Abstract
Background:
Transcranial direct current stimulation (tDCS) is a potential neuromodulation option for the management of cravings in patients with alcohol use disorder (AUD). This study aims to assess the efficacy and safety of tDCS in the management of craving for alcohol and to measure the change in subjective well-being in patients with AUD following tDCS intervention.
Methods:
Patients with AUD aged between 18 and 60 years were randomly assigned to active tDCS intervention and sham tDCS intervention groups, each consisting of 17 patients. Over the course of a week, five tDCS sessions were given, with the anode positioned on the scalp over the right dorsolateral prefrontal cortices (DLPFC) area, the cathode over the left DLPFC area, and a 2 mA current. After every session, a tDCS side effects checklist was used. Follow-up assessments were conducted at week 1, week 4, and week 8 of the recruitment using the alcohol urge questionnaire (AUQ) and the World Health Organization (WHO) (five) Well-Being Index (WHO-5).
Results:
A significantly greater reduction in AUQ scores was present in the active tDCS intervention group compared to the sham tDCS intervention at week 1 and week 4, but the difference was not significant at the end of 8 weeks. A significant improvement in WHO-5 scores was found in both groups; however, the difference between the groups was not significant at follow-ups. The side effects observed were mild to moderate in intensity, were short-lived, and did not require any active management.
Conclusions:
The tDCS may be useful in the acute reduction of craving in AUD. It is a safe and well-tolerated intervention modality.
Keywords: Alcohol use disorder, craving, transcranial direct current stimulation, neuromodulation, subjective wellbeing
Key Messages:
Transcranial direct current stimulation is a beneficial and safe treatment strategy in dealing with craving in alcohol use disorder.
The effects on craving are short-lasting and tend to fade over a few weeks following termination of intervention.
Alcohol use disorder (AUD) is a leading contributor to the global burden of disease. 1 Its negative consequences include increased morbidity and mortality rates, major economic losses, and social harms. 2 The chronic relapsing- remitting life course of AUD poses a major challenge. A substantial proportion of AUD patients rapidly relapse to alcohol use after treatment or the abstinence attempt.3,4 The biological treatment approaches for managing AUD focus on managing withdrawal symptoms and craving to prevent relapse.5,6 However, current pharmacological and psychosocial management approaches have limited effectiveness in managing the urge to consume alcohol.7,8 Recent research has suggested that noninvasive brain stimulation techniques (NIBS) could be useful in treating substance disorders, including AUD.9,10 Specific brain areas implicated in the development of addiction can be targeted through neuromodulation techniques to effect changes in their function. The NIBS can alter the neuronal excitability of superficial brain areas of interest and that of connected deeper brain regions.
Transcranial direct current stimulation (tDCS) is a form of NIBS employed in the treatment of diverse substance use disorders, including alcohol, nicotine, cocaine, opioids, and cannabis. 11 The brain areas commonly targeted are the prefrontal cortex (PFC) and inferior frontal gyrus.11,12 Also, tDCS has demonstrated a favorable safety profile, and only minor side effects have been reported with tDCS therapy. 13 The role of tDCS in AUD was initially examined in a double-blind, crossover, randomized controlled trial (RCT) where participants received single sessions of tDCS stimulation over both the dorsolateral prefrontal cortices (DLPFCs), anodal right/cathodal left and anodal left/cathodal right, as well as sham stimulation. A significant reduction was observed in craving measured using the alcohol urge questionnaire (AUQ) and visual analog scale in both the active groups (receiving DLPFC stimulation) compared to the sham group. 14 Subsequent studies targeting left DLPFC for anodal stimulation showed mixed results. Some studies revealed no difference in craving or relapse rates after tDCS sessions.15–18 On the contrary, several other studies observed a reduction in craving after anodal stimulation of left DLPFC.19,20 Further, the studies targeting the right DLPFC as anodal site have reported the benefits of tDCS intervention in terms of reduced craving or relapse rates or consumption of alcohol.21–25 Mostafavi et al. (2020) conducted a meta-analysis of 11 RCTs and reported no evidence of a beneficial effect of tDCS on alcohol craving. However, a subsequent meta-analysis comprising 18 studies identified a modest positive effect of tDCS. Specifically, the application of anodal tDCS over right DLPFC and cathodal tDCS over left DLPFC was revealed to have significant favorable effects on craving. Moreover, compared to a single session, the tDCS protocols involving multiple sessions were found to have better effects. The authors highlighted the need for further research to substantiate the findings. 26
For AUD, a variety of tDCS protocols is used till date, yet the evidence for effectiveness is mixed, and there is a need for more research on this topic. Thus, in the present study, our principal aim was to compare the change in alcohol craving scores between active tDCS versus sham tDCS in AUD patients. We hypothesized that AUD patients receiving active tDCS intervention would have a greater reduction in the measures of craving compared to those receiving sham tDCS. Furthermore, we sought to evaluate the differences between the active and sham tDCS groups in terms of change in subjective well-being scores and tDCS-related side effects.
Methods
Study Design
The study was a single-blind, RCT.
Settings and Participants
The participants were inpatients and patients attending the outpatient and emergency services of a tertiary care center for the treatment of psychiatric disorders in North India. Patients aged 18–60 years with AUD (diagnosed as per DSM-5), experiencing a craving for alcohol (AUQ ≥ 24), but only mild or no alcohol withdrawal (Clinical Institute Withdrawal Assessment for Alcohol Scale, revised [CIWA-Ar] score <10) and consenting to participate, were included in the study. Patients, who had any other psychiatric disorder except tobacco use disorder, history of seizure or delirium or any other complication during acute withdrawal state in the current episode, any major medical comorbidity requiring priority management, any contraindication for tDCS procedures, or were left-handed were excluded. Participants were considered dropouts if they had any of the following—(a) failed to complete five sessions over 1 week time period, (b) any alcohol use during the 5-day tDCS sessions, (c) withdrew consent during the course of therapy, (d) developed any severe intolerable side effects after the initiation of tDCS therapy, (e) missed first follow-up visit despite the attempts made to reach out to them.
The estimated sample size for this study was 30, computed using G*Power version 3.1.9.7, based on an effect size of 0.25, a power of 0.90, an α-error probability of 0.05, four measurement points, non-sphericity correction epsilon of one, and correlation between the repeated measures as 0.5. It was assumed that the sphericity assumption was met, and consequently, the non-sphericity correction was maintained at one. The data collection was done between January 2022 and November 2022. The study was approved by the institutional ethics committee (letter no 557/Ethics/2021 with reference code II PGTSC-IIA/-P23). Prospective registration of the trial was done in the Clinical Trials Registry, India (Registration number CTRI/2022/01/039412).
Study Groups
Based on the tDCS treatment protocol, the study included two groups—the “active group” and the “sham group.” Participants were randomly assigned to these treatment arms according to a computer-generated random number table. The allocation ratio was 1:1. The study participants were unaware of the tDCS protocol (single blinding).
tDCS Intervention Protocol
A tDCS device was used to deliver direct current with the anode placed over the right DLPFC and the cathode placed over the left DLPFC. The targets of interest were located using the BeamF3 software. 27 The electrodes (size 5 cm × 5 cm) were placed in sponge cases soaked in 0.9% NaCl solution and affixed to the scalp using elastic straps. An electric current, with an intensity of 2 mA, was administered for 20 minutes per session, preceded by a 10-second ramping-up time. This procedure was conducted once daily for a period of five consecutive days. For the sham group, the current did not flow except in the ramp phase. The tDCS was administered to patients in sitting positions between 10:00 am and 2:00 pm.
Study Procedure
Treatment-seeking adult inpatients and outpatients diagnosed with AUD were considered for inclusion in the study. The acute alcohol withdrawal was managed and those with at least 7 days of abstinence from alcohol, CIWA-Ar score <10, and off benzodiazepines for 48 hours were evaluated for enrolment in the study. The World Health Organization (WHO)-Alcohol, Smoking, and Substance Involvement Screening Test version 3.0 was used to screen for the use of alcohol, tobacco products, and other drugs and to assess the severity of alcohol use. Participants meeting the selection criteria were enrolled in the study and randomly allocated to one of two groups. One of the authors (SKK) prepared opaque envelopes containing a paper sheet specifying group information in accordance with the random number table generated beforehand. These envelopes were sequentially labeled and sealed. The participant’s group assignment was established by unsealing these envelopes, leading to participants being designated to either the active tDCS group or the sham tDCS group. The author who generated the random number table and took care of allocation concealment was not involved in the shortlisting or selection of study participants. Sociodemographic and clinical information (including the age at alcohol use onset, duration of illness, severity of dependence, prior quit attempts, and mean period of abstinence) was collected. CIWA-Ar was applied for monitoring alcohol withdrawal symptoms. AUQ was used for the assessment of cravings due to alcohol. The WHO (five) Well-Being Index (WHO-5) was applied to assess subjective well-being. Participants were administered either an active or sham tDCS protocol based on their respective study groups. The protocol was concealed from them; however, the investigator was aware of it. Over the course of a week, five (once daily) tDCS sessions were given. The tDCS side effects checklist was used every day to monitor the adverse effects. In case the patient developed any minor adverse effects due to tDCS, conservative management was done. In case of serious adverse effects, the patient was to be dropped out of the study and managed appropriately. Only trazodone up to 100 mg for sleep as rescue medication, along with vitamin supplementation (including thiamine) was allowed for the participants during the period of tDCS intervention. Follow-up assessments (on AUQ, WHO-5) were conducted at week 1 (±2 days), week 4 (±2 days), and week 8 (±2 days) for both groups, after recruitment in the study. Patients in both groups were prescribed disulfiram and anti-craving agents during the follow-up period if required. All necessary COVID-19-related precautions were followed during the study, and it was ensured that the participants and their caregivers did not have any symptoms of the same.
Statistical Analysis
The quantitative variables were expressed as mean ± standard deviation and categorical variables were presented as percentages. Shapiro–Wilk test was used for assessing data normality. Depending on the type of data the groups were compared using Fisher’s Exact and the Mann–Whitney U test. A repeated measures ANOVA (2 × 2) was conducted to determine the acute effect of tDCS on craving. The two study groups were considered as between- subject factors, whereas time (baseline and week 2 [post-tDCS]) was considered as within-subject factors. The AUQ score and WHO-5 were used as dependent variables. Repeated measures ANOVA was also used to determine the long-term effects of tDCS on craving and well-being (at week 4 and week 8). The effect sizes were represented by partial eta squared, and the relative risk was determined for side effects. The study data was analyzed using SPSS software version 25.0. 28
Result
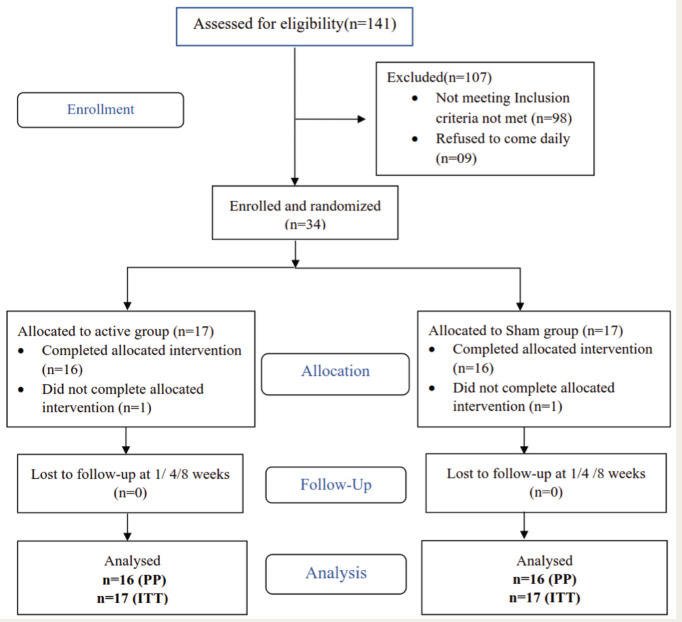
The total number of patients screened was 141 out of which 34 were selected to participate in the study. The common reasons for exclusion were the presence of other substance use disorders (n = 34), and the presence of other psychiatric illnesses (n = 26). Two patients dropped out during the 1 week of administrating therapy. Thus, a total of 32 patients (16 each in active and sham group) were evaluated for per protocol analysis and 34 patients (17 each in active and sham group) were evaluated as per intention to treat (ITT) analysis in the study (Figure 1).
Figure 1. Consolidated Standards of Reporting Trials (CONSORT) Flow Diagram for the Study.
Sociodemographic Profile
All the participants were male. No significant difference was present between the groups with respect to age, marital status, education, domicile, type of family, family income, and level of education (Table 1).
Table 1.
Socio-demographic and Clinical Profile of the Study Groups.
| Variable | Active Group (n = 17) Mean (SD) or n (%) |
Sham group (n = 17) Mean (SD) or n (%) |
U | p Value |
| Mean age (in years) | 38.11 (7.61) | 33.47 (6.8) | 94.5 | .08 |
| Age category | ||||
| 18–40 years | 9 (52.94) | 14 (82.35) | .14 | |
| 41–60 years | 8 (47.06) | 3 (17.65) | ||
| Education | ||||
| Up to high school | 5 (29.42) | 2 (11.76) | .39 | |
| Above high school | 12 (70.58) | 15 (88.24) | ||
| Occupation | ||||
| Unemployed | 3 (18.75) | 3 (18.75) | 1 | |
| Employed | 14 (81.25) | 14 (81.25) | ||
| Monthly family income (in rupees) | ||||
| <20,000 | 5 (29.42) | 2 (11.76) | .39 | |
| >20,000 | 12 (70.58) | 15 (88.34) | ||
| Marital status | ||||
| Married | 13 (76.47) | 12 (70.59) | 1 | |
| Single (unmarried/separated/widowed) | 4 (23.53) | 5 (29.41) | ||
| Domicile | ||||
| Urban | 13 (76.47) | 15 (88.24) | .65 | |
| Rural | 4 (23.53) | 2 (11.76) | ||
| Family type | ||||
| Joint/extended | 9 (52.94) | 4 (23.53) | .15 | |
| Nuclear | 8 (47.06) | 13 (76.47) | ||
| Comorbid tobacco use disorder | ||||
| Yes | 14 (81.25) | 16 (93.75) | .60 | |
| No | 3 (18.75) | 1 (6.25) | ||
| Age at alcohol use onset (years) | 20.7 (3.83) | 21.05 (3.11) | 141.5 | .92 |
| Duration of dependence (years) | 6.67 (4.01) | 5.67 (3.50) | 131.5 | .66 |
| Number of prior quit attempts | 3.24 (1.35) | 3.18 (1.54) | 137.5 | .82 |
| ASSIST score | 36.82 (2.04) | 35.7 (2.69) | 116 | .34 |
| CIWA-Ar scores | 2.06 (0.64) | 1.88 (0.89) | 130.5 | .64 |
| Period of abstinence (days) | 6.94 (0.24) | 7.17 (0.38) | 112 | .27 |
ASSIST—Alcohol, Smoking and Substance Involvement Screening Test version 3.0; CIWA-Ar—Clinical Institute Withdrawal Assessment for Alcohol Scale, revised; U—Mann–Whitney U value; SD—standard deviation.
Clinical Profile
The groups were comparable at baseline on all the clinical variables, including age at alcohol use onset, duration of alcohol dependence, severity of dependence, CIWA-Ar score, prior quit attempts, and mean period of abstinence before the tDCS intervention (Table 1). Also, the baseline AUQ (U = 151; p = .84) and WHO-5 scores (U = 163.5; p = .52) between the groups were not statistically different. Post-tDCS intervention, 10 patients in the active group and 12 in the sham group received disulfiram. Whereas, five patients in the active group and four in the sham group received anti-craving medication (naltrexone) after the termination of tDCS. The inter-group differences were not significant for either disulfiram (p = .72) and naltrexone (p = 1.0) use.
Urge to Drink
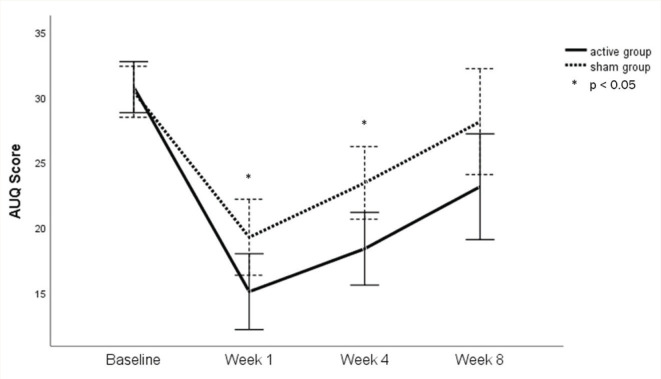
For the acute effect of tDCS, a comparison of scores at baseline and at week 1 revealed that there was a significant main effect of time on AUQ scores, F(1, 32) = 140.2, p = <.001, ηp2=0.81. However, the time × group interaction was not significant, F(1, 30) = 5.51, p = .055, ηp2=0.11 (Figure 2). There was no significant main effect of tDCS intervention on AUQ scores, F(62.13, 28.63) = 2.17, p = .15, ηp2 = 0.064.
Figure 2. Change in AUQ Scores in Study Groups.
ITT analysis of the long-term effects of tDCS through repeated measure ANOVA showed that the sphericity assumption was violated for the main effect of time. The Greenhouse–Geisser estimate for the departure from sphericity was ε = 0.70. A significant main effect for time was observed, F(2.11, 67.65) = 46.92, p = <.001. However, the time × group interaction was not significant, F(2.11, 67.65) = 2.30, p = .10, revealing that when comparing active to sham tDCS, there were no differences in craving over time. Simple effects analysis revealed that at week 1 and week 4, but not at baseline or week 8, AUQ scores were significantly lower in the active tDCS group when compared to the sham tDCS group (Table 2).
Table 2.
Change in Alcohol Urge Questionnaire Score and WHO-5 Well-being Index Scores in Study Groups.
| Clinical Variables | Active tDCS Group Mean (SD) | Sham tDCS Group Mean (SD) | F-Value | p Value | |
| AUQ | Baseline | ||||
| ITT | 30.76 (4.63) | 30.41 (3.16) | 0.06 | .79 | |
| PP | 31.00 (4.68) | 30.31 (3.24) | 0.23 | .63 | |
| 2 weeks | |||||
| ITT | 15.05 (5.40) | 19.23 (6.35) | 4.25 | .04* | |
| PP | 14.31 (4.59) | 18.44 (5.62) | 5.17 | .03* | |
| 4 weeks | |||||
| ITT | 18.35 (4.38) | 23.41 (6.67) | 6.82 | .01* | |
| PP | 17.81 (3.90) | 22.88 (6.50) | 7.13 | .01* | |
| 8 weeks | |||||
| ITT | 23.11 (8.71) | 28.11 (7.71) | 3.13 | .08 | |
| PP | 22.40 (9.05) | 27.88 (7.90) | 2.81 | .10 | |
| Within the group (F; p value) |
|||||
| ITT | 35.18; <.01* | 16.27; <.01* | |||
| PP | 51.06; <.01* | 23.47; <.01* | |||
| WHO-5 | Baseline | ||||
| ITT | 32.00 (16.06) | 35.76 (11.17) | 0.62 | .43 | |
| PP | 32.0 (16.06) | 35.5 (11.12) | 0.48 | .49 | |
| 2 weeks | |||||
| ITT | 65.41 (15.48) | 62.58 (17.14) | 0.25 | .61 | |
| PP | 67.5 (12.8) | 64.0 (16.12) | 0.43 | .51 | |
| 4 weeks | |||||
| ITT | 62.35 (14.83) | 53.17 (17.30) | 2.75 | .10 | |
| PP | 64.25 (12.69) | 53.8 (14.9) | 3.52 | .07 | |
| 8 weeks | |||||
| ITT | 59.52 (21.72) | 48.00 (17.26) | 2.93 | .09 | |
| PP | 59.75 (25.27) | 49.33 (17.13) | 3.41 | .07 | |
| Within the group (F; p value) | |||||
| ITT | 23.19; <.01* | 12.42; <.01* | |||
| PP | 29.16; <.01* | 15.46; <.01* | |||
F—F statistic for ANOVA; AUQ—alcohol urge questionnaire; WHO-5—WHO-5 well-being index score; ITT—intention to treat; PP—per-protocol; SD—standard deviation. *Significant at p value of <0.05.
Well-being Score
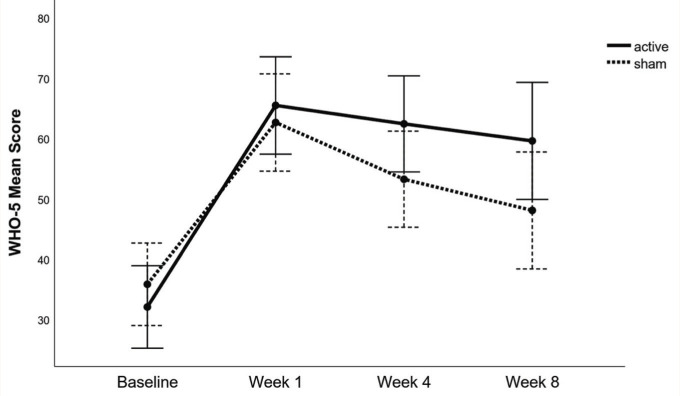
The Huynh–Feldt estimate of the departure from sphericity was ε = 0.94. The main effect of time on WHO-5 scores was significant, F(2.83, 90.56) = 45.10, p = <.001. The time × group interaction was also significant, F(2.83, 90.56) = 3.14, p = .03. However, simple effects analysis revealed that the WHO-5 scores were not significantly different between the active tDCS group and sham tDCS group at baseline, week 1, week 4, and week 8 (Figure 3).
Figure 3. Change in WHO 5 Well-being Scores in Study Groups.
Safety of tDCS
The most reported side effects included burning sensation, numbness, and pain at the stimulation site. Other reported side effects included headache, tingling sensation, and sedation (Table 3). The majority of the side effects observed in the active tDCS group were of “very mild” to “mild” intensity, except moderate sedation reported in one session. Side effects reported in all sessions of the sham group were “very mild” to “mild” in intensity. None of the groups reported “severe” or “very severe” side effects of tDCS. None of the side effects required any active medical intervention or led to dropouts.
Table 3.
Comparison of Adverse Effects in the Study Groups.
| Adverse Effects | Active Group (n = 17) N (%) | Sham Group (n = 17) N (%) | Relative risk (RR) |
| Numbness at stimulation site | 5 (29.41) | 2 (11.76)5 | 2.5 |
| Burning at stimulation site | 5 (29.41) | 1 (5.88) | 5 |
| Pain | 3 (17.64) | 2 (11.76) | 1.5 |
| Headache | 1 (5.88) | 1 (5.88) | 1 |
| Tingling sensation | 1 (5.88) | 2 (11.76) | 0.5 |
| Sedation | 2 (11.76) | 0 (0.00) | – |
Number Needed to Treat (NNT) and Number Needed to Harm (NNH)
Five patients (29.41%) in the sham group and three (17.64%) in the active group relapsed at the end of 8 weeks. Thus, the NNT was 8.5. The side effects were noticed in 21.9% (n = 18) of sessions in the active group, in contrast to 13.25% (n = 11) of sessions in the sham group. The NNH was 4.5.
Discussion
Craving constitutes a key feature of AUD and plays a pivotal role in sustaining the cycle of addiction. This single-blind, RCT was devised to evaluate the efficacy and safety of tDCS in reducing craving among patients with AUD. Also, it attempted to determine the impact of tDCS on subjective well-being. In this study, the DLPFC was the target region of interest, given the growing recognition of frontal dysfunction as a distinctive feature of alcoholism.29,30 It has a role in regulating behavioral (drinking behavior) and cognitive response (craving).31,32 We decided on cathode placement at the left DLPFC and anodal placement at the right DLPFC. Right DLPFC tDCS is especially suggested for intervention in substance use and has a role in craving control. 33 This study is an addition to the limited research assessing the efficacy of multisession tDCS applied over the right DLPFC in reducing alcohol craving.
The two groups in our study were comparable in sociodemographic and clinical profiles. Alcohol use is much more common among males compared to females in this geography, a reason why all the participants in our study were male. 34 The difference in withdrawal scores between the groups was insignificant, and the average scores were minimal, implying little effect, if any, of withdrawal severity on the craving scores.
There was a significantly greater reduction in craving scores in the active group compared to the sham group. Also, we found a significant decline in the AUQ scores after tDCS application at week 1 in both groups. This suggests that tDCS administration leads to an additional reduction in the urge for alcohol beyond what happens with time since abstaining from alcohol. Similar findings were reported by a previous study with multisession tDCS targeting the right DLPFC. 22 However, studies with single-session tDCS have shown mixed results.14,25,35 A single session may not be adequate to produce significant effects on craving. Literature suggests that the effects of single tDCS do not persist beyond an hour and are less likely to carry over the impact to the next day. 36 As has been reported, the improvement is gradual and likely dependent on the number of stimulations administered over time. Thus, the cumulative effect of repeated tDCS sessions should be considered. 37
In follow-up, although the mean AUQ scores increased between week 1 and week 4 in both the groups, the difference in scores continued to be statistically significant at week 4, with a greater reduction from baseline in the active group. This implies that active tDCS may positively affect craving; however, the effect is not persistent and starts weaning off after stimulation. At week 8, the mean AUQ scores of both groups were comparable, suggesting that the effect of tDCS does not last long (even though some patients in both groups received disulfiram and anti-craving medications). In our study, two patients in the active group and four in the sham group relapsed before the final assessment at 8 weeks. Klauss et al. reported three times fewer relapses in the active tDCS group compared to the sham tDCS group; however, they did not find any significant inter-group differences in craving. 23 In a subsequent study, craving scores were found to be significantly reduced following active tDCS compared to sham, and significantly fewer relapses up to 3 months in the active stimulation group were noticed. 22
There was an improvement in the WHO-5 scores between the baseline and week 1 in both groups; however, the difference between the group scores was not significant. Harmful drinking is associated with poor mental well-being and the relationship between levels of alcohol consumption and mental well-being may be bidirectional.38–40 As both the groups were abstinent during the period of intervention in this study, their well-being improved. At week 4 and week 8 follow-up, there was a decline in WHO-5 scores compared to week 1 in both groups. However, the inter-group differences of well-being continue to remain non-significant. This is understandable as well-being is a complex phenomenon and various psycho-social factors are known to influence it.
In our study, the tDCS side effect checklist was used to evaluate subjects for adverse effects. 41 The most commonly reported side effects experienced by individuals included burning at the stimulation site, numbness, and pain at the stimulation site. In both groups, the side effects were mostly temporary, lasting only a few seconds or minutes after beginning tDCS. Similar findings of mild and tolerable side effects have been reported in other studies.33,42 The NNH was 4.5. However, considering the low intensity and transient nature of side effects, the tDCS can be considered a safe procedure for AUD craving in accordance with previous literature, where serious side effects have rarely been observed. 13
One major limitation of the study is the use of disulfiram in 22 patients (65%) and anti-craving agents in nine patients (26%) during the follow-ups. The use of both, anti-craving agents and disulfiram, is expected to influence relapse rates. Besides, we could not ensure adherence to the medications; so, the differential effect of medicines cannot be ruled out. As it was a single-blinded study, observer bias could be a potential study limitation. Patients were given only five sessions of tDCS on a daily basis, administration of a higher number of sessions and multiple sessions per day could have given different results and should be explored in future research.
Conclusion
The availability of limited alcohol craving management options underscores the need to explore new strategies and regimens. This randomized, single-blind controlled trial supports the use of tDCS for the early reduction of craving in AUD. There were significant differences in the craving till 4 weeks of treatment in a naturalistic setting. However, the effects were not sustained for long. Thus, the role of tDCS in the long-term management of AUD as maintenance treatment or regular booster sessions can be explored in future studies. Also, tDCS is well tolerated. Hence, tDCS could be considered as an additional option in the management of AUD.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Trial Registration: Clinical Trials Registry, India (Registration number CTRI/2022/01/039412).
References
- 1.Rehm J and Shield KD. Global Burden of Disease and the impact of mental and addictive disorders. Curr Psychiatry Rep, 2019; 21: 10. [DOI] [PubMed] [Google Scholar]
- 2.WHO. Alcohol. https://www.who.int/news-room/fact-sheets/detail/alcohol.
- 3.Maddux JF and Desmon DP. Addiction or dependence? Addiction, 2000; 95: 661–665. [DOI] [PubMed] [Google Scholar]
- 4.Swift RM. Drug therapy for alcohol dependence. N Engl J Med, 1999; 340: 1482–1490. [DOI] [PubMed] [Google Scholar]
- 5.Klimas J, Tobin H, Field C-A, et al. Psychosocial interventions to reduce alcohol consumption in concurrent problem alcohol and illicit drug users. Cochrane Database Syst Rev. Epub ahead of print 3 December 2014. doi: 10.1002/14651858.CD009269.pub3. [DOI] [PubMed] [Google Scholar]
- 6.Soyka M, Kranzler HR, Hesselbrock V, et al. Guidelines for biological treatment of substance use and related disorders, part 1: Alcoholism, first revision. World J Biol Psychiatry, 2017; 18: 86–119. [DOI] [PubMed] [Google Scholar]
- 7.Miller PM, Book SW, and Stewart SH. Medical treatment of alcohol dependence: A systematic review. Int J Psychiatry Med, 2011; 42: 227–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association Practice Guideline for the pharmacological treatment of patients with alcohol use disorder. Am J Psychiatry, 2018; 175: 86–90. [DOI] [PubMed] [Google Scholar]
- 9.Naish KR, Vedelago L, MacKillop J, et al. Effects of neuromodulation on cognitive performance in individuals exhibiting addictive behaviors: A systematic review. Drug Alcohol Depend, 2018; 192: 338–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azevedo CA and Mammis A.. Neuromodulation therapies for alcohol addiction: A literature review. Neuromodulation Technol Neural Interface, 2018; 21: 144–148. [DOI] [PubMed] [Google Scholar]
- 11.Lupi M, Martinotti G, Santacroce R, et al. Transcranial direct current stimulation in substance use disorders: A systematic review of scientific literature. J ECT, 2017; 33: 203–209. [DOI] [PubMed] [Google Scholar]
- 12.Koob GF and Volkow ND. Neurobiology of addiction: A neurocircuitry analysis. Lancet Psychiatry, 2016; 3: 760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bikson M, Grossman P, Thomas C, et al. Safety of transcranial direct current stimulation: Evidence based update 2016. Brain Stimulat, 2016; 9: 641–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boggio PS, Sultani N, Fecteau S, et al. Prefrontal cortex modulation using transcranial DC stimulation reduces alcohol craving: A double-blind, sham-controlled study. Drug Alcohol Depend, 2008; 92: 55–60. [DOI] [PubMed] [Google Scholar]
- 15.da Silva MC, Conti CL, Klauss J, et al. Behavioral effects of transcranial direct current stimulation (tDCS) induced dorsolateral prefrontal cortex plasticity in alcohol dependence. J Physiol-Paris, 2013; 107: 493–502. [DOI] [PubMed] [Google Scholar]
- 16.den Uyl TE, Gladwin TE, Lindenmeyer J, et al. A clinical trial with combined transcranial direct current stimulation and attentional bias modification in alcohol-dependent patients. Alcohol Clin Exp Res, 2018; 42: 1961–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.den Uyl TE, Gladwin TE, Rinck M, et al. A clinical trial with combined transcranial direct current stimulation and alcohol approach bias retraining: Clinical trial tDCS and CBM. Addict Biol, 2017; 22: 1632–1640. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura-Palacios EM, Lopes IBC, Souza RA, et al. Ventral medial prefrontal cortex (vmPFC) as a target of the dorsolateral prefrontal modulation by transcranial direct current stimulation (tDCS) in drug addiction. J Neural Transm, 2016; 123: 1179–1194. [DOI] [PubMed] [Google Scholar]
- 19.den Uyl TE, Gladwin TE, and Wiers RW. Electrophysiological and behavioral effects of combined transcranial direct current stimulation and alcohol approach bias retraining in hazardous drinkers. Alcohol Clin Exp Res, 2016; 40: 2124–2133. [DOI] [PubMed] [Google Scholar]
- 20.den Uyl Tess E, Gladwin TE, and Wiers RW. Transcranial direct current stimulation, implicit alcohol associations and craving. Biol Psychol, 2015; 105: 37–42. [DOI] [PubMed] [Google Scholar]
- 21.Holla B, Biswal J, Ramesh V, et al. Effect of prefrontal tDCS on resting brain fMRI graph measures in alcohol use disorders: A randomized, double-blind, sham- controlled study. Prog Neuropsychopharmacol Biol Psychiatry, 2020; 102: 109950. [DOI] [PubMed] [Google Scholar]
- 22.Klauss J, Anders QS, Felippe LV, et al. Multiple sessions of transcranial direct current stimulation (tDCS) reduced craving and relapses for alcohol use: A randomized placebo-controlled trial in alcohol use disorder. Front Pharmacol, 2018; 9: 716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klauss J, Penido Pinheiro LC, Silva Merlo BL, et al. A randomized controlled trial of targeted prefrontal cortex modulation with tDCS in patients with alcohol dependence. Int J Neuropsychopharmacol, 2014; 17: 1793–1803. [DOI] [PubMed] [Google Scholar]
- 24.Vanderhasselt M-A, Allaert J, De Raedt R, et al. Bifrontal tDCS applied to the dorsolateral prefrontal cortex in heavy drinkers: Influence on reward-triggered approach bias and alcohol consumption. Brain Cogn, 2020; 138: 105512. [DOI] [PubMed] [Google Scholar]
- 25.Wietschorke K, Lippold J, Jacob C, et al. Transcranial direct current stimulation of the prefrontal cortex reduces cue- reactivity in alcohol-dependent patients. J Neural Transm, 2016; 123: 1173–1178. [DOI] [PubMed] [Google Scholar]
- 26.Kim HJ and Kang N.. Bilateral transcranial direct current stimulation attenuated symptoms of alcohol use disorder: A systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry, 2021; 108: 110160. [DOI] [PubMed] [Google Scholar]
- 27.Beam W, Borckardt JJ, Reeves ST, et al. An efficient and accurate new method for locating the F3 position for prefrontal TMS applications. Brain Stimulat, 2009; 2: 50–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.IBM. SPSS for Windows. Version 25.0. Chicago, IL: IBM; 2017. [Google Scholar]
- 29.Duka T, Trick L, Nikolaou K, et al. Unique brain areas associated with abstinence control are damaged in multiply detoxified alcoholics. Biol Psychiatry, 2011; 70: 545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moselhy HF. Frontal lobe changes in alcoholism: A review of the literature. Alcohol, 2001; 36: 357–368. [DOI] [PubMed] [Google Scholar]
- 31.Zilverstand A, Huang AS, Alia-Klein N, et al. Neuroimaging impaired response inhibition and salience attribution in human drug addiction: A systematic review. Neuron, 2018; 98: 886–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zilverstand A, Parvaz MA, Moeller SJ, et al. Cognitive interventions for addiction medicine: Understanding the underlying neurobiological mechanisms. Prog Brain Res, 2016; 224: 285–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lefaucheur J-P, Antal A, Ayache SS, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol, 2017; 128: 56–92. [DOI] [PubMed] [Google Scholar]
- 34.Ambekar A, Agrawal A, Rao R, et al. Magnitude of substance use in India. New Delhi: Ministry of Social Justice and Empowerment; 2019. [Google Scholar]
- 35.Lien R. The effects of transcranial direct current stimulation of the prefrontal cortex on alcohol craving [dissertation]. Ghent University; 2018. [Google Scholar]
- 36.Nitsche MA, Fricke K, Henschke U, et al. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J Physiol, 2003; 553: 293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loo CK and Mitchell PB. A review of the efficacy of transcranial magnetic stimulation (TMS) treatment for depression, and current and future strategies to optimize efficacy. J Affect Disord, 2005; 88: 255–267. [DOI] [PubMed] [Google Scholar]
- 38.Appleton A, James R, and Larsen J.. The association between mental wellbeing, levels of harmful drinking, and drinking motivations: A cross-sectional study of the UK adult population. Int J Environ Res Public Health, 2018; 15: 1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Awaworyi Churchill S, and Farrell L.. Alcohol and depression: Evidence from the 2014 health survey for England. Drug Alcohol Depend, 2017; 180: 86–92. [DOI] [PubMed] [Google Scholar]
- 40.Lang I, Wallace RB, Huppert FA, et al. Moderate alcohol consumption in older adults is associated with better cognition and well-being than abstinence. Age Ageing, 2007; 36: 256–261. [DOI] [PubMed] [Google Scholar]
- 41.Eryılmaz G, Sayar GH, Ünsalver BÖ, et al. Adverse effects of transcranial direct current stimulation (tDCS) in a group of psychiatric patients. Sch J App Med Sci, 2014; 2: 294–297. [Google Scholar]
- 42.Chhabra H, Bose A, Shivakumar V, et al. Tolerance of transcranial direct current stimulation in psychiatric disorders: An analysis of 2000+ sessions. Psychiatry Res, 2020; 284: 112744. [DOI] [PubMed] [Google Scholar]