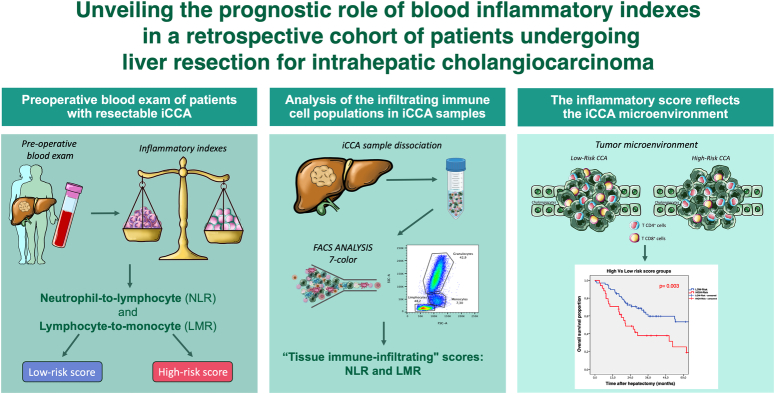
Abstract
Background:
Systemic inflammation is relevant in intrahepatic cholangiocarcinoma (iCCA), but controversial results exist on the prognostic role of inflammatory indexes and their correlation with tumor microenvironment. The authors aimed to explore the biological and prognostic values of these indexes.
Materials and methods:
A retrospective cohort study involving iCCA patients who underwent hepatic resection between 2010 and 2021 was conducted. The neutrophil-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR), and clinic-pathological factors were recorded. Immune-cell subpopulations, isolated from surgical specimens, were analyzed by flow cytometry. NLR and LMR cut-offs were calculated by X-Tile software. Linear regression, Kaplan–Meier, and Cox regression analyses were conducted.
Results:
A total of 101 iCCA patients were considered. NLR ≥3.83 and LMR <2.28 correlated with worse survival. Patients were divided into groups: 67 (66.3%) in the low-risk and 34 (33.7%) in the high-risk (having at least one worse prognostic ratio). The 5-year overall survival was 49.8 and 18.9% for low- and high-risk groups, respectively (P=0.003). An elevated CA19-9 in the high-risk group gives 2.148 HR (95% CI: 1.060–4.349) of mortality and 2.182 HR (95% CI: 1.206–3.948) of disease recurrence. Flow cytometry analysis of 20 surgical specimens highlighted that NLR was associated with tumor-derived NLR (P=0.026) and LMR with tumor-infiltrating lymphocytes (P=0.002). In a subset of five high-risk vs five low-risk patients, T-cell evaluation showed a higher prevalence of CD4+ compared to CD8+ cells in the high-risk group (78.5 vs. 21.5%, P<0.0001). Conversely, low-risk patients demonstrated a noteworthy infiltration of CD8+ cells compared to the high-risk group (21.5 vs. 48.7%, P=0.037).
Conclusions:
The combination of blood inflammatory indexes determined two survival-risk profiles. The correlation between the blood scores and the iCCA microenvironment suggests a link between immune-cell infiltration and the risk group. These findings open the possibility of patient stratification with the chance to identify subgroups suitable for dedicated follow-up and targeted immuno-chemotherapy protocols.
Keywords: biliary tract cancer, intrahepatic cholangiocarcinoma, lymphocyte-to-monocyte ratio, neutrophil-to-lymphocyte ratio, tumor-infiltrating lymphocyte
Introduction
Highlights
Elevated neutrophil-to-lymphocyte ratio and decreased lymphocyte-to-monocyte ratio identify high-risk patients with worse prognosis.
Tumor microenvironment analysis revealed correlations between inflammatory indexes and tumor-infiltrating leukocytes.
Blood inflammatory indexes may serve as prognostic markers for survival-risk stratification in intrahepatic cholangiocarcinoma patients.
The link with immune cell infiltration opens possibilities for targeted immuno-chemotherapy protocols and tailored follow-up strategies for specific risk groups.
Intrahepatic cholangiocarcinoma (iCCA) is a rare tumor representing the second most common primary liver cancer, with an increasing incidence and mortality1. While surgical resection is the mainstay of curative treatment, the challenges of late diagnosis and high rates of recurrence, despite advancements in systemic treatment protocols, make survivals dismal2,3.
Over the past decades, numerous tumoral prognostic factors have been identified, with most of them relying on histopathological characteristics (e.g. vascular invasion, tumor margin, and nodal status), which are only available after surgical resection4,5. Consequently, significant efforts have been dedicated to exploring and validating preoperative criteria to stratify tumor aggressiveness and guide the decision process. In this context, several scores based on preoperative immune and nutritional laboratory values have been proposed6–8, yet their precise role in characterizing tumor behavior remains controversial9. Numerous reports on iCCA prognostic scores have been derived from studies concerning other solid tumors, such as lung10, breast11, colon-rectal12, and hepatocellular carcinoma13. Despite exhibiting some fluctuating association with survival data, no comprehensive explanation has been provided regarding how these systemic scores may be influenced by the tumoral mass itself and whether any association exists. While inflammation is closely linked to tumor onset and progression14, no conclusive evidence has demonstrated links between the inflammatory score and cancer-associated leukocyte populations.
This study aimed to investigate the role of systemic inflammatory indexes in iCCA and explore how tumor biology may lead to systemic leukocyte alterations, thereby affecting patient recurrence and prognosis. To achieve this, a single-center iCCA patient cohort was analyzed, focusing on the possible cause-effect relationship between the inflammation identified locally in the tumor and systemically, as reflected by the inflammatory indexes.
Methods
Study design and patients’ selection
Patients undergoing liver resection for iCCA in a tertiary referral center between February 2010 and February 2021 were retrospectively identified from a prospectively maintained database. iCCA was defined following the Japanese classification of biliary tract cancer15 and staged in conformity with the American Joint Committee against Cancer (AJCC) staging system, 8th edition5. Demographic, clinical, and laboratory data of the patients were analyzed as common baseline characteristics.
The study was approved by the local Independent Ethics Committee at the enrolling center (registration number 1341/14). Written informed consent was obtained from the patients. Data collection and analysis were conducted following the ethical guidelines of the Declaration of Helsinki16. The results are reported following the principles of Strengthening the Reporting of cohort, cross-sectional, and case–control studies in surgery (STROCSS, Supplemental Digital Content 1, http://links.lww.com/JS9/D47)17.
Study endpoint
The primary endpoint of the study was defining the role of inflammatory indexes in stratifying patients in terms of overall survival (OS) and disease-free survival (DFS). The secondary endpoint was deciphering the associations between immune cell infiltration in both tumoral and peritumoral liver tissue with the corresponding blood indexes.
Study eligibility criteria
The study included patients undergoing resection for iCCA. The inclusion criteria were: 1) confirmed histological diagnosis of iCCA, 2) age ≥18 years, and 3) a minimum follow-up of 12 months, unless an earlier death event occurred. The exclusion criteria encompassed: 1) any systemic inflammatory condition before the surgical resection, and 2) incomplete laboratory data within 1 month before surgery. Patient surveillance was closed at the end of June 2022.
Surgical technique and follow-up protocol
All patients were deemed resectable at diagnosis. Treatment decisions were made in a multidisciplinary setting. Each patient’s case was thoroughly evaluated, considering their underlying condition, oncologic and medical history, and local protocols. In our center, liver surgery follows a strict parenchyma-sparing surgical policy18.
After resection, patients were monitored with regular measurements of serum tumor markers (CA19-9 and CEA), abdominal ultrasound, and computed tomography scan or MRI, according to local outpatient visit protocols.
Inflammatory blood indexes
The inflammatory blood indexes analyzed in this study were the Neutrophil-to-Lymphocyte Ratio (NLR), Lymphocyte-to-Monocyte Ratio (LMR), and Platelet-to-Lymphocyte ratio (PLR), as previously reported in the literature9.
Sample collection, flow cytometry, and immunohistochemistry
The iCCA surgical samples (n=20) were prospectively collected between 2018 and 2020. Patients were included in the study based on the availability of sufficient material from surgical specimens of both healthy and tumoral tissues. This small cohort corresponded to 20% of the whole patients’ cohort and was thought to be representative at least for a preliminary study. The samples were digested as previously described19,20.
After the complete digestion of the healthy and tumor specimens, the immunophenotyping of circulating intrahepatic immune cells, intrahepatic immune cells, and tumor-infiltrating immune cells was performed using the 7-Color Immunophenotyping kit (Miltenyi Biotec) according to manufacturer protocol.
Flow cytometry data were analyzed using BD FACSCanto II (BD Biosciences), and the results were analyzed using FlowJo software (v10.8.2).
The schematic representation of sample digestion and the gating strategy used to analyze the immune cells are provided in SDC Fig. S1 (Supplemental Digital Content 2, http://links.lww.com/JS9/D48).
Immunohistochemistry (IHC) was performed on paraffin-embedded iCCA tissue from samples obtained from the Pathology Department, as already described19,20. The tissue sections were incubated with the following primary antibody: antihuman CD4 (1:300, clone UMAB64; Origene) and antihuman CD8 (1:500, clone C8/144B; Dako).
Statistical analysis
Normal distribution was assessed using the Kolmogorov–Smirnov test. Continuous variables were reported as median and interquartile range (IQR), while categorical variables were presented as frequency and percentage. The cut-off values for NLR, LMR, and PLR were determined using the bioinformatic tool X-Tile Software (Copyright: Yale University, Version 3.6.1). The choice of this software was based on its proven validity through the examination of several other established prognostic markers21. Baseline characteristics between the groups were compared using Mann–Whitney and χ 2 or Fisher tests, as appropriate. The OS was measured as the difference in months between the date of surgery and the date of the death or last follow-up available. The DFS was measured as the difference in months between the date of treatment and the date of the disease recurrence. In case of no recurrence, or death, data were censored at the date of the last available follow-up visit. The analyses were made by the Kaplan–Meier method and comparisons among the two groups were assessed using the log-rank test. The adjusted Cox proportional hazard model was utilized to identify independent prognostic factors for survival, and the results were reported as hazard ratio (HR) with the corresponding 95% CI. Univariate and multivariate linear regression analyses were conducted to examine the association between systemic score, tumoral histopathological data, and leukocyte infiltrating cells. All the variables resulting in association with the dependent variable with a P-value <0.10 at the univariate analysis were inserted in the multivariate models. All statistical tests were two-tailed and a 5% significance level was adopted. All the analyses were computed by using the GraphPad Prism software (v. 9.5.1) and IBM-SPSS statistics software (v. 24.0).
Results
Patients
Between February 2010 and February 2021, a total of 101 patients met the selection criteria and were enrolled in the study. The median age at the time of surgery was 70.6 years (IQR 62.9–75.3). Of these patients, 49 (48.5%) were male. Fifty-three (52.5%) patients were affected by T2-T4 iCCA and 29 (28.7%) cases were N0. Notably, a total of 42 (41.6%) patients underwent complete lymph node dissection. A negative resection margin (R0) was achieved in 66 (65.3%) patients. The median tumor size was 5.7 cm (IQR 3.9–8.7), and 28 (27.7%) cases showed multinodular disease. Additionally, 42 (41.6%) patients exhibited an elevated level of CA19-9. The baseline clinical and pathological characteristics of the population are summarized in SDC Table S1 (Supplemental Digital Content 2, http://links.lww.com/JS9/D48). After a median follow-up time of 44.6 (95% CI: 25.9–63.2) months the cohort had a median OS of 52.6 (95% CI: 31.5–73.7) months with a 1-year, 3-year, and 5-year OS rate of 82.2, 52.4, and 41.7%, respectively (SDC Fig. S2A, Supplemental Digital Content 2, http://links.lww.com/JS9/D48). The median DFS was 12.6 (95% CI: 10.0–15.2) with a 1-year and 3-year DFS rate of 54.6 and 21.9% (SDC Fig. S2B, Supplemental Digital Content 2, http://links.lww.com/JS9/D48).
Inflammatory score assessment and prognostic significance
According to the preoperative laboratory exam conducted within a month before the surgical procedure, the population was classified based on the aforementioned blood inflammatory scores, namely: NLR, LMR, and PLR. The optimal cut-off values were determined using the graphical method of X-Tile Software, depending on OS, as shown in SDC Figure S3 (Supplemental Digital Content 2, http://links.lww.com/JS9/D48). The resulting cut-offs were as follows: 3.83 for NLR, 2.28 for LMR, and 285.00 for PLR. Details regarding the distribution of patients according to their respective scores are provided in SDC Table S2 (Supplemental Digital Content 2, http://links.lww.com/JS9/D48). Univariate comparisons between groups in terms of OS and DFS were run. The NLR was found to be significantly associated with OS: patients with a preoperative NLR score <3.83 had a favorable median OS of 54.4 (95% CI: 26.2–82.6) months compared to 26.0 (95% CI: 12.9–39.1) months for others (P=0.036) (SDC Fig. S4A, Supplemental Digital Content 2, http://links.lww.com/JS9/D48). Similarly, an LMR score of ≥2.28 resulted in a better median OS of 54.4 (95% CI: 21.3–87.4) months compared to 20.7 (95% CI: 7.2–34.1) months for the other group (P=0.035) (SDC Fig. S4B, Supplemental Digital Content 2, http://links.lww.com/JS9/D48). However, no significant association was found between NLR and LMR scores with DFS (SDC Fig. S4C,D, Supplemental Digital Content 2, http://links.lww.com/JS9/D48), and there was no association observed by dividing the population based on the PLR score cut-off (data not shown). Subsequently, a comprehensive score considering both NLR and LMR cut-offs was calculated. The high-risk score (HRS) group was considered comprehensive of patients presenting with at least one among the prognostic unfavorable score values (NLR≥3.83 or LMR <2.28) with 34 (33.7%) patients belonging to this group (SDC Table S2, Supplemental Digital Content 2, http://links.lww.com/JS9/D48). The HRS and low-risk score (LRS) groups were then compared based on characteristics known to better represent tumor aggressiveness (Table 1). The HRS patients showed an increased number of tumor sizes >5 cm (P=0.001), as well as higher tumor stages (P=0.011). Conversely, patients in the LRS group presented with a higher proportion of well-differentiated (G stage) tumors (P=0.001).
Table 1.
Tumor aggressiveness characteristics and population’s score.
| Histopathological data and tumor markers | Low-risk score n 67 (%a) | High-risk score n 34 (%a) | P |
|---|---|---|---|
| T stage | 0.011 | ||
| T1a-b | 37 (55.2) | 9 (26.5) | |
| T2-4 | 30 (44.8) | 23 (67.5) | |
| G stage | 0.001 | ||
| G1-2 | 54 (80.6) | 16 (47.1) | |
| G3 | 13 (19.4) | 17 (50) | |
| N stageb | 0.481 | ||
| N0 | 21 (50.0) | 8 (19.1) | |
| N1 | 8 (19.1) | 5 (11.9) | |
| Microvascular invasion | |||
| No | 45 (67.2) | 19 (55.9) | 0.448 |
| Yes | 22 (32.8) | 13 (38.2) | |
| Tumor nodule >1 | 0.687 | ||
| No | 49 (73.1) | 24 (70.6) | |
| Yes | 18 (26.9) | 10 (29.4) | |
| Tumor size >5 cm | 0.001 | ||
| No | 39 (58.2) | 8 (23.5) | |
| Yes | 28 (41.8) | 26 (76.5) | |
| Increased CA19-9 | 1 | ||
| No | 36 (53.7) | 18 (52.9) | |
| Yes | 28 (41.8) | 14 (41.2) | |
| Increased CEA | 0.344 | ||
| No | 54 (80.6) | 29 (85.3) | |
| Yes | 8 (11.9) | 2 (5.9) | |
Percentages refer to the total amount of patients considering missing values.
N stage is based on 42 patients undergone to lymphadenectomy.
CA 19.9, carbohydrate antigen 19.9; CEA, carcinoembryonic antigen; G, grade; N, lymph nodal; n, number; T, tumor.
Prognostic value of high-risk profile and survival analyses
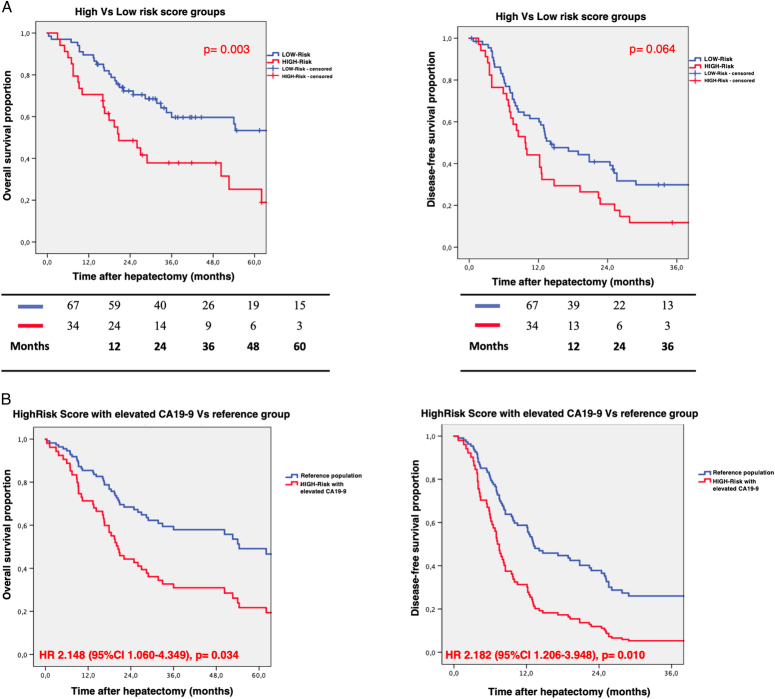
The HRS population exhibited OS rates of 70.6, 37.8, and 18.9% at 1-year, 3-year, and 5-year, respectively, while the LRS group had higher OS rates of 88.1, 59.6, and 49.8% at the same intervals (P=0.003). In terms of recurrence, the 1-year and 3-year DFS were 38.2 and 11.8% for the HRS group and 60.0 and 27.7% for the LRS group, although the difference did not reach statistical significance (P=0.064). The survival curves are depicted in Figure 1A. Following the univariate analysis, several factors were identified as associated with OS, including T stage, tumor size, risk score (referring to HRS vs. LRS), and CA19-9. The multivariate analysis recognized an increased CA19-9 (HR: 1.969, 95% CI: 1.072–3.618, P=0.029) and the HRS (HR: 2.138, 95% CI: 1.153–3.967, P=0.016) as statistically significant factors associated with OS. The results of Cox univariate and multivariate regression analyses for OS are reported in Table 2. When the referred outcome was DFS, only multinodular disease was confirmed to be a relevant prognostic factor in terms of recurrence in the multivariate analysis (SDC Table S2, Supplemental Digital Content 2, http://links.lww.com/JS9/D48). As depicted in Figure 1B, a combination of the HRS population with an abnormal CA19-9 level identified a specific subgroup at increased risk, not only for OS (HR: 2.148, 95% CI: 1.060–4.349, P=0.034) but also in terms of recurrence (HR: 2.182, 95% CI: 1.206–3.948, P=0.010).
Figure 1.
Survival of the analyzed cohort according to the risk score. A) Overall survival and disease-free survival comparisons between the high-risk and the low-risk score populations. B) Overall survival and disease-free survival comparisons between high-risk and CA19-9pos versus reference group.
Table 2.
Univariate and multivariate Cox-regression analysis for overall survival (OS).
| Variable | Univariate (OS) | Multivariate (OS) | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age >70 years | 1.219 (0.717–2.073) | 0.463 | ||
| Sex (male) | 0.907 (0.536–1.534) | 0.716 | ||
| G stage | ||||
| G1 | Ref | 0.916 | ||
| G2 | 1.053 (0.406–2.732) | 0.143 | ||
| G3 | 2.104 (0.778–5.690) | |||
| T stage | ||||
| T1a | Ref | 0.178 | Ref | 0.970 |
| T1b | 1.794 (0.767–4.194) | 0.011 | 1.022 (0.332–3.149) | 0.332 |
| T2 | 2.575 (1.244–5.329) | 0.009 | 1.646 (0.602–4.505) | 0.527 |
| T3 | 3.019 (1.325–6.880) | 0.031 | 1.451 (0.459–4.591) | 0.446 |
| T4 | 5.367 (1.165–24.720) | 2.019 (0.332–12.293) | ||
| N stagea | ||||
| N0 | Ref | 0.181 | ||
| N1 | 0.563 (0.242–1.307) | |||
| MVI | 1.403 (0.814–2.416) | 0.223 | ||
| Surgical margins (R1) | 1.243 (0.708–2.184) | 0.449 | ||
| n nodule (>1) | 1.445 (0.814–2.565) | 0.209 | ||
| Size (>5 cm) | 2.085 (1.204–3.610) | 0.009 | 1.266 (0.576–2.782) | 0.558 |
| RiskScore (High) | 2.055 (1.197–3.530) | 0.009 | 2.138 (1.153–3.967) | 0.016 |
| Increased CEA | 1.702 (0.755–3.837) | 0.200 | ||
| Increased CA19-9 | 2.191 (1.279–3.753) | 0.004 | 1.969 (1.072–3.618) | 0.029 |
| Liver substrateb | 0.691 (0.407–1.172) | 0.171 | ||
| Viral infectionc (yes) | 1.057 (0.546–2.048) | 0.869 | ||
N stage is based on patients undergone to lymphadenectomy.
Liver substrate is intended for patients having normal liver background versus those with hepatopathy (from steatosis to cirrhosis).
Viral infections are considered both HBV/HCV.
CA 19.9, carbohydrate antigen 19.9; CEA, carcinoembryonic antigen; G, grade; HR, Hazard Ratio; MVI, microvascular invasion; N, lymph nodal; n, number; R1, microscopic residual tumor; T, tumor.
The inflammatory score reflects the tumor microenvironment in iCCA patients
To evaluate the relationship between blood inflammatory indexes and tumor characteristics, an in-depth analysis was conducted on the immune infiltrate derived from circulating intrahepatic blood, intrahepatic, and intratumoral tissue, isolated from 20 patients’ surgical specimens (SDC Fig. S1, Supplemental Digital Content 2, http://links.lww.com/JS9/D48). In this subpopulation, seven (35%) patients belong to the HRS group. Using the infiltrating lymphocytes, monocytes, and neutrophils from FACS analysis, we computed ‘tissue immune-infiltrating’ scores for NLR and LMR in the circulating, intrahepatic, and intratumoral samples. Univariate and multivariate linear regression analyses were employed to explore the potential association between the systemic inflammatory score and the infiltrating immune cell populations.
In multivariate analysis, aside from the tumor size >5 cm, the infiltrating immune cells demonstrated a significant association with both the systemic NLR and LMR (Table 3a-b). Specifically, an increase in the systemic NLR was positively associated with the tumor NLR, with a beta coefficient of 0.424 (B=0.568 95% CI: 0.075–1.061, P=0.026). Conversely, the increase in systemic LMR showed a positive correlation with tumor lymphocyte infiltration, with a beta coefficient of 1.118 (B=0.090, 95% CI: 0.039–0.141, P=0.002), other than tumor neutrophils with a beta coefficient of 0.872 (B=0.077, 95% CI: 0.017–0.138, P=0.016). In both cases, the presence of a tumor size >5 cm was associated with the NLR (P=0.015) and the LMR (P=0.001), with a positive or negative correlation depending on the respective prognostic significance of the two ratios.
Table 3.
Univariate and multivariate regression analyses for a) neutrophil-to-lymphocyte ratio (NLR) and b) lymphocyte-to-monocyte ratio (LMR) with healthy, peri-tumoral, and tumoral immune infiltrate
| Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | B | Beta | 95% CI | P | B | Beta | 95% CI | P |
| a. Univariate and multivariate regression analyses for neutrophil-to-lymphocyte ratio (NLR) with healthy, peri-tumoral, and tumoral immune infiltrate. | ||||||||
| Tumor lymphocytes | −0.034 | −0.533 | −0.060–−0.007 | 0.016 a | ||||
| Tumor neutrophils | 0.035 | 0.507 | 0.006–0.065 | 0.023 a | ||||
| Tumor monocytes | 0.079 | 0.267 | −0.065–0.221 | 0.255 | ||||
| Healthy tissue lymphocytes | 0.007 | 0.106 | −0.024–0.037 | 0.657 | ||||
| Healthy tissue neutrophils | 0.003 | 0.052 | −0.025–0.031 | 0.826 | ||||
| Healthy tissue monocytes | −0.021 | −0.065 | −0.180–0.138 | 0.785 | ||||
| Circulating lymphocytes | −0.004 | −0.094 | −0.028–0.020 | 0.711 | ||||
| Circulating neutrophils | 0.011 | 0.222 | −0.015–0.038 | 0.376 | ||||
| Circulating monocytes | −0.043 | −0.186 | −0.162–0.077 | 0.461 | ||||
| G stage | 0.428 | 0.233 | −0.457–1.312 | 0.323 | ||||
| T stage | 0.217 | 0.176 | −0.383–0.818 | 0.457 | ||||
| N stageb | 0.044 | 0.019 | −2.023–2.111 | 0.961 | ||||
| MVI | 0.331 | 0.157 | −0.703–1.364 | 0.510 | ||||
| Tumor nodule >1 | −0.800 | −0.349 | −1.864–0.264 | 0.132 | ||||
| Size >5 cm | 1.398 | 0.634 | 0.554–2.241 | 0.003 | 1.045 | 0.474 | 0.234–1.855 | 0.015 |
| Liver substrate | −0.762 | −0.355 | −1.755–0.231 | 0.124 | ||||
| Increased CEA | −0.763 | −0.301 | −2.147–0.620 | 0.275 | ||||
| Increased CA19-9 | −0.454 | −0.209 | −1.603–0.695 | 0.414 | ||||
| NLRscore (tumor) | 0.808 | 0.603 | 0.279–1.336 | 0.005 | 0.568 | 0.424 | 0.075–1.061 | 0.026 |
| NLRscore (Healthy tissue) | −0.060 | −0.131 | −0.287–0.166 | 0.582 | ||||
| NLRscore circulating | 0.027 | 0.068 | −0.183–0.237 | 0.790 | ||||
| b. Univariate and Multivariate regression analyses for lymphocyte-to-monocyte ratio (LMR) with healthy, peri-tumoral, and tumoral immune infiltrate. | ||||||||
| Tumor lymphocytes | 0.045 | 0.566 | 0.013–0.078 | 0.009 | 0.090 | 1.118 | 0.039–0.141 | 0.002 |
| Tumor neutrophils | −0.039 | −0.439 | −0.078–0.001 | 0.053 | 0.077 | 0.872 | 0.017–0.138 | 0.016 |
| Tumor monocytes | −0.022 | −0.058 | −0.208–0.165 | 0.808 | ||||
| Healthy tissue lymphocytes | −0.004 | −0.055 | −0.043–0.034 | 0.817 | ||||
| Healthy tissue neutrophils | 0.001 | 0.012 | −0.034–0.036 | 0.960 | ||||
| Healthy tissue monocytes | 0.046 | 0.113 | −0.155–0.247 | 0.636 | ||||
| Circulating lymphocytes | 0.004 | 0.071 | −0.025–0.033 | 0.780 | ||||
| Circulating neutrophils | −0.008 | −0.130 | −0.041–0-025 | 0.608 | ||||
| Circulating monocytes | −0.013 | −0.045 | −0.160–0.135 | 0.860 | ||||
| G stage | −0.256 | −0.110 | −1.402–0.890 | 0.644 | ||||
| T stage | −0.195 | −0.125 | −0.962–0.573 | 0.601 | ||||
| N stagec | 0.949 | 0.303 | −1.719–3.617 | 0.428 | ||||
| MVI | 0.384 | 0.144 | −0.928–1.697 | 0.546 | ||||
| Tumor nodule >1 | 0.604 | 0.208 | −0.804–2.013 | 0.379 | ||||
| Size >5 cm | −1.750 | −0.626 | −2.828–−0.671 | 0.003 | −1.961 | −0.702 | −2.930–−0.991 | 0.001 |
| Liver substrate | −0.026 | −0.010 | −1.373–1.321 | 0.968 | ||||
| Increased CEA | 1.371 | 0.396 | −0.454–3.195 | 0.129 | ||||
| Increased CA19-9 | 0.028 | 0.010 | −1.457–1.514 | 0.968 | ||||
| LMRscore (tumor) | 0.017 | 0.224 | −0.020–0.054 | 0.342 | ||||
| LMRscore (Healthy tissue) | −0.018 | −0.162 | −0.073–0.037 | 0.496 | ||||
| LMRscore circulating | −0.006 | −0.201 | −0.023–0.010 | 0.424 | ||||
Data not considered for multivariate linear regression analysis since multicollinearity with NLRscore (tumor).
Calculated on patients undergone lymphadenectomy.
Calculated on patients undergone lymphadenectomy.
CA 19.9, carbohydrate antigen 19.9; CEA, carcinoembryonic antigen; G, grade; MVI, microvascular invasion; N, lymph nodal; T, tumor.
To gain deeper insights into the prognostic significance of the identified systemic scores concerning the immune cell composition within the tumor microenvironment (TME), we evaluated the percentages of CD4+ and CD8+ T cells in high-risk (n=5) and low-risk (n=5) groups, as determined by systemic scores. As shown in Figure 2, low-risk patients demonstrated a comparable percentage of CD4+ and CD8+ T cells within the tumor niche (51.6 vs. 48.7%, P=0.998). On the contrary, high-risk patients displayed a significantly higher prevalence of CD4+ T cells, compared to the CD8+ T cells (78.5 vs. 21.5%, P<0.0001). Moreover, low-risk patients exhibited a noteworthy infiltration of CD8+ cells in comparison to the high-risk group (21.5 vs. 48.7%, P=0.037), suggesting a potential association between the distinct infiltration patterns of CD4+ and CD8+ cells and the risk stratification observed in iCCA patients based on the identified inflammatory scores.
Figure 2.
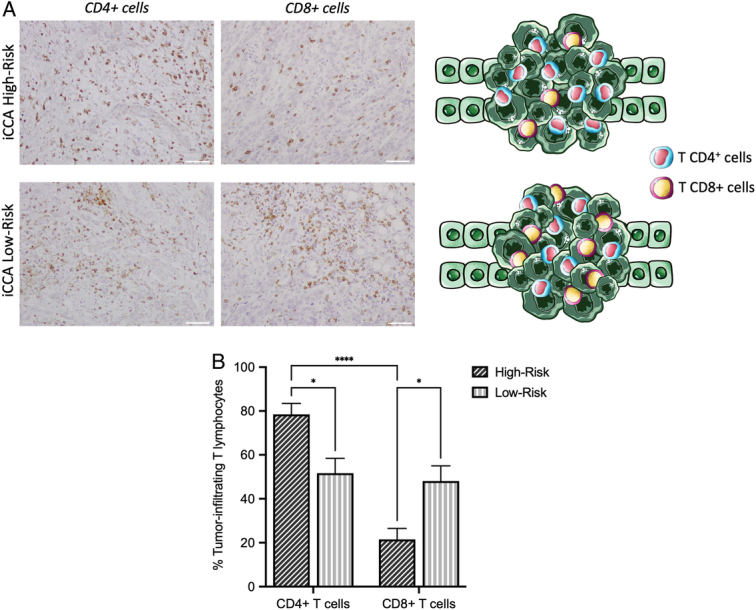
T cell subpopulation analysis of iCCA high-risk and low-risk patients. A) Immunohistochemistry (IHC) analysis and graphical representation of CD4+ and CD8+ cells in iCCA high-risk and low-risk patients. Scale bar 50 μm. B) Percentage of infiltrating T cell subpopulations in iCCA high-risk (n=5) and low-risk (n=5) patients. Two-way ANOVA (mean±SEM). *P<0.05; ****P<0.0001.
Discussion
iCCA is an aggressive tumor associated with short-term and long-term survival even after surgical resection3,22. Efforts have been made to stratify the iCCA prognosis by using biomarkers2,23. Traditionally, CEA and CA 19-9 have been correlated with clinical outcomes with disparate results24. Recently, attention has been concentrated on the role of inflammation, the immune system, and the TME in the development and progression of cancer25,26. Indeed, inflammatory and immune biomarkers are emerging to predict the clinical outcome of cancer patients.
This study demonstrates the efficacy of a novel score in predicting the prognosis of patients undergoing resection for iCCA. It stratified the OS and, combined with CA19-9, identified patients at higher risk of recurrence. The prediction was based on preoperative information, clinically available to every patient awaiting surgery for iCCA. This practicability represents an added value to this work. Besides, for the first time, the significance of this preoperative laboratory score was correlated with the immune contexture of the corresponding TME, as measured with FACS and IHC in the surgical specimens of the same patients.
Prior research has extensively emphasized the prognostic value of blood biomarkers in various types of cancer10,13, and also in iCCA9. In particular, Cui et al.9 validated the role of preoperative scores but also revealed a significant association between higher NLR and poorer overall and recurrence-free survival. Moreover, further corroborating our results, despite some heterogeneity among the reported studies, recent meta-analysis findings underscore that low LMR is associated with poor overall and recurrence-free survival27. Over the years, other laboratory scores, such as the LabScore8, IFS7, and IS28, based on these simple ratios, have been reported as prognostic tools for iCCA, each exhibiting different performance characteristics.
Despite the recognition of the role of inflammation in iCCA prognosis, the biological explanation behind those scores has never been thoroughly investigated. The herein reported study represents the first attempt to comprehend the physio-pathological value of those prognostic scores highlighting that the blood inflammatory indexes closely reflect the iCCA TME, thus suggesting a rationale beyond their potential application for stratifying patients’ prognoses. These results further suggest that, in iCCA, the blood peripheral circulating immune cells could be influenced by the TME, explaining the prognostic significance of laboratory-derived scores.
As said, inflammation plays a crucial role in tumor growth, development, and cancer progression, with different cell populations exerting distinct roles in the systemic inflammatory response14,29. In detail, neutrophils have been shown to promote the survival and proliferation of cancer cells by releasing inflammatory mediators such as tumor necrosis factor, interleukin 1, interleukin 6, and vascular endothelial growth factor30,31. Conversely, tumor-infiltrating lymphocytes (TILs), particularly T CD8+, have a recognized protective role, in inducing cancer cell apoptosis through the release of cytotoxic factors32,33. As previously reported by our group and other studies, higher levels of CD8+ TILs have been associated with improved OS in iCCA patients34,35. Our analysis revealed a significant increase in the abundance of CD8+ T cells within the TME of iCCA patients with low-risk profiles compared to those in the high-risk group. These findings suggest that the balance between CD4+ and CD8+ T cell infiltration in the TME plays a crucial role in determining survival. Altogether these findings reflect the presence of very complex interactions among the different immune cells in the TME that might be responsible for the wide array of clinical presentations and responsiveness to treatments. Understanding these interactions in general, and on an individual patient, is the way to investigate and apply immunotherapy as a therapeutic option for a larger number of iCCA patients36.
In terms of advantages, it is important to acknowledge that the proposed score holds strengths in clinical practice, generalizability, and personalized medicine. First, it is easily obtainable in clinical as it is cost-effective, derived from a standard blood examination that is affordable, accessible, and already performed on all patients at diagnosis or just before surgery. Second, it could significantly impact patient management by facilitating a truly personalized approach. This would enable a more aggressive postoperative treatment and/or dedicated follow-up protocol, particularly for patients at a higher risk of recurrence. Third, unraveling the immune landscape within the TME presents tremendous translational opportunities. This includes gaining insights into the pathophysiological mechanisms influencing the controversial responsiveness to immunotherapy and uncovering novel treatment strategies37. In this context, there is a significant advantage in having the surgical specimen available for translational research. Utilizing cutting-edge omics technologies, comprehensive translational research should be conducted to understand the relevant implications and identify potential predictive biomarkers that may help select which patients may truly benefit from immunotherapy.
Despite these encouraging future perspectives, our study has some limitations. This is a single-center, retrospective study with a relatively small sample size, which might have attenuated the association between prognostic factors and survival outcomes with potential biases from unmeasured confounders. The long-time frame of the study may also underestimate the impact of recent treatment advancements, such as the newest adjuvant protocols, and recent surgical implementations, as routine lymphadenectomy. Yet, the analyses of infiltrating immune cells were performed on a subpopulation of iCCA patients. Lastly, the study included all iCCAs without stratifying the disease based on the liver pathological substrate (e.g. metabolic and viral hepatitis) or pathological division of iCCA categories (e.g. large vs. small duct), which warrants further investigation focusing on specific pathological subtypes. Importantly, our findings require to be confirmed on an external larger patients’ cohort. Nevertheless, the prognostic score developed in the context of this study allows for a prospective prognostic assignment of iCCA patients.
Conclusion
In conclusion, this study represents substantial progress in predicting iCCA prognosis by introducing a novel predictive risk score able to determine patients’ sub-cohort with different clinical outcomes. The findings from this study offer a valuable understanding of how laboratory-derived scores are influenced by the TME, filling the gap between the clinical features and TME composition, and suggesting potential implications for enhancing personalized medicine approaches in managing iCCA patients.
Ethical approval
The study protocol was in accordance with the ethical guidelines established in the 1975 Declaration of Helsinki and compliant to the procedures of the local ethical committee of IRCCS Humanitas Research Hospital (Milan, Italy – registration number 1341/14).
Consent
Written informed consent was obtained from the patients.
Source of funding
This work was supported by ‘PRIN: Progetti Di Ricerca Di Rilevante Interesse Nazionale (ID=2022SZT583). The funding agency had no role in the design of the study or collection and analysis of data.
Author contribution
F.M., M.A.P., M.D., and G.T.: conceptualization; F.M., M.A.P., C.S., B.F., L.D.T., L.M.T., and A.L.: data curation; F.M., M.A.P., and S.F.: formal analysis; A.L., M.D., and G.T.: funding acquisition; F.M., M.A.P., C.S., B.F., and S.F.: investigation; F.M., M.A.P., M.D., and G.T.: methodology; M.D. and G.T.: project administration; M.A.P., C.S., B.F., and L.D.T.: resources; F.M., M.A.P., and S.F.: software; A.L., M.D., and G.T.: supervision; F.M. and M.A.P.: validation and visualization; F.M., M.A.P., and M.D.: writing – original draft; M.D. and G.T.: writing – review and editing. Then all authors read and approved the manuscript.
Conflicts of interest disclosure
The authors declare that they have no financial conflict of interest with regard to the content of this report.
Research registration unique identifying number (UIN)
Not applicable (retrospective observational study).
Guarantor
All the authors serve as guarantor.
Data availability statement
The data that support the findings of this study are available on request from the corresponding authors, [MD, GT]. The data are not publicly available.
Provenance and peer review
Not commissioned.
Supplementary Material
Acknowledgements
Assistance with the study: none.
Footnotes
Flavio Milana and Michela A. Polidoro contributed equally to this work.
Matteo Donadon and Guido Torzilli jointly supervised this work.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal's website, www.lww.com/international-journal-of-surgery.
Published online 5 July 2024
Contributor Information
Flavio Milana, Email: milana.flavio@gmail.com.
Michela A. Polidoro, Email: polid.michela@gmail.com.
Cristiana Soldani, Email: cristiana.soldani@humanitas.it.
Barbara Franceschini, Email: barbara.franceschini@humanitas.it.
Simone Famularo, Email: simone.famularo@gmail.com.
Luca Di Tommaso, Email: luca.di_tommaso@hunimed.eu.
Luigi M. Terracciano, Email: luigi.terracciano@hunimed.eu.
Ana Lleo, Email: ana.lleo@humanitas.it.
Matteo Donadon, Email: matteo.donadon@uniupo.it.
Guido Torzilli, Email: guido.torzilli@hunimed.eu.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7–30. [DOI] [PubMed] [Google Scholar]
- 2. European Association for the Study of the Liver . Electronic address: easloffice@easloffice.eu, European Association for the Study of the Liver. EASL-ILCA Clinical Practice Guidelines on the management of intrahepatic cholangiocarcinoma. J Hepatol 2023;79:181–208. [DOI] [PubMed] [Google Scholar]
- 3. Moris D, Palta M, Kim C, et al. Advances in the treatment of intrahepatic cholangiocarcinoma: An overview of the current and future therapeutic landscape for clinicians. CA Cancer J Clin 2023;73:198–222. [DOI] [PubMed] [Google Scholar]
- 4. Izquierdo-Sanchez L, Lamarca A, La Casta A, et al. Cholangiocarcinoma landscape in Europe: Diagnostic, prognostic and therapeutic insights from the ENSCCA Registry. J Hepatol 2022;76:1109–1121. [DOI] [PubMed] [Google Scholar]
- 5. Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin 2017;67:93–99. [DOI] [PubMed] [Google Scholar]
- 6. Choi WJ, Perez FM, Gravely A, et al. Preoperative neutrophil-to-lymphocyte ratio is prognostic for early recurrence after curative intrahepatic cholangiocarcinoma resection. Ann Hepatobiliary Pancreat Surg 2023;27:158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fu J, Chen Q, Lai Z, et al. A novel preoperative inflammation score system established for postoperative prognosis predicting of intrahepatic cholangiocarcinoma. BMC Cancer 2023;23:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tsilimigras DI, Mehta R, Aldrighetti L, et al. Development and validation of a laboratory risk score (LabScore) to predict outcomes after resection for intrahepatic cholangiocarcinoma. J Am Coll Surg 2020;230:381. [DOI] [PubMed] [Google Scholar]
- 9. Cui H, Li Y, Li S, et al. Prognostic utility of preoperative inflammatory markers in patients with intrahepatic cholangiocarcinoma after hepatic resection: a systematic review and meta-analysis. Cancer Med 2023;12:99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Winther-Larsen A, Aggerholm-Pedersen N, Sandfeld-Paulsen B. Inflammation scores as prognostic biomarkers in small cell lung cancer: a systematic review and meta-analysis. Syst Rev 2021;10:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dirican A, Kucukzeybek BB, Alacacioglu A, et al. Do the derived neutrophil to lymphocyte ratio and the neutrophil to lymphocyte ratio predict prognosis in breast cancer? Int J Clin Oncol 2015;20:70–81. [DOI] [PubMed] [Google Scholar]
- 12. Kwon HC, Kim SH, Oh SY, et al. Clinical significance of preoperative neutrophil-lymphocyte versus platelet-lymphocyte ratio in patients with operable colorectal cancer. Biomarkers 2012;17:216–222. [DOI] [PubMed] [Google Scholar]
- 13. Xiao WK, Chen D, Li SQ, et al. Prognostic significance of neutrophil-lymphocyte ratio in hepatocellular carcinoma: a meta-analysis. BMC Cancer 2014;14:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity 2019;51:27–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Miyazaki M, Ohtsuka M, Miyakawa S, et al. Classification of biliary tract cancers established by the Japanese Society of Hepato-Biliary-Pancreatic Surgery: 3(rd) English edition. J Hepatobiliary Pancreat Sci 2015;22:181–196. [DOI] [PubMed] [Google Scholar]
- 16. World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191–2194. [DOI] [PubMed] [Google Scholar]
- 17. Mathew G, Agha R, for the STROCSS Group . STROCSS 2021: strengthening the Reporting of cohort, cross-sectional and case-control studies in Surgery. Int J Surg 2021;96:106165. [DOI] [PubMed] [Google Scholar]
- 18. Torzilli G, Viganò L, Gatti A, et al. Twelve-year experience of “radical but conservative” liver surgery for colorectal metastases: impact on surgical practice and oncologic efficacy. HPB 2017;19:775–784. [DOI] [PubMed] [Google Scholar]
- 19. Polidoro MA, Ferrari E, Soldani C, et al. Cholangiocarcinoma-on-a-chip: a human 3D platform for personalised medicine. JHEP Rep 2024;6:100910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vitali E, Franceschini B, Milana F, et al. Filamin A is involved in human intrahepatic cholangiocarcinoma aggressiveness and progression. Liver Int 2024;44:518–531. [DOI] [PubMed] [Google Scholar]
- 21. Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res 2004;10:7252–7259. [DOI] [PubMed] [Google Scholar]
- 22. Torzilli G, Viganò L, Fontana A, et al. Oncological outcome of R1 vascular margin for mass-forming cholangiocarcinoma. A single center observational cohort analysis. HPB 2020;22:570–577. [DOI] [PubMed] [Google Scholar]
- 23. Macias RIR, Kornek M, Rodrigues PM, et al. Diagnostic and prognostic biomarkers in cholangiocarcinoma. Liver Int 2019;39(Suppl 1):108–122. [DOI] [PubMed] [Google Scholar]
- 24. Loosen SH, Roderburg C, Kauertz KL, et al. CEA but not CA19-9 is an independent prognostic factor in patients undergoing resection of cholangiocarcinoma. Sci Rep 2017;7:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fabris L, Sato K, Alpini G, et al. The tumor microenvironment in cholangiocarcinoma progression. Hepatology 2021;73(Suppl 1(Suppl 1)):75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Roy S, Glaser S, Chakraborty S. Inflammation and progression of cholangiocarcinoma: role of angiogenic and lymphangiogenic mechanisms. Front Med 2019;6:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dotto-Vasquez G, Villacorta-Ampuero AK, Ulloque-Badaracco JR, et al. Lymphocyte-to-monocyte ratio and clinical outcomes in cholangiocarcinoma: a systematic review and meta-analysis. Diagnostic (Basel) 2022;12:2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ohira M, Yoshizumi T, Yugawa K, et al. Association of inflammatory biomarkers with long-term outcomes after curative surgery for mass-forming intrahepatic cholangiocarcinoma. Surg Today 2020;50:379–388. [DOI] [PubMed] [Google Scholar]
- 29. Alvisi G, Termanini A, Soldani C, et al. Multimodal single-cell profiling of intrahepatic cholangiocarcinoma defines hyperactivated Tregs as a potential therapeutic target. J Hepatol 2022;77:1359–1372. [DOI] [PubMed] [Google Scholar]
- 30. Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 2011;331:1565–1570. [DOI] [PubMed] [Google Scholar]
- 31. Hofman PM. Pathobiology of the neutrophil-intestinal epithelial cell interaction: role in carcinogenesis. World J Gastroenterol 2010;16:5790–5800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zikos TA, Donnenberg AD, Landreneau RJ, et al. Lung T-cell subset composition at the time of surgical resection is a prognostic indicator in non-small cell lung cancer. Cancer Immunol Immunother 2011;60:819–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang M, Yang H, Wan L, et al. Single-cell transcriptomic architecture and intercellular crosstalk of human intrahepatic cholangiocarcinoma. J Hepatol 2020;73:1118–1130. [DOI] [PubMed] [Google Scholar]
- 34. Vigano L, Soldani C, Franceschini B, et al. Tumor-infiltrating lymphocytes and macrophages in intrahepatic cholangiocellular carcinoma. impact on prognosis after complete surgery. J Gastrointest Surg 2019;23:2216–2224. [DOI] [PubMed] [Google Scholar]
- 35. Chen L, Huang H, Huang Z, et al. Prognostic values of tissue-resident CD8T cells in human hepatocellular carcinoma and intrahepatic cholangiocarcinoma. World J Surg Oncol 2023;21:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Donadon M, Marchesi F, Rimassa L, et al. Immunotherapy in hepatobiliary tumors: search for the missing pieces of the puzzle. Hepatobiliary Surg Nutr 2020;9:86–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fiste O, Ntanasis-Stathopoulos I, Gavriatopoulou M, et al. The emerging role of immunotherapy in intrahepatic cholangiocarcinoma. Vaccines (Basel) 2021;9:422. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding authors, [MD, GT]. The data are not publicly available.