Abstract
BACKGROUND
Esophageal melanosis (EM) is a rare condition characterized by melanin pigmentation in the esophageal mucosa. It is not well understood and has been documented in less than 100 cases worldwide.
CASE SUMMARY
We report two cases of African American patients who complained of significant weight loss (over 20 pounds in approximately six months) and abdominal pain during their first visit. The first case involves a 54-year female with a history of hepatic steatosis and polysubstance abuse, who also experiences nausea and vomiting. The second case is a 59-year-old male with hypertension and gastroesophageal reflux disease (GERD), who was diagnosed with esophageal squamous cell carcinoma. Both cases show benign melanocytes in the basal layer on the esophagus biopsy and are diagnosed as EM.
CONCLUSION
It is important to note that EM has been associated with malignancies such as carcinoma and melanoma. Therefore, accurate diagnosis and appropriate management are crucial. Patients with EM, especially those with concurrent risk factors (e.g., GERD, smoking), should be carefully monitored for any signs of malignancy.
Keywords: Esophagus, Melanoblasts, Esophageal melanosis, Gastroesophageal reflux disease, Case report
Core Tip: Esophageal melanosis is a rare condition. Its causes and natural progression are not fully understood. Some studies have reported an association with malignancy and as a potential precursor for malignancy. Extended research is required to establish an additional correlation for this rare entity.
INTRODUCTION
De La Pava et al[1] first described melanoblasts in esophageal mucosa in 1963, with its prevalence reported as 4% in autopsy cases. The recognition of this condition has been emphasized over the past few decades. While there is some correlation between microscopic and endoscopic findings, microscopic diagnosis is more common than endoscopic diagnosis due to the requirement of a large number of melanocytes to be present for it to be visible during endoscopy[2]. The specific cause of this condition and its connection to other medical conditions is still not fully understood. Some reports have suggested a link between esophageal melanosis (EM), esophageal malignancies, and esophageal injury[1,3,4]. While there is a strong connection between gastroesophageal reflux disease (GERD) and chronic esophagitis, the association with Barrett’s esophagus has not been confirmed[5]. Recent reviews identified only fifty-five reported cases of EM[6-8]. This exceptionally rare condition significantly hinders the ability of endoscopists to diagnose and follow up. We aim to raise awareness of this rare condition by presenting two cases of EM and reviewing the existing literature.
This study retrospectively assessed the data of patients who underwent upper gastrointestinal endoscopy at our hospital between March 2021 and June 2024. Of these, two patients of EM were identified. The demographic, clinical, endoscopic, medication uses, histopathologic and immunohistochemical stains evaluation was conducted by two gastrointestinal pathologists independently. This retrospective study was approved by our institutional review board, which waived the requirement for informed consent. Patient anonymity was ensured prior to assessment of the data.
A review of the literature was conducted with the use of PubMed (https://pubmed.ncbi.nlm.nih.gov/?term=esophageal%20melanosis&filter=years.1979-2024&page=8). Search terms EM were used and yielded 80 results; dates of publications were between 1980-2024. The titles and abstracts of these publications were screened, and only articles containing actual case reports were included. A total of 55 case reports were identified, and literature on EM was reviewed. Full texts of all 33 articles containing 55 reported cases were examined (Table 1)[3,5-7,9-33]. From the data collected, we proposed updates on EM’s clinical, histopathologic, and immunohistochemical features.
Table 1.
Review of published cases of clinical and pathological characteristics of esophageal melanosis
|
Ref.
|
Age
|
Sex
|
Esophagus location
|
Exact location of the lesion
|
Country
|
Associated clinical diagnosis
|
Clinical symptoms
|
Medication
|
Smoking
|
Alcohol drinking
|
| Kuo et al[9], 2011 | 76 | F | NA | Not specified | Australia | Celiac disease | NA | Azathioprine | NA | NA |
| Dubail et al[7], 2022 | 57 | F | Middle/lower esophagus | Middle and lower esophagus | Beljium | Peptic ulcer disease | NA | PPI | Yes | No |
| Dubail et al[7], 2022 | 66 | M | Upper esophagus | Upper esophagus | Beljium | Gastritis | NA | NA | No | Yes |
| Horowitz et al[10], 1998 | 49 | M | NA | Not specified | Brazil | Anal melanoma | NA | NA | NA | NA |
| Dumas et al[11], 1990 | 66 | F | Lower esophagus | 32-35 cm from incisor | Cedax | Mental anorexia | NA | NA | NA | NA |
| Bogomoletz et al[12], 1997 | 75 | M | Middle esophagus | 20 cm below Killian’s line | France | NA | Epigastric pain, dysphagia | NA | NA | NA |
| Maroy et al[13], 2013 | 65 | M | Middle/lower esophagus | Mid esophagus 19 cm (1st lesion) and distal esophagus 38 cm (2nd lesion) | France | Tonsil squamous cell carcinoma | NA | NA | Yes | Yes |
| Sarbia et al[14], 2012 | 69 | M | Middle/lower esophagus | Middle and the lower thirds of the esophagus | Germany | Adenocarcinoma of the esophagogastric junction, acanthosis nigricans of the esophagus | Pain | NA | NA | NA |
| Kleikamp et al[15], 2006 | 48 | M | NA | No data | Germany | Adenocarcinoma of the esophagogastric junction, acanthosis nigricans of the esophagus | Pain | NA | NA | NA |
| Agarwal et al[6], 2022 | 53 | F | Middle/lower esophagus | Mid and lower esophagus | India | Iron deficiency anemia | NA | Iron | NA | NA |
| Sharma et al[5], 1991 | 50 | M | Lower esophagus | Lower third of esophagus | India | Peptic ulcer disease | NA | NA | NA | NA |
| Sharma et al[5], 1991 | 60 | M | Lower esophagus | Lower third of esophagus | India | Peptic ulcer disease | NA | NA | NA | NA |
| Sharma et al[5], 1991 | 68 | M | Middle esophagus | Middle third of esophagus | India | Peptic ulcer disease | NA | NA | NA | NA |
| Sharma et al[5], 1991 | 51 | F | Middle esophagus | Middle third of esophagus | India | Peptic ulcer disease | NA | NA | NA | NA |
| Sharma et al[5], 1991 | 57 | F | Middle esophagus | Middle third of esophagus | India | Peptic ulcer disease | NA | NA | NA | NA |
| Sharma et al[5], 1991 | 70 | M | Middle esophagus | Middle third of esophagus | India | Peptic ulcer disease | NA | NA | NA | NA |
| Sharma et al[5], 1991 | 45 | M | Upper esophagus | Upper third of esophagus | India | Peptic ulcer disease | NA | NA | NA | NA |
| Sharma et al[5], 1991 | 55 | M | Upper esophagus | Upper third of the esophagus | India | Peptic ulcer disease | NA | NA | NA | NA |
| Sharma et al[5], 1991 | 60 | M | Middle esophagus | Middle third of esophagus | India | Peptic ulcer disease | Non-ulcer dyspepsia | NA | NA | NA |
| Sharma et al[5], 1991 | 50 | F | Middle esophagus | Middle third of esophagus | India | NA | Non-ulcer dyspepsia | NA | NA | NA |
| Sharma et al[5], 1991 | 49 | M | Lower esophagus | Lower third of esophagus | India | NA | Non-ulcer dyspepsia | NA | NA | NA |
| Sharma et al[5], 1991 | 40 | F | Middle esophagus | Middle third of esophagus | India | NA | Non-ulcer dyspepsia | NA | NA | NA |
| Sharma et al[5], 1991 | 43 | F | Middle esophagus | Middle third of esophagus | India | NA | Non-ulcer dyspepsia | NA | NA | NA |
| Sharma et al[5], 1991 | 48 | M | Middle esophagus | Middle third of esophagus | India | NA | Non-ulcer dyspepsia | NA | NA | NA |
| Sharma et al[5], 1991 | 50 | M | Middle esophagus | Middle third of esophagus | India | NA | Non-ulcer dyspepsia | NA | NA | NA |
| Sharma et al[5], 1991 | 58 | M | Middle esophagus | Middle third of esophagus | India | NA | Non-ulcer dyspepsia | NA | NA | NA |
| Sharma et al[5], 1991 | 59 | M | Middle esophagus | Middle third of esophagus | India | NA | Non-ulcer dyspepsia | NA | NA | NA |
| Kumari et al[16], 2016 | 66 | M | NA | No data | India | NA | Chest pain and dysphagia | NA | NA | NA |
| Sharma et al[5], 1991 | 50 | M | Upper esophagus | Upper third of esophagus | India | NA | Non-ulcer dyspepsia | NA | NA | NA |
| Sharma et al[5], 1991 | 56 | M | Middle esophagus | Middle third of esophagus | India | Gastritis | NA | NA | NA | NA |
| Sharma et al[5], 1991 | 59 | M | Middle esophagus | Middle third of esophagus | India | Gastritis | NA | NA | NA | NA |
| Sharma et al[5], 1991 | 48 | M | Middle esophagus | Middle third of esophagus | India | Gastritis | NA | NA | NA | NA |
| Vincent Comraj et al[17], 2023 | 70 | M | NA | No data | India | Gastritis | NA | NA | No | No |
| Geramizadeh et al[18], 2014 | 75 | M | Middle esophagus | Middle part of the esophagus | Iran | NA | Epigastric pain and dysphagia | NA | Yes | No |
| Kinugasa et al[19], 2020 | 77 | M | Lower esophagus | Lower part of the esophagus | Japan | Eosinophilic esophagitis | NA | Antihypertensive medications | NA | NA |
| Mori et al[20], 2005 | 70 | M | Lower esophagus | 35 cm from the incisor teeth | Japan | None | NA | NA | NA | NA |
| Mori et al[20], 2005 | 60 | F | Lower esophagus | 30 cm from the incisor | Japan | NA | Dysphagia | NA | NA | NA |
| Yamazaki et al[21], 1991 | 56 | M | Lower esophagus | 30-33 cm from incisor | Japan | NA | NA | NA | NA | NA |
| Yamazaki et al[21], 1991 | 62 | M | Lower esophagus | 35 cm from incisor | Japan | NA | NA | NA | NA | NA |
| Yamazaki et al[21], 1991 | 70 | F | Lower esophagus | Lower third of esophagus | Japan | NA | NA | NA | NA | NA |
| Yamazaki et al[21], 1991 | 66 | M | Lower esophagus | Lower third of esophagus | Japan | NA | NA | NA | NA | NA |
| Yamamoto et al[22], 1999 | 62 | F | Lower esophagus | Middle esophagus 30 cm from the incisor | Japan | Laugier-Hunziker syndrome | NA | NA | NA | NA |
| Kaneko et al[23], 2022 | 62 | M | Middle esophagus | Middle thoracic esophagus | Japan | Hypopharyngeal cancer, | NA | NA | NA | NA |
| Kato et al[24], 2013 | 71 | M | Middle esophagus | Middle thoracic esophagus | Japan | Esophageal melanoma | NA | NA | NA | NA |
| Oshiro et al[25], 2007 | 71 | M | Lower esophagus | 35 cm away from the incisor | Japan | Esophageal melanoma | NA | PPI | NA | NA |
| Suzuki et al[26], 2008 | 67 | M | Lower esophagus | Lower thoracic esophagus | Japan | Esophageal melanoma | NA | NA | Yes | No |
| Suzuki et al[26], 2008 | 62 | M | Upper/middle esophagus | Upper to middle thoracic esophagus | Japan | Esophageal melanoma | NA | NA | Yes | Yes |
| Walter et al[27], 2000 | 64 | F | Middle/lower esophagus | Lower half of the esophagectomy specimen | Netherlands | Esophageal SCC | NA | PPI | Yes | Yes |
| Destek et al[28], 2016 | 55 | F | Middle/lower esophagus | Middle, the front incisor teeth from 20 cm to the starting 30 cm | Turkey | Gastritis | NA | NA | No | No |
| Chang et al[3], 2006 (unpublished data) | 80 | F | Lower esophagus | Distal esophagus 30-34 cm from the incisor teeth | United Kingdom | Gastritis | NA | NA | NA | NA |
| Berry et al[29], 1995 | 51 | F | NA | NA | United States | NA | NA | NA | NA | NA |
| Nagra et al[30], 2020 | 57 | F | Middle esophagus | Middle esophagus | United States | Iron deficiency anemia | NA | NA | NA | NA |
| Jones et al[31], 2005 | 22 | M | Lower esophagus | 38 cm from the incisor | United States | Addison’s disease | Dysphagia | NA | NA | NA |
| Dinneen et al[32], 2014 | 65 | F | Upper/middle esophagus | Upper and middle esophagus | United States | Esophageal wall thickening on CT | Abdominal pain | NA | NA | NA |
| Changela et al[33], 2017 | 48 | F | Upper esophagus | Upper third of the esophagus | United States | NA | Weight loss and dyspepsia | NA | Yes | NA |
| Current study, 2024 | 59 | M | Upper and lower esophagus | Upper esophagus and lower esophagus | United States | Esophageal SCC | NA | PPI | No | No |
| Current study, 2024 | 54 | F | Middle esophagus | Middle esophagus | United States | None | Weight loss | Losartan | Yes | Yes |
NA: Not available; PPI: Proton pump inhibitor; SCC: Squamous cell carcinoma.
CASE PRESENTATION
Chief complaints
Case 1: An African American female patient, 54 years old, arrived at the emergency department with sharp left-sided abdominal/chest pain, an unintentional weight loss of 20 pounds over the last six months, as well as nausea, vomiting, and poor appetite.
Case 2: A 59-year-old African American male came to the emergency department with main complaints of right upper quadrant abdominal pain and a 20-pound weight loss despite maintaining a good appetite.
History of present illness
Case 1: Patient presents to the emergency department due to hypotension, altered mental status, left-sided sharp abdominal/chest pain, and an unintentional weight loss of 20 pounds over the span of the last six months. She reported increased nausea and vomiting, along with a poor appetite, which has progressively worsened.
Case 2: The patient presented to the emergency department with chief complaints of right upper quadrant abdominal pain, which he describes as constant, sharp, and non-radiating, intermittently worsening and 20 pounds of weight loss despite maintaining a good appetite.
History of past illness
Case 1: Past medical history includes hepatic steatosis, iron deficiency anemia, polysubstance abuse (cocaine, alcohol, and marijuana), tobacco use, and hypertension (HTN).
Case 2: HTN, GERD, and seizures.
Personal and family history
Case 1: Family history of colon cancer in her grandfather. No personal and family history of melanocytic lesions and malignancy.
Case 2: Family history of HTN in his mother.
Physical examination
Case 1: Normal appearance. Abdomen is flat soft on palpation with tenderness in the left lower quadrant. Bowel sounds are normal.
Case 2: He is not in acute distress. Abdomen is flat, soft and abnormal tenderness on right side and no guarding.
Laboratory examinations
Case 1: White blood cell counts 8.85 × 109/L (3.50-9.50 × 109/L); hematocrit 29.7% (40%-50%); hemoglobin 9.40 g/dL, platelets 135 K/μL (150-450 K/μL); serum calcium 8.20 mg/dL (8.70-10.50 mg/dL); alanine aminotransferase 100 U/L (0-40 U/L); aspartate aminotransferase 159 U/L (0-40 U/L); serum triglycerides 568 mg/dL (30-150 mg/dL).
Case 2: White blood cell counts 10.02 × 109/L (3.50-9.50 × 109/L); hematocrit 34.6% (40.0%-50.0%); serum calcium 10.80 mg/dL (8.70-10.50 mg/dL); alanine aminotransferase 7 U/L (0-40 U/L); aspartate aminotransferase 13 U/L (0-40 U/L).
Imaging examinations
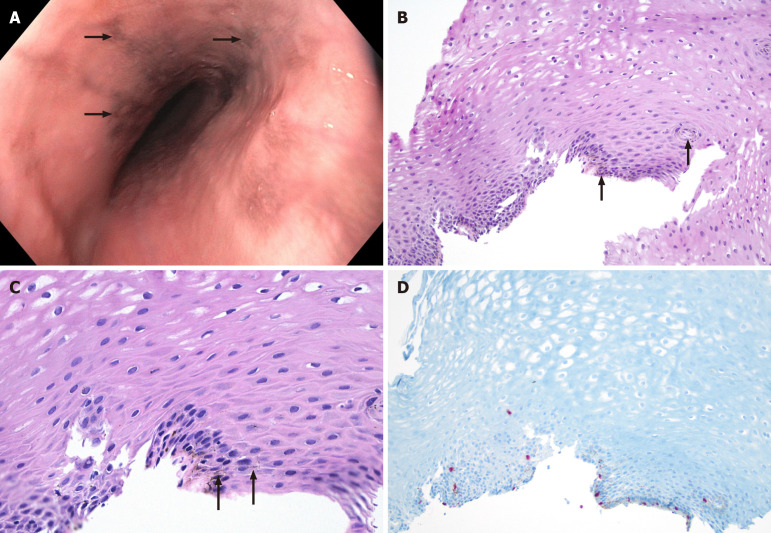
Case 1: During the upper gastrointestinal endoscopy, a normal Z-line was observed at 36 cm from the incisors, and brown/black pigmentation was seen in the middle one-third of the esophagus (Figure 1A). The abdominal X-ray showed normal results. A computed tomography (CT) scan of the abdomen/pelvis revealed the presence of uterine fibroids and bilateral hydroureteronephrosis. Additionally, an upper abdominal ultrasound indicated the presence of hepatic steatosis.
Figure 1.
Imaging examinations. A: Endoscopic poorly delineated pigmented lesion (arrow) on the mucosa; B and C: Hematoxylin and eosin stain shows basal cell hyperplasia, intercellular edema, and presence on non-atypical cells with melanin deposition (arrow) along the basal layer of the epithelium, 10 × and 40 × magnification; D: SRY-related HMG box 10 immunohistochemistry red nuclear positivity in the melanocytes.
Case 2: During an upper gastrointestinal endoscopy, a narrowing was found in the upper part of the esophagus. Additionally, a single raised, cancerous-looking, and ulcerated mass measuring 50 mm in size was discovered in the lower esophagus, located 35 cm from the incisors. A CT scan of the abdomen revealed thickening of the esophageal wall in the middle to lower part of the esophagus.
FINAL DIAGNOSIS
Case 1
On the targeted biopsies, histopathologic examination of the specimen with hematoxylin and eosin staining showed basal cell hyperplasia, intracellular edema, and melanin pigment deposition in the basal layer without atypia (Figure 1B and C). Immunochemistry stains show scattered SRY-related HMG box 10 (SOX10) (Figure 1D) and S100-positive melanocytes in the basal layer, consistent with EM. The iron stain was negative.
Case 2
The esophageal biopsies from the stricture in the upper esophagus show squamous mucosa with melanin pigment deposition in the basal layer, without atypia. Immunohistochemistry stains show scattered SOX10-positive melanocytes in the basal layer, consistent with EM. The iron stain was negative. The biopsies from the mass show moderately differentiated squamous cell carcinoma.
TREATMENT
Case 1
Antihypertensive (losartan and carvedilol) and selective serotonin reuptake inhibitors (paroxetine).
Case 2
Neoadjuvant chemoradiation therapy for squamous cell carcinoma.
OUTCOME AND FOLLOW-UP
Case 1
The patient’s upper gastrointestinal symptoms have resolved at the one-year follow-up, and no follow-up endoscopy data is available.
Case 2
One year follow up imaging showed metastatic squamous cell carcinoma in thoracic vertebra.
DISCUSSION
A review of 55 reported cases and a cohort of 2 cases from our hospital (total n = 57) was performed (Table 1). Patients had a mean age of 59 (range 22-80) years, with male predominance (M:F = 1.9:1.0). Endoscopically visible pigmented lesions were present in 89% (51 out of 57) of the patients. In 84 % (n = 43/51) of the patients, the lesion is present in the middle one-third or lower one-third of the esophagus, six patients have lesions in the upper one-third, two patients have lesions in the upper and middle one-third of esophagus. A clinical association with various diseases has been reported in 38 of 57 patients. The most frequent association was present in 45 % (n = 17/38) of the patients with peptic ulcer diseases and gastritis. Other less frequent associations have been reported with esophageal melanoma (n = 4), anal melanoma (n = 1), esophageal squamous cell carcinoma (n = 3), hypopharyngeal squamous cell carcinoma (n = 1), gastroesophageal junction adenocarcinoma (n = 2), eosinophilic esophagitis (n = 1), and esophageal wall thickening (n = 1). There is a rare association with systemic disorders like celiac disease (n = 1), Addison’s disease (n = 1), Laugier-Hunziker syndrome (n = 1), and mental anorexia (n = 1). No association was reported in two cases (n = 2). Medication history is available for eight patients, and four of these patients are on proton pump inhibitors, two are on antihypertensive medications (losartan), one is on iron medication, and one is on azathioprine. A clinical history of alcohol use is present in 55 % (6/11), and smoking is present in 67% (8/12) of patients.
EM is a relatively rare condition characterized by the presence of melanin pigment and melanocytes at the basal layer of the esophagus. EM was described for the first time in 1963 by De La Pava et al[1], and its prevalence was reported to be 4% in autopsy series. While EM is generally considered a benign condition, its clinical significance and potential association with other gastrointestinal disorders have been subjects of interest in medical literature.
Although the pathogenesis of melanocytosis is not clearly defined, there are two theories. The first is the aberrant migration of neural crest cells during embryogenesis, and the second is the differentiation of stem cells located in the basal layer of the epithelium into melanoblasts due to various injuries. The second theory seems to be preferred as melanocytosis has been associated with esophagitis and gastroesophageal reflux diseases, described in patients with a history of alcohol abuse and smoking and in the surrounding esophageal carcinomas[13-28]. One case from our cohort (case 2) also showed an association with stricture of the upper esophagus and squamous cell carcinoma of the lower esophagus. It is worth noting that this patient does not have a history of smoking or alcohol consumption. This finding may suggest that EM could potentially be considered an independent risk factor for the development of malignancy. Walter et al[27] reported EM associated with esophageal squamous cell carcinoma, and Dinneen et al[32] reported an association with esophageal wall thickness on CT scans. Sarbia et al[14] and Kleikamp et al[15] reported EM associated with gastroesophageal junction adenocarcinoma. Maroy et al[13] reported EM associated with tonsillar squamous cell carcinoma and esophageal melanoma. The association with other rare systemic disorders reported in the literature include Laugier-Hunziker syndrome, Addison’s disease, and celiac disease[3,28,34].
The other case in our cohort (case 1) shows the presence of melanin pigment and melanocytes in the basal layer, along with basal cell hyperplasia and intercellular edema. Case series of Sharma et al[5] have shown an association of histological features of esophageal injury (such as basal cell proliferation, intercellular edema, and esophagitis) with EM in seven of twenty-one cases. Destek et al[28] and Vincent Comraj et al[17] reported EM in association with esophageal injury. The observed association between histological features of injury may provide support for the hypothesis that the stem cells situated in the basal layer differentiate towards the melanocytes in response to injury as one of the initial events.
The differential diagnosis of EM includes pseudomelanosis, tumoral melanocytic lesion, and other rare entities such as blue rubber bleb nevus syndrome. Pseudo melanosis is an accumulation of various substances in macrophages, fibroblasts, or epithelial cells and is caused by exogenous deposition (dye injection and anthracosis), hemosiderosis or hemochromatosis, and lipofuscin pigment deposition. Periodic-Acid Schiff and Prussian blue iron stains highlight lipofuscin and iron pigments accumulation, respectively. The most important differential diagnosis of esophageal melanocytosis has to be made with tumoral melanocytic lesions. The two main entities described in the literature are blue nevus and primary malignant esophageal melanoma (PMME). The blue nevus is rare and shows the presence of heavily pigmented melanocytes in the lamina propria without junctional activity and cytonuclear atypia. PMME is also sparce and appears mainly as a pigmented mass or a polypoid pigmented lesion in the middle or lower esophagus. Although PMME can occasionally present as flat and poorly delineated macules exhibiting the same endoscopic picture as melanosis, histologically in PMME, there is invasion and destruction of the basal lamina and nests of epithelioid cells or spindle cells with cytonuclear atypia, abnormal mitotic figures, and junctional activity[3,35,36].
CONCLUSION
EM is a rare but well-characterized condition. Although it can be treated conservatively, it is essential to consider it when diagnosing esophageal pigmented lesions. The causes and natural progression of the condition are not fully understood, but various studies have reported an association with malignancy and as a potential precursor for malignancy. We recommend following up with a gastroenterologist for further evaluation. Extended research is necessary to establish additional correlations for the condition.
Footnotes
Informed consent statement: Informed written consent was obtained from the patients for the publication of this report and any accompanying images.
Conflict-of-interest statement: The authors declare that they have no conflict of interest to disclose.
CARE Checklist (2016) statement: The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report’s classification
Scientific Quality: Grade B, Grade B
Novelty: Grade B, Grade B
Creativity or Innovation: Grade B, Grade B
Scientific Significance: Grade B, Grade C
P-Reviewer: Popovic DD; Yan JX S-Editor: Chen YL L-Editor: A P-Editor: Zheng XM
Contributor Information
Liubou Kazacheuskaya, Department of Pathology, Louisiana State University Health, Shreveport, LA 71103, United States.
Kshitij Arora, Department of Pathology, Louisiana State University Health, Shreveport, LA 71103, United States. kshitij.arora@lsuhs.edu.
References
- 1.De La Pava S, Nigogosyan G, Pickren JW, Cabrera A. Melanosis of the esophagus. Cancer. 1963;16:48–50. doi: 10.1002/1097-0142(196301)16:1<48::aid-cncr2820160107>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 2.Özden A, Seven G, Savaş B, Üstün Y, Ensari A, Yusifova A. Özofageal melanositozis - Üç olgu ve literatürün gözden geçirilmesi. 2008. [cited 20 September 2024]. Available from: https://akademik.tgv.org.tr/index.asp?islem=makaledetay&sayfa=281 .
- 3.Chang F, Deere H. Esophageal melanocytosis morphologic features and review of the literature. Arch Pathol Lab Med. 2006;130:552–557. doi: 10.5858/2006-130-552-EMMFAR. [DOI] [PubMed] [Google Scholar]
- 4.Yokoyama A, Omori T, Yokoyama T, Tanaka Y, Mizukami T, Matsushita S, Higuchi S, Takahashi H, Maruyama K, Ishii H, Hibi T. Esophageal melanosis, an endoscopic finding associated with squamous cell neoplasms of the upper aerodigestive tract, and inactive aldehyde dehydrogenase-2 in alcoholic Japanese men. J Gastroenterol. 2005;40:676–684. doi: 10.1007/s00535-005-1610-3. [DOI] [PubMed] [Google Scholar]
- 5.Sharma SS, Venkateswaran S, Chacko A, Mathan M. Melanosis of the esophagus. An endoscopic, histochemical, and ultrastructural study. Gastroenterology. 1991;100:13–16. doi: 10.1016/0016-5085(91)90576-7. [DOI] [PubMed] [Google Scholar]
- 6.Agarwal S A, Gnanamoorthy K, K A, Athani AV. Esophageal Melanosis: An Unknown Entity. Cureus. 2022;14:e29064. doi: 10.7759/cureus.29064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dubail A, Dano H, de Suray N, Hassaini H, Jouret-Mourin A. Esophageal Melanocytosis: report of two cases and review of a rare and misunderstood entity. Acta Gastroenterol Belg. 2022;85:390–392. doi: 10.51821/85.2.10126. [DOI] [PubMed] [Google Scholar]
- 8.Voltaggio L, Montgomery EA. Biopsy Interpretation of Gastrointestinal Tract mucosa. Philadelphia: Wolter Kluwer, 2018: 8-9. [Google Scholar]
- 9.Kuo P, Takahashi H, Ruszkiewicz A, Schoeman M. Education and imaging. Gastrointestinal: esophageal melanocytosis--the esophagus that seemed "off-color". J Gastroenterol Hepatol. 2011;26:1463. doi: 10.1111/j.1440-1746.2011.06696.x. [DOI] [PubMed] [Google Scholar]
- 10.Horowitz M, Nobrega MM. Primary anal melanoma associated with melanosis of the upper gastrointestinal tract. Endoscopy. 1998;30:662–665. doi: 10.1055/s-2007-1001373. [DOI] [PubMed] [Google Scholar]
- 11.Dumas O, Barthélémy C, Billard F, Dumollard JM, Boucheron S, Calmard P, Rousset H, Audigier JC. Isolated melanosis of the esophagus: systematic endoscopic diagnosis. Endoscopy. 1990;22:94–95. doi: 10.1055/s-2007-1012807. [DOI] [PubMed] [Google Scholar]
- 12.Bogomoletz WV, Lecat M, Amoros F. Melanosis of the oesophagus in a Western patient. Histopathology. 1997;30:498–499. doi: 10.1046/j.1365-2559.1997.00540.x. [DOI] [PubMed] [Google Scholar]
- 13.Maroy B, Baylac F. Primary malignant esophageal melanoma arising from localized benign melanocytosis. Clin Res Hepatol Gastroenterol. 2013;37:e65–e67. doi: 10.1016/j.clinre.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Sarbia M, Ringelhan M, Siveke J, Bettstetter M, Karimi D. [Paraneoplastic acanthosis nigricans of the esophagus: a case report] Z Gastroenterol. 2012;50:680–683. doi: 10.1055/s-0031-1299503. [DOI] [PubMed] [Google Scholar]
- 15.Kleikamp S, Böhm M, Frosch P, Brinkmeier T. [Acanthosis nigricans, papillomatosis mucosae and "tripe palms" in a patient with metastasized gastric carcinoma] Dtsch Med Wochenschr. 2006;131:1209–1213. doi: 10.1055/s-2006-941753. [DOI] [PubMed] [Google Scholar]
- 16.Kumari NS, Srujana S, Sireesha A, Krishna L, Kumar OS. Esophageal Melanocytosis - An Unusual Melanocytic Entity. J Assoc Physicians India. 2016;64:75–76. [PubMed] [Google Scholar]
- 17.Vincent Comraj D, Zainab A, Arthur M, Chauhan J, Pandurangan V, Srinivasan D. A Case Report of Hereditary Palmoplantar Keratoderma with Esophageal Melanosis. Middle East J Dig Dis. 2023;15:141–143. doi: 10.34172/mejdd.2023.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geramizadeh B, Asadian F, Taghavi A. Esophageal melanocytosis in oral opium consumption. Iran Red Crescent Med J. 2014;16:e7820. doi: 10.5812/ircmj.7820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kinugasa H, Tanaka T, Okada H. Esophageal melanosis with eosinophilic esophagitis. Gastrointest Endosc. 2020;91:1203–1204. doi: 10.1016/j.gie.2019.12.006. [DOI] [PubMed] [Google Scholar]
- 20.Mori A, Tanaka M, Terasawa K, Hayashi S, Shimada Y. A magnified endoscopic view of esophageal melanocytosis. Gastrointest Endosc. 2005;61:479–481. doi: 10.1016/s0016-5107(04)02638-0. [DOI] [PubMed] [Google Scholar]
- 21.Yamazaki K, Ohmori T, Kumagai Y, Makuuchi H, Eyden B. Ultrastructure of oesophageal melanocytosis. Virchows Arch A Pathol Anat Histopathol. 1991;418:515–522. doi: 10.1007/BF01606502. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto O, Yoshinaga K, Asahi M, Murata I. A Laugier-Hunziker syndrome associated with esophageal melanocytosis. Dermatology. 1999;199:162–164. doi: 10.1159/000018227. [DOI] [PubMed] [Google Scholar]
- 23.Kaneko Y, Miyoshi K, Tsurui K, Matsumoto M, Enomoto M, Iwasaki K, Ota Y, Katsumata K, Nagakawa Y. [A Case of Esophageal Malignant Melanoma during Follow-Up of Esophageal Melanosis Metastasis] Gan To Kagaku Ryoho. 2022;49:1998–2000. [PubMed] [Google Scholar]
- 24.Kato T, Harano M, Ono S, Sato D. [A case of superficial primary malignant melanoma of the esophagus] Gan To Kagaku Ryoho. 2013;40:2109–2111. [PubMed] [Google Scholar]
- 25.Oshiro T, Shimoji H, Matsuura F, Uchima N, Kinjo F, Nakayama T, Nishimaki T. Primary malignant melanoma of the esophagus arising from a melanotic lesion: report of a case. Surg Today. 2007;37:671–675. doi: 10.1007/s00595-006-3444-x. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki H, Nakanishi Y, Taniguchi H, Shimoda T, Yamaguchi H, Igaki H, Tachimori Y, Kato H. Two cases of early-stage esophageal malignant melanoma with long-term survival. Pathol Int. 2008;58:432–435. doi: 10.1111/j.1440-1827.2008.02249.x. [DOI] [PubMed] [Google Scholar]
- 27.Walter A, van Rees BP, Heijnen BH, van Lanschot JJ, Offerhaus GJ. Atypical melanocytic proliferation associated with squamous cell carcinoma in situ of the esophagus. Virchows Arch. 2000;437:203–207. doi: 10.1007/s004280000220. [DOI] [PubMed] [Google Scholar]
- 28.Destek S, Gul VO, Ahioglu S, Erbil Y. A Rare Disease of the Digestive Tract: Esophageal Melanosis. Gastroenterology Res. 2016;9:56–60. doi: 10.14740/gr670w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berry MA, DiPalma JA. Esophageal melanosis. J Clin Gastroenterol. 1995;21:79. [PubMed] [Google Scholar]
- 30.Nagra N, Tolentino L, Singhvi G. Esophageal Melanosis: A Rare Condition of Undetermined Significance. Clin Gastroenterol Hepatol. 2020;18:e59. doi: 10.1016/j.cgh.2019.03.025. [DOI] [PubMed] [Google Scholar]
- 31.Jones BH, Fleischer DE, De Petris G, Heigh RI, Shiff AD. Esophageal melanocytosis in the setting of Addison’s disease. Gastrointest Endosc. 2005;61:485–487. doi: 10.1016/s0016-5107(04)02845-7. [DOI] [PubMed] [Google Scholar]
- 32.Dinneen HS, Protopapas G, Fitzhugh V, Ahlawat S. Darkest before dawn: esophageal melanocytosis mimicking primary esophageal melanoma. Gastrointest Endosc. 2014;80:1203–1205. doi: 10.1016/j.gie.2014.07.042. [DOI] [PubMed] [Google Scholar]
- 33.Changela K, Reddy M. Smoker’s melanosis: Isolated pigmented lesion in the laryngopharynx and esophagus. Turk J Gastroenterol. 2017;28:524–525. doi: 10.5152/tjg.2017.17186. [DOI] [PubMed] [Google Scholar]
- 34.Ohashi K, Kato Y, Kanno J, Kasuga T. Melanocytes and melanosis of the oesophagus in Japanese subjects--analysis of factors effecting their increase. Virchows Arch A Pathol Anat Histopathol. 1990;417:137–143. doi: 10.1007/BF02190531. [DOI] [PubMed] [Google Scholar]
- 35.Lam KY, Law S, Chan GS. Esophageal blue nevus: an isolated endoscopic finding. Head Neck. 2001;23:506–509. doi: 10.1002/hed.1068. [DOI] [PubMed] [Google Scholar]
- 36.Ohnuma H, Ishikawa K, Hirakawa M, Kikuchi S, Sato Y, Miyanishi K, Kato J. Cases of primary malignant melanoma and melanocytosis of the esophagus observed by magnifying endoscopy: Application to differential diagnosis: case series. Medicine (Baltimore) 2017;96:e6701. doi: 10.1097/MD.0000000000006701. [DOI] [PMC free article] [PubMed] [Google Scholar]