Abstract
BACKGROUND
Cerebral small vessel disease (CSVD) is a prevalent cerebrovascular disease in clinical practice that is often associated with macrovascular disease. A clear understanding of the underlying causes of CSVD remains elusive.
AIM
To explore the association between intercellular adhesion molecule-1 (ICAM-1) and blood-brain barrier (BBB) penetration in CSVD.
METHODS
This study included patients admitted to Fuyang People’s Hospital and Fuyang Community (Anhui, China) between December 2021 and March 2022. The study population comprised 142 patients, including 80 in the CSVD group and 62 in the control group. Depression was present in 53 out of 80 patients with CSVD. Multisequence magnetic resonance imaging (MRI) and dynamic contrast-enhanced MRI were applied in patients to determine the brain volume, cortical thickness, and cortical area of each brain region. Moreover, neuropsychological tests including the Hamilton depression scale, mini-mental state examination, and Montreal cognitive assessment basic scores were performed.
RESULTS
The multivariable analysis showed that age [P = 0.011; odds ratio (OR) = 0.930, 95% confidence interval (CI): 0.880-0.983] and ICAM-1 levels (P = 0.023; OR = 1.007, 95%CI: 1.001-1.013) were associated with CSVD. Two regions of interest (ROIs; ROI3 and ROI4) in the white matter showed significant (both P < 0.001; 95%CI: 0.419-0.837 and 0.366-0.878) differences between the two groups, whereas only ROI1 in the gray matter showed significant difference (P = 0.046; 95%CI: 0.007-0.680) between the two groups. ICAM-1 was significantly correlated (all P < 0.05) with cortical thickness in multiple brain regions in the CSVD group.
CONCLUSION
This study revealed that ICAM-1 levels were independently associated with CSVD. ICAM-1 may be associated with cortical thickness in the brain, predominantly in the white matter, and a significant increase in BBB permeability, proposing the involvement of ICAM-1 in BBB destruction.
Keywords: Cerebral small vessel disease, Intercellular adhesion molecule-1, Blood-brain barrier penetration, Cortical thickness, White matter
Core Tip: Cerebral small vessel disease (CSVD) is a prevalent cerebrovascular disease in clinical practice that is often associated with macrovascular disease. A clear understanding of the underlying causes of CSVD remains elusive. Several studies have reported the relationship between intercellular adhesion molecule-1 (ICAM-1) and blood-brain barrier (BBB) penetration in neurological disorders. Thus, this study examined the association between ICAM-1 and BBB penetration in CSVD.
INTRODUCTION
Cerebral small vessel disease (CSVD) is a cluster of clinical, imaging, and pathological symptoms resulting from multiple factors that affect the cerebral arterioles, capillaries, venules, and veins[1,2]. It is a prevalent cerebrovascular disease in clinical practice, is often associated with macrovascular disease, and accounts for 20%-30% of all ischemic stroke and cerebral hemorrhage[3]. Given its high incidence, CSVD is thought to play a key role in stroke, dementia, and aging[3,4]. Therefore, it should be considered a major public health problem, particularly among aging populations. Despite extensive research, a clear understanding of the causes of CSVD remains elusive[5]. Numerous studies have identified inflammation as an etiological factor in CSVD development and progression[6]. A community-based cohort study also demonstrated a correlation between inflammatory biomarkers in CSVDs and magnetic resonance imaging (MRI)[7].
Dysregulation of cell adhesion molecules (CAMs), which coordinate leukocyte trafficking, may affect blood-brain barrier (BBB) permeability and promote inflammation and immune-mediated transport to the central nervous system (CNS), thereby connecting peripheral and neuroinflammation in severe psychiatric disorders[8,9]. Intercellular adhesion molecule-1 (ICAM-1) is a protein found on different cell surfaces, including endothelial, epithelial, and white blood cells. White blood cell adhesion and migration are critical processes in immune response and inflammation, both involving ICAM. In addition, ICAM-1 is associated with cardiovascular disease, cancer, and autoimmune disorders. Several studies have reported the relationship between ICAM-1 and BBB penetration in neurological disorders[10,11]. ICAM-1 and BBB penetration correlate with Alzheimer’s disease[12]. Integrin receptors on leukocytes bind to CAMs and promote their migration across the BBB[13,14]. Although the BBB does not deteriorate in severe mental illness as in neurologic diseases and neurodegenerative disorders, postmortem transcriptomic studies have shown increased permeability in schizophrenia, revealing the dysregulated expression of tight junction-related genes in the prefrontal cortex[15,16] and BBB transcripts enriched in brain endothelial cells such as ICAM1[13] as well as aberrant immunoreactivity of claudin-5 in brain sections from the parietal lobe[17]. A recent study also revealed a correlation between ICAM-1 expression and CSVD development[18]. Endothelial dysfunction and subsequent BBB damage are key pathophysiological mechanisms in CSVD. Dynamic contrast-enhancement MRI (DCE-MRI) is the preferred imaging technique for evaluating BBB leakage in CSVD[11]. It can assess BBB damage in specific areas and quantify permeability changes.
Although the aforementioned studies discussed the relationships between ICAM-1 and CSVD, more studies are required to elucidate the precise mechanisms through which inflammatory processes contribute to the pathology of CSVD.
At present, the pathogenesis of CSVD remains unclear, no standard treatment or plan has been established, and its diagnosis mainly depends on brain MRI; thus, early diagnostic markers are needed. Accordingly, this study aimed to examine the association between ICAM-1 and BBB penetration in CSVD. The results of this study could identify surrogate markers that could help screen for patients with radiological CSVD before the onset of symptoms. Indeed, blood biomarkers are easier to assess than markers observed via MRI. Early disease detection may allow physicians to develop a tailored, individualized treatment plan for each patient with CSVD. This approach can also help avoid unnecessary treatments and lower healthcare costs, ultimately improving patients’ quality of life.
MATERIALS AND METHODS
Study design and participants
This study included patients admitted to Fuyang People’s Hospital and Fuyang Community (Anhui, China) between December 2021 and March 2022. The inclusion criteria were as follows: (1) Patients who met the diagnostic criteria for cerebrovascular disease and were confirmed by head MRI[19]; (2) Age > 50 years; (3) Lack of apparent subjective neurological symptoms; (4) Normal cognitive function with normal mini-mental state examination (MMSE) and Montreal cognitive assessment basic (MoCA-B) scale scores; (5) No history of brain trauma; (6) No long-term use of antibiotics, antiplatelet drugs, and statins; and (7) All underwent multisequence MRI. The exclusion criteria were as follows: (1) Presence of intracranial macrovascular lesions; (2) Abnormal liver and kidney function; (3) History of a brain tumor or brain trauma, CNS infection, thyroid function abnormality, vitamin B1/B12 deficiency, alcoholic brain injury, syphilis, malnutrition, or other diseases that may cause brain injury; (4) Cognitive impairment, pconsciousness impairment, visual-auditory impairment, aphasia, and inability to cooperate with scale testing; (5) Vascular infarction or severe hemorrhagic stroke or patients with white matter hyperintensity (WMH) and leukodystrophy, CNS inflammatory demyelinating disease and vasculitis on MRI, or the presence of white matter lesions from other causes; (6) Current infectious disease/autoimmune disease; (7) Susceptibility weighted imaging (SWI) sequence indicated severe micro bleeding lesions; (8) Intake of nonsteroidal anti-inflammatory drugs or corticosteroids; (9) Presence of malignant tumor; and (10) Any condition or treatment that could affect the study outcomes according to the investigators.
The study was approved by the Ethics Committee of Fuyang People’s Hospital, and all participants signed an informed consent form for participation.
In addition, individuals who did not present with such symptoms and remained undiagnosed with CSVD were included in the control group.
Diagnostic criteria for CSVD
At least one CSVD marker (lacunar infarction, high signal in the cerebral white matter, microhemorrhage, and perivascular gap) must be present on MRI. MRI findings are as follows: (1) Lacunar infarction is characterized by a well-defined round, oval, or lacunar lesion, 3-15 mm in diameter, with isosignal to the cerebrospinal fluid. T2-fluid attenuation inversion recovery (FLAIR) sequences show cerebrospinal fluid-like low signals in the center and high signals around it, and T1 and T2 sequences usually show cerebrospinal fluid-like signal signals; (2) High cerebral in the white matter is characterized by abnormal signal in the cerebral white matter area, high signal on T2 or T2-FLAIR, micro-equal or low signal on T1, and lack of cavity in the lesion, which is classified into deep white matter high signal and paraventricular white matter high signal, and early fusion of deep white matter high signal or irregular paraventricular white matter high signal extending to the deep white matter is positive for cerebral white matter high signal; (3) The perivascular gap is characterized by a basal ganglia region, enlarged perivascular gap, direct < 3 mm, isosignal with cerebrospinal fluid, linear (when imaging plane is parallel to vessel alignment) or round-like (when perpendicular to vessel alignment), and positive if the number of perivascular gaps in unilateral basal ganglia region is > 10; and (4) Microhemorrhages are small round or ovoid, well-defined, homogeneous foci of signal loss, 2-5 mm in diameter, surrounded by brain parenchyma, and shown on (SWI) sequences; one or more microhemorrhages are positive.
Procedures
All participants underwent cognitive assessment using the MMSE and MoCA-B scales during the routine course of their clinical workup. The normal ranges of MMSE are > 17, > 20, and > 24 points for those who are illiterate, completed primary school, and completed junior high school or higher, respectively. Normal MoCA-B scores were > 19, > 22, and > 24 for those who were illiterate or had primary school education, secondary school education, and university education, respectively[20].
All patients also underwent multisequence MRI and DCE-MRI for clinical indications. Conventional axial and sagittal T1-weighted imaging [repetition time (TR) 7.9 ms, echo time (TE) 3.5 ms, layer thickness 1 mm, layer spacing 0 mm, 140 layers], T2WI (TR 3000 ms, TE 90 ms, section thickness of 5 mm, section gap of 0 mm, 24 layers), and DCE-MRI (TR 3.5 ms, TE 1.64 ms, section thickness of 3 mm, section gap of 0 mm, 36 layers) were performed using an Ingenia CX 3.0-T MRI system (Philips, Best, The Netherlands). Four physicians, including two imaging specialists and two neurovascular experts, independently evaluated the imaging results. If discrepancies occurred, the four physicians engaged in collaborative discussions to reach a diagnosis under the guidance of a senior imaging specialist. T2-weighted FLAIR WMH was quantified using the Fazekas scale[21].
Venous blood (3 mL) was collected in the fasting state and left at room temperature for 10-20 minutes. The blood samples were centrifuged at 3000 rpm for 15 minutes, and the supernatant was collected. Serum was analyzed using the human ICAM-1 enzyme-linked immunosorbent assay kit according to the manufacturer’s instructions.
T1-weighted images were preprocessed using SPM8 in Matlab R2012b. Image normalization, segmentation, modulation, and smoothing were performed according to standard procedures. The multiple regression approach in SPM8 was used to analyze the correlation between ICAM-1 expression and brain volume. A significance level of P < 0.001 and identified clumps > 10 pixels were defined as regions of interest (ROIs). Professional imaging physicians analyzed the ROIs on DCE-MRI using the Philips Intellispace Portal (v 7.0.5.40155) on a workstation with a Philips Ingenia CX scanner to obtain average Ktrans values for the ROIs.
FreeSurfer was used to process the structural magnetic resonance images of all patients. “Recon-all” processing was used with default parameters to derive the brain volume, cortical thickness, and cortical area of each brain region.
Data collection
Demographic and clinical data, including sex, age, education, personal history, medical history, and medication history, were extracted from patient charts.
Statistical analysis
Statistical analyses were performed using SPSS 24.0 (IBM, Armonk, NY, United States). Continuous variables are presented as means ± SD and were analyzed using the independent samples t-test. Categorical variables were presented as n (%) and analyzed using the χ2 test. Logistic regression analysis was used to identify factors independently associated with CSVD. Voxel-based morphometry (VBM) analysis was performed using SPM8 on Matlab R2012b. Multiple regression analysis was performed to identify associations between ROIs and ICAM-1.
RESULTS
This study included 142 patients. Their average age was 60.36 ± 6.83 years. Sixty-one were males. Compared with the control group, the CSVD group was older (61.69 ± 7.13 vs 58.71 ± 6.10 years, P = 0.008). Sex, hypertension, diabetes, smoking, and drinking were comparable between the two groups (all P > 0.05; Table 1). Depression and cognitive assessment results by Hamilton depression scale (HAMD), MMSE, and MoCA-B were significantly different (all P < 0.001) between the CSVD and control groups.
Table 1.
Clinical characteristics of the patients
|
Characteristics
|
All (n = 142)
|
Control (n = 62)
|
CSVD (n = 80)
|
P value
|
| Age (years) | 60.39 ± 6.84 | 58.71 ± 6.10 | 61.69 ± 7.13 | 0.008 |
| Male, n (%) | 61 (43.0) | 28 (45.2) | 33 (41.3) | 0.641 |
| Hypertension, n (%) | 63 (44.4) | 23 (37.1) | 40 (50.0) | 0.125 |
| Diabetes, n (%) | 19 (13.4) | 5 (8.1) | 14 (17.5) | 0.101 |
| Smoking, n (%) | 40 (28.2) | 16 (25.8) | 24 (30.0) | 0.582 |
| Drinking, n (%) | 56 (39.4) | 23 (37.1) | 33 (41.3) | 0.615 |
| ICAM-1 (ng/mL) | 258.11 ± 62.78 | 272.85 ± 63.90 | 246.69 ± 59.83 | 0.013 |
| VCAM-1 (ng/mL) | 285.77 ± 59.99 | 286.11 ± 55.08 | 285.51 ± 63.88 | 0.953 |
| HAMD | 16.01 ± 6.50 | 11.39 ± 4.03 | 19.60 ± 5.74 | < 0.001 |
| MMSE | 26.36 ± 3.14 | 27.35 ± 2.72 | 25.59 ± 3.24 | 0.001 |
| MoCA-B | 25.51 ± 2.67 | 26.37 ± 2.21 | 24.85 ± 2.81 | < 0.001 |
| CRP (mg/L) | 2.65 ± 4.03 | 2.43 ± 3.97 | 2.81± 4.09 | 0.578 |
CSVD: Cerebral small vessel disease; ICAM-1: Intercellular adhesion molecule-1; VCAM-1: Vascular cell adhesion molecule 1; HAMD: Hamilton depression scale; MMSE: Mini-mental state examination; MoCA-B: Montreal cognitive assessment basic; CRP: C-reactive protein.
Depression was present in 53 of 80 patients with CSVD and absent in 27. The HAMD, MMSE, and MoCA-B scores of the two groups significantly differed (all P < 0.05, Table 2).
Table 2.
Correlation between depression and cognitive functioning among cerebral small vessel disease patients
|
Characteristics
|
Depression (n = 53)
|
Non depression (n = 27)
|
P value
|
| Age (years) | 62.28 ± 7.83 | 60.52 ± 5.47 | 0.298 |
| Male, n (%) | 21 (39.6) | 12 (44.4) | 0.679 |
| Hypertension, n (%) | 28 (52.8) | 12 (44.4) | 0.478 |
| Diabetes, n (%) | 10 (18.9) | 4 (14.8) | 0.652 |
| Smoking, n (%) | 15 (28.3) | 9 (33.3) | 0.642 |
| Drinking, n (%) | 22 (41.5) | 11 (40.7) | 0.947 |
| ICAM-1 (ng/mL) | 244.14 ± 60.41 | 251.69 ± 59.50 | 0.597 |
| VCAM-1 (ng/mL) | 286.35 ± 63.82 | 283.87 ± 65.19 | 0.871 |
| HAMD | 22.92 ± 3.30 | 13.07 ± 3.41 | < 0.001 |
| MMSE | 24.62 ± 2.96 | 27.52 ± 2.83 | < 0.001 |
| MoCA-B | 24.26 ± 2.77 | 26.04 ± 2.47 | 0.006 |
| CRP (mg/L) | 3.09 ± 4.61 | 2.26 ± 2.82 | 0.393 |
ICAM-1: Intercellular adhesion molecule-1; VCAM-1: Vascular cell adhesion molecule 1; HAMD: Hamilton depression scale; MMSE: Mini-mental state examination; MoCA-B: Montreal cognitive assessment basic; CRP: C-reactive protein.
The multivariable analysis showed that age [P = 0.011; odds ratio (OR) = 0.930, 95% confidence interval (95%CI) = 0.880-0.983] and ICAM-1 levels (P = 0.023; OR = 1.007, 95%CI: 1.001-1.013) were independently associated with CSVD (Table 3).
Table 3.
Multivariable analysis on cerebral small vessel disease
|
Characteristics
|
B
|
SE
|
Wald
|
P value
|
OR
|
95%CI
|
|
|
Lower
|
Upper
|
||||||
| Sex | 0.653 | 0.487 | 1.793 | 0.181 | 1.921 | 0.739 | 4.993 |
| Age | -0.072 | 0.028 | 6.545 | 0.011 | 0.930 | 0.880 | 0.983 |
| Hypertension | -0.309 | 0.384 | 0.647 | 0.421 | 0.734 | 0.346 | 1.559 |
| Diabetes | -0.476 | 0.579 | 0.678 | 0.410 | 0.621 | 0.200 | 1.930 |
| Smoking | -0.500 | 0.558 | 0.802 | 0.370 | 0.606 | 0.203 | 1.812 |
| Drinking | -0.002 | 0.471 | 0.000 | 0.996 | 0.998 | 0.396 | 2.510 |
| ICAM-1 | 0.007 | 0.003 | 5.141 | 0.023 | 1.007 | 1.001 | 1.013 |
OR: Odds ratio; CI: Confidence interval; ICAM-1: Intercellular adhesion molecule-1.
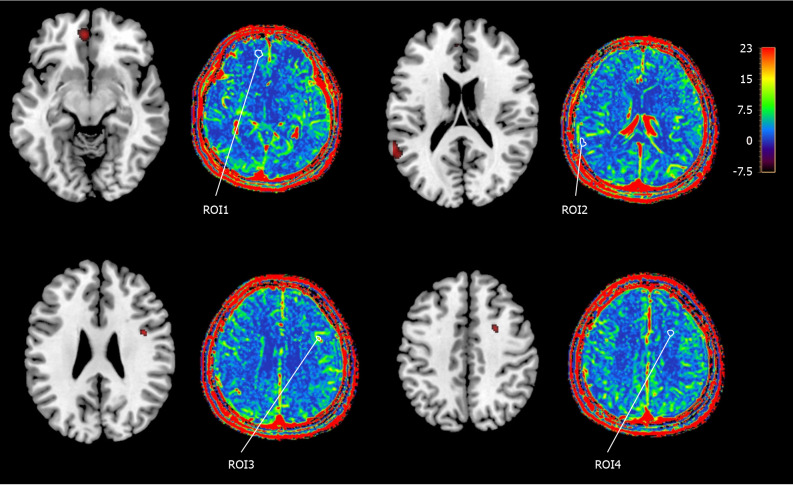
Using the VBM method, brain regions significantly correlated with ICAM-1 levels were identified. ROI1 and ROI2 from the gray matter and ROI3 and ROI4 from the white matter were selected for analysis. The average Ktrans value of each ROI was tested (Figure 1). The two ROIs in the white matter showed significant intergroup differences (both P < 0.001; 95%CI: 0.419-0.837 and 0.366-0.878), whereas only one ROI in the gray matter (P = 0.04; 95%CI: 0.007-0.680) showed significant difference (Table 4).
Figure 1.
Color and grayscale images of the regions of interest and their average Ktrans values. ROI: Region of interest.
Table 4.
Comparison of the mean Ktrans values of the region of interest regions between the cerebral small vessel disease group and the control group
|
Regions
|
CSVD group
|
Control group
|
Significance
|
Mean difference
|
95%CI
|
|
|
Lower
|
Upper
|
|||||
| ROI1 | 2.509 ± 1.082 | 2.166 ± 0.897 | 0.046 | 0.343 | 0.007 | 0.680 |
| ROI2 | 2.698 ± 0.987 | 2.695 ± 1.287 | 0.988 | 0.003 | -0.374 | 0.380 |
| ROI3 | 1.529 ± 0.748 | 0.901 ± 0.51 | < 0.001 | 0.628 | 0.419 | 0.837 |
| ROI4 | 1.571 ± 0.888 | 0.949 ± 0.654 | < 0.001 | 0.622 | 0.366 | 0.878 |
CI: Confidence interval; CSVD: Cerebral small vessel disease; ROI: Region of interest.
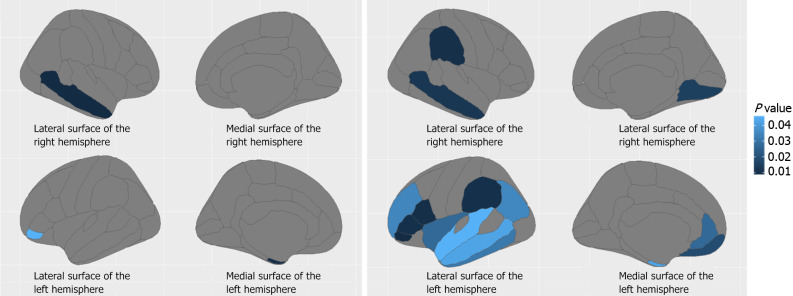
The DKTAtlas portioning scheme results showed that ICAM-1 was significantly correlated (all P < 0.05) with cortical thickness in multiple brain regions in the CSVD group, whereas only limited correlations were observed in the control group (Figure 2 and Table 5).
Figure 2.
Brain regions (blue area) where the cortical thickness was significantly correlated with intercellular adhesion molecule-1 in the control and cerebral small vessel disease groups. A: Regions where cortical thickness correlated with intercellular adhesion molecule-1 (ICAM-1) in healthy control group (independent sample t-test); B: Regions where cortical thickness correlated with ICAM-1 in cerebral small vessel disease group (independent sample t-test).
Table 5.
Brain regions where cortical thickness correlates with intercellular adhesion molecule-1
|
Group
|
Region
|
Correlation
|
Significance
|
| CSVD | Right lingual | 0.292 | 0.009 |
| Right middle temporal | 0.310 | 0.005 | |
| Right supramarginal | 0.329 | 0.003 | |
| Left entorhinal | 0.230 | 0.040 | |
| Left inferior parietal | 0.240 | 0.032 | |
| Left inferior temporal | 0.247 | 0.027 | |
| Left medial orbitofrontal | 0.278 | 0.013 | |
| Left middle temporal | 0.230 | 0.040 | |
| Left pars opercularis | 0.337 | 0.002 | |
| Left pars orbitalis | 0.363 | 0.001 | |
| Left pars triangularis | 0.331 | 0.003 | |
| Left rostral anterior cingulate | 0.256 | 0.022 | |
| Left rostral middle frontal | 0.241 | 0.031 | |
| Left superior temporal | 0.226 | 0.044 | |
| Left supramarginal | 0.331 | 0.003 | |
| Left insula | 0.256 | 0.022 | |
| Left mean thickness | 0.235 | 0.036 | |
| Control | Right middle temporal | 0.329 | 0.009 |
| Left entorhinal | -0.325 | 0.010 | |
| Left pars orbitalis | 0.254 | 0.046 |
CSVD: Cerebral small vessel disease.
DISCUSSION
Age and ICAM-1 levels were independently associated with CSVD. ROIs in the white matter were significantly different between the two groups, whereas only one ROI in the gray matter region was different between the two groups. Thus, serum ICAM-1 Levels can be used to screen for early CSVD. The four main clinical manifestations of CSVD are stroke, cognitive impairment, recurrent lacunar infarction, and white matter lesions[22,23]. Acute phase lesions increase the risk of lacunar infarction and cerebral parenchymal hemorrhage. Chronic phase lesions cause persistent brain tissue damage, leading to cognitive impairment, abnormal physical function, and emotional and personality disorders[24-27]. CSVD is common among individuals aged > 60 years, and its incidence is close to 100% in individuals aged > 90 years[27,28]. The multivariable analysis showed that age was independently associated with CSVD.
ICAM-1, as a regulator of the inflammatory response to vascular endothelial injury, can induce the release of toxic substances, causing vascular endothelial damage, which in turn leads to the adhesion and aggregation of inflammatory cells and microvasculature obstruction, ultimately leading to CSVD[29]. ICAM-1 expression in endothelial and epithelial cells is associated with the induction of inflammatory responses[3]. Another study reported that an increase in ICAM-1 also enhances the levels of interleukin (IL)-6 and causes asymptomatic cerebral infarction and white matter lesions[30,31]. Moreover, IL-6 and other proinflammatory cytokines aggravate vascular endothelial cell damage and BBB destruction[7,32]. ICAM-1 is predominantly expressed in endothelial cells, is overexpressed in many pathological conditions, and has functions similar to those of chemokines. In patients with CSVD, ICAM-1 can induce leukocyte aggregation and infiltration from the circulation to the inflammation site and increase the permeability of endothelial cells, resulting in pathological manifestations such as BBB damage, insufficient cerebral perfusion, and increases in the total cranial MRI load in patients with CSVD[33,34].
In addition, by analyzing the BBB permeability of ICAM-1 and associated brain regions, this study quantitatively assessed the correlation between ICAM-1 and BBB damage. Previous studies have shown that BBB penetration in the CNS is more common in patients with CSVD than in healthy patients[23,35,36]. Correlation analyses between ICAM-1 and cortical thickness were performed to determine whether BBB damage can cause changes in cortical thickness. The results showed that ICAM-1 displayed significant intergroup differences and was possibly associated with the pathogenesis of CSVD. Brain regions associated with ICAM-1, particularly the white matter, showed a significant increase in BBB permeability, suggesting the involvement of ICAM-1 in BBB damage. Moreover, inflammatory response is a crucial factor leading to BBB destruction in the early stages of CSVD, and white matter damage occurs earlier. ICAM-1 is expressed on endothelial cells and binds leukocytes through lymphocyte function-associated antigen 1, which mediates leukocyte adhesion to epithelial cells and promotes transendothelial migration[37-39]. Vascular permeability is tightly regulated by adhesion molecules between endothelial cells, which are activated at inflammation sites and perilymphatic endothelial cells, leading to increased expression of adhesion molecules during leukocyte infiltration. When leukocyte infiltration is initiated, these adhesion molecules activate endothelial cell signaling, which then alters the shape of the endothelium and opens pathways for leukocyte migration[40,41]. Blood vessels are pathways for blood flow within the body, including arteries, veins, and capillaries[42]. Capillaries comprise approximately 85% of the total cerebral vascular length and are the main sites of the BBB. The blood vessels in the CNS are continuous and non-enclosed and strictly regulate the movement of cells, molecules, and ions between CNS tissues and the bloodstream[43]. The strict confinement of the BBB precisely regulates internal homeostasis in the nervous system, which protects the brain’s neural tissues from invasion by diseases, injuries, inflammation, toxins, and pathogens and safeguards the normal function of neurons[44]. Therefore, normal BBB function is essential for the CNS.
The BBB is located between the brain parenchyma and blood vessels and is a dynamic interface between the brain parenchyma and blood, which is a composite organization composed of endothelial cells, basement membranes, pericytes, and their tight junctions[45]. Maintaining the structural and functional integrity of the BBB is essential for controlling the chemical composition of the extracellular fluid in the brain, which helps ensure normal synaptic information processing and neuronal connectivity. When the structural integrity of the BBB is disrupted, dysfunction is manifested as increased vascular permeability, and toxic cytokines, immune molecules and cells, microorganisms, and viruses in the bloodstream enter the brain, causing various ionic imbalances, signaling homeostasis, and activation of immune cells in the brain, resulting in various inflammatory reactions, which can lead to different neurodegenerative changes and sequelae[27,46]. Thus, ICAM-1 Levels predict BBB permeability and may serve as a biomarker for the early detection of CSVD. Nevertheless, this study had a cross-sectional design, and causal relationships could not be determined. Moreover, previous studies have highlighted the early development of cerebral microangiopathy with high BBB leakage in individuals with diabetes before the emergence of CSVD[47,48]; however, it was not observed in the present study. The conflicting results could be attributed to the study population and patient characteristics. CSVD affects several cognitive domains in asymptomatic individuals and in those with stroke or dementia[1,49]. In this study, the CSVD group had lower MMSE and MoCA-B scores than the control group.
However, this study has limitations. First, this study followed a cross-sectional design, causal relationships could not be determined, and selection bias was possible. Second, the patients were limited to those with asymptomatic CSVD given that the study aimed to examine ICAM-1 in early-onset CSVD and explore its utility as a surrogate marker of MRI-detected CSVD before symptom onset. ICAM-1 changes in the CSVD course were not detected. Third, diffusion tensor imaging can more effectively clarify the damage to white matter fiber bundles, which was not investigated in this study. In addition, DCE-MRI is time-consuming and mainly depends on patient cooperation, and patient movements during the procedure may compromise the accuracy of the results. Thus, a well-designed, large-sample longitudinal study is needed. Fourth, age was independently associated with CSVD, and older individuals typically experience chronic sterile low-grade inflammation, which were attributed to multiple cellular and biological mechanisms. In older individuals, inflammation predicts the risk of various age-related chronic noncommunicable diseases, including age-related CSVD. The close relationship between inflammation and CSVD presents the potential for inflammation-related therapies. However, few studies have focused on inflammation-related therapies and their effects. This gap is an important area of unmet medical need and warrants further translational research.
CONCLUSION
This study found that age and ICAM-1 levels were independently associated with CSVD. ICAM-1 may cause cortical thickness in the brain, predominantly in the white matter, and a significant increase in BBB permeability, demonstrating the involvement of ICAM-1 in BBB damage. The potential clinical utility of ICAM-1 as a CSVD biomarker is important for its early detection and thus the prevention of its progression.
Footnotes
Institutional review board statement: The study was approved by the Ethics Committee of Fuyang People’s Hospital ([2022]26).
Informed consent statement: All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest statement: All authors declare that they have no conflicts of interest.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Psychiatry
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade B, Grade C
Novelty: Grade B, Grade B
Creativity or Innovation: Grade C, Grade C
Scientific Significance: Grade B, Grade B
P-Reviewer: Fumia A; Sosa SEY S-Editor: Lin C L-Editor: A P-Editor: Yuan YY
Contributor Information
Ju-Luo Chen, Cheeloo College of Medicine, Shandong University, Jinan 250012, Shandong Province, China; The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei 230001, Anhui Province, China; Department of Neurology, Fuyang People’s Hospital, Fuyang 236000, Anhui Province, China.
Rui Wang, The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei 230001, Anhui Province, China.
Pei-Qi Ma, Department of Neurology, Fuyang People’s Hospital, Fuyang 236000, Anhui Province, China.
You-Meng Wang, Department of Neurology, Fuyang People’s Hospital, Fuyang 236000, Anhui Province, China.
Qi-Qiang Tang, Cheeloo College of Medicine, Shandong University, Jinan 250012, Shandong Province, China; The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei 230001, Anhui Province, China. tqq1995@126.com.
Data sharing statement
All data generated or analyzed during this study are included in this published article.
References
- 1.Salvadori E, Brambilla M, Maestri G, Nicotra A, Cova I, Pomati S, Pantoni L. The clinical profile of cerebral small vessel disease: Toward an evidence-based identification of cognitive markers. Alzheimers Dement. 2023;19:244–260. doi: 10.1002/alz.12650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith EE, Beaudin AE. New insights into cerebral small vessel disease and vascular cognitive impairment from MRI. Curr Opin Neurol. 2018;31:36–43. doi: 10.1097/WCO.0000000000000513. [DOI] [PubMed] [Google Scholar]
- 3.Thompson CS, Hakim AM. Living beyond our physiological means: small vessel disease of the brain is an expression of a systemic failure in arteriolar function: a unifying hypothesis. Stroke. 2009;40:e322–e330. doi: 10.1161/STROKEAHA.108.542266. [DOI] [PubMed] [Google Scholar]
- 4.Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol. 2013;12:483–497. doi: 10.1016/S1474-4422(13)70060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Low A, Mak E, Rowe JB, Markus HS, O'Brien JT. Inflammation and cerebral small vessel disease: A systematic review. Ageing Res Rev. 2019;53:100916. doi: 10.1016/j.arr.2019.100916. [DOI] [PubMed] [Google Scholar]
- 6.Altendahl M, Maillard P, Harvey D, Cotter D, Walters S, Wolf A, Singh B, Kakarla V, Azizkhanian I, Sheth SA, Xiao G, Fox E, You M, Leng M, Elashoff D, Kramer JH, Decarli C, Elahi F, Hinman JD. An IL-18-centered inflammatory network as a biomarker for cerebral white matter injury. PLoS One. 2020;15:e0227835. doi: 10.1371/journal.pone.0227835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu D, Qin R, Li M, Shen J, Mao Y, Tang K, Zhang A, Wang D, Shi Y. Identification of a novel cell cycle-related risk signature predicting prognosis in patients with pancreatic adenocarcinoma. Medicine (Baltimore) 2022;101:e29683. doi: 10.1097/MD.0000000000029683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dietrich JB. The adhesion molecule ICAM-1 and its regulation in relation with the blood-brain barrier. J Neuroimmunol. 2002;128:58–68. doi: 10.1016/s0165-5728(02)00114-5. [DOI] [PubMed] [Google Scholar]
- 9.Wu F, Liu L, Zhou H. Endothelial cell activation in central nervous system inflammation. J Leukoc Biol. 2017;101:1119–1132. doi: 10.1189/jlb.3RU0816-352RR. [DOI] [PubMed] [Google Scholar]
- 10.Kaiser D, Weise G, Möller K, Scheibe J, Pösel C, Baasch S, Gawlitza M, Lobsien D, Diederich K, Minnerup J, Kranz A, Boltze J, Wagner DC. Spontaneous white matter damage, cognitive decline and neuroinflammation in middle-aged hypertensive rats: an animal model of early-stage cerebral small vessel disease. Acta Neuropathol Commun. 2014;2:169. doi: 10.1186/s40478-014-0169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thrippleton MJ, Backes WH, Sourbron S, Ingrisch M, van Osch MJP, Dichgans M, Fazekas F, Ropele S, Frayne R, van Oostenbrugge RJ, Smith EE, Wardlaw JM. Quantifying blood-brain barrier leakage in small vessel disease: Review and consensus recommendations. Alzheimers Dement. 2019;15:840–858. doi: 10.1016/j.jalz.2019.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bowman GL, Dayon L, Kirkland R, Wojcik J, Peyratout G, Severin IC, Henry H, Oikonomidi A, Migliavacca E, Bacher M, Popp J. Blood-brain barrier breakdown, neuroinflammation, and cognitive decline in older adults. Alzheimers Dement. 2018;14:1640–1650. doi: 10.1016/j.jalz.2018.06.2857. [DOI] [PubMed] [Google Scholar]
- 13.Cai HQ, Weickert TW, Catts VS, Balzan R, Galletly C, Liu D, O'Donnell M, Shannon Weickert C. Altered levels of immune cell adhesion molecules are associated with memory impairment in schizophrenia and healthy controls. Brain Behav Immun. 2020;89:200–208. doi: 10.1016/j.bbi.2020.06.017. [DOI] [PubMed] [Google Scholar]
- 14.Ormel PR, Böttcher C, Gigase FAJ, Missall RD, van Zuiden W, Fernández Zapata MC, Ilhan D, de Goeij M, Udine E, Sommer IEC, Priller J, Raj T, Kahn RS, Hol EM, de Witte LD. A characterization of the molecular phenotype and inflammatory response of schizophrenia patient-derived microglia-like cells. Brain Behav Immun. 2020;90:196–207. doi: 10.1016/j.bbi.2020.08.012. [DOI] [PubMed] [Google Scholar]
- 15.Enwright Iii JF, Huo Z, Arion D, Corradi JP, Tseng G, Lewis DA. Transcriptome alterations of prefrontal cortical parvalbumin neurons in schizophrenia. Mol Psychiatry. 2018;23:1606–1613. doi: 10.1038/mp.2017.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greene C, Hanley N, Campbell M. Blood-brain barrier associated tight junction disruption is a hallmark feature of major psychiatric disorders. Transl Psychiatry. 2020;10:373. doi: 10.1038/s41398-020-01054-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greene C, Kealy J, Humphries MM, Gong Y, Hou J, Hudson N, Cassidy LM, Martiniano R, Shashi V, Hooper SR, Grant GA, Kenna PF, Norris K, Callaghan CK, Islam MD, O'Mara SM, Najda Z, Campbell SG, Pachter JS, Thomas J, Williams NM, Humphries P, Murphy KC, Campbell M. Dose-dependent expression of claudin-5 is a modifying factor in schizophrenia. Mol Psychiatry. 2018;23:2156–2166. doi: 10.1038/mp.2017.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma C, Yang L, Wang L. Correlation of Serum C-Peptide, Soluble Intercellular Adhesion Molecule-1, and NLRP3 Inflammasome-Related Inflammatory Factor Interleukin-1β after Brain Magnetic Resonance Imaging Examination with Cerebral Small Vessel Disease. Contrast Media Mol Imaging. 2022;2022:4379847. doi: 10.1155/2022/4379847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rost NS, Etherton M. Cerebral Small Vessel Disease. Continuum (Minneap Minn) 2020;26:332–352. doi: 10.1212/CON.0000000000000841. [DOI] [PubMed] [Google Scholar]
- 20.Chen KL, Xu Y, Chu AQ, Ding D, Liang XN, Nasreddine ZS, Dong Q, Hong Z, Zhao QH, Guo QH. Validation of the Chinese Version of Montreal Cognitive Assessment Basic for Screening Mild Cognitive Impairment. J Am Geriatr Soc. 2016;64:e285–e290. doi: 10.1111/jgs.14530. [DOI] [PubMed] [Google Scholar]
- 21.Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol. 1987;149:351–356. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- 22.Kwon SM, Choi KS, Yi HJ, Ko Y, Kim YS, Bak KH, Chun HJ, Lee YJ, Lee JY. Impact of brain atrophy on 90-day functional outcome after moderate-volume basal ganglia hemorrhage. Sci Rep. 2018;8:4819. doi: 10.1038/s41598-018-22916-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Markus HS, de Leeuw FE. Cerebral small vessel disease: Recent advances and future directions. Int J Stroke. 2023;18:4–14. doi: 10.1177/17474930221144911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S American Heart Association Stroke Council, Council on Epidemiology and Prevention, Council on Cardiovascular Nursing, Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nam KW, Kwon HM, Lim JS, Han MK, Nam H, Lee YS. The presence and severity of cerebral small vessel disease increases the frequency of stroke in a cohort of patients with large artery occlusive disease. PLoS One. 2017;12:e0184944. doi: 10.1371/journal.pone.0184944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryu WS, Woo SH, Schellingerhout D, Jang MU, Park KJ, Hong KS, Jeong SW, Na JY, Cho KH, Kim JT, Kim BJ, Han MK, Lee J, Cha JK, Kim DH, Lee SJ, Ko Y, Cho YJ, Lee BC, Yu KH, Oh MS, Park JM, Kang K, Lee KB, Park TH, Lee J, Choi HK, Lee K, Bae HJ, Kim DE. Stroke outcomes are worse with larger leukoaraiosis volumes. Brain. 2017;140:158–170. doi: 10.1093/brain/aww259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang X, Tang Y, Xie Y, Ding C, Xiao J, Jiang X, Shan H, Lin Y, Li C, Hu D, Li T, Sheng L. Total magnetic resonance imaging burden of cerebral small-vessel disease is associated with post-stroke depression in patients with acute lacunar stroke. Eur J Neurol. 2017;24:374–380. doi: 10.1111/ene.13213. [DOI] [PubMed] [Google Scholar]
- 28.Cannistraro RJ, Badi M, Eidelman BH, Dickson DW, Middlebrooks EH, Meschia JF. CNS small vessel disease: A clinical review. Neurology. 2019;92:1146–1156. doi: 10.1212/WNL.0000000000007654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Troncoso MF, Ortiz-Quintero J, Garrido-Moreno V, Sanhueza-Olivares F, Guerrero-Moncayo A, Chiong M, Castro PF, García L, Gabrielli L, Corbalán R, Garrido-Olivares L, Lavandero S. VCAM-1 as a predictor biomarker in cardiovascular disease. Biochim Biophys Acta Mol Basis Dis. 2021;1867:166170. doi: 10.1016/j.bbadis.2021.166170. [DOI] [PubMed] [Google Scholar]
- 30.Walker KA, Power MC, Hoogeveen RC, Folsom AR, Ballantyne CM, Knopman DS, Windham BG, Selvin E, Jack CR Jr, Gottesman RF. Midlife Systemic Inflammation, Late-Life White Matter Integrity, and Cerebral Small Vessel Disease: The Atherosclerosis Risk in Communities Study. Stroke. 2017;48:3196–3202. doi: 10.1161/STROKEAHA.117.018675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao MZ, Guo X, Sun B, Sun XF, Pang GF, Yang LY, Zhao X, Sun LX, Zhang Q. HA of H1N1 enhanced the expression of ICAM-1 and IL-6 in HUVECs and pathological injury in the lungs in mice. Gene. 2021;801:145854. doi: 10.1016/j.gene.2021.145854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ren B, Tan L, Song Y, Li D, Xue B, Lai X, Gao Y. Cerebral Small Vessel Disease: Neuroimaging Features, Biochemical Markers, Influencing Factors, Pathological Mechanism and Treatment. Front Neurol. 2022;13:843953. doi: 10.3389/fneur.2022.843953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang L, Chen Y, Feng D, Wang X. Serum ICAM-1 as a Predictor of Prognosis in Patients with Acute Ischemic Stroke. Biomed Res Int. 2021;2021:5539304. doi: 10.1155/2021/5539304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klausz K, Cieker M, Kellner C, Rösner T, Otte A, Krohn S, Lux A, Nimmerjahn F, Valerius T, Gramatzki M, Peipp M. Fc-engineering significantly improves the recruitment of immune effector cells by anti-ICAM-1 antibody MSH-TP15 for myeloma therapy. Haematologica. 2021;106:1857–1866. doi: 10.3324/haematol.2020.251371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang CE, Wong SM, van de Haar HJ, Staals J, Jansen JF, Jeukens CR, Hofman PA, van Oostenbrugge RJ, Backes WH. Blood-brain barrier leakage is more widespread in patients with cerebral small vessel disease. Neurology. 2017;88:426–432. doi: 10.1212/WNL.0000000000003556. [DOI] [PubMed] [Google Scholar]
- 36.Nation DA, Sweeney MD, Montagne A, Sagare AP, D'Orazio LM, Pachicano M, Sepehrband F, Nelson AR, Buennagel DP, Harrington MG, Benzinger TLS, Fagan AM, Ringman JM, Schneider LS, Morris JC, Chui HC, Law M, Toga AW, Zlokovic BV. Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med. 2019;25:270–276. doi: 10.1038/s41591-018-0297-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elangbam CS, Qualls CW Jr, Dahlgren RR. Cell adhesion molecules--update. Vet Pathol. 1997;34:61–73. doi: 10.1177/030098589703400113. [DOI] [PubMed] [Google Scholar]
- 38.Yang L, Froio RM, Sciuto TE, Dvorak AM, Alon R, Luscinskas FW. ICAM-1 regulates neutrophil adhesion and transcellular migration of TNF-alpha-activated vascular endothelium under flow. Blood. 2005;106:584–592. doi: 10.1182/blood-2004-12-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Collins RG, Velji R, Guevara NV, Hicks MJ, Chan L, Beaudet AL. P-Selectin or intercellular adhesion molecule (ICAM)-1 deficiency substantially protects against atherosclerosis in apolipoprotein E-deficient mice. J Exp Med. 2000;191:189–194. doi: 10.1084/jem.191.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 41.Kisler K, Nelson AR, Montagne A, Zlokovic BV. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat Rev Neurosci. 2017;18:419–434. doi: 10.1038/nrn.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fleischer S, Tavakol DN, Vunjak-Novakovic G. From arteries to capillaries: approaches to engineering human vasculature. Adv Funct Mater. 2020;30 doi: 10.1002/adfm.201910811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lochhead JJ, Yang J, Ronaldson PT, Davis TP. Structure, Function, and Regulation of the Blood-Brain Barrier Tight Junction in Central Nervous System Disorders. Front Physiol. 2020;11:914. doi: 10.3389/fphys.2020.00914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sweeney MD, Sagare AP, Zlokovic BV. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol. 2018;14:133–150. doi: 10.1038/nrneurol.2017.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lacoste B, Prat A, Freitas-Andrade M, Gu C. The Blood-Brain Barrier: Composition, Properties, and Roles in Brain Health. Cold Spring Harb Perspect Biol. 2024 doi: 10.1101/cshperspect.a041422. [DOI] [PubMed] [Google Scholar]
- 46.Allen BD, Limoli CL. Breaking barriers: Neurodegenerative repercussions of radiotherapy induced damage on the blood-brain and blood-tumor barrier. Free Radic Biol Med. 2022;178:189–201. doi: 10.1016/j.freeradbiomed.2021.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen YC, Lu BZ, Shu YC, Sun YT. Spatiotemporal Dynamics of Cerebral Vascular Permeability in Type 2 Diabetes-Related Cerebral Microangiopathy. Front Endocrinol (Lausanne) 2021;12:805637. doi: 10.3389/fendo.2021.805637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou H, Hu J, Xie P, Dong Y, Chen W, Wu H, Jiang Y, Lei H, Luo G, Liu J. Lacunes and type 2 diabetes mellitus have a joint effect on cognitive impairment: a retrospective study. PeerJ. 2022;10:e13069. doi: 10.7717/peerj.13069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hamilton OKL, Cox SR, Okely JA, Conte F, Ballerini L, Bastin ME, Corley J, Taylor AM, Page D, Gow AJ, Muñoz Maniega S, Redmond P, Valdés-Hernández MDC, Wardlaw JM, Deary IJ. Cerebral small vessel disease burden and longitudinal cognitive decline from age 73 to 82: the Lothian Birth Cohort 1936. Transl Psychiatry. 2021;11:376. doi: 10.1038/s41398-021-01495-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.