Abstract
Background:
R0 rates have increased as neoadjuvant treatment (NAT) has become the primary treatment for pancreatic ductal adenocarcinoma (PDAC) with venous involvement, suggesting a decrease in venous tumor infiltration. The aim of this study was to investigate the clinical outcomes of preserving the portal/superior mesenteric vein (PV/SMV) during pancreaticoduodenectomy (PD) in PDAC patients who underwent NAT.
Material and methods:
The 113 patients with resectable and borderline resectable PDAC with venous involvement who responded to NAT and underwent curative PD between 2012 and 2022 were retrospectively reviewed.
Results:
Among the 113 patients, PV/SMV preservation (PVP) was performed in 68 patients (60.2%), and PV/SMV resection (PVR) was performed in 45 patients (39.8%). There was no significant difference in the R0 rate, 5-year overall survival (OS) and recurrence-free survival between the two groups. PV/SMV stenosis within 3 months after surgery was more common in the PVR group than in the PVP group (1.5% versus 22.2%; P<0.001), and 5-year PV/SMV stenosis-free survival was significantly higher in the PVP group than in the PVR group (76.5% versus 53.4%; P=0.014). Multivariate analysis showed that gemcitabine-based neoadjuvant chemotherapy was associated with poor OS. PVR, clinically relevant postoperative pancreatic fistula, and locoregional recurrence were independent risk factors for PV/SMV stenosis.
Conclusion:
The PVP group had similar oncologic outcomes and better vessel-functional outcomes than the PVR group. Therefore, if dissection is possible and there is a high likelihood of achieving R0 resection after NAT, routine PVR may be unnecessary in PDAC patients with venous involvement.
Keywords: neoadjuvant, pancreatic cancer, portal vein, stenosis, superior mesenteric vein, survival
Introduction
Highlights
There is a lack of evidence on the need to perform portal/superior mesenteric vein (PV/SMV) resection routinely in pancreatic ductal adenocarcinoma (PDAC) patients with venous involvement who responded to neoadjuvant treatment (NAT).
There is no significant differences in R0 rate, 5-year overall survival and recurrence-free survival between the PV/SMV preservation (PVP) group and the PV/SMV resection (PVR) group.
The PVP group showed significantly better 5-year PV/SMV stenosis-free survival than the PVR group.
We propose that if dissection is possible and there is a high likelihood of achieving R0 resection after NAT, routine PVR may be unnecessary in PDAC patients with venous involvement.
As R0 resection is associated with good prognosis in pancreatic cancer, the International Study Group of Pancreatic Surgery suggests that the partial resection of the portal/superior mesenteric vein (PV/SMV) should be performed to achieve curative resection in cases with suspected venous involvement1. Technological advancements improved our understanding of the correlation between preoperative images and vessel involvement in pancreatic cancer, expanding the indications for PV/SMV resection (PVR) during pancreaticoduodenectomy (PD)2–7. Nakao group classified the grades according to radiographic findings of PV/SMV involvement in pancreatic head cancer and showed that the actual tumor-vessel invasion rate was higher with more advanced grades of vascular contact3. However, the accuracy of preoperative images in predicting pathological PV/SMV invasion is not very high. For instance, histopathological findings confirmed tumor invasion of the PV/SMV in only 51–93% of patients3,8. Additionally, PVR is associated with an increased risk of adverse postoperative outcomes, including PV/SMV stenosis9–13.
Neoadjuvant treatment (NAT) using chemotherapy with or without additional radiation has become the standard treatment for pancreatic ductal adenocarcinoma (PDAC) with venous involvement. NAT improves overall survival (OS) and increases the likelihood of achieving R0 resection and having negative lymph nodes compared with upfront surgery14–17. Therefore, PV/SMV preservation (PVP) during PD can be considered in PDAC patients with venous involvement who responded to NAT and can detach the tumor from the vessel. Some hospitals routinely perform PVR using no-touch techniques, while other hospitals attempt to dissect the vessel to preserve it. However, there is a lack of evidence on the need to perform PVR routinely in PDAC patients with improvement in the tumor-vessel relationship after NAT based on preoperative images and intraoperative findings. The aim of this study was to investigate the oncologic and vessel-functional outcomes of PVP during PD in PDAC patients who underwent NAT.
Material and methods
Patient selection
This study retrospectively included 264 pancreatic head cancer patients who underwent surgery after NAT between January 2012 and December 2022 at a tertiary hospital in South Korea. Clinicopathological data and radiological images were collected prospectively from electronic medical records.
Based on computed tomography (CT) and magnetic resonance imaging images, 79 patients with metastatic unresectable and locally advanced pancreatic cancer (LAPC) and 28 patients with resectable pancreatic cancer (RPC) without PV/SMV invasion were excluded. Ten patients were excluded because of cancer aggravation after NAT, such as progressive disease in the Response Evaluation Criteria In Solid Tumors18 and aggravation of the tumor-vessel relationship. Six patients with PV/SMV encasing and long segment narrowing after NAT were excluded from the analysis because of strongly suspected vascular invasion, and all underwent PVR. Seven patients who underwent palliative surgery (open biopsy or bypass surgery) because of peritoneal seeding or liver metastasis, non-PDAC (n=6), death within 30 days of surgery (n=1), and loss to follow-up (n=2) and 12 patients who underwent resection for suspected main artery and adjacent organ invasion were also excluded.
Among 264 patients, we included 113 patients with resectable and borderline resectable PDAC with venous involvement who responded to NAT and underwent curative PD.
This study was conducted in compliance with the Declaration of Helsinki. This retrospective study has been reported in line with the strengthening the reporting of cohort, cross-sectional, and case–control studies in surgery (STROCSS, Supplemental Digital Content 1, http://links.lww.com/JS9/D469) criteria19.
Perioperative evaluation and surgical techniques
Tumor size and the degree of tumor-vessel contact before and after NAT were evaluated by CT or magnetic resonance imaging. Based on the extent of contact between the PV/SMV and the tumor, the resectability was classified into resectable (contact of ≤180° without contour irregularity) and borderline resectable (contact of >180° or ≤180° with contour irregularity) according to the National Comprehensive Cancer Network guideline20. Furthermore, the Nakao classification was reassessed, defining type A (normal), type B (unilateral narrowing of PV/SMV), and type C (bilateral narrowing of PV/SMV)3. For the evaluation of tumor-vessel contact, both axial and coronal images were carefully reviewed. All cases were reviewed in multidisciplinary treatment meetings, including radiologists.
PVR was performed as one of the final steps of PD, after transection of the pancreatic parenchyma and dissection of the pancreatic head plexus, at which stage the only attachment of the pancreatic head was to the PV/SMV. If the pancreatic mass attached to the PV/SMV was successfully dissected with the soft tissue layer preserved, PVP was performed without the need for additional examination. However, if the surrounding soft tissue was firm or venous tumor infiltration could not be completely ruled out even after dissection, confirmation was achieved via a frozen biopsy of the vessel groove. When PV/SMV invasion was strongly suspected and the tumor could not be detached from the vessel, the proximal and distal portions of the involved vein were clamped, and en-bloc tumor resection was performed, with both proximal and distal vessel margins checked by frozen biopsy. Reconstructive surgery included wedge resection, primary end-to-end anastomosis, and bovine patch interposition, depending on the vein involved and the extent of resection21.
The margin status was classified as R0 or R1, with R1 defined as the presence of microscopic residual tumor when the distance between the tumor and any of the surgical margins was 0 mm, based on the final pathological report.
During postoperative follow-up, serial CT scans were performed 4 days after surgery, every 3 months until 2 years, and thereafter every 4–5 months until 5 years after surgery to evaluate for recurrence and PV/SMV stenosis. PV/SMV stenosis was defined as a reduction of more than 50% in vessel diameter compared to the first postoperative CT scan, and clinically relevant stenosis was defined as the presence of clinical features such as bleeding or ascites, or stenoses that required interventions.
Neoadjuvant and adjuvant treatment
In our institution, most patients with RPC underwent upfront surgery. However, if the patient was reluctant to undergo surgery or had a high carbohydrate antigen 19-9 (CA 19-9) level, considered biologically borderline resectable pancreatic cancer (BRPC), or when the PV/SMV involvement was suspected, NAT was performed first. A multidisciplinary team decided whether to commence NAT, considering each patient’s age, general condition, laboratory findings, and the Korean national health insurance system.
The neoadjuvant chemotherapy (NAC) regimens were FOLFIRINOX or gemcitabine-based combination. FOLFIRINOX consisted of 85 mg/m2 oxaliplatin, followed by 400 mg/m2 leucovorin, administered as a 2 h intravenous infusion with an additional 90 min intravenous infusion 180 mg/m2 irinotecan after 30 min. This treatment was followed by 5-fluorouracil (FU) at a dose of 400 mg/m2 administered as an intravenous bolus, followed by a continuous infusion of 2400 mg/m2 for a 46 h period every 2 weeks. Gemcitabine was administered as a 30 min intravenous infusion once weekly for 3 of every 4 weeks at a dose of 1000 mg/m2. If there was a change in regimen during NAC, patients were considered to have been treated with the main treatment regimen.
Some patients received neoadjuvant radiotherapy. Concurrent chemoradiotherapy (CCRT) with intravenous gemcitabine or 5-FU, radiation at 44–58 Gy was delivered in 28 fractions. Recently, stereotactic body radiation therapy (SBRT), which consisted of 50 Gy in five fractions, was delivered after FOLFIRINOX.
Adjuvant treatment (AT) was recommended for all patients who underwent surgery. Postoperative adjuvant chemotherapy was basically applied for 6 months with the same regimen as the NAC protocol. However, some patients did not receive AT because of their performance status or recovery after surgery.
Statistical analysis
All statistical analyses were performed using SPSS version 27.0 (IBM Corp.) for Windows. Categorical variables were analyzed using the χ 2 test and Fisher’s exact test, and continuous variables were analyzed using Student’s t-test. OS, recurrence-free survival (RFS), and PV/SMV stenosis-free survival curves were constructed using the Kaplan–Meier method and compared using the log-rank test. Multivariate Cox proportional hazard analysis was used to find independent prognostic factors for survival and logistic regression analysis was used to identify independent risk factors for PV/SMV stenosis. Variables with P<0.15 in the univariate analysis were included in the multivariate model. P values of less than 0.05 were considered statistically significant.
Results
Patient characteristics
The mean age was 61.3±9.6 years, and 60 patients (53.1%) were male. FOLFIRINOX and gemcitabine-based NAT was administered to 94 (83.2%) and 19 (16.8%) patients, respectively. Seventy-one patients (62.8%) underwent preoperative radiotherapy of which 58 (81.7%) underwent SBRT and 13 (18.3%) underwent CCRT. One hundred-four patients (92%) underwent R0 resection, and 108 (95.6%) underwent adjuvant chemotherapy. PVP and PVR were performed in 68 (60.2%) and 45 patients (39.8%), respectively.
The baseline, surgical, and perioperative characteristics of our patients are shown in Table 1. The PVR group had a larger clinical tumor size and higher venous involvement (angle of tumor-vessel contact and according to the Nakao classification) after NAT. Other than that, there were no demographic differences, including resectability, tumor marker, NAT and AT ratio between two groups. The PVR group had a higher pathological T stage. However, there was no significant between-group difference in 30-day major morbidity, defined as Clavien–Dindo grade ≥III22, including clinically relevant postoperative pancreatic fistula (CR-POPF)23, R0, and recurrence rate. Nonetheless, the PVR group had a higher incidence of postoperative PV/SMV stenosis (12 of 68 [17.6%] vs 16 of 45 [35.6%], P=0.031) and clinically relevant stenosis (2 of 68 [2.9%] vs 7 of 45 [15.6%], P=0.015) than the PVP group.
Table 1.
Baseline, surgical, and perioperative characteristics of PVP group and PVR group.
| Preservation of PV/SMV (PVP), n=68 | Resection of PV/SMV (PVR), n=45 | P | |
|---|---|---|---|
| Age(years), mean±SD | 62.2±9.6 | 60.6±9.6 | 0.378 |
| Sex ratio (M:F) | 36:32 | 24:21 | 0.967 |
| Clinical tumor size (cm), mean±SD | |||
| Initial | 2.6±0.7 | 3.0±0.6 | 0.005 |
| After NAT | 1.9±0.5 | 2.2±0.7 | 0.024 |
| Resectability (R/BR), n | |||
| Initial | 26/42 | 15/30 | 0.596 |
| After NAT | 59/9 | 36/9 | 0.336 |
| Angle of TVC (°), mean±SD | |||
| Initial | 135.0±56.0 | 146.7±57.3 | 0.286 |
| After NAT | 69.4±50.2 | 96.1±30.0 | 0.002 |
| *Nakao classification (Type A/B/C), n | |||
| Initial | 41/18/9 | 21/13 /11 | 0.235 |
| After NAT | 62 / 6 / 0 | 32 / 13 / 0 | 0.005 |
| CA 19-9 (U/ml), median (IQR) | |||
| Initial | 242 (944) | 639 (1325.5) | 0.955 |
| After NAT | 31.8 (88) | 46.3 (124.6) | 0.857 |
| Neoadjuvant chemotherapy, n (%) | 0.771 | ||
| FOLFIRINOX | 56 (82.4) | 38 (84.4) | |
| Gemcitabine-based | 12 (17.6) | 7 (15.6) | |
| Neoadjuvant radiotherapy, n (%) | 43 (63.2) | 28 (62.2) | 0.913 |
| CCRT | 10 (23.3) | 3 (10.7) | 0.182 |
| SBRT | 33 (76.7) | 25 (89.3) | |
| Adjuvant chemotherapy, n (%) | 67 (98.5) | 41 (91.1) | 0.060 |
| Adjuvant radiotherapy, n (%) | 13 (19.1) | 6 (13.3) | 0.421 |
| Operation method, n (%) | 0.334 | ||
| PD/PPPD | 65 (95.6) | 41 (91.1) | |
| TP | 3 (4.4) | 4 (8.9) | |
| Operation time (mins), mean±SD | 270.0±74.6 | 318.5±70.3 | 0.001 |
| Estimated blood loss (ml), mean±SD | 586.3±591.0 | 725.3±642.9 | 0.246 |
| Postoperative stay (days), mean±SD | 11.8±5.0 | 12.7±7.6 | 0.438 |
| Major morbidity (CD ≥ III), n (%) | 14 (20.6) | 7 (15.6) | 0.501 |
| CR-POPF, n (%) | 5 (7.7) | 0 (0.0) | 0.069 |
| PV/SMV stenosis, n (%) | 12 (17.6) | 16 (35.6) | 0.031 |
| Locoregional recurrence related | 8 (66.7) | 5 (31.3) | 0.017 |
| Postoperative change related | 4 (33.3) | 11 (68.8) | |
| Clinical relevant PV/SMV stenosis, n (%) | 2 (2.9) | 7 (15.6) | 0.015 |
| Margin status, n (%) | 0.768 | ||
| R0 | 63 (92.6) | 41 (91.1) | |
| R1 | 5 (7.4) | 4 (8.9) | |
| Harvested lymph nodes (n), mean±SD | 21.2±9.4 | 24.0±10.4 | 0.126 |
| Positive lymph nodes (n), mean±SD | 0.6±1.0 | 0.8±1.6 | 0.464 |
| Pathological T stage, n (%) | 0.001 | ||
| ypT1 | 32 (47.1) | 10 (22.2) | |
| ypT2 | 36 (52.9) | 29 (64.4) | |
| ypT3 | 0 (0.0) | 6 (13.3) | |
| Pathological N stage, n (%) | 0.788 | ||
| ypN0 | 44 (64.7) | 28 (62.2) | |
| ypN+ | 24 (35.3) | 17 (37.8) | |
| Recurrence, n (%) | 38 (55.9) | 26 (57.8) | 0.842 |
| Locoregional | 7 (18.4) | 11 (42.3) | 0.177 |
| Systemic | 24 (63.2) | 13 (50.0) | |
| Both | 7 (18.4) | 2 (7.7) |
BR, borderline resectable; CA 19-9, carbohydrate antigen 19-9; CCRT, concurrent chemoradiotherapy; CD, Clavien–Dindo classification; CR-POPF, clinically relevant postoperative pancreatic fistula; NAT, neoadjuvant treatment; PD, pancreaticoduodenectomy; PPPD, pyrolus preserving pancreaticoduodenectomy; PV/SMV, portal/superior mesenteric vein; R, resectable; SBRT, stereotactic body radiation therapy; TP, total pancreatectomy; TVC, tumor-vessel contact.
Nakao classification: Type A: normal, Type B: unilateral narrowing of PV/SMV, Type C: bilateral narrowing of PV/SMV.
Long-term oncologic outcomes
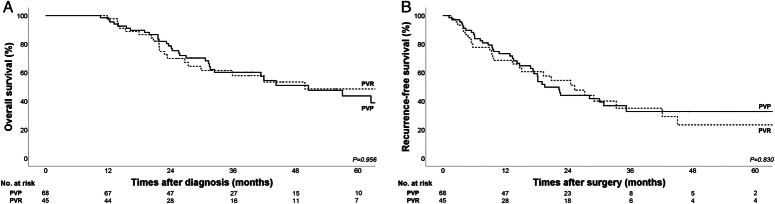
The median follow-up period was 29.9 months in the entire cohort. OS and RFS rates according to PVR are shown in Figure 1. The 2-year and 5-year OS rates were 78.7 and 43.7% in the PVP group and 69.9 and 48.6% in the PVR group. There were no significant between-group differences in OS (median, 50.5 vs 49.8 months; P=0.956) and RFS (median, 19.6 vs 25.3 months; P=0.830) (Fig. 1A, B).
Figure 1.
Kaplan -Meier curves of overall survival and recurrence-free survival in the PVP and PVR groups A. 5-year overall survival (OS) in the PVP and PVR groups (43.7% versus 48.6%; median, 50.5 vs 49.8 months; P = 0.956) B. 5-year postoperative recurrence-free survival (RFS) in the PVP and PVR groups (median, 19.6 vs 25.3 months; P = 0.830) PVP, PV/SMV preservation; PVR, PV/SMV resection; PV/SMV, portal/superior mesenteric vein.
Among the 45 patients with PVR, 24 (53.3%) had inflammation, resulting in ‘false negative’ resection (PVR pv0). There was no significant difference in perioperative outcomes (major morbidity, R0 rate, and recurrence pattern) between the PVP and PVR pv0 groups (Supplementary Table 1, Supplemental Digital Content 2, http://links.lww.com/JS9/D470). Furthermore, there was no significant difference in 5-year OS (43.7% versus 64.4%; median, 50.5 vs not applicable months; P=0.262) and RFS (median, 19.6 vs 33.3 months; P=0.417) between the PVP and PVR pv0 groups (Supplementary Fig 1, Supplemental Digital Content 3, http://links.lww.com/JS9/D471).
The multivariate analysis of the prognostic factors for OS is shown in Table 2. The gemcitabine-based NAC was significantly associated with poor OS [hazard ratio (HR) 2.03, 95% CI: 1.05–3.90; P=0.034].
Table 2.
Prognostic factors for overall survival.
| Univariable | Multivariable | |||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age (year) ≥70 | 1.45 (0.75–2.80) | 0.267 | ||
| Sex | ||||
| M | 1 | |||
| F | 0.86 (0.49–1.51) | 0.597 | ||
| Degree of tumor-vessel contact >90 | ||||
| Initial | 0.59 (0.28–1.22) | 0.154 | ||
| After NAT | 0.94 (0.53–1.67) | 0.836 | ||
| *Nakao classification (Initial) | ||||
| Type A | 1 | |||
| Type B | 1.14 (0.59–2.18) | 0.697 | ||
| Type C | 1.08 (0.50–2.31) | 0.847 | ||
| *Nakao classification (after NAT) | ||||
| Type A | 1 | |||
| Type B | 1.59 (0.81–3.12) | 0.176 | ||
| CA 19-9 >37 U/ml | ||||
| Initial | 1.10 (0.53–2.30) | 0.796 | ||
| After NAT | 1.13 (0.65–1.99) | 0.663 | ||
| Neoadjuvant chemotherapy | ||||
| FOLFIRINOX | 1 | |||
| Gemcitabine-based | 2.08 (1.09–4.01) | 0.028 | 2.03 (1.05–3.90) | 0.034 |
| Margin status | ||||
| R0 | 1 | |||
| R1 | 1.40 (0.56–3.54) | 0.474 | ||
| T stage | ||||
| ypT1 | 1 | |||
| ≥ ypT2 | 1.61 (0.87–2.99) | 0.133 | 1.51 (0.80–2.83) | 0.203 |
| N stage | ||||
| ypN0 | 1 | |||
| ypN+ | 1.50 (0.85–2.64) | 0.158 | ||
| Adjuvant chemotherapy | 0.49 (0.15–1.16) | 0.241 | ||
| Pathological vessel invasion | 1.64 (0.86–3.16) | 0.136 | 1.48 (0.76–2.88) | 0.246 |
CA 19-9, carbohydrate antigen 19-9; HR, hazard ratio.
Nakao classification: Type A: normal, Type B: unilateral narrowing of PV/SMV, Type C: bilateral narrowing of PV/SMV.
Causes and timing of PV/SMV stenosis
After a median CT follow-up of 21 months, 28 of 113 patients (24.8%) showed PV/SMV stenosis, with 9 of these 28 patients (32.1%) developing clinically relevant stenosis. The main causes of PV/SMV stenosis were locoregional recurrence (13 of 28, 46.4%) around the PV/SMV and postoperative changes (15 of 28, 53.6%), such as granulation tissue formation without recurrence. As it is difficult to distinguish postoperative granulation tissue from early local recurrence, serial CT images were analyzed with a shorter follow-up period to assess changes in soft tissue density around the PV/SMV in conjunction with other evidence of tumor recurrence. In the PVP group, 8 of 12 patients (66.7%) developed PV/SMV stenosis due to locoregional recurrence, while 4 of 12 (33.3%) developed it as a result of postoperative changes. In the PVR group, locoregional recurrence caused PV/SMV stenosis in 5 of 16 patients (31.3%), while 11 of 16 (84.6%) developed it as a result of postoperative changes. In the PVR group, the postoperative PV/SMV stenosis rate was more than twice as high as that in the PVP group (17.6% vs 35.6%), and clinically relevant stenosis was more than five times as high (2.9% vs 15.6%) (Table 1). The method of venous reconstruction was not associated with PV/SMV stenosis: 37.5% (3 of 8) for wedge resection versus 36.4% (12 of 33) for segmental resection and end-to-end anastomosis versus 25% (1 of 4) for bovine patch interposition (P=0.897).
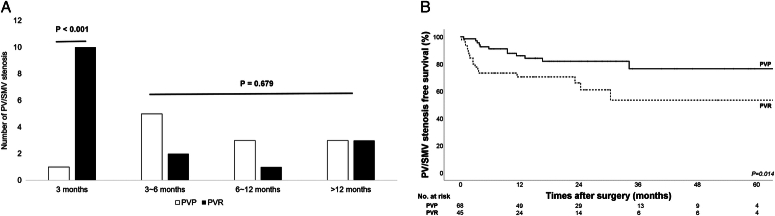
PV/SMV stenosis within 3 months after surgery was more common in the PVR group than in the PVP group (1 of 68 [1.5%] vs 10 of 45 [22.2%], P < 0.001). Most short-term stenoses (within 3 months) were caused by postoperative changes (9 of 11, 81.8%). The incidence rates of PV/SMV stenosis from 3 months to 1 year and after 1 year, in the PVP and PVR groups were 11.8% versus 6.7% and 4.4% versus 6.7%, respectively. After 3 months, there was no between-group difference in the incidence of stenosis and the leading causes of PV/SMV stenosis were locoregional recurrence (11 of 17, 64.7%) (Fig. 2A). Furthermore, 5-year PV/SMV stenosis-free survival was significantly higher in the PVP group than in the PVR group (76.5% versus 53.4%; P=0.014) (Fig. 2B).
Figure 2.
PV/SMV stenosis in the PVP and PVR groups. A. Time of PV/SMV stenosis occurrence in the PVP and PVR groups. B. 5-year postoperative PV/SMV stenosis-free survival in the PVP and PVR groups (76.5% versus 53.4%; P = 0.014) PV/SMV, portal/superior mesenteric vein; PVP, PV/SMV preservation; PVR, PV/SMV resection.
Multivariate analysis showed that PVR [odds ratio (OR) 3.17, 95% CI: 1.05–9.59; P=0.041], CR-POPF (OR 21.86, 95% CI: 1.47–324.35; P=0.025), and locoregional recurrence (OR 3.95, 95% CI: 1.30–11.97; P=0.015) were independent risk factors for PV/SMV stenosis (Table 3).
Table 3.
Risk factors for PV/SMV stenosis.
| Univariable | Multivariable | |||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Age (year) ≥70 | 0.75 (0.30–1.87) | 0.537 | ||
| Sex | ||||
| M | 1 | |||
| F | 0.66 (0.28–1.58) | 0.353 | ||
| Neoadjuvant radiotherapy | 1.34 (0.54–3.31) | 0.527 | ||
| Resection of PV/SMV | 2.58 (1.08–6.16) | 0.034 | 3.17 (1.05–9.59) | 0.041 |
| Method of venous reconstruction | ||||
| Wedge resection | 1 | |||
| End-to-end anastomosis | 0.91 (0.18–4.48) | 0.907 | ||
| Bovine patch interposition | 0.56 (0.04–8.09) | 0.667 | ||
| Margin status | ||||
| R0 | 1 | |||
| R1 | 1.58 (0.37–6.78) | 0.538 | ||
| Major morbidity (CD ≥III) | 2.88 (1.06–7.84) | 0.038 | 1.54 (0.34–6.90) | 0.575 |
| CR-POPF | 15.24 (1.62–143.62) | 0.017 | 21.86 (1.47–324.35) | 0.025 |
| Locoregional recurrence | 5.46 (2.12–14.09) | < 0.001 | 3.95 (1.30–11.97) | 0.015 |
| Adjuvant chemotherapy | 0.07 (0.01–0.67) | 0.021 | 0.1 (0.01–1.04) | 0.054 |
| Adjuvant radiotherapy | 1.51 (0.51–4.44) | 0.454 | ||
CD, Clavien–Dindo classification; CR-POPF, clinically relevant postoperative pancreatic fistula; OR, odds ratio; PV/SMV, portal/superior mesenteric vein.
Discussion
NAT is the current accepted standard treatment for patients with BRPC and LAPC. Even among cases of RPC with venous involvement, there are variations in tumor aggressiveness, which correlate with higher risks of recurrence and poorer prognosis24,25. While the effectiveness of NAT for patients with RPC is controversial, a recent retrospective study showed that NAT was associated with better R0 resection rate (86.3% vs 77.1%; P=0.004), lymph node negativity rate (57.6% vs 34.2%; P=0.002), and longer survival (median 33 vs 23 months; P=0.003) in patients with resectable PDAC with venous involvement26. R0 rates have increased as NAT has become the primary treatment for PDAC with venous involvement, suggesting a decrease in venous tumor infiltration. It is challenging to distinguish true venous tumor infiltration from inflammation or fibrosis after NAT using preoperative images, which may not accurately show resectability27–29. Nonetheless, there is controversy regarding whether the PV/SMV should be resected during PD in PDAC patients who responded to NAT. While oncologic outcome after surgery is very important, postoperative vessel-functional outcome is also clinically important because PV/SMV stenosis can cause PV hypertension, potentially leading to severe complications.
NAT is associated with downstaging of the tumor. In this study, the mean clinical tumor size (cm) decreased from 2.7 to 2.0 and angle of tumor-vessel contact (°) decreased from 126 to 87 after NAT. Fourty-one patients with RPC and 72 patients with BRPC were reevaluated after NAT, with 95 patients as RPC and 18 as BRPC. Furthermore, the ratio of Nakao classification (type A: type B: type C) changed from 62: 31: 20 to 94: 19: 0 after NAT.
We found no significant differences in R0 rates and 5-year OS and RFS between the PVP and PVR groups. As the pathological invasion of PV/SMV is a poor prognostic factor for survival2,3,30–32, we performed additional subgroup analysis to compare oncologic outcomes between the PVP and PVR pv0 groups. There were no significant differences in R0 rates and 5-year OS and RFS between these two groups. Some studies have shown that PVR is feasible only when intraoperative findings suggest PV/SMV invasion, despite preoperative image findings suggesting PV/SMV involvement. Turrini et al.33 compared clinical outcomes between PVP and PVR pv0 groups and showed that OS was significantly higher in the PVR pv0 group than in the PVP group (median, 22 vs 42 months; P=0.040), suggesting that PV/SMV dissection from tumors increased the risk of noncurative resection. In contrast, Klein et al.34 showed that OS was significantly worse in the PVR pv0 group than in the PVP group (median, 558 vs 311 days; P=0.001), whereas Kishi et al. found no significant difference in RFS (median, 15.5 vs 14.7 months; P=0.557) and OS (median, 32.4 vs 32.1 months; P=0.780) between the PVP and PVR pv0 groups. These results suggest that extensive PVR is not always required and that PVR is needed only when the tumor cannot be detached from the vessel during surgery35. Given the discrepancy in the results, the potential benefit of routine PVR is debatable, and further evaluation is needed. However, in contrast to our study, these three studies evaluated patients who did not undergo NAT. As NAT provides good local control through early systemic treatment for undetected micrometastasis and increases R0 resection, a new approach is needed to determine whether to resect PV/SMV after NAT.
Our results showed that PVP was associated with better vessel-functional outcomes and PVR affects PV/SMV stenosis. Among patients with PV/SMV stenosis, the median time to stenosis caused by local recurrence, postoperative changes was 11.3 and 2.5 months, respectively (P=0.012). Postoperative changes were the primary cause of PV/SMV stenosis in the PVR group (11 of 16, 68.8%), and many patients developed stenosis within 3 months after surgery (10 of 16, 62.5%). Eight of ten patients with short-term stenosis (within 3 months) developed it as a result of postoperative changes. Furthermore, the higher incidence of clinically relevant stenosis in the PVR group (2 of 12 [16.7%] vs 7 of 16 [43.8%]) seemed to be associated with postoperative changes resulting from vascular manipulations during surgery. Kang et al. also showed that 28 out of 55 patients (51%) who underwent PVR developed PV stenosis and 5-year PV patency rates were significantly lower than in PVP group (72.7% vs 17%; P<0.001). Furthermore, among the ten patients who developed PV stenosis within the first month after surgery, nine patients underwent PVR and PVR was an independent risk factor for PV stenosis (OR 3.28, 95% CI: 1.80–6.00; P<0.001) regardless of the vascular reconstruction method10. Therefore, most technical problems seem to occur in the early stage after surgery, and PVR should be carefully considered as it affects vessel-functional outcomes and can induce clinically relevant stenosis, leading to PV hypertension.
In the PVP group, locoregional recurrence was the primary cause of PV/SMV stenosis (8 of 12, 66.7%). Considering that R status after PD can affect overall recurrence rates36,37, the PV/SMV stenosis ratio may differ depending on the R status. Among the PVP group, only one patient had an R1 resection in the PV/SMV stenosis group, and there was no demographic difference in R1 resection (4 of 56 [7.1%] vs 1 of 12 [8.3%], P=0.886). In multivariate analysis, R1 resection was not an independent risk factor for PV/SMV stenosis. Furthermore, there was no demographic difference in R1 resection between the PVP and PVR groups (5 of 68 [7.4%] vs 4 of 45 [8.9%], P=0.768), and the same result was seen within the PV/SMV stenosis group (1 of 12 [8.3%] vs 2 of 16 [12.5%], P=0.724). Therefore, there seemed to be no association between R status and PV/SMV stenosis.
Surgical technique with aggressive local dissection is critical for achieving R0 and complete tumor remnant resection, which can affect locoregional recurrence, but it has limitations. A review of prospective randomized trials concerning the value of extended surgery concludes that circumferential dissection of the SMA is no longer recommended considering its morbidity and oncological necessity38. In the resectable stage, however, extended dissection of perineural tissues, so called as triangular resection, may offer advantages in achieving R0 resection and reducing local recurrence. In cases of BRPC and LAPC, besides surgical extent, NAT including radiotherapy is also very important for local control. Recent reviews of SBRT have shown that it is an effective modality for patients with pancreatic cancer39,40. Future efforts will be needed to expand NAT using radiotherapy, which could potentially improve outcomes for pancreatic cancer patients.
The rate of pathological PV/SMV invasion (21 of 45, 46.7%) in the resected vein was lower than previously reported (51–93%). In contrast to previous studies, our study revealed no significant difference in 5-year OS (43.7% versus 30.9%; median, 50.5 vs 29.9 months; P=0.247) and RFS (median, 19.6 vs 15.1 months; P=0.210) when comparing the PVP and PVR pv(+) groups. These results suggested that NAT may have the effect of reducing venous tumor infiltration and extensive PVR does not compensate for the aggressive biology of PDAC, especially in the era of NAT.
In the PV/SMV invasion group, preoperative images tended to be more severe (Supplementary Table 2, Supplemental Digital Content 2, http://links.lww.com/JS9/D470) and often displayed asymmetric vessel contours or significant long segment narrowing of the vessels, compared to the noninvasion group, even after NAT. This information can be useful in surgical planning, including decisions on whether to attempt minimally invasive pancreatic surgery (MIPS) or to perform vessel resection. MIPS has recently been considered an important part of current pancreatic surgery practice and there is an increasing effort to implement MIPS for BRPC patients. Even though, there is insufficient evidence to define a venous resection and anastomosis technique during MIPS41, it might be considered for patients who have responded to NAT, except in cases of extensive vessel invasion by experienced surgeons in high-volume centers. Although preoperative images can be useful in surgical planning, they may not accurately show the extent of venous tumor infiltration. Therefore, the final decision on whether to perform PVR was made based on the surgical findings; however, there is a need to better justify the subjectivity in intraoperative decision-making. As artificial intelligence is increasingly being used in medical practice and has demonstrated growing applicability, we can expect it to improve preoperative planning and operative execution in the future42–44.
This study has several limitations. First, the experimental design was retrospective, single-center study, and selection bias could not be avoided. Second, there is still a lack of evidence regarding the effectiveness of NAT in RPC patients, and high-level evidence is still needed. Third, the absence of objective preoperative measures following NAT makes intraoperative decisions regarding PVR challenging and often subjective, varying depending on the individual surgeon. Lastly, the PVP group might have included patients with pathological venous infiltration because true venous infiltration is confirmed only following PVR. However, the R1 rate, the sites of resection margin positivity, and postoperative prognosis were similar between the two groups. Five of 68 patients (7.4%) in the PVP group had an R1 resection, and only one patient (1.5%) had margin positivity in the PV/SMV groove.
In conclusion, the PVP group had similar oncologic outcomes and better vessel-functional outcomes than the PVR group. Therefore, if dissection is possible and there is a high likelihood of achieving R0 resection after NAT, routine PVR may be unnecessary in PDAC patients with venous involvement.
Ethical approval
This study was exempted from deliberation by the Institutional Review Board (IRB) of Seoul National University Hospital (IRB no. 2402-147-1516).
Consent
Informed consent for enrolled patients was waived by the Institutional Review Board of Seoul National University Hospital.
Source of funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2022R1A2C2011122) and a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (No. HI23C1591).
Author contribution
Y.S.C.: conceptualization, formal analysis, writing – original draft, and writing – review and editing; H.-S.J.: methodology and validation; W.-G.Y. and Y.H.: investigation; Y.J.C. and M.L.: data collection; W.K. and J.S.P.: validation; J.-Y.J.: supervision and writing – review and editing.
Conflicts of interest disclosure
The authors declare no conflicts of interest.
Research registration unique identifying number (UIN)
Clinicaltrials.gov : NCT06372886.
Guarantor
Yoon Soo Chae and Jin-Young Jang.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy and ethical restrictions.
Provenance and peer review
Not applicable.
Assistance with the study
Not applicable.
Presentation
This study was presented at the HBP surgery week 2024 and the 60th Annual Congress of the Korean Association of HBP Surgery.
Supplementary Material
Acknowledgements
Not applicable.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this articles.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal's website, www.lww.com/international-journal-of-surgery.
Published online 23 September 2024
Contributor Information
Yoon Soo Chae, Email: codbstnsla@gmail.com.
Hye-Sol Jung, Email: hyesoljung@gmail.com.
Won-Gun Yun, Email: wkeonyun@gmail.com.
Youngmin Han, Email: vickijoa@gmail.com.
Young Jae Cho, Email: whdudwo85@naver.com.
Mirang Lee, Email: effly5026@gmail.com.
Wooil Kwon, Email: willdoc78@gmail.com.
Joon Seong Park, Email: jspark330@gmail.com.
Jin-Young Jang, Email: jangjy4@snu.ac.kr.
References
- 1. Bockhorn M, Uzunoglu FG, Adham M, et al. Borderline resectable pancreatic cancer: a consensus statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2014;155:977–988. [DOI] [PubMed] [Google Scholar]
- 2. Ishikawa O, Ohigashi H, Imaoka S, et al. Preoperative indications for extended pancreatectomy for locally advanced pancreas cancer involving the portal vein. Ann Surg 1992;215:231–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nakao A, Kanzaki A, Fujii T, et al. Correlation between radiographic classification and pathological grade of portal vein wall invasion in pancreatic head cancer. Ann Surg 2012;255:103–108. [DOI] [PubMed] [Google Scholar]
- 4. Fuhrman GM, Leach SD, Staley CA, et al. Rationale for en bloc vein resection in the treatment of pancreatic adenocarcinoma adherent to the superior mesenteric-portal vein confluence. Pancreatic Tumor Study Group Ann Surg 1996;223:154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ramacciato G, Mercantini P, Petrucciani N, et al. Does portal-superior mesenteric vein invasion still indicate irresectability for pancreatic carcinoma? Ann Surg Oncol 2009;16:817–825. [DOI] [PubMed] [Google Scholar]
- 6. Zhou Y, Zhang Z, Liu Y, et al. Pancreatectomy combined with superior mesenteric vein-portal vein resection for pancreatic cancer: a meta-analysis. World J Surg 2012;36:884–891. [DOI] [PubMed] [Google Scholar]
- 7. Yu XZ, Li J, Fu DL, et al. Benefit from synchronous portal-superior mesenteric vein resection during pancreaticoduodenectomy for cancer: a meta-analysis. Eur J Surg Oncol 2014;40:371–378. [DOI] [PubMed] [Google Scholar]
- 8. Buchs NC, Chilcott M, Poletti PA, et al. Vascular invasion in pancreatic cancer: imaging modalities, preoperative diagnosis and surgical management. World J Gastroenterol 2010;16:818–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Worni M, Castleberry AW, Clary BM, et al. Concomitant vascular reconstruction during pancreatectomy for malignant disease: a propensity score-adjusted, population-based trend analysis involving 10,206 patients. JAMA Surg 2013;148:331–338. [DOI] [PubMed] [Google Scholar]
- 10. Kang MJ, Jang JY, Chang YR, et al. Portal vein patency after pancreatoduodenoectomy for periampullary cancer. Br J Surg 2015;102:77–84. [DOI] [PubMed] [Google Scholar]
- 11. Groen JV, Michiels N, van Roessel S, et al. Venous wedge and segment resection during pancreatoduodenectomy for pancreatic cancer: impact on short- and long-term outcomes in a nationwide cohort analysis. Br J Surg 2021;109:96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Giovinazzo F, Turri G, Katz MH, et al. Meta-analysis of benefits of portal-superior mesenteric vein resection in pancreatic resection for ductal adenocarcinoma. Br J Surg 2016;103:179–191. [DOI] [PubMed] [Google Scholar]
- 13. Kleive D, Sahakyan MA, Berstad AE, et al. Trends in indications, complications and outcomes for venous resection during pancreatoduodenectomy. Br J Surg 2017;104:1558–1567. [DOI] [PubMed] [Google Scholar]
- 14. Versteijne E, van Dam JL, Suker M, et al. Neoadjuvant chemoradiotherapy versus upfront surgery for resectable and borderline resectable pancreatic cancer: long-term results of the Dutch Randomized PREOPANC Trial. J Clin Oncol 2022;40:1220–1230. [DOI] [PubMed] [Google Scholar]
- 15. Jang JY, Han Y, Lee H, et al. Oncological benefits of neoadjuvant chemoradiation with gemcitabine versus upfront surgery in patients with borderline resectable pancreatic cancer: a prospective, randomized, open-label, multicenter phase 2/3 trial. Ann Surg 2018;268:215–222. [DOI] [PubMed] [Google Scholar]
- 16. Jung HS, Kim HS, Kang JS, et al. Oncologic benefits of neoadjuvant treatment versus upfront surgery in borderline resectable pancreatic cancer: a systematic review and meta-analysis. Cancers (Basel) 2022;14:4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cloyd JM, Heh V, Pawlik TM, et al. Neoadjuvant therapy for resectable and borderline resectable pancreatic cancer: a meta-analysis of randomized controlled trials. J Clin Med 2020;9:1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 19. Mathew G, Agha R, Albrecht J, et al. STROCSS 2021: strengthening the reporting of cohort, cross-sectional and case-control studies in surgery. Int J Surg 2021;96:106165. [DOI] [PubMed] [Google Scholar]
- 20. Tempero MA, Malafa MP, Al-Hawary M, et al. Pancreatic adenocarcinoma, version 2.2021, NCCN Clinical Practice Guidelines in oncology. J Natl Compr Canc Netw 2021;19:439–457. [DOI] [PubMed] [Google Scholar]
- 21. Kim SM, Min SK, Park D, et al. Reconstruction of portal vein and superior mesenteric vein after extensive resection for pancreatic cancer. J Korean Surg Soc 2013;84:346–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009;250:187–196. [DOI] [PubMed] [Google Scholar]
- 23. Bassi C, Marchegiani G, Dervenis C, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery 2017;161:584–591. [DOI] [PubMed] [Google Scholar]
- 24. Kim HS, Lee M, Han Y, et al. Role of neoadjuvant treatment in resectable pancreatic cancer according to vessel invasion and increase of CA19-9 levels. J Hepatobiliary Pancreat Sci 2023;30:924–934. [DOI] [PubMed] [Google Scholar]
- 25. Kang MJ, Jang JY, Kwon W, et al. Clinical significance of defining borderline resectable pancreatic cancer. Pancreatology 2018;18:139–145. [DOI] [PubMed] [Google Scholar]
- 26. Jung HS, Han Y, Yun WG, et al. Examining neoadjuvant treatment candidates in resectable pancreatic cancer based on tumor-vessel interactions and CA 19-0 levels: a retrospective cohort study. Int J Surg 2024;110:2883–2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang Y, Huang ZX, Song B. Role of imaging in evaluating the response after neoadjuvant treatment for pancreatic ductal adenocarcinoma. World J Gastroenterol 2021;27:3037–3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cassinotto C, Sa-Cunha A, Trillaud H. Radiological evaluation of response to neoadjuvant treatment in pancreatic cancer. Diagn Interv Imaging 2016;97:1225–1232. [DOI] [PubMed] [Google Scholar]
- 29. Katz MH, Fleming JB, Bhosale P, et al. Response of borderline resectable pancreatic cancer to neoadjuvant therapy is not reflected by radiographic indicators. Cancer 2012;118:5749–5756. [DOI] [PubMed] [Google Scholar]
- 30. Wang J, Estrella JS, Peng L, et al. Histologic tumor involvement of superior mesenteric vein/portal vein predicts poor prognosis in patients with stage II pancreatic adenocarcinoma treated with neoadjuvant chemoradiation. Cancer 2012;118:3801–3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ramacciato G, Nigri G, Petrucciani N, et al. Pancreatectomy with mesenteric and portal vein resection for borderline resectable pancreatic cancer: multicenter study of 406 patients. Ann Surg Oncol 2016;23:2028–2037. [DOI] [PubMed] [Google Scholar]
- 32. Mierke F, Hempel S, Distler M, et al. Impact of portal vein involvement from pancreatic cancer on metastatic pattern after surgical resection. Ann Surg Oncol 2016;23(suppl 5):730–736. [DOI] [PubMed] [Google Scholar]
- 33. Turrini O, Ewald J, Barbier L, et al. Should the portal vein be routinely resected during pancreaticoduodenectomy for adenocarcinoma? Ann Surg 2013;257:726–730. [DOI] [PubMed] [Google Scholar]
- 34. Klein F, Berresheim F, Felsenstein M, et al. Routine portal vein resection for pancreatic adenocarcinoma shows no benefit in overall survival. Eur J Surg Oncol 2018;44:1094–1099. [DOI] [PubMed] [Google Scholar]
- 35. Kishi Y, Nara S, Esaki M, et al. Feasibility of resecting the portal vein only when necessary during pancreatoduodenectomy for pancreatic cancer. BJS Open 2019;3:327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ghaneh P, Kleeff J, Halloran CM, et al. The impact of positive resection margins on survival and recurrence following resection and adjuvant chemotherapy for pancreatic ductal adenocarcinoma. Ann Surg 2019;269:520–529. [DOI] [PubMed] [Google Scholar]
- 37. Tummers WS, Groen JV, Sibinga Mulder BG, et al. Impact of resection margin status on recurrence and survival in pancreatic cancer surgery. Br J Surg 2019;106:1055–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kang MJ, Jang JY, Kim SW. Surgical resection of pancreatic head cancer: what is the optimal extent of surgery? Cancer Lett 2016;382:259–265. [DOI] [PubMed] [Google Scholar]
- 39. de la Pinta C. Stereotactic body radiotherapy in pancreatic adenocarcinoma. Hepatobiliary Pancreat Dis Int 2024;23:14–19. [DOI] [PubMed] [Google Scholar]
- 40. Burkoň P, Trna J, Slávik M, et al. Stereotactic body radiotherapy (SBRT) of pancreatic cancer-a critical review and practical consideration. Biomedicines 2022;10:2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Abu Hilal M, van Ramshorst TME, Boggi U, et al. The Brescia Internationally Validated European Guidelines on minimally invasive pancreatic surgery (EGUMIPS). Ann Surg 2024;279:45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stott M, Kausar A. Can 3D visualisation and navigation techniques improve pancreatic surgery? A systematic review. Art Int Surg 2023;3:207–216. [Google Scholar]
- 43. Shapey IM, Sultan M. Machine learning for prediction of postoperative complications after hepato-biliary and pancreatic surgery. Art Int Surg 2023;3:1–13. [Google Scholar]
- 44. De Robertis R, Todesco M, Autelitano D, et al. The role of radiomics in hepato-bilio-pancreatic surgery: a literature review. Art Int Surg 2023;3:166–179. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy and ethical restrictions.