Reyniès A, Laurent-Puig P, Van Laethem JL, Bachet JB, Maréchal R. Stratification of Pancreatic Ductal Adenocarcinomas Based on Tumor and Microenvironment Features. Gastroenterology. 2018;155:1999–2013.e3. doi: 10.1053/j.gastro.2018.08.033. [DOI] [PubMed] [Google Scholar]
- 31.Kim S, Woo KJ, Yang CM, Park SH, Hwang JC, Yoo BM, Kim JH, Lee D, Yang MJ. Simultaneous establishment of pancreatic cancer organoid and cancer-associated fibroblast using a single-pass endoscopic ultrasound-guided fine-needle biopsy specimen. Dig Endosc. 2023;35:918–926. doi: 10.1111/den.14648. [DOI] [PubMed] [Google Scholar]
- 32.Hogenson TL, Xie H, Phillips WJ, Toruner MD, Li JJ, Horn IP, Kennedy DJ, Almada LL, Marks DL, Carr RM, Toruner M, Sigafoos AN, Koenig-Kappes AN, Olson RL, Tolosa EJ, Zhang C, Li H, Doles JD, Bleeker J, Barrett MT, Boyum JH, Kipp BR, Mahipal A, Hubbard JM, Scheffler Hanson TJ, Petersen GM, Dasari S, Oberg AL, Truty MJ, Graham RP, Levy MJ, Zhu M, Billadeau DD, Adjei AA, Dusetti N, Iovanna JL, Bekaii-Saab TS, Ma WW, Fernandez-Zapico ME. Culture media composition influences patient-derived organoid ability to predict therapeutic responses in gastrointestinal cancers. JCI Insight. 2022;7 doi: 10.1172/jci.insight.158060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hennig A, Wolf L, Jahnke B, Polster H, Seidlitz T, Werner K, Aust DE, Hampe J, Distler M, Weitz J, Stange DE, Welsch T. CFTR Expression Analysis for Subtyping of Human Pancreatic Cancer Organoids. Stem Cells Int. 2019;2019:1024614. doi: 10.1155/2019/1024614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geyer M, Queiroz K. Microfluidic Platforms for High-Throughput Pancreatic Ductal Adenocarcinoma Organoid Culture and Drug Screening. Front Cell Dev Biol. 2021;9:761807. doi: 10.3389/fcell.2021.761807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van den Berg A, Mummery CL, Passier R, van der Meer AD. Personalised organs-on-chips: functional testing for precision medicine. Lab Chip. 2019;19:198–205. doi: 10.1039/c8lc00827b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maloney E, Clark C, Sivakumar H, Yoo K, Aleman J, Rajan SAP, Forsythe S, Mazzocchi A, Laxton AW, Tatter SB, Strowd RE, Votanopoulos KI, Skardal A. Immersion Bioprinting of Tumor Organoids in Multi-Well Plates for Increasing Chemotherapy Screening Throughput. Micromachines (Basel) 2020;11 doi: 10.3390/mi11020208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duan X, Zhang T, Feng L, de Silva N, Greenspun B, Wang X, Moyer J, Martin ML, Chandwani R, Elemento O, Leach SD, Evans T, Chen S, Pan FC. A pancreatic cancer organoid platform identifies an inhibitor specific to mutant KRAS. Cell Stem Cell. 2024;31:71–88.e8. doi: 10.1016/j.stem.2023.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perelló-Reus CM, Rubio-Tomás T, Cisneros-Barroso E, Ibargüen-González L, Segura-Sampedro JJ, Morales-Soriano R, Barceló C. Challenges in precision medicine in pancreatic cancer: A focus in cancer stem cells and microbiota. Front Oncol. 2022;12:995357. doi: 10.3389/fonc.2022.995357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Casamitjana J, Espinet E, Rovira M. Pancreatic Organoids for Regenerative Medicine and Cancer Research. Front Cell Dev Biol. 2022;10:886153. doi: 10.3389/fcell.2022.886153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Datasets generated during the current study are available upon reasonable request.