Abstract
This study aimed to comprehensively explore the intricacies of corneal neurotization (CN) and the nuanced factors that set it apart from routine clinical practice, exerting a substantial influence on its success. A symbiotic relationship is evident between corneal innervation and ocular surface health. The loss of corneal innervation results in a potentially challenging corneal condition known as neurotrophic keratopathy (NK). The majority of treatments are primarily focused on preventing epithelial breakdown rather than addressing the underlying pathogenesis. Consequently, to address the impaired corneal sensation (underlying etiology), a novel surgical approach has emerged, namely CN, which involves transferring healthy sensory nerve axons to the affected cornea. This review offers valuable insights into the existing body of supporting evidence for CN, meticulously examining clinical studies, case reports, and experimental findings. The aim is to enhance our understanding of the effectiveness and potential outcomes associated with this innovative surgical technique. The exploration of innovative therapeutic avenues holds promise for revolutionizing the management of NK, offering a potentially permanent solution to a condition once deemed incurable and severely debilitating.
Keywords: Corneal neurotization, donor nerve, insensate cornea, interpositional nerve graft, neurotrophic keratopathy, reinnervation
Neurotrophic keratopathy (NK) represents a rare challenging corneal condition characterized by altered corneal sensations leading to the breakdown of the corneal epithelium.[1] Various etiologies contribute to its development, including infectious keratitis, inflammation, chemical/thermal eye injury, iatrogenic causes (such as corneal and neurosurgery), metabolic disease (e.g., diabetes), radiation keratopathy, and neurological conditions affecting the trigeminal nerve (e.g., cerebellopontine angle tumor).[1] Corneal sensation loss in NK can manifest as partial (hypoesthesia) or complete (anesthesia). In post-viral cases, the sensory deficit often presents in a patchy manner, while anesthesia of the entire corneal surface is frequently observed following tumor resection and in congenital or developmental diseases.[2,3]
Historically, management depending on the cause, severity, and laterality aimed to protect the cornea, facilitate healing, and prevent NK progression, which may lead to corneal melting and perforation. These interventions included conservative measures such as topical lubricants, antibiotics, cautiously administered steroids, contact lenses, and surgical procedures such as tarsorrhaphy and amniotic membrane transplant.[1] However, the effectiveness of these treatment modalities is often limited or temporary, primarily focusing on maintaining ocular surface homeostasis rather than directly addressing the underlying pathophysiology of NK, which is the loss of corneal innervation.
In 2018, a novel medication received approval from the US Food and Drug Administration for its use in NK- topically administered recombinant nerve growth factor (NGF) known as cenegermin.[4] This approval was based on the positive outcomes observed in two multicenter, double-masked, randomized, vehicle-controlled phase-2 clinical trials focused on the treatment of neurotrophic keratitis (NK). The trials demonstrated the efficacy of cenegermin in promoting the healing of persistent epithelial defects.[4,5,6] Specifically, the REPARO study conducted in Europe[5] and the US trial[6] treated patients with cenegermin at concentrations of 10 or 20 g/mL. During both studies, patients diligently applied their assigned treatment six times daily over an 8-week period.[5,6] At the conclusion of the 8-week treatment phase, corneal healing was attained by 65.2% of the 23 patients in the US trial and by 72% of the 52 patients in the REPARO study.
It is noteworthy, however, that these recombinant NGFs did not demonstrate a significant improvement in central corneal sensation or best-corrected visual acuity.
To address this limitation, a novel surgical technique called corneal neurotization (CN) was introduced to overcome the perpetuation of the disease and its principal pathogenesis.
We conducted a comprehensive literature search using PubMed and Google Scholar, utilizing combinations of the following keywords: corneal neurotization, neurotrophic keratopathy, neurotrophic keratitis, recombinant human nerve growth factor, therapies for neurotrophic keratopathy, minimally invasive corneal neurotization, direct and indirect CN, donor nerve, interpositional nerve graft, corneal reinnervation, restoration of corneal sensation, and update on corneal neurotization. Relevant publications were selected based on their pertinence to the subject matter, without adherence to a systematic approach. We excluded non-English publications. The timeframe considered for inclusion spanned from 2008 to 2023. Hence, this review provides an overview of the prevailing status of advancements, supporting evidence, and cumulated expertise within the respective field.
Corneal Neurotization – Evolution
The CN technique, originally introduced by Terzis et al. in 2009,[7] has witnessed a rapid expansion of varied methodologies in the literature, as emphasized by numerous studies.
Definition CN is a surgical technique in which intact sensory nerve axons from a healthy donor are transferred to the insensate cornea with the aim of restoring corneal innervation.
Donor nerve selection
Optimal selection of a donor nerve for for CN requires a thorough assessment encompassing both preoperative and intraoperative evaluations. Criteria for this selection include factors such as nerve viability, proximity to the affected cornea, possession of the highest number of axons, ease of accessibility, minimal donor site morbidity, and the size/compatibility of the nerve for an interposition graft.[8,9] When possible, preference is given to ipsilateral donor nerves due to their proximity to the target site, with contralateral nerves considered when the former is unsuitable or unavailable.
Terzis et al.[7] established a cutoff of 900 myelinated axons for effective motor facial nerve reanimation. The same number of axons was extrapolated for CN. Notably, the distal branches of the supratrochlear and supraorbital nerves, emerging from the orbital rim, contain more than 2000 myelinated axons, making them ideal donor nerves without causing end-organ damage.[10] In an anatomic and histomorphometric feasibility study, Catapano et al.[11] reported that the infraorbital nerve contains 975 myelinated fibers, a number considered sufficient for contemplation as a donor nerve. Hence, the supraorbital, supratrochlear, or infraorbital nerve can serve as a donor nerve for CN.
However, the infraorbital nerve is slightly less favored due to its need for intricate orbital dissection, requiring bone removal. In addition, transection of the infraorbital nerve results in more bothersome sensory loss, especially in the oral mucosa, compared to other options.[8]
The supratrochlear and supraorbital nerves, branches of the frontal nerve, the largest branch of the ophthalmic division of the trigeminal nerve, play a crucial role. After entering the orbit through the superior orbital fissure, the ophthalmic nerve gives off the frontal nerve, which then splits into the supratrochlear and supraorbital nerves midway through the orbit. These nerves exit the orbit superiorly, with the supraorbital nerve passing through either the supraorbital notch or foramen. The supratrochlear nerve, the smaller of the two, provides sensory innervation to the skin of the lower forehead and contributes to the sensory innervation of the conjunctiva.[12] In most cases, the supratrochlear nerve seems not to pass through the frontal notch but under it. Haładaj et al.[13] observed diverse presentations and characteristics of the supraorbital foramen or notch, specifically in relation to the content of branches of the supraorbital nerve. Their findings emphasize the significance of understanding the anatomy in the context of surgical techniques for CN.
Anatomical localization
In clinical practice, we observed that the course of supraorbital neurovascular bundle can be reliably determined based on the topographical anatomy of the eye, with the medial limbus corresponding to its location in the orbit,[14] while the supratrochlear nerve exits at 21 mm from the midline between the supraorbital notch and the pulley of the superior oblique.[15]
Although the supraorbital nerve has a tendency to divide upon exiting the orbital rim, making it more challenging to localize and dissect, its greater density of myelinated nerve fibers near the orbital rim, almost double the axonal load of the supratrochlear nerve, suggests it as a robust source for coaptation. However, it might be the surgeon’s preference to select one over the other. Due to the dual supply to the forehead, harvesting one of these nerves is feasible without significant end-organ morbidity.[15]
Surgical techniques
The surgical approach in CN can be broadly categorized into direct and indirect CN.
Direct corneal neurotization
The original technique involves the transplantation of contralateral supratrochlear and supraorbital nerves to the ipsilateral perilimbal area of the neurotrophic cornea. While this technique has demonstrated favorable outcomes in multiple studies, its drawback lies in the requirement for a sizable bicoronal incision and extensive nerve dissection, potentially impacting cosmetic appearance and resulting in an extended recovery period.[16]
Indirect corneal neurotization
In 2014, Elbaz et al.[17] presented an innovative indirect technique, offering a distinct advancement. An indirect microincision CN (MICN) technique utilized a reversed sural nerve graft coapted to either the supratrochlear or supraorbital nerve. Nerve fascicles were sutured subconjunctivally to the perilimbal region. Access to the supratrochlear nerve was achieved through a transverse sub-brow incision, facilitating end-to-side coaptation of the sural nerve graft through the creation of an epineural window. The coaptation was performed using fibrin glue and 10-0 nylon sutures. In unilateral cases, the contralateral supratrochlear nerve was employed, necessitating subcutaneous tunneling of the reversed nerve graft over the nasal bridge to reach the perilimbal area of the cornea. Distally, the epineurium was removed, and individual fascicles were separated. Approximately five fascicles were then placed around the entire limbal circumference and secured to the sclera with 10-0 nylon sutures. The representative case is illustrated in Fig. 1.
Figure 1.
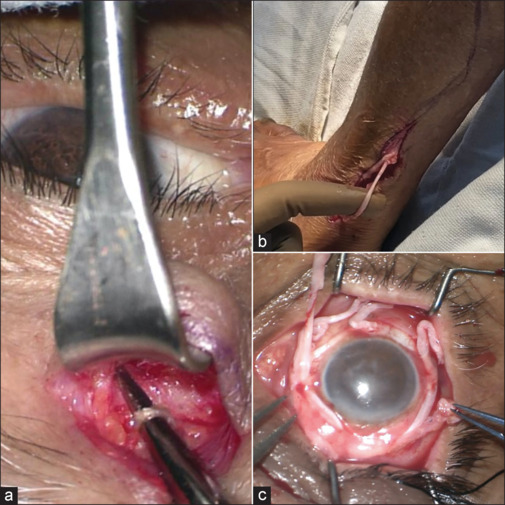
Represents Indirect corneal neurotization surgical Steps; (a) Isolated Supratrochlear nerve as donor Nerve; (b) Sural nerve harvesting in the leg as Interpositional nerve graft; (c) Fascicle securement of harvested Sural nerve around the limbus
This technique eliminates the necessity for a bicoronal incision, presenting a significant improvement in terms of cosmetic outcomes. Moreover, its applicability extends to the treatment of bilateral NK cases, enhancing its clinical utility.[17]
Interpositional nerve graft
The interpositional nerve graft is positioned between the donor nerve and the affected cornea, serving as a bridge or conduit allowing the transmission of sensory nerve signals from the donor nerve to the affected insensate cornea. Various interpositional nerve grafts have been employed in the indirect CN procedure; the details are tabulated in Table 1. Nevertheless, it remains uncertain whether any of these nerve grafts exhibit superior reliability and efficacy compared to the others.
Table 1:
Analysis of interpositional nerve graft: insights from the existing literature
| Interpositional nerve graft selection | Authors | Advantage | Disadvantage | Clinical outcome* |
|---|---|---|---|---|
| Sural nerve | Elbaz et al.[17] were the first to report the use of the medial cutaneous branch of the sural nerve for indirect CN. Bains et al.[42] Weis et al.[46] | Provides over 20 cm length, which expands the donor nerve options to maxillary and mandibular branches of the trigeminal nerve in CN Smaller incision and can be used for bilateral cases |
Donor site morbidity associated with harvesting of the sural nerve includes Sensation loss or allodynia to the distal lower leg and dorsum of the foot | Elbaz et al. reported successful outcomes in 2/3 patients. Partial success in 1/3 patients Bainz et al. noted successful outcomes in 4/4 patients Wies et al. observed successful in 6/6 patient |
| Acellular nerve allograft | Leyngold et al.[8] | Commercially available nonimmunogenic scaffold guides regenerating donor nerve fibers to the insensate cornea, offering benefits of indirect CN without the added invasiveness and potential subsequent morbidity of nerve autograft harvesting This approach results in shorter operative and recovery times |
Acellular nerve allografts lack viable Schwann cells crucial for supporting axon regeneration, studies have demonstrated axonal regrowth over distances of 3–4 cm only[19] Cost, risk of graft failure |
Successful in 7/7 patients |
| Greater Auricular Nerve graft | Benkhatar et al.[20] | Minimally invasive, Shorter operative time, better end-to-end coaptation due to better size compatibility of graft | Not commonly performed, less data in the literature, only partial success reported | Partial success in 1 patient |
| Jovett et al.[47] | Theoretical potential for greater sensory recovery is attributed to higher axonal count in the graft Approximately 7 cm of the GAN can be harvested Single surgical field allows to obtain the desired graft length needed to cover the interpupillary distance and a good anatomical match with the Suptratroachlear nerve, and a limited area of postoperative anesthesia to the earlobe was observed |
Limited space around the patient’s head, which could increase the overall operating time | Successful in 2/2 patients | |
| Lateral antebrachial cutaneous nerve | Bourcier et al.[21] | The lateral antebrachial cutaneous nerve provides sensation to one-third of the anterior and posterior forearm, and it can be readily accessed under the skin, yielding a 12-cm nerve graft. The advantages of the LACN graft include its substantial length with multiple terminal branches, aligning well with the superior orbital nerve. The temporary loss of sensation in a noncritical cutaneous region of the anterolateral forearm is mitigated by the radial sensory nerve’s co-innervation. | No data supporting the outcomes | Successful in 1/1 patient |
*Success was defined as improvement in corneal sensation
Determinants Influencing Successful Outcomes
Factors influencing the successful outcomes of CN are multifaceted and encompass a range of determinants. These determinants play pivotal roles in ascertaining the effectiveness of the procedure and include considerations such as patient-specific factors, age, associated systemic morbidities, duration, and etiology of corneal denervation, surgical techniques, graft selection, meticulous coaptation methods, and postoperative prophylactic measures. The intricate interplay of these elements contributes significantly to the overall success of CN procedures, highlighting the importance of a comprehensive understanding and optimization of these determinants for favorable patient outcomes.
Patient SPECIFIC FACTORS
In the existing literature, a prevalent observation suggests that younger age is linked with more favorable outcomes in CN procedures.[22] This association is attributed to heightened corneal sensation and increased sub-basal nerve fiber density observed in younger eyes at baseline. These factors potentially contribute to a more comprehensive restoration of vision and sensation through the reestablishment of trophic functions following CN. Supporting this notion is evidence indicating that aging has a detrimental impact on neural plasticity, peripheral nerve function, and regeneration. This includes the loss of myelinated and unmyelinated nerve fibers, slower axonal regeneration, and reduced secretion of tropic factors by reactive Schwann cells during the regenerative process after nerve injury. However, it is important to note that pococurante findings exist as some authors were unable to establish a discernible association between age and outcomes[23] following CN procedures.
Furthermore, it was proposed that performing CN earlier in the disease course, as determined by Mackie staging, could lead to more substantial sensory and visual recovery.[24]
In the context of the duration of denervation as a potential factor influencing clinical outcomes, it is well-established that the regenerative potential of affected peripheral nerves decreases with time. A study conducted by Ting et al.[25] wherein both participants experienced significant surgical injuries affecting the proximal segment of the fasciculus of the trigeminal nerve, with a prolonged denervation time of 23 years. This extended denervation time might have adversely impacted the prognosis for corneal sensation improvement following CN. It was suggested that individuals with more proximal trigeminal nerve injuries following surgical insult may have less “functional reserve” compared to those with more distal trigeminal nerve insults such as herpes simplex keratitis (HSK). Consequently, the surgical insult NK patients in Ting et al.’s study might have a lower likelihood of improvement in corneal sensation following CN. In contrast, studies reporting positive outcomes following CN typically involve patients with significantly shorter denervation times, ranging from 1 to 6 years.
On a different note, Lin et al.[26] included 13 patients diagnosed with HSK, with an average denervation time of 15.2 years, and demonstrated a positive impact on alleviating the severity of NK in 11 out of 13 patients (84.6%). However, the comprehensive details regarding the extent of improvement in corneal sensation following CN were not fully reported.
Nonetheless, there is a lack of head-to-head comparison of outcomes based on NK etiology. In the available literature, no conclusive distinction has been established in clinical outcomes related to the underlying causes or origins of the conditions under consideration.[23]
Surgical insights for corneal neurotization
The proximal segments of donor nerves exhibit a higher density of axons than their distal counterparts, potentially leading to increased innervation and enhanced corneal sensation.[15]
In contrast, proponents of direct nerve transfer (direct CN) argue that despite the lower axon count in the distal nerve portions, the shorter regeneration distance could result in greater success. Conversely, advocates for indirect approaches highlight the use of a more proximal section of the donor nerve, offering a higher number of axons for growth through the graft and subsequent corneal reinnervation.[27]
Nevertheless, in a prospective comparative case series, successful reinnervation was observed in 80% for direct and 83% for indirect neurotization[28] at 1 year.
For the indirect CN approach, the identification of a consolidated donor nerve and the separation of interpositional nerve graft fascicles without collateral damage are essential prerequisites for success. A modification was attempted to expedite the process and prevent collateral damage by performing fascicle separation of the interpositional nerve graft in a taut position before severing.[23] In addition, the caliber of the selected donor nerve is crucially important as a smaller caliber implies fewer axons available to support the regeneration and functioning of the harvested nerve graft. Consequently, the results could be adversely affected.
During the anastomosis between the donor nerve and the graft, caution should be exercised to prevent tension on the repair. In addition, it is essential to engage only the epineurium to avoid fascicular damage and the potential formation of a neuroma.[29] When the diameter of the interpositional graft exceeds the selected diameter of the donor sensory nerve, opting for an end-to-side neurorrhaphy (coaptation) seems appropriate. One significant advantage of end-to-side neurorrhaphy is the ability to perform coaptation while preserving sensation in the distribution of the donor nerve. The application of fibrin glue and/or amniotic membrane wraps at the neurorrhaphy site has been utilized to augment the process of regeneration.[30] Notably, the average postoperative corneal sensibility did not appear to be influenced by the number of nerve bundles utilized during the CN procedure.[7]
Special attention should be dedicated to donor nerve selection and the timing of surgery in patients with herpetic keratitis, considering the theoretical risk of spreading viral particles during nerve transfer. In a retrospective study involving six adult patients with herpetic keratitis who underwent CN, five patients received prophylactic antiviral therapy before surgery, and none of the patients exhibited clinical evidence of active herpetic disease at the time of the procedure.[24]
Axonal regeneration timeline
Following coaptation, there is an average latency period of 2–4 weeks before the initiation of axonal regeneration into the graft, acting as a conduit for axons to reach the denervated cornea.[31] Subsequently, the axons grow at an average rate of 1 mm per day, corresponding to the slow transport rate of the neurofilament protein, though this is likely faster in children.[29] Given the pace of axonal regeneration, one can anticipate a delay of at least 2–3 months or longer for the re-establishment of sensibility following neurotization.[29]
Comparative analysis: nerve fascicle securement and coaptation type selection
Various techniques are employed in securing interpositional nerve graft fascicles to the insensate cornea during CN. Some authors opt for securing fascicles to the corneoscleral limbus by using fibrin glue exclusively or a combination of methods.[32] Another consideration involves placing fascicles within perilimbal scleral tunnels, a technique that brings nerve fascicles into closer proximity with the cornea but poses technical challenges and may result in nerve fascicle damage, potentially compromising the structural integrity of the cornea.[32]
An innovative approach involves directly placing fascicles into corneal tunnels, potentially increasing the available axons for corneal reinnervation. However, this method may diminish the likelihood of restoring sensation to the bulbar conjunctiva, a complication associated with paralimbal fixation. Addressing this concern, Malhotra et al.[33] employed a dual strategy of corneoscleral tunnel fixation along with one or two supplementary fascicles left in the perilimbal sub-Tenon’s space, secured with fibrin glue within the palpebral aperture region.
In a comparative study, Catapano et al.[32] investigated cases where fascicles were positioned in the perilimbal subconjunctival space versus those where fascicles were secured within corneoscleral tunnels. The latter group exhibited a swifter recovery of central corneal sensation, typically observed within the first 3 months postoperatively. However, no significant differences between the two groups were noted at the 6-month follow-up.
Similarly, statistical analysis revealed no significant outcome variations based on coaptation type (end-to-end or end-to-side), donor nerve selection (supraorbital or infraorbital), or laterality of donor nerve (ipsilateral or contralateral).[34] It is essential to note that the cohort size was limited, preventing definitive conclusions from being drawn.
Clinical Measurement and Outcomes of Corneal Neurotization
Corneal sensation
The reinnervation of an insensate cornea following CN adheres to a well-defined timeline. Observable neurotization near the limbus occurs at 8 weeks postoperatively, followed by superficial central CN between 3 to 7 months postoperatively. Complete neurotization, encompassing the basal layers of the central cornea, becomes apparent within the timeframe of 6 months to 2 years postoperatively. However, this timeline may be influenced by factors affecting the regenerative potential of rerouted nerves, such as the patient’s age and preoperative health.
The achieved levels of sensibility in most studies, regardless of age, were noted to be sufficient to maintain a healthy epithelium and elicit the blink reflex in response to various stimuli, protecting the eye.[35,36,37] However, unintentional injury to the long donor axons during surgery may lead to prolonged regeneration following Wallerian degeneration.[7]
The median time for the objective return of corneal sensation was noted to be 0.5 years (standard deviation: 1 year, range: 0.1–5 years). Although early restoration of corneal sensation has been documented, Elbaz U et al.[17] observed a noticeable improvement in corneal sensibility after just 3 months of follow-up in 3 out of 4 eyes. Similarly, Malhotra et al.[33] performed indirect CN in six eyes and demonstrated improved sensation at 3 months follow-up. Saini et al.[23] also observed corneal sensation improvement at 3 months postoperatively in 11 eyes following indirect CN.
Park et al.[22] conducted a comprehensive systematic review in 2020, examining the clinical outcomes of CN by incorporating all published articles and meeting abstracts from December 2008 to February 2019. Their analysis encompassed 54 eyes that underwent SCN, revealing a baseline corneal sensitivity of 2.18 ± 5.37 mm measured with Cochet-Bonnet esthesiometry (CBA). Following SCN, there was a significant enhancement in sensitivity to 40.10 ± 18.95 mm, reflecting a mean filament length improvement of 38.00 mm.
For children under 2 years of age, surrogate outcomes have been employed to gauge successful sensitization, such as the regression of corneal vascularization, enhancement in fluorescein staining, and improvement in corneal clarity.[18]
Corneal nerve imaging by confocal microscopy
The improvement in corneal sensation was evident through an increase in parameters related to the sub-basal nerve fiber plexus observed on confocal microscopy. The morphology of these nerves was also studied and documented in the literature.
Ting et al.[25] included two patients who underwent direct CN, as described by Terzis et al., and observed objective improvement in corneal sensation at 9 months and 15 months postoperatively. The authors noted an abundant presence of corneal nerves in the neurotization cases compared to the normal eye, with larger-caliber nerves. In neurotrophic corneas,[38] nerves observed were more beaded and attenuated on in vivo confocal microscopy.
Giannaccare et al.[39] observed thin and attenuated nerve fibers 3 months postoperatively in three cases undergoing direct CN. Over time, there was an improvement in morphology, with in vivo confocal microscopy (IVCM) metrics comparable to the contralateral unaffected eye but lower electrical activity compared to the unaffected eye at 1 year.
Fung et al.[29] conducted indirect CN in two cases and, through IVCM, demonstrated corneal reinnervation at the stromal and sub-basal levels in a pattern different from the normal cornea, although quantification was not performed.
Lin and Lai et al.[26] performed ipsilateral direct CN in 13 patients with herpes simplex virus (HSV). The subepithelial corneal nerve plexus was found at 9 months postoperatively, accompanied by a decrease in corneal thickness and an increase in corneal endothelial count.
In the available literature, documentation of improvement in sub-basal nerve plexus through in vivo confocal microscopy (IVCM) was observed as early as 3 months post-surgery, and this escalation persisted for up to 1 year or beyond. In addition, the chronological sequence of corneal innervation was recorded in the literature, revealing an initial increase in preexisting sub-basal nerve fiber length observed as early as 1 month post surgery. Subsequently, there was an objective perception of corneal sensation by the 3-month follow-up, and a notable improvement in sub-basal nerve fiber density was noted from 6 months to 1 year following CN.[23]
Several studies have reported a progressive restoration of corneal transparency coupled with an enhancement in visual acuity following CN.[7,18,19,21,23,32,40] In contrast, some studies have documented no improvement in visual acuity.[9,20] Furthermore, CN has been observed to be effective following Descemet’s anterior lamellar keratoplasty (DALK). In a study conducted by Giannaccare et al.,[39] DALK was performed 18 months after the neurotization procedure. Remarkably, the corneal graft achieved clarity and complete epithelialization within a mere 3 months post surgery.
Anterior segment OCT: Imaging epithelium and corneal nerves
Lathrop et al.[41] investigated the clinical progression of corneal limbal epithelium following indirect CN (ICN) using optical coherence tomography (OCT). The 2-year follow-up post-neurotization revealed a restoration of normal epithelial thickness across the cornea and limbus. The study proposed that the positive outcomes of neurotization might be attributed to improvements in the anatomy of the palisades of Vogt. The potential impact of preferentially placing nerve fascicles at the 12 and 6 o’clock positions, where corneal limbal stem cells are most abundant, on achieving better outcomes remains to be determined.
Subjective perception post corneal neurotization
The subjective perception following CN exhibited variability in the literature. However, initially, patients may experience discomfort once sensation is established, attributed to the damaged epithelium, making innervation potentially painful. These individuals commonly describe the mechanical stimulation of the cornea as akin to stimulating the cutaneous skin territory of the donor nerve. Over the subsequent months, there is a perceptual shift among patients who come to recognize mechanical corneal stimulation as genuine corneal sensation. This transition in perception implies a likely occurrence of central nervous system remodeling.[42]
In a documented case, the neurophysiological pathways involved in corneal reinnervation were explored using magnetoencephalography (MEG).[32] Initially, MEG failed to detect any sensory response during stimulation of the neuropathic cornea. However, 8 months post microinvasive CN (MICN), an evoked response was noted in the right somatosensory cortex during mechanical corneal stimulation on the right side. This area corresponded to the region supplied by the left supratrochlear nerve, affirming that the corneal response was generated and perceived through relearning via the donor’s left supratrochlear nerve. This insight suggests the potential benefit of employing analogous exercises, such as using cold or warm eye drops or gentle corneal stimulation, aimed at modulating cortical remapping to enhance clinical outcomes after CN.[32]
Ex Vivo Histopathological Investigations
Ex vivo histopathological findings were gleaned from the literature to validate the presence of newly formed nerve fibers in the corneas affected by NK. Giannaccare et al.[39] conducted ex vivo histopathological analysis on neurotized corneal buttons excised during Descemet’s anterior lamellar keratoplasty (DALK). PGP 9.5 immunofluorescence staining revealed multiple fascicles of nerve fibers beneath the epithelium, while transmission electron microscopy (TEM) demonstrated amyelinated nerve axons and normal-appearing nerve endings.
Catapano et al.[43] developed a rat model of NK and CN to demonstrate corneal epithelium healing and maintenance. Retrograde labeling illustrated that the newly formed nerves in the cornea originated from the donor sensory nerve rather than preexisting corneal nerves. Notably, the observed axons in the neurotized cornea appeared thinner, and there was an absence of the typical whorl pattern in the sub-basal nerve plexus. The authors suggested that the cornea may selectively permit the growth of unmyelinated nerve fibers with a specific phenotype.
In contrast to the highly regulated network of unmyelinated C fibers and thinly myelinated (Ad) fibers in corneal innervation, donor nerves used in CN encompass a more diverse population, including a substantial number of myelinated fibers.[44] The authors propose that to preserve corneal clarity, the growth of myelinated axons into the cornea may be restricted. In addition, the corneal receptors guiding axon regeneration following neurotization may become saturated, limiting further axon growth once a certain number of axons successfully regenerate into the corneal periphery.[43]
In an interpositional graft model in rats, Catapano found approximately 8000 axons growing into the nerve graft segment, with only a small fraction, around 200, appearing to innervate the cornea. The precise number of nerves required to fully restore corneal sensory function remains unknown.[43]
Physiology of Corneal Reinnervation
Two prominent theories have emerged concerning corneal reinnervation: one involving the secondary generation of nerve fibers directly sprouting from the donor nerve, and the other positing native corneal nerve regeneration facilitated by the paracrine trophic support provided by the donor nerve.[25] Given the rate of nerve growth, which can reach up to 1 mm/day, a paracrine-like effect has been suggested to account for the relatively rapid subjective recovery observed in many patients.[25]
However, several lines of evidence lend support to the second theory of direct axonal sprouting. Clinically, stimulation of the affected cornea induced a referred tactile sensation in the territory of the contralateral donor nerve.[17] Magnetoencephalography revealed that stimulation of the operated cornea elicited a response in the brain area corresponding to the location of the contralateral donor nerve, rather than the ipsilateral trigeminal nerve.[32] In addition, in a rat model, labelled corneal nerves post-neurotization were traced back to the contralateral donor nerve, indicating a continuous axonal connection to the donor neurons.[43]
While Ting et al.[25] demonstrated the absence of a direct connection of nerves from perilimbal fascicles, suggesting that the regeneration of corneal nerves may be primarily attributed to paracrine trophic support rather than direct sprouting from perilimbal fascicles, further research is required to better elucidate the precise pathophysiological mechanism behind corneal reinnervation.
Contraindications
Conditions that might pose challenges to CN are classified into relative and absolute contraindications. Relative contraindications encompass conditions such as extensive conjunctival scarring, uncontrolled diabetes, patients with compromised systemic health or those deemed unsuitable for surgery, and individuals on anticoagulation therapy, along with those harboring unrealistic expectations. In contrast, absolute contraindications include active or uncontrolled inflammatory or infectious ocular surface diseases, the presence of perineural malignancy in the donor nerve region, the absence of healthy sensory donor nerves, and ongoing external beam radiation to the orbit or eye.[45]
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Dua HS, Said DG, Messmer EM, Rolando M, Benitez-Del-Castillo JM, Hossain PN, et al. Neurotrophic keratopathy. Prog Retin Eye Res. 2018;66:107–31. doi: 10.1016/j.preteyeres.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Liu S, Pavan-Langston D, Colby KA. Pediatric herpes simplex of the anterior segment: Characteristics, treatment, and out- comes. Ophthalmology. 2012;119:2003–8. doi: 10.1016/j.ophtha.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg ML. Congenital trigeminal anaesthesia: A review and classification. Brain. 1984;107:1073–82. doi: 10.1093/brain/107.4.1073. [DOI] [PubMed] [Google Scholar]
- 4.US Food and Drug Administration FDA approves first drug for neurotrophic keratitis, a rare eye disease. 2018 August 22; Accessed March 13, 2020. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-neurotrophic-keratitis-rare-eye-disease . [Google Scholar]
- 5.Bonini S, Lambiase A, Rama P, Sinigaglia F, Allegretti M, Chao W, et al. Phase II randomized, double-masked, vehicle-controlled trial of recombinant human nerve growth factor for neurotrophic keratitis. Ophthalmology. 2018;125:1332–43. doi: 10.1016/j.ophtha.2018.02.022. [DOI] [PubMed] [Google Scholar]
- 6.Pflugfelder SC, Massaro-Giordano M, Perez VL, Hamrah P, Deng SX, Espandar L, et al. Topical recombinant human nerve growth factor (cenegermin) for neurotrophic keratopathy: A multicenter randomized vehicle- controlled pivotal trial. Ophthalmology. 2020;127:14–26. doi: 10.1016/j.ophtha.2019.08.020. [DOI] [PubMed] [Google Scholar]
- 7.Terzis JK, Dryer MM, Bodner BI. Corneal neurotization: A novel solution to neurotrophic keratopathy. Plast Reconstr Surg. 2009;123:112–20. doi: 10.1097/PRS.0b013e3181904d3a. [DOI] [PubMed] [Google Scholar]
- 8.Leyngold IM, Yen MT, Tian J, Leyngold MM, Vora GK, Weller C. Minimally invasive corneal neurotization with acellular nerve allograft: Surgical technique and clinical outcomes. Ophthalmic Plast Reconstr Surg. 2019;35:133–40. doi: 10.1097/IOP.0000000000001181. [DOI] [PubMed] [Google Scholar]
- 9.Sweeney AR, Wang M, Weller CL, Burkat C, Kossler AL, Lee BW, et al. Outcomes of corneal neurotisation using processed nerve allografts: A multicentre case series. Br J Ophthalmol. 2022;106:326–30. doi: 10.1136/bjophthalmol-2020-317361. [DOI] [PubMed] [Google Scholar]
- 10.Gyori E, Tzou CH, Weninger WJ, Reissig L, Schmidt-Erfurth U, Radtke C, et al. Axon numbers and landmarks of trigeminal donor nerves for corneal neurotization. PLoS One. 2018;13:e0206642. doi: 10.1371/journal.pone.0206642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Catapano J, Demsey DR, Ho ES, Zuker RM, Borschel GH. Cross-face nerve grafting with infraorbital nerve pathway protection: Anatomic and histomorphometric feasibility study. Plast Reconstr Surg Glob Open. 2016;4:e1037. doi: 10.1097/GOX.0000000000001037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janis JE, Hatef DA, Hagan R, Schaub T, Liu JH, Thakar H, et al. Anatomy of the supratrochlear nerve: Implications for the surgical treatment of migraine headaches. Plast Reconstr Surg. 2013;131:743–50. doi: 10.1097/PRS.0b013e3182818b0c. [DOI] [PubMed] [Google Scholar]
- 13.Haładaj R, Polguj M, Topol M. Anatomical variations of the supraorbital and supratrochlear nerves: Their intraorbital course and relation to the supraorbital margin. Med Sci Monit. 2019;25:5201–10. doi: 10.12659/MSM.915447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cuzalina AL, Holmes JD. A simple and reliable landmark for identification of the supraorbital nerve in surgery of the forehead: An in vivo anatomical study. J Oral Maxillofac Surg. 2005;63:25–7. doi: 10.1016/j.joms.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 15.Domeshek LF, Hunter DA, Santosa K, Couch SM, Ali A, Borschel GH, et al. Anatomic characteristics of supraorbital and supratrochlear nerves relevant to their use in corneal neurotization. Eye. 2018;33:398–403. doi: 10.1038/s41433-018-0222-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu CY, Arteaga AC, Fung SE, Cortina MS, Leyngold IM, Aakalu VK. Corneal neurotization for neurotrophic keratopathy: Review of surgical techniques and outcomes. Ocul Surf. 2021;20:163–72. doi: 10.1016/j.jtos.2021.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elbaz U, Bains R, Zuker RM, Borschel GH, Ali A. Restoration of corneal sensation with regional nerve transfers and nerve grafts: A new approach to a difficult problem. JAMA Ophthalmol. 2014;132:1289–95. doi: 10.1001/jamaophthalmol.2014.2316. [DOI] [PubMed] [Google Scholar]
- 18.Sepehripour S, Lloyd MS, Nishikawa H, Richard B, Parulekar M. Surrogate outcome measures for corneal neurotization in infants and children. J Craniofac Surg. 2017;28:1167–70. doi: 10.1097/SCS.0000000000003677. [DOI] [PubMed] [Google Scholar]
- 19.Jacinto F, Espana E, Padilla M, Ahmad A, Leyngold I. Ipsilateral supraorbital nerve transfer in a case of recalcitrant neurotrophic keratopathy with an intact ipsilateral frontal nerve: A novel surgical technique. Am J Ophthalmol Case Rep. 2016;4:14–7. doi: 10.1016/j.ajoc.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benkhatar H, Levy O, Goemaere I, Borderie V, Laroche L, Bouheraoua N. Corneal neurotization with a great auricular nerve graft: Effective reinnervation demonstrated by in vivo confocal microscopy. Cornea. 2018;37:647–50. doi: 10.1097/ICO.0000000000001549. [DOI] [PubMed] [Google Scholar]
- 21.Bourcier T, Henrat C, Heitz A, Kremer SF, Labetoulle M, Liverneaux P. Lateral antebrachial cutaneous nerve as autologous graft for mini-invasive corneal neurotization (MICORNE) Cornea. 2019;38:1029–32. doi: 10.1097/ICO.0000000000002004. [DOI] [PubMed] [Google Scholar]
- 22.Park JK, Charlson ES, Leyngold I, Kossler AL. Corneal neurotization: A review of pathophysiology and outcomes. Ophthalmic Plast Reconstr Surg. 2020;36:431–7. doi: 10.1097/IOP.0000000000001583. [DOI] [PubMed] [Google Scholar]
- 23.Saini M, Kalia A, Jain AK, Gaba S, Malhotra C, Gupta A, et al. Clinical outcomes of corneal neurotization using sural nerve graft in neurotrophic keratopathy. PLoS One. 2023;18:e0294756. doi: 10.1371/journal.pone.0294756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim JS, Rafailov L, Leyngold IM. Corneal neurotization for postherpetic neurotrophic keratopathy: Initial experience and clinical outcomes. Ophthalmic Plast Reconstr Surg. 2021;37:42–50. doi: 10.1097/IOP.0000000000001676. [DOI] [PubMed] [Google Scholar]
- 25.Ting DSJ, Figueiredo GS, Henein C, Barnes E, Ahmed O, Mudhar HS, et al. Corneal neurotization for neurotrophic keratopathy: Clinical outcomes and in vivo confocal microscopic and histopathological findings. Cornea. 2018;37:641–6. doi: 10.1097/ICO.0000000000001522. [DOI] [PubMed] [Google Scholar]
- 26.Lin CH, Lai LJ. Herpetic corneal keratopathy management using ipsilateral supratrochlear nerve transfer for corneal neurotization. Ann Plast Surg. 2019;83:553–7. doi: 10.1097/SAP.0000000000002120. [DOI] [PubMed] [Google Scholar]
- 27.Wolkow N, Habib LA, Yoon MK, Freitag SK. Corneal neurotization: Review of a new surgical approach and its developments. Semin Ophthalmol. 2019;34:473–87. doi: 10.1080/08820538.2019.1648692. [DOI] [PubMed] [Google Scholar]
- 28.Fogagnolo P, Giannaccare G, Bolognesi F, Digiuni M, Tranchina L, Rossetti L, et al. Direct versus indirect corneal neurotization for the treatment of neurotrophic keratopathy: A multicenter prospective comparative study. Am J Ophthalmol. 2020;220:203–14. doi: 10.1016/j.ajo.2020.07.003. [DOI] [PubMed] [Google Scholar]
- 29.Fung SSM, Catapano J, Elbaz U, Zuker RM, Borschel GH, Ali A. In vivo confocal microscopy reveals corneal reinnervation after treatment of neurotrophic keratopathy with corneal neurotization. Cornea. 2018;37:109–12. doi: 10.1097/ICO.0000000000001315. [DOI] [PubMed] [Google Scholar]
- 30.Rosenblatt TR, Sears CM, Park JK, Kossler AL. Corneal neurotization and novel medical therapies for neurotrophic keratopathy. Curr Ophthalmol Rep. 2020;8:252–66. [Google Scholar]
- 31.Colen KL, Choi M, Chiu DT. Nerve grafts and conduits. Plast Reconstr Surg. 2009;124:e386–94. doi: 10.1097/PRS.0b013e3181bf8430. [DOI] [PubMed] [Google Scholar]
- 32.Catapano J, Fung SSM, Halliday W, Jobst C, Cheyne D, Ho ES, et al. Treatment of neurotrophic keratopathy with minimally invasive corneal neurotisation: Long-term clinical outcomes and evidence of corneal reinnervation. Br J Ophthalmol. 2019;103:1724–31. doi: 10.1136/bjophthalmol-2018-313042. [DOI] [PubMed] [Google Scholar]
- 33.Malhotra R, Elalfy MS, Kannan R, Nduka C, Hamada S. Update on corneal neurotisation. Br J Ophthalmol. 2019;103:26–35. doi: 10.1136/bjophthalmol-2018-312104. [DOI] [PubMed] [Google Scholar]
- 34.Dragnea DC, Krolo I, Koppen C, Faris C, Van den Bogerd B, Ní Dhubhghaill S. Corneal neurotization-indications, surgical techniques and outcomes. J Clin Med. 2023;12:2214. doi: 10.3390/jcm12062214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kauffmann T, Bodanowitz S, Hesse L, Kroll P. Corneal re-innervation after photorefractive keratectomy and laser in situ keratomileusis: An in vivo study with a confocal video- microscope. Ger J Ophthalmol. 1996;5:508–12. [PubMed] [Google Scholar]
- 36.Fuchsluger TA, Steuhl KP, Meller D. Neurotrophic keratopathy: A post-LASIK case report [in German] Klin Monatsbl Augenheilkd. 2005;222:901–4. doi: 10.1055/s-2005-858800. [DOI] [PubMed] [Google Scholar]
- 37.Gallar J, Acosta MC, Moilanen JAO, Holopainen JM, Belmonte C, Tervo TMT. Recovery of corneal sensitivity to mechanical and chemical stimulation after laser in situ kerato-mileusis. J Refract Surg. 2004;20:229–35. doi: 10.3928/1081-597X-20040501-06. [DOI] [PubMed] [Google Scholar]
- 38.Cruzat A, Qazi Y, Hamrah P. In vivo confocal microscopy of corneal nerves in health and disease. Ocul Surf. 2017;15:15–47. doi: 10.1016/j.jtos.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giannaccare G, Bolognesi F, Biglioli F, Marchetti C, Mariani S, Weiss JS, et al. In vivo and ex vivo comprehensive evaluation of corneal reinnervation in eyes neurotized with contralateral supratrochlear and supraorbital nerves. Cornea. 2020;39:210–4. doi: 10.1097/ICO.0000000000002083. [DOI] [PubMed] [Google Scholar]
- 40.Allevi F, Fogagnolo P, Rossetti L, Biglioli F. Eyelid reanimation, neurotisation, and transplantation of the cornea in a patient with facial palsy. BMJ Case Rep. 2014;2014:bcr2014205372. doi: 10.1136/bcr-2014-205372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lathrop KL, Duncan K, Yu J, Shah PR, Goldstein J, Nischal KK. Development of corneal sensation with remodeling of the epithelium and the palisades of Vogt after corneal neurotization. Cornea. 2020;39:657–60. doi: 10.1097/ICO.0000000000002269. [DOI] [PubMed] [Google Scholar]
- 42.Bains RD, Elbaz U, Zuker RM, Ali A, Borschel GH. Corneal neurotization from the supratrochlear nerve with sural nerve grafts: A minimally invasive approach. Plast Reconstr Surg. 2015;135:397e–400e. doi: 10.1097/PRS.0000000000000994. [DOI] [PubMed] [Google Scholar]
- 43.Catapano J, Antonyshyn K, Zhang JJ, Gordon T, Borschel GH. Corneal neurotization improves ocular surface health in a novel rat model of neurotrophic keratopathy and corneal neurotization. Invest Ophthalmol Vis Sci. 2018;59:4345–54. doi: 10.1167/iovs.18-24843. [DOI] [PubMed] [Google Scholar]
- 44.Belmonte C. Response of sensory units with unmyelinates fibres to mechanical, thermal and chemical stimulation of the cat’s cornea. J Physiol. 1993;468:609–22. doi: 10.1113/jphysiol.1993.sp019791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wisely CE, Rafailov L, Cypen S, Proia AD, Boehlke CS, Leyngold IM. Clinical and morphologic outcomes of minimally invasive direct corneal neurotization. OPRS. 2020;36:451–7. doi: 10.1097/IOP.0000000000001586. [DOI] [PubMed] [Google Scholar]
- 46.Weis E, Rubinov A, Al-ghoul AR, Yau FM. Sural nerve graft for neurotrophic keratitis: early results. Can J Ophthalmol. 2018;53:23–8. doi: 10.1016/j.jcjo.2017.10.044. [DOI] [PubMed] [Google Scholar]
- 47.Jowett N, Pineda R II. Corneal neurotisation by great auricular nerve transfer and scleral-corneal tunnel incisions for neurotrophic keratopathy. Br J Ophthalmol. 2019;103:1235–8. doi: 10.1136/bjophthalmol-2018-312563. [DOI] [PubMed] [Google Scholar]


