Abstract
Background:
Patients with aspirin-exacerbated respiratory disease (AERD) frequently experience symptoms consistent with eustachian tube dysfunction (ETD), which can substantially impair patient quality of life.
Methods:
We analyzed a cohort of 98 adult patients with AERD who participated in a longitudinal, survey-based study.
Results:
By assessing data over 1 year, we established that, in patients with AERD, the ear/facial subdomain of the 22-item Sino-Nasal Outcome Test (SNOT-22) questionnaire could predict performance on the 7-item Eustachian Tube Dysfunction Questionnaire, a validated instrument for the diagnosis of ETD. We then performed a re-analysis of data from a prospective, open-label study of 22 adult patients with AERD treated with dupilumab for 3 months. We found that treatment with dupilumab was associated with a significant decrease in the SNOT-22 ear/facial subdomain score, which reflects a substantial reduction in otologic symptoms and ETD within 1 month of initiating dupilumab and was sustained for 3 months afterward.
Conclusion:
Our findings provide evidence that dupilumab significantly improved ETD and otologic symptoms in AERD, evidenced by changes in the SNOT-22 ear/facial subdomain score. The presence of ETD and otologic symptoms should be considered when determining the optimal therapeutic course for patients with AERD.
Keywords: aspirin-exacerbated respiratory disease, respiratory biologic, aspirin desensitization, eustachian tube dysfunction, otologic symptoms, dupilumab, chronic rhinosinusitis with nasal polyps, eosinophilic otitis media
Aspirin-exacerbated respiratory disease (AERD) is a syndrome characterized by chronic rhinosinusitis with nasal polyps (CRSwNP), severe asthma, and respiratory reactions to cyclooxygenase-1 inhibitors. The chronic inflammation and obstruction of the sinonasal passages and eustachian tubes that characterize AERD are known to contribute to the development of eustachian tube dysfunction (ETD), ear infections, and conductive hearing loss.1 Otologic symptoms described in the setting of CRSwNP include ear fullness, dizziness, and ear pain.2,3
The mainstays of treatment for CRSwNP in the setting of AERD have traditionally included intranasal corticosteroids, endoscopic sinus surgery (ESS), and aspirin therapy after desensitization (ATAD). ATAD is a therapeutic option specific for patients with AERD, which requires patients to undergo aspirin desensitization followed by daily aspirin therapy and can lead to improvements in upper- and lower-airway symptoms.4 ESS has been shown to improve otologic-specific 22-item Sino-Nasal Outcome Test (SNOT-22) scores in AERD and CRSwNP,5,6 and the improvement in the SNOT-22 otologic-specific domain scores remained stable for patients with AERD when ATAD was used after ESS.6 Recently, biologics that target type 2 inflammation have also emerged as effective treatment options for AERD7–9 because these agents are effective for treating both asthma and CRSwNP. Two international phase III studies of dupilumab compared with placebo for severe CRSwNP showed that patients who received dupilumab had a reduction in the endoscopic nasal polyp score, improved sinonasal symptoms, and improved smell.10 Dupilumab is also efficacious for upper- and lower-airway symptoms in patients with AERD specifically and can be efficacious in patients who do not respond to ATAD.9,11,12
It has recently been shown that dupilumab has a benefit in treating ETD in patients with CRSwNP.13 Dupilumab has also been shown to significantly improve the SNOT-22 ear/facial subdomain score in patients with asthma and chronic rhinosinusitis.14 However, the role of biologics in treating otologic symptoms and ETD in AERD has not yet been characterized. In this study, we sought to understand the effects of dupilumab, a monoclonal antibody that targets interleukin-4Rα, on otologic symptoms and ETD in AERD.
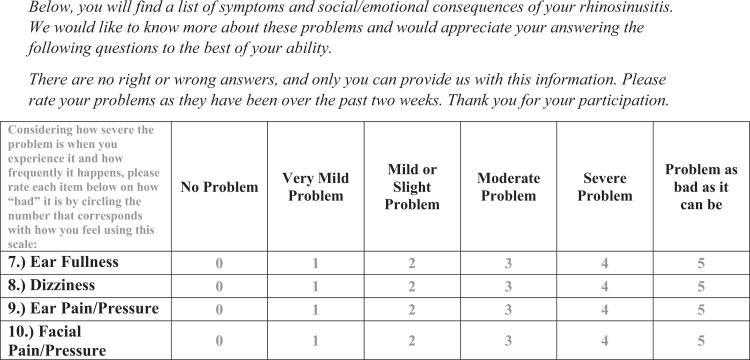
We first investigated the utility of the SNOT-22 in assessing otologic symptoms in AERD. The SNOT-22 ear/facial subdomain is a subset of four questions on the standard SNOT-22 form validated for assessing otologic and facial symptoms in CRSwNP,15,16 with a score ranging from 0 to 20, with a minimal clinically important difference of 3.2 points (Fig. 1).17 The 7-item Eustachian Tube Dysfunction Questionnaire (ETDQ-7), a validated questionnaire for the diagnosis of ETD, has been shown to correlate with the SNOT-22 total score and ear/facial subdomain score in studies that included patients with chronic rhinosinusitis18,19 although this correlation has not been previously specifically investigated in patients with AERD. ETDQ-7 scores range from 7 to 49, and scores ≥14.5 are strongly predictive of clinically meaningful ETD,20 with a difference of 3.5 points defined as a minimal clinically important difference.21
Figure 1.
The SNOT-22 ear/facial subdomain questions, validated for the assessment of otologic and facial symptoms in chronic rhinosinusitis. The instructions, question form, and rating scale, as provided to patients, are presented in the figure. Each individual question is scored from 0 to 5; the SNOT-22 ear/facial subdomain score can range from 0 to 20. SNOT-22 = 22-Item Sino-Nasal Outcome Test.
METHODS
To investigate whether the ETDQ-7 and the SNOT-22 ear/facial subdomain scores are correlated in patients with AERD, we examined previously collected data from a cohort of 98 adult patients with AERD who participated in a single-center, cross-sectional survey-based study at the Brigham and Women’s Hospital, as described previously.12 The study was approved by the Mass General Brigham institutional review board, and all the participants provided informed consent. All participants had a physician-confirmed diagnosis of CRSwNP and AERD. Eighty seven of the 98 participants (89%) had undergone physician-observed aspirin challenge to confirm the diagnosis. The other 11 participants had not undergone formal drug challenge to confirm the diagnosis but had convincing clinical histories of upper and/or lower respiratory reactions to a cyclooxygenase-1 inhibitor. The patients completed the SNOT-22 and ETDQ-7 questionnaires every 3 months for 1 year via an electronic survey, and the association between the SNOT-22 ear/facial subdomain score and the ETDQ-7 score was analyzed by using repeated measures analysis of variance with the subdomain score as a time-changing continuous variable.
We then analyzed the four individual SNOT-22 ear/facial subdomain questions by using repeated measures analysis of variance, with each question as a categorical covariate and the responses as a four-category, time-changing variable. The responses “moderate (3),” “severe (4),” and “problem as bad as it can be (5)” were combined and defined as “moderate or greater,” given the distribution of responses.
After establishing that the SNOT-22 ear/facial subdomain score correlates with the ETDQ-7 score in patients with AERD, we assessed the impact of dupilumab therapy on otologic symptoms and ETD in AERD. We performed a re-analysis of previously collected data from a prospective, open-label study of 22 adults with AERD, with the diagnosis confirmed by physician-observed aspirin challenge, treated with dupilumab for 3 months, as described previously.22 Notably, 8 of the 22 patients were on ATAD during the observational study, 10 of the 22 had tried ATAD but discontinued aspirin due to lack of efficacy or adverse effects before enrollment in the study. The SNOT-22 scores were collected at a pre-dupilumab baseline and at 1 and 3 months after dupilumab initiation, and were analyzed with a mixed-effects model.
RESULTS AND DISCUSSION
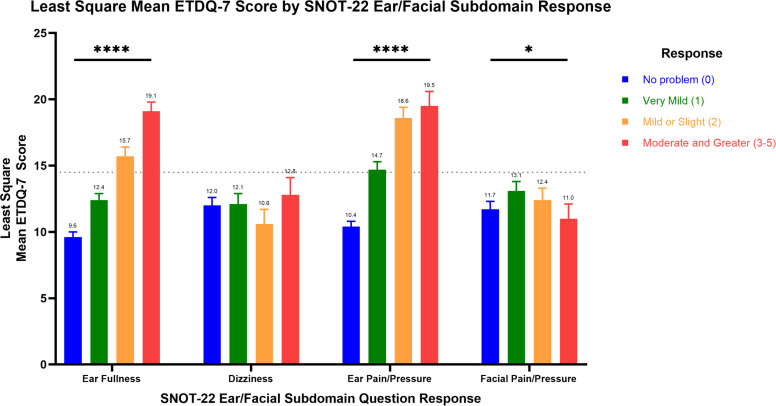
In assessing the correlation of ETDQ-7 and the SNOT-22 ear/facial subdomain scores in patients with AERD, we found that a one-point increase in the SNOT-22 ear/facial subdomain score was associated with an increase in the mean ETDQ-7 score of 1.2 ± standard error (SE) of 0.1 points (p < 0.0001). Combining all timepoints for the specific subdomain questions, we found that, for the “ear fullness” question (p < 0.0001), the “ear pain/pressure” question (p < 0.0001), and “facial pain/pressure” question (p = 0.03), each of the three questions were significant predictors of the ETDQ-7 score. Individual responses to the “dizziness” question were not significant predictors of ETDQ-7. The least square mean ETDQ-7 score associated with specific responses to each SNOT-22 ear/facial subdomain question is illustrated in Figure 2.
Figure 2.
Least square mean ETDQ-7 score by response to SNOT-22 ear/facial subdomain questions. Least square mean with standard error bar ETDQ-7 score by SNOT-22 ear/facial subdomain response. Repeated measures ANOVA: n = 98, *p < 0.05, ****p < 0.0001. Responses “moderate,” “severe,” and “problem as bad as it can be” were combined as “moderate and greater.” The dotted line indicates an ETDQ-7 score diagnostic for ETD. SNOT-22 questionnaire responses to “ear fullness,” “ear pain/pressure,” and “facial pain/pressure” were significant independent predictors of the ETDQ-7 score. ETDQ-7 = 7-Item Eustachian Tube Dysfunction Questionnaire; SNOT-22 = 22-item Sino-Nasal Outcome Test; ANOVA = analysis of variance.
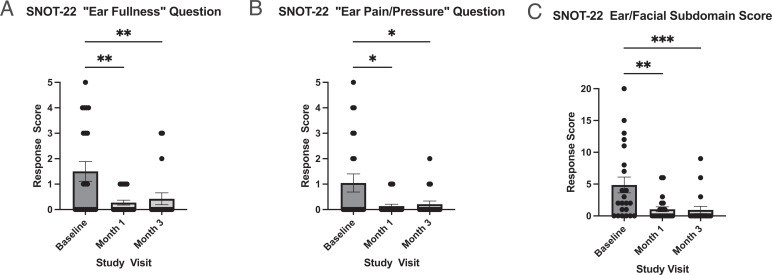
By using a mixed-effects model, we found a significant decrease in mean± SE SNOT-22 ear/facial subdomain score after initiation of dupilumab, from 4.9 ± 1.2 at baseline to 1.0 ± 0.4 at month 1 and 0.9 ± 0.5 at month 3 (p < 0.001) (Fig. 3). The difference in the mean subdomain score from baseline to month 1 (3.9) was more than the minimal clinically important difference for this subdomain. The magnitude of decrease in SNOT-22 ear/facial subdomain scores was not associated with age, gender, baseline peripheral blood absolute eosinophil count, or ATAD use.
Figure 3.
Decreases in otologic SNOT-22 scores after 1 and 3 months of treatment with dupilumab. The SNOT-22 otologic questions score (range, 0–5) and SNOT-22 ear/facial subdomain score (range, 0–20) 1 month and 3 months after initiating dupilumab are shown. Dupilumab treatment significantly reduced the mean SNOT-22 ear/facial subdomain score (p < 0.001), “ear fullness” (p = 0.001), and “ear pain/pressure” (p = 0.01) response scores (mixed-effects model, n = 22). Columns reflect mean score ± SE, with individual data points plotted. Pairwise comparisons on graph reflect post hoc Tukey multiple comparisons test (*p < 0.05, **p < 0.01, ***p < 0.001). SNOT-22 = 22-Item Sino-Nasal Outcome Test; SE = standard error.
We separately analyzed the otologic-specific SNOT-22 ear/facial subdomain questions (“ear fullness” and “ear pain/pressure”) by using a mixed-effects model (Fig. 3). After starting dupilumab, there was a significant decrease in mean score for both “ear fullness” (1.5 ± 0.4 to 0.3 ± 0.1 [month 1] and 0.4 ± 0.2 [month 3], p = 0.001) and “ear pain/pressure” (1.0 ± 0.4 to 0.1 ± 0.1 [month 1] and 0.2 ± 0.1 [month 3], p = 0.01, reported as mean ± SE).
Analysis of our findings suggests that dupilumab is effective at treating otologic symptoms and ETD in AERD. This effect is rapid (within 1 month), sustained for at least 3 months after starting dupilumab, and corresponds with the previously reported dupilumab-induced improvement in objective smell measurements, nasal polyp score, and overall sinonasal symptoms in this cohort of participants.22 Dupilumab is known to be efficacious for the treatment of asthma and sinonasal symptoms in AERD,9,22 and analysis of these data suggests another therapeutic benefit of this medication. Given the substantial impact of ETD on overall quality of life,19 the treatment of ETD is an important clinical consideration. A reduction in anatomic obstruction and improvement in sinonasal patency are likely central to improving middle ear dysfunction in AERD. Whereas ESS accomplishes this surgically, we hypothesize that dupilumab achieves this immunologically through mitigation of type 2 inflammation central to disease pathogenesis. In practice, analysis of these data suggests that patients with AERD and who are experiencing ETD may benefit from treatment with dupilumab, especially those ineligible for ESS or who prefer a nonsurgical treatment option.
Analysis of these data also demonstrates that the SNOT-22 ear/facial subdomain scores can predict the ETDQ-7 scores among patients with AERD, specifically the “ear fullness” and “ear pain/pressure” questions. Notably, even a response of “mild or slight problem” to either “ear fullness” or “ear pain/pressure” was associated with an ETDQ-7 score above the threshold for clinically meaningful ETD symptoms. This suggests that even mild, minimally bothersome otologic symptoms in AERD can be indicative of ETD. Importantly, these data also demonstrate that the SNOT-22 score can be used to help identify ETD in a clinical setting. Although the SNOT-22 ear/facial subdomain score has been demonstrated to correlate with the ETDQ-7 score in previous studies, our data now also confirm the relationship between these instruments in the AERD population. Although the SNOT-22 is likely to be familiar to clinicians who treat CRSwNP and AERD, questionnaires specifically for the diagnosis of ETD and other otologic symptoms are less commonly used in routine clinical allergy/immunology practice. These data support the use of the SNOT-22 for identification of diverse AERD symptoms.
This study does have limitations given its scope. Most notably, this study was limited and observational in nature; thus, the lack of a matched control group for patients on dupilumab limits the interpretation of the findings. In addition, because this study focused on patient-reported outcome measures, objective measures of otologic symptoms (such as otoscopic examination or audiologic evaluation) were not available. Future prospective, randomized studies are required to establish the effects of dupilumab treatment on otologic symptoms in AERD and CRSwNP, and should correlate patient reports of symptomatic improvement with objective assessment of anatomy and function. Nonetheless, patient-reported symptomatic improvements are an important clinical goal. We did not identify a significant relationship between patient demographics or biomarkers and dupilumab-induced improvement in otologic symptoms, perhaps due to the small sample size. Larger future studies should assess for possible predictive biomarkers to help phenotype patients most likely to respond to therapy.
CONCLUSION
Results of the study suggest that dupilumab significantly reduces otologic symptoms and likely improves eustachian tube symptoms in AERD, an effect that can be measured clinically by changes in the SNOT-22 ear/facial subdomain scores. For clinicians, the presence of ETD and otologic symptoms should be considered when determining the optimal treatment for AERD.
Footnotes
M. Lundberg has served on scientific advisory boards and received consultancy fees from Sanofi, Astra-Zeneca, Glaxo-Smith-Klein, and Chordate LTD. T.M. Laidlaw has served on scientific advisory boards for AstraZeneca, Regeneron, Sanofi, GlaxoSmithKline, and Eli Lilly. K. Buchheit has served on scientific advisory boards for AstraZeneca, Regeneron, Sanofi, and GlaxoSmithKline; has received personal consulting fees from Genentech; and receives royalties from UpToDate. J. Mullur and R. Maurer have no conflicts of interest to declare pertaining to this article
This work was supported by Regeneron, the National Institutes of Health (grants U19AI095219, K23AI139352, T32AI007306), and by generous contributions from the Vinik and Kaye Families
REFERENCES
- 1.Mullur J, Singer J, Roditi R, et al. Hearing loss and middle ear symptoms in aspirin-exacerbated respiratory disease. J Allergy Clin Immunol Pract. 2019; 7:1671–1672.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parietti-Winkler C, Baumann C, Gallet P, et al. Otitis media with effusion as a marker of the inflammatory process associated to nasal polyposis. Rhinology. 2009; 47:396–399. [DOI] [PubMed] [Google Scholar]
- 3.Hong S-N, Lee WH, Lee SH, et al. Chronic rhinosinusitis with nasal polyps is associated with chronic otitis media in the elderly. Eur Arch Otorhinolaryngol. 2017; 274:1463–1470. [DOI] [PubMed] [Google Scholar]
- 4.Stevens WW, Jerschow E, Baptist AP, et al. The role of aspirin desensitization followed by oral aspirin therapy in managing patients with aspirin-exacerbated respiratory disease: a Work Group Report from the Rhinitis, Rhinosinusitis and Ocular Allergy Committee of the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2021; 147:827–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teo NW, Mace JC, Smith TL, et al. Impact of endoscopic sinus surgery on otologic symptoms associated with chronic rhinosinusitis. World J Otorhinolaryngol Head Neck Surg. 2017; 3:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naples JG, Corr A, Tripathi S, et al. Endoscopic sinus surgery and aspirin desensitization improve otologic-specific SNOT-22 scores. World J Otorhinolaryngol Head Neck Surg. 2020; 6:248–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buchheit KM, Laidlaw TM, Levy JM. Immunology-based recommendations for available and upcoming biologics in aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2021; 148:348–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laidlaw TM, Mullol J, Fan C, et al. Dupilumab improves nasal polyp burden and asthma control in patients with CRSwNP and AERD. J Allergy Clin Immunol Pract. 2019; 7:2462–2465.e1. [DOI] [PubMed] [Google Scholar]
- 9.Mullol J, Laidlaw TM, Bachert C, et al. Efficacy and safety of dupilumab in patients with uncontrolled severe chronic rhinosinusitis with nasal polyps and a clinical diagnosis of NSAID-ERD: results from two randomized placebo-controlled phase 3 trials. Allergy. 2022; 77:1231–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bachert C, Han JK, Desrosiers M, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet. 2019; 394:1638–1650. [DOI] [PubMed] [Google Scholar]
- 11.Wangberg H, Spierling Bagsic SR, Osuna L, et al. Appraisal of the real-world effectiveness of biologic therapies in aspirin-exacerbated respiratory disease. J Allergy Clin Immunol Pract. 2022; 10:478–484.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mullur J, Steger CM, Gakpo D, et al. Aspirin desensitization and biologics in aspirin-exacerbated respiratory disease: efficacy, tolerability, and patient experience. Ann Allergy Asthma Immunol. 2022; 128:575–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang MT, Roozdar P, Lin Y-T, et al. Effect of dupilumab on Eustachian tube dysfunction in patients with chronic rhinosinusitis with nasal polyposis. Int Forum Allergy Rhinol. 2023; 13:1561–1563. [DOI] [PubMed] [Google Scholar]
- 14.Hopkins C, Buchheit KM, Heffler E, et al. Improvement in health-related quality of life with dupilumab in patients with moderate-to-severe asthma with comorbid chronic rhinosinusitis with/without nasal polyps: an analysis of the QUEST study. J Asthma Allergy. 2022; 15:767–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan AH, Reaney M, Guillemin I, et al. Development of Sinonasal Outcome Test (SNOT-22) domains in chronic rhinosinusitis with nasal polyps. Laryngoscope. 2022; 132:933–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng AL, Wesely NC, Hoehle LP, et al. A validated model for the 22-item Sino-Nasal Outcome Test subdomain structure in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2017; 7:1140–1148. [DOI] [PubMed] [Google Scholar]
- 17.Chowdhury NI, Mace JC, Bodner TE, et al. Investigating the minimal clinically important difference for SNOT-22 symptom domains in surgically managed chronic rhinosinusitis. Int Forum Allergy Rhinol. 2017; 7:1149–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tangbumrungtham N, Patel VS, Thamboo A, et al. The prevalence of Eustachian tube dysfunction symptoms in patients with chronic rhinosinusitis. Int Forum Allergy Rhinol. 2018; 8:620–623. [DOI] [PubMed] [Google Scholar]
- 19.Wu AW, Walgama ES, Higgins TS, et al. Eustachian tube quality of life and severity of disease in patients with chronic rhinosinusitis. Am J Rhinol Allergy. 2020; 34:532–536. [DOI] [PubMed] [Google Scholar]
- 20.McCoul ED, Anand VK, Christos PJ. Validating the clinical assessment of eustachian tube dysfunction: the Eustachian Tube Dysfunction Questionnaire (ETDQ-7). Laryngoscope. 2012; 122:1137–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins TS, Cappello ZJ, Wu AW, et al. Predictors of eustachian tube dysfunction improvement and normalization after endoscopic sinus surgery. Laryngoscope. 2020; 130:E721–E726. [DOI] [PubMed] [Google Scholar]
- 22.Buchheit KM, Sohail A, Hacker J, et al. Rapid and sustained effect of dupilumab on clinical and mechanistic outcomes in aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2022; 150:415-424. [DOI] [PMC free article] [PubMed] [Google Scholar]