Abstract
BACKGROUND:
Mitral annular calcification with valve dysfunction remains a challenging syndrome. Operative risk is high, and available transcatheter therapies are limited.
METHODS:
This study describes our initial experience with a novel procedure to address large mitral annuli when no surgical or trial-based transcatheter mitral valve replacement device is available. The rationale was to shorten the intercommissural distance using commissural mitral transcatheter edge-to-edge repair (TEER) followed by valve-in-mitral annular calcification transcatheter mitral valve replacement with a balloon-expandable aortic valve platform. Patients with long intercommissural distances and large mitral annulus areas were selected based on a high perceived risk of transcatheter valve embolization. Patients underwent mitral TEER with MitraClip in a commissural position, followed immediately by transseptal transcatheter mitral valve replacement with a 29 mm SAPIEN 3 valve.
RESULTS:
Thirteen patients were included. Median intercommissural distance and annular area were 39.1 mm and 930 mm2, respectively. Commissural mitral TEER was successful in all patients with no instances of single leaflet detachment. In 10 of 13 instances, an NTW device size was used. In 12 of 13 patients, valve implantation was successful, including 1 case that required a second valve for atrial positioning of the first valve. In 1 case, frank valve embolization into the left atrium occurred. Among the 12 successful cases, paravalvular leak was 1+ or less, and there were no instances of paravalvular leak adjacent to the TEER device.
CONCLUSIONS:
In patients with large annuli and sufficient annular calcium, a hybrid mitral TEER and valve replacement with the SAPIEN platform can be successfully used to facilitate transcatheter mitral valve replacement.
Keywords: heart valve diseases, humans, mitral valve, mitral valve insufficiency, mitral valve stenosis
WHAT IS KNOWN
Transcatheter mitral valve replacement in mitral annular calcification with a balloon-expandable valve is often limited by annular size and calcium distribution.
WHAT THE STUDY ADDS
In large mitral annuli, edge-to-edge repair devices can be placed in the commissure to optimize annular sizing and device anchoring.
Mitral annular calcification (MAC) increases in prevalence in an aging population. Up to 40% of elderly adults have echocardiographic evidence of MAC.1,2 Mitral valve (MV) dysfunction (both mitral stenosis and regurgitation) is significantly more common in patients with MAC.2 Risk of operative management is frequently prohibitively high, and 1-year mortality in patients with MAC-related MV dysfunction undergoing transcatheter MV replacement (TMVR) is up to 53.7%. These sobering data highlight the need for novel, safer approaches to treat this high-risk population. There are no commercially available transcatheter valves designed specifically for the mitral annulus shape. TMVR platforms currently in various stages of clinical trial include Medtronic Intrepid (APOLLO trial [Transcatheter Mitral Valve Replacement With the Medtronic Intrepid (TMVR) System in Patients With Severe Symptomatic Mitral Regurgitation], NCT03242642, Minneapolis, MN), Edwards Lifesciences SAPIEN M3 (ENCIRCLE trial [SAPIEN M3 System Transcatheter MItral Valve Replacement via Transseptal Access], NCT04153292, Irvine, CA), and Abbott Tendyne (SUMMIT trial [Clinical Trial to Evaluate the Safety and Effectiveness of Using the Tendyne Transcatheter Mitral Valve System for the Treatment of Symptomatic Mitral Regurgitation], NCT03433274, North Plymouth, MN). However, screen failure rates among potential trial patients remain high, frequently related to issues of predicted left ventricular outflow tract (LVOT) obstruction. Furthermore, these trials have many important exclusion criteria, such as advanced kidney disease, which is a well-known risk factor for the development of MAC.3
In patients with small predicted neo-LVOT,4 there has been success using the SAPIEN 3 valve platform (Edwards LifeSciences, Irvine, CA) combined with procedures to mitigate LVOT obstruction, including (1) alcohol septal ablation,5,6 (2) the newly developed septal myectomy procedure (SESAME),7 and (3) intraprocedural management of the displaced anterior mitral leaflet via electrosurgical laceration (LAMPOON [Laceration of the Anterior Mitral Leaflet to Prevent Outflow Tract Obstruction]). This approach also allows for the use of commercialized technologies in patients who are otherwise ineligible for trial-based devices. However, there are key limitations to commercial valve-in-MAC with the SAPIEN platform. First, the mitral annulus is larger than the aortic annulus with an oblong shape. Thus, large annuli, particularly with long intercommissural distances, are challenging to address given the sizing of the SAPIEN 3 valve platform. In the MITRAL I and II trials (Mitral Implantation of Transcatheter Valves), the only prospective trials of the SAPIEN 3 valve platform for valve-in-MAC TMVR, annulus size >810 mm2, or a high perceived risk of valve embolization were exclusion criteria. Second, MAC distribution around the annulus is heterogeneous and often mainly located along the posterior annulus.8 Since the SAPIEN 3 valve prostheses have no active fixation and remain in place only via friction, near circumferential MAC is typically desired. Thus, in patients for whom surgical replacement is deemed too high risk, MAC-related MV disease remains a challenging anatomic subset to treat unless they have favorable anatomy and calcium distribution or qualify for a trial-based mitral-specific device.
While percutaneous mitral transcatheter edge-to-edge repair (TEER) with the MitraClip device (Abbott Vascular, Plymouth, MN) is an established therapy for patients with severe mitral regurgitation, known limitations of this intervention include MAC in the grasping zone and unacceptable elevation in diastolic transmitral pressure gradient.9 For patients with calcified MV leaflets or preexisting mitral stenosis, percutaneous mitral TEER alone is not a viable solution. With this in mind, we sought to combine the technologies of mitral TEER and TMVR with the SAPIEN 3 valve platform to manage nonoperative patients with MAC-related MV dysfunction and large mitral annulus. Here, we present the initial experience using the ARCTIC procedure (Annular Reduction by Cinching With TEER in the Commissure), a novel technique for TMVR in MAC facilitated by mitral TEER to prevent valve embolization or migration and paravalvular leak in the setting of large annuli and specifically long intercommissural distances.
Methods
Patients
The data that support the findings of this study are available from the corresponding author upon reasonable request. This is a single-center retrospective review of the initial clinical experience with ARCTIC TMVR. Patients consented to clinical treatment despite the novelty and attendant high risk once all other options for treatment were exhausted. All patients who underwent ARCTIC TMVR without additional exploratory annular modification techniques were included. Data were collected retrospectively in the course of clinical care, not research, and therefore some data are missing and not imputed. Clinical data were abstracted from hospital records. Institutional international review board approval was obtained for this analysis.
Preprocedural Computed Tomography Parameters
All patients underwent cardiac-gated computed tomography angiography for preprocedural planning. Reconstructions of the MV annulus and post-TMVR predicted neo-LVOT area were performed using the 3Mensio Structural Heart module (Pie Medical Imaging, Maastricht, the Netherlands). Annular size and estimated neo-LVOT measurements were performed in an end-systolic (≈40%) phase. Thick slice reconstructions of the MV en face were used to define the extent and distribution of annular calcium (Figure 1). MAC scores were calculated in standard fashion as previously described.10
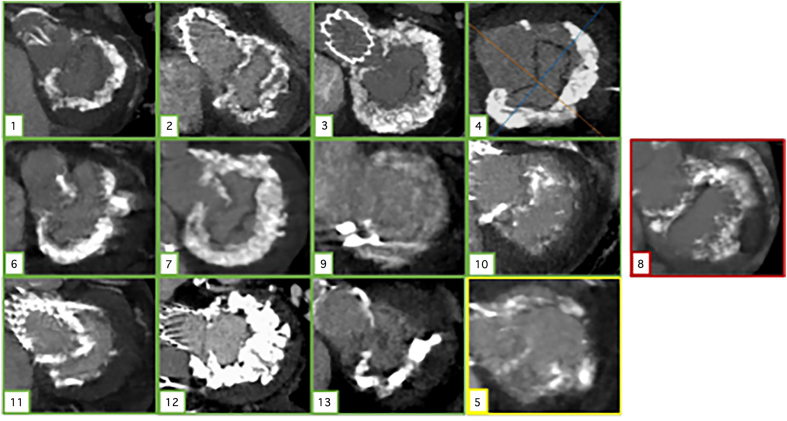
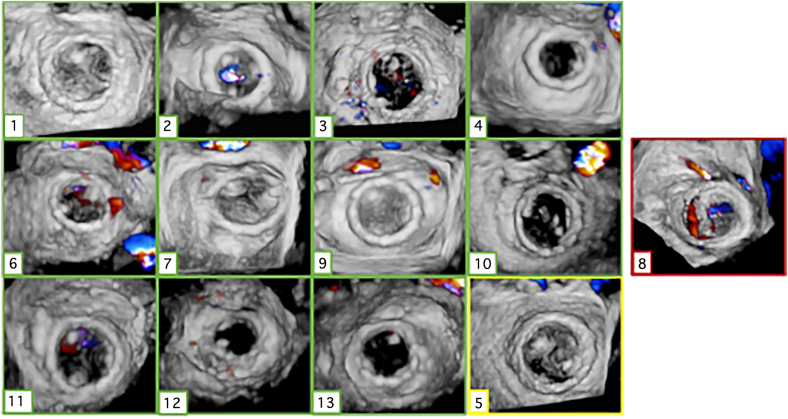
Figure 1.
En face thick slice reconstruction of mitral valve annulus showing distribution of calcium. Ten cases outlined in green (cases 1–4, 6–7, and 9–13) had successful valve implantation after ARCTIC (Annular Reduction by Cinching With TEER in the Commissure) procedure, case 5 (in yellow) required a second valve for atrial migration but ultimately did well, and case 8 (in red) experienced frank atrial embolization.
Patients were only considered for ARCTIC TMVR in the setting of large mitral annular dimensions. Based on prior experience and judgment, we considered an intercommissural distance greater than ≈35 mm to be at significantly high risk for embolization if the anteroposterior dimension was also >25 mm or the area was >850 mm, particularly if the annulus was not circumferentially calcified. Assessment was performed on a case-by-case basis by expert operators.
Procedure Overview
Briefly, patients were brought to the hybrid catheterization laboratory, and access points were secured in the following locations: right femoral vein (for transseptal access and TEER device/valve delivery), right femoral artery (if needed for aortic snare catheter for LAMPOON), and the left femoral vein (for temporary transvenous pacing wire). In some circumstances, we also placed the SENTINEL cerebral embolic protection system (Boston Scientific, Marlborough, MA) via the right radial artery and a prophylactic intra-aortic balloon pump when performing LAMPOON via a second femoral arterial access.
Next, transseptal puncture was performed in a suitable position for the TEER device (typically mid and posterior to ensure adequate height above the valve), and the TEER device steerable guide system was introduced into the left atrium. Careful interrogation of the MV was performed with multiplanar transesophageal echocardiography to determine which commissural leaflets appeared most suitable for TEER device implantation. The medial commissure was preferred due to a perceived reduced risk of dislodgement during valve delivery, but the degree of MAC and leaflet pathology frequently necessitated lateral commissure TEER device placement. The choice of laterality for the TEER device was dictated by the procedural transesophageal echocardiography and where the most viable leaflets for grasping were appreciated. Typically, 1 commissure would be less heavily calcified, and that side was chosen for the TEER implant. In 2 cases, TEER devices were placed in both commissures. This scenario was reserved for MVs with less MAC where the devices were felt to meaningfully improve points of frictional contact and not simply reduce the intercommissural distance. The NTW MitraClip was the preferred TEER device of choice to minimize risks of chordal entanglement in the commissure while still achieving a significant cinching effect (eg, reduction of the intercommissural distance).
Adjunctive LAMPOON was performed in 5 cases: 2 before TEER and 3 after TEER. In each case, antegrade base-to-tip LAMPOON was performed as previously described11 via a 26 French DrySeal (Gore, Newark, DE) venous sheath. This sheath was then exchanged for the TEER device steerable guide system if performed in advance or used for valve delivery if TEER was already completed.
Through the 26 French DrySeal sheath, an atrial septostomy was performed to facilitate delivery of a 29 mm SAPIEN 3 valve into the mitral position. Appropriate fluoroscopic angles (steep right anterior oblique) were predetermined with cardiac computed tomography angiography to view the valve with minimal parallax. The valve was then deployed with 3 to 4 mL of additional balloon volume with rapid ventricular pacing.
Analysis and Statistics
Symptoms were assessed using the World Health Organization-modified New York Heart Association Classification that incorporates death. Technical success and procedural success and outcomes were defined by Mitral Valve Academic Research Consortium criteria.12 This is a descriptive analysis of an initial experience. As such, summary statistics are provided for qualitative assessment. This retrospective analysis was approved by the local international review board.
Results
Patient Characteristics
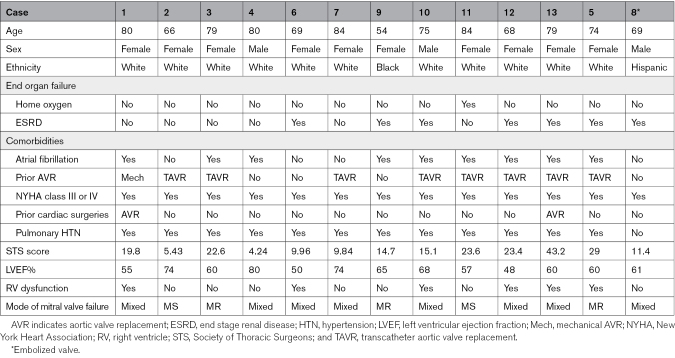
Thirteen patients underwent the ARCTIC procedure to facilitate TMVR from May 2021 to June 2024. (Table 1; Figure 2) This represents ≈15% of all valve-in-MAC patients treated commercially during the corresponding period at our institution. Patients were elderly, with a median age of 75 (interquartile range [IQR], 69–80) years, and felt to be inoperable candidates for surgical valve replacement after heart team discussion. The median Society of Thoracic Surgeons score was 15.1% (IQR, 10.0%–23.4%). All patients had New York Heart Association class III or IV symptoms. All but 2 patients (cases 6 and 12) had left ventricular ejection fraction of 50% or greater, but most (12/13) had either right ventricular dysfunction or pulmonary hypertension. The mechanism of MV dysfunction was mixed (at least moderate mitral stenosis and regurgitation) in 8 cases, primarily regurgitation in 3 cases, and primarily stenosis in 2 cases. One patient (case 9) had severe mitral regurgitation due to recent, but sterilized, endocarditis.
Table 1.
Patient Characteristics
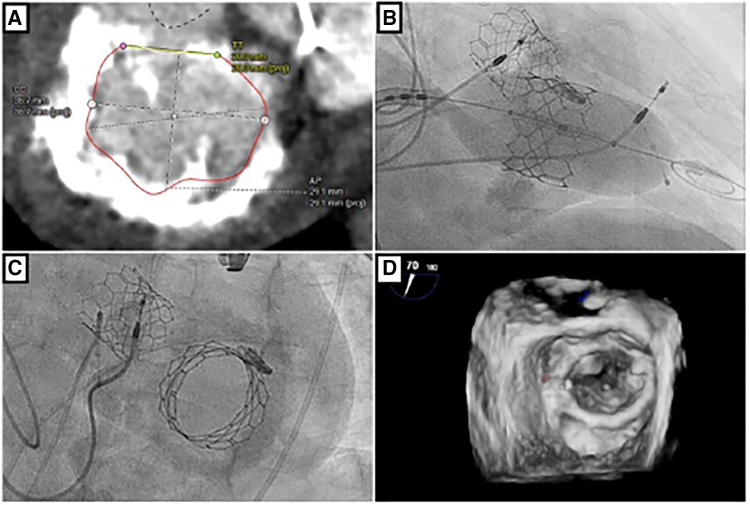
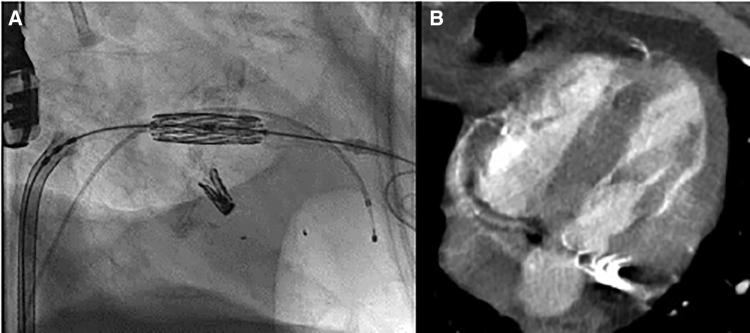
Figure 2.
Example patient computed tomography scan, ARCTIC (Annular Reduction by Cinching With TEER in the Commissure) transcatheter mitral valve replacement procedure and result. Preprocedural cardiac-gated computed tomography angiography with en face reconstruction of the mitral valve showing severe, circumferential calcium but large intercommissural distance (A). Following placement of a transcatheter edge-to-edge device (Mitraclip NTW, Abbott Vascular) in a commissural position (B), a 29 mm SAPIEN 3 valve (Edwards LifeSciences) is implanted via a transseptal approach (C). Three-dimensional transesophogeal echocardiography acquisition with color Doppler shows a stable valve implantation with trivial paravalvular leak (D).
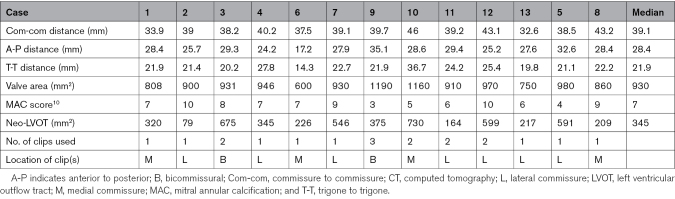
MV intercommissural lengths ranged from 32.6 to 46 mm (median, 39.1 [IQR, 38.2–40.2] mm; Table 2). Annular area ranged from 600 to 1190 mm2 (median, 930 [IQR, 860–970] mm2). Annular calcification was substantial in most patients, with a median MAC score of 7. The median neo-LVOT size for the population was 345 (IQR, 217–591) mm2. Neo-LVOT assessment predicted a high risk of LVOT obstruction in 5 of 13 patients, with predicted neo-LVOT under 250 mm2. These 5 patients underwent antegrade LAMPOON at the time of TMVR.
Table 2.
Preprocedural CT Parameters
ARCTIC Outcomes
TEER device deployment was successful in all patients (total of 19 TEER devices). The TEER devices were placed in the medial commissure in 4 of 13, lateral commissure in 7 of 13, and both commissures in 2 of 10 cases. The majority were NTW TEER devices (11/19). One patient (case 9) received 3 total TEER devices, 2 XTW TEER devices medially, and 1 XTW TEER device laterally. There were no instances of TEER embolization or dislodgement during valve deployment.
TMVR Outcomes
In 11 of 13 cases, the first valve deployment was successful, meeting Mitral Valve Academic Research Consortium criteria for stable valve placement with less than a moderate paravalvular leak. In 1 case (case 5), a second valve was placed due to skirt leak from a slightly atrial deployment of the first prosthesis, after which the system was stable without significant leak. In the 12 successful cases, the mitral inflow gradient was 3 mm Hg or less, the paravalvular leak was 1+ or less, and there were no instances of an increase in the LVOT gradient by >10 mm Hg.
Interestingly, there was only 1 case of commissural leak on the side of the ARCTIC procedure. Case 12 required plugging of moderate PVL with a 27-mm Gore atrial septal defect occluder. Figure 3 demonstrates the transesophageal echocardiography en face view with color demonstrating the pattern of paravalvular leak for all patients. One patient (case 9) had indolent but ultimately clinically significant hemolysis in the setting of a mild anterior paravalvular leak (distant from the commissural clips) and underwent successful paravalvular leak plugging about 5 weeks after TMVR with resolution.
Figure 3.
Three-dimensional transesophogeal acquisitions of the mitral valve in surgeon’s view with color Doppler in early to mid-systole. All successful cases demonstrated at most 1+ paravalvular leak at the conclusion of the procedure. Case 12 is shown after closure of a paravalvular leak with a 27-mm Gore atrial septal defect occluder. Case 5 is shown after the implantation of a second valve to manage atrial migration of the first valve deployment. Case 8 shows unstable atrial position of the system despite the placement of a second valve with a significant leak.
Case 8 was unsuccessful due to atrial embolization despite placement of a second valve while the first was still in the annulus. As determined before the procedure, there was no available surgical bailout, and the valves were pinned to the interatrial septum using a large atrial septal defect occluder. The patient ultimately died on postoperative day 2. On retrospective review, it is possible that hypertrophy of the subvalvular structures played a role in the acute embolization as both valves migrated atrially upon balloon inflation despite appropriate position and coaxiality (Figure 4). Ultimately, in hospital mortality in this extreme-risk population was 15% (2/13) with the other death (case 3) occurring suddenly on postoperative day 1 for unclear reasons (stat bedside echo demonstrated a well-functioning and positioned MV prosthesis and new right ventricular dysfunction).
Figure 4.
A case of atrial migration. A, Cine acquisition of initial valve in deployment-ready position in steep right anterior oblique projection following medial commissure transcatheter edge-to-edge device placement. As the delivery balloon inflates, the system appears to be driven toward the atrium, resulting in an atrial implantation. On retrospective review, the patient had significant papillary muscle hypertrophy (B), which may have interacted with the ventricular shoulder of the delivery balloon.
Discussion
Proof of Concept
This series of 13 patients with symptomatic calcific MV disease and large annuli with long intercommissural distances demonstrates the feasibility of an ARCTIC TMVR approach to reduce intercommissural length and facilitate valve anchoring before transcatheter valve deployment. By a recent state-of-the-art review, all of these patients would otherwise be considered at high or prohibitive risk for valve-in-MAC TMVR via a transseptal approach.13 Given that they would be considered high-risk for an already high-risk procedure, this population represents perhaps the most challenging cohort possible. Nevertheless, 12 of the 13 patients had an excellent procedural result with minimal paravalvular leak and appropriate transvalvular gradients. Furthermore, to the best of our knowledge, there have been no episodes of late valvular migration, valve embolization, or clip detachment. Additionally, paravalvular leak closure was required in only 2 patients.
Patient Selection
Selection criteria for determining who might benefit from an ARCTIC procedure have evolved and often remain predicated on expert judgment. Thus far, patients with large mitral annular areas due to long intercommissural distances have been the primary focus, but the decision matrix rests on multiple factors, including the distribution and severity of annular calcium, the calcification and mobility of the leaflets themselves, and the anterior-posterior diameter of the valve. Generally, the reported experience suggests a preemptive TEER device can facilitate anchoring in large annuli even when calcification is absent in 1 anterior quadrant of the valve (from trigone to medial or lateral commissure) or when the intercommissural length is >34 mm (it is generally not considered necessary when the intercommissural distance is <34 mm unless calcium is absent). As the length of the intercommissural axis grows, the number of TEER devices required is debatable. Each successive device is an opportunity for error, and as such, we generally sought to use as few TEER devices as possible.
During the study period in question, patients were turned down for ARCTIC TMVR or offered different novel concepts to facilitate valve-in-MAC based on anatomy. These patients were not systematically tracked, but the primary reasons patients were not offered ARCTIC TMVR were too little annular calcium, particularly anteriorly, and too large an anterior-posterior annular dimension. This remains a matter of opinion, but we feel caution should be applied when the anterior-posterior diameter approaches the diameter of the intended prosthesis unless there is substantial calcification of the anterior mitral leaflet and subvalvular structures.
Importantly, a broad argument can be made for futility in this patient population. Survival at 5 years can be as low as 20% in patients with MAC and elevated transmitral gradient. However, it is important to note the symptom burden of this population, with all classified as New York Heart Association III and IV. Most patients had multiple hospitalizations for heart failure prior to their procedures. It is also notable that the process by which all these patients were considered for this procedure was deliberate and protracted. All patients were submitted for trial device consideration unless they had clear exclusion criteria. Several also had septal modification in advance of trial submission and screen failed before and after this procedure. They were extensively counseled that the process commonly takes months and can require staged procedures. Despite this, these patients still made the choice that their quality of life was poor enough to submit to a high-risk and time-consuming management strategy.
ARCTIC Technique
It is notable that in our series (14 TEER device implants), we experienced no instances of single leaflet detachment despite commissural device location and calcified annuli. Dislodgement of the freshly implanted TEER device during delivery or deployment of the valve prosthesis is a hypothetical, though not observed, risk. We took care to minimize this risk by placing the TEER device(s) as commissural as possible such that they were buttressed by the ventricular wall, thereby limiting any potential displacement. Similarly, the laterality of the TEER device was predicated on leaflet health and where grasping felt most robust between the extreme medial and lateral commissures. When both options are available, the medial commissure was preferred since the valve delivery complex invariably leans on the lateral commissure during delivery. Thus far, this has proven to be only a potential concern, as most TEER devices were placed laterally without subsequent issue. Choosing the size of TEER device to implant was also a matter of judgment. In general, we used the NTW device as our default size given the extreme commissural placement of the devices and the calcific nature of the leaflets. In scenarios where the commissural leaflets were longer and less calcified, we chose to use the longer XTW devices. No outcome differences have been appreciated between the device sizes used, though the case volume to date does not lend itself to extensive analysis yet.
In the development of this strategy, a particular concern was the risk of developing a paravalvular leak in the commissure with the TEER device. It is notable that this occurred in only 1 case, consistent with the lack of single leaflet detachment. The only case (case 9) to develop clinically significant hemolysis had a mild, anterior leak, anatomically distant from the commissures. Particular attention was paid to placing the TEER devices as deep into the commissure as possible; however, on a few instances, a commissural orifice relative to the TEER device(s) could be seen before valve implantation. These orifices were all obliterated following valve deployment when the clips were further displaced towards the commissure.
A major limitation for any TMVR has been the risk of LVOT obstruction. We saw no clinically significant elevations in the LVOT gradient in patients with successful implants. In certain instances, this technique was chosen specifically because it facilitated LAMPOON at the time of valve deployment. With more aggressive and novel septal modification strategies in development, this may not be as much of a deciding factor in the future, particularly if novel TMVR devices continue to use fully closed cell structures that largely limit the efficacy of LAMPOON.
While the case series is too small to make clear statistical associations, some patterns have emerged that may demonstrate limitations that cannot be overcome with the ARCTIC technique. Of the 2 cases for which there was valve migration or embolism, case 5 was one of only 3 with an annulus area >980 mm2. The patients with a larger annulus (case 9, area 1190 mm2, and case 10, area 1160 mm2) were successful, albeit with 3 TEER devices in case 9 (2 medial and 1 lateral) and 2 TEER devices in case 10 (2 medial). The case of valve embolism (case 8) interestingly had an annular area below the mean (860 mm2), however, did have the second largest intercommissural distance at 43.2 mm and, as noted, appeared to have marked papillary muscle hypertrophy that may have been a key contributing factor.
Study Limitations
This is a small, observational study of a novel procedure performed at a single institution with a great deal of shared experience in this space. The results are not necessarily generalizable to all facilities. Additionally, patient selection criteria remain uncodified, though general considerations have been outlined. The article also lacks long-term follow-up; however, the intention of the article was to demonstrate concept feasibility rather than valve-in-MAC outcomes, particularly in such a small sample size. Finally, it remains unclear if a technique such as this one will remain an important tool in the treatment of MV disease related to MAC in the long term or just until new dedicated devices come online. Thus far, enrollment for such dedicated devices has been sluggish, and the importance of leaflet modification with LAMPOON for outflow tract preservation has been paramount, something not feasible with the current generation of dedicated mitral transcatheter prostheses.
Perspectives
At present, there are limited options for the management of MV dysfunction in the setting of severe MAC and high operative risk. Elevation of the mitral inflow gradient frequently precludes use of TEER devices, and use of specific devices designed for the mitral position remains available only through clinical trials. Use of the SAPIEN 3 valve platform in MAC is well described but frequently limited by annular size. We have demonstrated the feasibility and acute safety of the novel ARCTIC TMVR procedure that employs TEER to facilitate anchoring and valve stability and minimize paravalvular leak during valve-in-MAC TMVR in large annuli.
ARTICLE INFORMATION
Sources of Funding
This study was supported indirectly via the David and Nancy Auth Endowed Chair but was otherwise not financially supported.
Disclosures
Dr McCabe receives honoraria from Abbott Vascular and Edwards LifeSciences. The other authors report no conflicts.
Nonstandard Abbreviations and Acronyms
- ARCTIC
- Annular Reduction by Cinching With TEER in the Commissure
- CCTA
- cardiac computed tomography angiography
- LAMPOON
- Laceration of the Anterior Mitral Leaflet to Prevent Outflow Tract Obstruction
- LVOT
- left ventricular outflow tract
- MAC
- mitral annular calcification
- MV
- mitral valve
- TEER
- transcatheter edge-to-edge repair
- TMVR
- transcatheter mitral valve replacement
This manuscript was sent to Frederick G. Welt, Senior Guest Editor, for review by expert referees, editorial decision, and final disposition.
For Sources of Funding and Disclosures, see page 1047.
Contributor Information
David Elison, Email: davide5@uw.edu.
Rafael Harari, Email: rafharari@gmail.com.
Cristina Sanina, Email: csanina@gmail.com.
Srdjan Jelacic, Email: sjelacic@uw.edu.
Richard Sheu, Email: sheurich@uw.edu.
G. Burkhard Mackensen, Email: gbmac@uw.edu.
References
- 1.Sticchi A. Mitral valve stenosis: epidemiology and causes in elderly patients. Eur J Cardiol Practice. 2018;16:1–8. [Google Scholar]
- 2.Okura H, Nakada Y, Nogi M, Ishihara S, Okamura A, Okayama S, Watanabe M, Kawakami R, Saito Y. Prevalence of mitral annular calcification and its association with mitral valvular disease. Echocardiogr. 2021;38:1907–1912. doi: 10.1111/echo.15236 [DOI] [PubMed] [Google Scholar]
- 3.Abd Alamir M, Radulescu V, Goyfman M, Mohler ER, 3rd, Gao YL, Budoff MJ; CRIC Study Investigators. Prevalence and correlates of mitral annular calcification in adults with chronic kidney disease: results from CRIC study. Atherosclerosis. 2015;242:117–122. doi: 10.1016/j.atherosclerosis.2015.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoon SH, Bleiziffer S, Latib A, Eschenbach L, Ancona M, Vincent F, Kim WK, Unbehaum A, Asami M, Dhoble A, et al. Predictors of left ventricular outflow tract obstruction after transcatheter mitral valve replacement. JACC Cardiovasc Interv. 2019;12:182–193. doi: 10.1016/j.jcin.2018.12.001 [DOI] [PubMed] [Google Scholar]
- 5.Tiwana J, Aldea G, Levin DB, Johnson K, Don CW, Dvir D, Mackensen GB, Reisman M, McCabe JM. Contemporary transcatheter mitral valve replacement for mitral annular calcification or ring. JACC Cardiovasc Interv. 2020;13:2388–2398. doi: 10.1016/j.jcin.2020.07.007 [DOI] [PubMed] [Google Scholar]
- 6.Khan JM, Babaliaros VC, Greenbaum AB, Foerst JR, Yazdani S, McCabe JM, Paone G, Eng MH, Leshnower BG, Gleason PT, et al. Anterior leaflet laceration to prevent ventricular outflow tract obstruction during transcatheter mitral valve replacement. J Am Coll Cardiol. 2019;73:2521–2534. doi: 10.1016/j.jacc.2019.02.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greenbaum AB, Khan JM, Bruce CG, Hanzel GS, Gleason PT, Kohli K, Inci EK, Guyton RA, Paone G, Rogers T, et al. Transcatheter myotomy to treat hypertrophic cardiomyopathy and enable transcatheter mitral valve replacement: first-in-human report of septal scoring along the midline endocardium. Circ Cardiovasc Interv. 2022;15:e012106. doi: 10.1161/CIRCINTERVENTIONS.122.012106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carpentier AF, Pellerin M, Fuzellier JF, Relland JY. Extensive calcification of the mitral valve anulus: pathology and surgical management. J Thorac Cardiovasc Surg. 1996;111:718–29; discussion 729. doi: 10.1016/s0022-5223(96)70332-x [DOI] [PubMed] [Google Scholar]
- 9.Mack MJ, Abraham WT, Lindenfeld J, Bolling SF, Feldman TE, Grayburn PA, Kapadia SR, McCarthy PM, Lim DS, Udelson JE, et al. Cardiovascular outcomes assessment of the MitraClip in patients with heart failure and secondary mitral regurgitation: design and rationale of the COAPT trial. Am Heart J. 2018;205:1–11. doi: 10.1016/j.ahj.2018.07.021 [DOI] [PubMed] [Google Scholar]
- 10.Guerrero M, Wang DD, Pursnani A, Eleid M, Khalique O, Urena M, Salinger M, Kodali S, Kaptzan T, Lewis B, et al. A cardiac computed tomography-based score to categorize mitral annular calcification severity and predict valve embolization. JACC Cardiovasc Imaging. 2020;13:1945–1957. doi: 10.1016/j.jcmg.2020.03.013 [DOI] [PubMed] [Google Scholar]
- 11.Lisko JC, Greenbaum AB, Khan JM, Kamioka N, Gleason PT, Byku I, Condado JF, Jadue A, Paone G, Grubb KJ, et al. Antegrade intentional laceration of the anterior mitral leaflet to prevent left ventricular outflow tract obstruction: a simplified technique from bench to bedside. Circ Cardiovasc Interv. 2020;13:e008903. doi: 10.1161/CIRCINTERVENTIONS.119.008903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stone GW, Adams DH, Abraham WT, Kappetein AP, Généreux P, Vranckx P, Mehran R, Kuck KH, Leon MB, Piazza N, et al. ; Mitral Valve Academic Research Consortium (MVARC). Clinical trial design principles and endpoint definitions for transcatheter mitral valve repair and replacement: part 2: endpoint definitions: a consensus document from the Mitral Valve Academic Research Consortium. Eur Heart J. 2015;36:1878–1891. doi: 10.1093/eurheartj/ehv333 [DOI] [PubMed] [Google Scholar]
- 13.Guerrero ME, Grayburn P, Smith RL, 2nd, Sorajja P, Wang DD, Ahmad Y, Blusztein D, Cavalcante J, Tang GHL, Ailawadi G, et al. Diagnosis, classification, and management strategies for mitral annular calcification: a heart valve collaboratory position statement. JACC Cardiovasc Interv. 2023;16:2195–2210. doi: 10.1016/j.jcin.2023.06.044 [DOI] [PubMed] [Google Scholar]