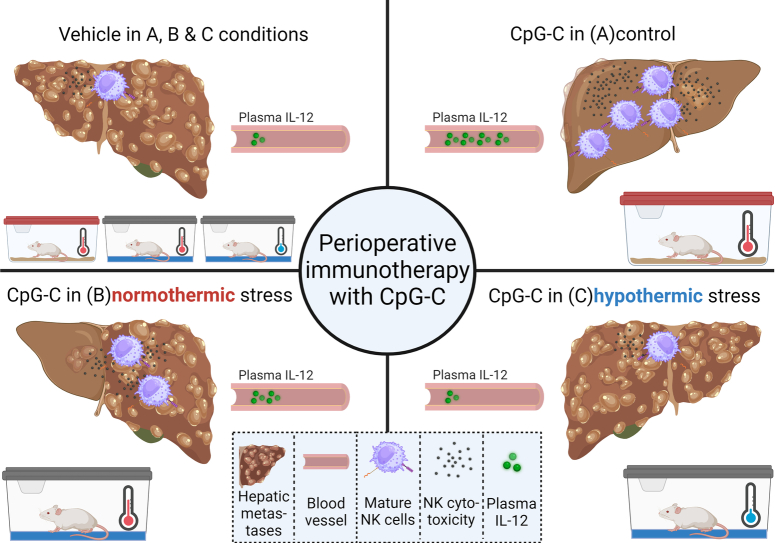
Abstract
Background:
The perioperative period often involves stress responses and surgery-induced hypothermia, which were suggested to hinder antimetastatic immunity and promote cancer metastasis. During this critical period, immunotherapies are rarely used, given contraindications to surgery. However, recent preclinical studies support the feasibility of perioperative TLR-9 activation using CpG-C.
Materials and methods:
Herein, we employed hypothermic-stress and normothermic-stress paradigms to assess their impact on perioperative CpG-C immune stimulation and resistance to experimental hepatic metastasis of CT26 colorectal cancer in BALB/c mice.
Results:
Perioperative hypothermic wet-cage stress markedly abrogated CpG-C-induced increase in plasma IL-12 levels, a persistent deleterious effect across different CpG-C doses and administration routes. These effects were not attenuated by blocking glucocorticoids, adrenergic, or opioid signaling, nor by adrenalectomy, suggesting a direct immunosuppressive impact of hypothermia on immunocytes. Indeed, normothermic wet-cage stress, which induced a similar corticosterone response, caused significantly less deleterious effects on IL-12 levels, hepatic NK cell maturation and cytotoxicity, and CT26 metastasis. Additionally, in-vitro exposure of PBMCs to 33°C markedly decreased CpG-C-induced IL-12 production. Last, two normothermic stress paradigms, tilt&light and restraint, did not jeopardize CpG-C-induced IL-12 response nor resistance to CT26 metastases. Interestingly, attenuating glucocorticoid signaling under tilt&light conditions improved CpG-C efficacy.
Conclusions:
Overall, these findings suggest that perioperative hypothermic stress can jeopardize antimetastatic immunity and resistance to metastasis, and prevent perioperative response to immune stimulation and its beneficial antimetastatic impacts, effects that are not mediated through classical neuroendocrine stress responses, but potentially through direct hypothermic impact on leukocytes. These findings may have clinical implications in operated cancer patients, many of whom suffer hypothermic stress.
Keywords: metastases, perioperative hypothermia, perioperative immunotherapy, stress, Toll-like receptor 9
Introduction
Highlights
Perioperative mild hypothermic stress jeopardizes antimetastatic immunity, immune activation, and resistance to cancer metastasis.
Direct hypothermic impact on immunocytes, rather than HPA, SNS, or opioid signaling, is sufficient to mediate the deleterious in-vivo impact of hypothermic stress conditions.
Perioperative immunotherapy, using the TLR-9 agonist CpG-C, is highly effective in preventing hepatic metastases, under no stress and normothermic stress conditions.
The immediate perioperative period (IPP), days before and after surgery, is known to profoundly influence long-term cancer outcomes through numerous physiological responses that (i) hinder antimetastatic immunity and/or (ii) directly affect the malignant tissue1. Accordingly, effective anticancer immunotherapy during this short timeframe was suggested as a prophylactic means to improve long-term cancer outcomes2,3. However, immunotherapies are rarely studied during the short perioperative period, given known and speculated contraindications to surgery, and may be rendered ineffective given the prevalent phenomenon of perioperative immune suppression2–5.
Despite limited preclinical research, few antimetastatic immunotherapies were shown to be effective when administered during the IPP, reducing metastasis and improving long-term cancer outcomes6–10. However, most of these studies did not adequately simulate the perioperative setting, or employed immunodeficient animal models, and thus their clinical relevance is limited11. Clinical studies on antimetastatic immunotherapies during the IPP are scarce2,3, and published clinical trials are typically small, low-phase, often single-arm, and commonly assess safety profiles and biomarkers rather than long-term cancer outcomes. Furthermore, these studies generally do not systematically address critical aspects of perioperative treatments, such as timing relative to surgery, duration of therapy, and drug combinations, which could mitigate adverse effects manifesting during the IPP. Nonetheless, these studies indicate tolerable safety profiles and promising biomarker results, with few randomized controlled trials assessing long-term cancer outcomes, but mostly underpowered for survival assessments. Specifically, administration of IL-2 during the IPP improved long-term cancer outcomes, including 5-year disease-free and overall survival, in pancreatic, renal cell, and colorectal cancer patients, with no serious adverse events12–14. Additionally, immune checkpoint inhibitors used during the IPP were shown to (i) demonstrate tolerable safety profiles15,16, (ii) increase plasma IL-12 and tumor IFN-gamma levels16, and (iii) promote early expansion of tumor-specific T cell subsets15. Overall, additional research is warranted to study the safety and efficacy of immunotherapy during the IPP to harness the immune system for improved long-term cancer outcomes.
In the last decade, Toll-like receptor (TLR) activation has shown potential as an antimetastatic immune-stimulating approach17. TLR-9 agonists are studied as monotherapy or in conjunction with other types of immune-, chemo- and/or radio- therapies against various cancer types18, and some have reached clinical testing18 and FDA approval19. The TLR-9 agonist CpG-oligodeoxynucleotide 2395 (CpG-C) was shown effective while exerting minimal adverse effects20, thus suggested as a potential perioperative immunotherapy2. Specifically, CpG-C was shown to elevate plasma IL-12 levels21, and when administered during the IPP to increase NK cytotoxicity, and significantly reduce metastasis in several rodent models6,9,22,23. IL-12 is a prominent activator of NK cells24, which are known to control the development of metastasis25, and high levels of NK activity are associated with long-term recurrence-free survival in patients harboring various cancers26,27.
Immunosuppression during and following the IPP is a well-documented and intricate phenomenon4,5, which may commence while awaiting surgery28,29. Cancer patients undergoing surgery are subjected to psychological stress and anxiety1,30,31, surgical procedures5, tissue damage1,5, anesthesia32,33, hypothermia34,35, blood loss and/or transfusion36, and the consequent postoperative resolution of inflammatory processes1–3,37. These experiences and physiological responses, which are predominantly characterized by excessive release of catecholamines (CAs) and prostaglandins (PGs), collectively contribute to immunosuppression2–5, and may also directly accelerate the transformation of minimal residual disease (MRD) into clinically manifested metastatic disease37,38. Overall, the IPP encompasses numerous processes that often orchestrate a strong and lasting perioperative immunosuppressive state in cancer patients2,4,39. Theoretically, such suppression may accelerate metastasis1,37. Importantly, immune-activating agents, such as CpG-C, may counteract these deleterious effects when administered during the IPP10, potentially improving long-term cancer outcomes2,3.
Hypothermia is a prevalent side effect of surgery and of anesthetic and analgesic agents, and is defined as a decline in core body temperatures below 36°C40. Hypothermia is categorized as mild (36–32°C), moderate (32–28°C), or severe (<28°C), and even mild hypothermic conditions were shown to have major deleterious effects on patient’s health and well-being40. Studies have shown that mild hypothermia: (i) increases the incidence of surgical site infection41; (ii) escalates intraoperative blood loss and postoperative ischemic myocardial events42,43; (iii) hinders CMI, including the suppression of lymphocyte activation and cytokine production34; and (iv) prolongs patients’ postoperative recovery44.
In our previous preclinical studies, we have shown that CpG-C can be safely and effectively used during the IPP6,9,22,23. Considering the aforementioned deleterious effects of hypothermia and stress, in this study we aimed to assess the efficacy of CpG-C immune stimulation under hypothermic-stress and normothermic-stress conditions, and to study the involvement of specific physiological mechanisms in modulating its efficacy under these conditions. Most cancer patients exhibit perioperative stress-inflammatory responses and/or suffer from perioperative hypothermia. Thus, understanding environmental and physiological factors that jeopardize or improve immunity and perioperative immunotherapy may advance the perioperative use of such approaches and their potential clinical benefits in cancer patients.
Materials and methods
Animals
C57BL/6J olaHSD and BALB/c olaHSD mice (Envigo Laboratories, Israel), 12–16 weeks old, housed four per cage with cotton ball enrichment, and free access to food and water, on a 12:12 light:dark cycle at 22–24°C. Drug administration and tumor inoculation were counterbalanced across groups in each experiment. Housing conditions are regularly monitored by the Institutional Animal Care and Use Committee, which approved all studies described herein (P-12-003, 31/12/2016; 10-18-005, 09/07/2018; 10-18-008, 06/12/2018).
This study has been reported in accordance with the ARRIVE guidelines (Animals in Research: Reporting In Vivo Experiments)45.
Drugs
CpG-C: CpG-C (Sigma, Israel), a TLR-9 agonist (ODN 2395: 50-TCGTCGTTTTCGGCGCGCGCCG-30), with a phosphorothioate backbone, was dissolved in PBS and administered intraperitoneally (i.p.) at a dose of 50/100 µg per mouse23.
RU486: RU486 (Sigma, Israel), progesterone and glucocorticoid receptor antagonist, was dissolved in corn oil and administered subcutaneously (s.c.) at a dose of 25 mg/kg.
Propranolol: Propranolol (Sigma, Israel), a non-selective β-adrenergic blocker, was dissolved in a slow-release vehicle (see below) and administered s.c. at a dose of 5 mg/kg.
Slow-release vehicle: an emulsion comprised of four parts PBS, three parts mineral oil (Sigma, Israel), and one part mannide monooleate (Sigma, Israel).
Naltrexone: Naltrexone (Sigma, Israel), a wide-range opioid receptor antagonist, was dissolved in PBS and administrated i.p. at a dose of 2 mg/kg46.
Tumor cell lines and their maintenance
CT26: murine colon carcinoma cell line chemically-induced undifferentiated carcinoma, syngeneic to the BALB/c strain47. Cells were grown in monolayer cultures in complete media at 37°C, 100% humidity, and 5% CO2. Cells were removed from the culture flask with a 0.25% trypsin solution in PBS, washed once in PBS containing 0.1 mg/ml BSA (335 g for 10 min) and adjusted to a final concentration of 1×105/ml in PBS-BSA for spleen injection at a volume of 100 µl per animal.
Stress paradigms
Hypothermic wet-cage: Mice were placed in cages filled with room-temperature water to the height of 1 cm, in a fully lighted room and with free access to food and water. This protocol was initiated 2 h after the onset of the dark period.
Normothermic wet-cage: Mice went through the same setup of wet-cage (see above); however, the cages were placed in a heated room (29–30°C) and were filled with heated water (36–37°C).
Tilt&light: Mice cages were placed in a 45° tilted position, in a fully lighted room maintained at 18°C, with free access to food and water, for 12 h. The stress protocol was initiated 3–5 h before the onset of the dark period.
Restraint: Mice were placed in a transparent cylinder with ventilation, with no access to food or water for 8 h. This stress protocol was initiated 3–5 h before the onset of the dark period.
Adrenalectomy and sham operation
This procedure was described elsewhere and adapted herein to mice (1 mg/kg corticosterone per injection to expedite recovery from surgery)48. Briefly, mice were anesthetized and both adrenal glands were removed using standard surgical techniques. Sham-operated mice underwent the exact same procedure without removal of the adrenal glands.
CT26 tumor cell intrasplenic inoculation
This procedure was described elsewhere9. Briefly, mice were anesthetized and 1×104 CT26 tumor cells in 100 µl PBS were injected into the spleen followed by a splenectomy, were the injection not successful, the animal was sacrificed according to ethical constraints.
Assessment of metastatic development: Animals were monitored daily for general well-being after tumor injection, and euthanized with an overdose of isoflurane on the 20th day for males and the 25th day for females. Livers were then harvested and weighed, and surface-hepatic metastases were counted by an experimenter blind to the experimental group of each animal. Metastases were identified as being bigger than 1 mm in diameter, forming a spherical solid and distinct formation.
Assessment of plasma corticosterone levels
Blood was drawn from the heart into heparinized test tubes. Plasma levels were measured employing ELISA (AssayPro, MO), per the manufacturer’s instructions.
Assessment of IL-12 p70 levels using ELISA
IL-12 p70 ELISA kit (eBioscience, Thermo Fischer Scientific, CA) was used to assess IL-12 levels from plasma and supernatants, based on the manufacturer’s instructions.
Blood draw and plasma collection
Mice were euthanized with isoflurane, and blood was drawn from the heart, within less than 3 min of approaching the animals, using EDTA-containing syringes (1.8 mg/1ml blood). Blood was then centrifuged for 20 min at 2000g, 4°C, for plasma separation, which was collected and stored at −20°C until assayed for IL-12 and/or CORT levels.
In-vitro CpG-C-induced production of IL-12
Half milliliter of pooled whole blood was washed once with PBS (4-fold dilution, 10 min at 456 g, followed by supernatant removal to restore the original volume) and twice with complete media to discard endogenous IL-12. A 500 μl washed blood aliquot was then added to a well containing 500 μl of complete media with CpG-C, reaching a final concentration of 5 μg CpG-C/ml. Samples were incubated at 100% humidity, 5% CO2, at 33°C or 37°C for 10 or 20 h. Supernatants were then harvested and stored at −20°C until assayed for IL-12 levels.
Harvesting of hepatic leukocytes
Mice were sacrificed with an overdose of isoflurane, and hepatic leukocytes were harvested by perfusing the liver with heparinized PBS (30 U/ml), described in detail elsewhere49.
Assessment of NK cytotoxicity
The standard 4-h 51Cr release assay was used without any cell enrichment procedures, described in detail elsewhere50.
Flow cytometry
Standard procedures were used to prepare cells for flow cytometry analysis51. NK cells were identified as being PE-conjugated anti-mouse NKp46 (clone 195314, R&D), FITC-conjugated CD49b (clone DX5, Peprotech), PC5.5-conjugated CD27 (clone LG3A10, BioLegend), and APC-Alexa Fluor 750-conjugated CD11b (clone M1/70, BioLegend).
Statistical analysis
Prism (version 8.4.2) was used for statistical analysis, and G*power (version 3.1.9.7) software was used for sample size analysis when needed52. Levene’s F-test was used to test homogeneity of variance, and when met, two-way or three-way ANOVA was used, followed by paired Tukey post-hocs to account for multiple comparisons. For data not meeting homogeneity of variance, Welch’s corrected unpaired t-test for unequal variances was employed to perform N−1 (N=number of experimental groups) independent comparisons. Repeated measures three-way ANOVA was performed, employing the Greenhouse–Geisser adjustment of sphericity, given interconnectedness between dependent variables. Sample size calculations were based on size effects and SD from published and preliminary experiments to ensure 80% power. For instance, the sample sizes for (i) NK cytotoxicity assay (Fig. 4) and (ii) CT26 metastases numbers (Figs. 3 and 6) were calculated based on effect sizes and variance evident in Sorski et al.9. Specifically, (i) NK cells’ cytotoxicity levels and numbers demonstrated an omega-squared of 0.394 ( ), and an effect size of 0.806 ( ), and the required sample size was calculated using G*power to be 4 per group (4–6 per group was chosen herein), (ii) CT26 metastases numbers demonstrated a weighted pooled SD of 43.6 ( ), and ‘Hedges’ g’ effect size of 2.223 ( ), and the required sample size was calculated using G*power to be 3–4 per group. However, we employed 10–11 per group to enable the recognition of much smaller effect sizes in the context of stress. Omega-squared, pooled SD, and Hedges’ g were chosen as they are considered less biased estimators for effect size, especially when the ‘n’ of the groups are unequal. All statistical tests were two-tailed, and P values smaller than 5% were considered significant.
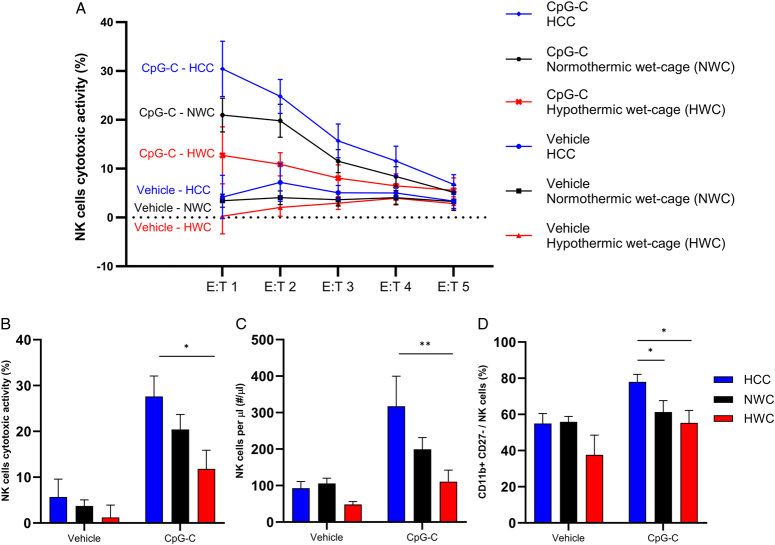
Figure 4.
Comparison between the effects of hypothermic (HWC) and normothermic (NWC) stress paradigm on hepatic NK cells activity, numbers, and maturation (n=31, n=4–6 per group). Mice were subjected to 8 h of HWC, NWC, or served as HCC and were administered with CpG-C or vehicle 2 h after the onset of stress. Hepatic leukocytes were harvested at the end of stress. (A) Percentage of cytotoxic activity against CT26 cells in different effector-to-target (E:T) ratios. (B) Average of E:T 1 and 2 in each animal. (C) NK cells count per microliter of liver perfusate. (D) CD11b+ CD27− NK cell percentages of the entire NK cell population. CpG-C significantly (B) elevated NK cells cytotoxicity (main effect, P<0.0001); (C) increased NK cell numbers (main effect, P=0.0027); and (D) increased CD11b+ CD27− NK cell percentages of the entire NK cell population (main effect, P=0.0018). In animals that were administered with CpG-C, HWC but not NWC significantly reduced (B) NK cell cytotoxicity (P=0.033) and (C) numbers (P=0.03) compared to HCC. Additionally, (D) both HWC (P=0.031) and NWC (P=0.027) significantly reduced the percentages of CD11b+ CD27− NK cells compared to HCC (*P<0.05; **P<0.001). Graphs represent mean±SEM.
Figure 3.
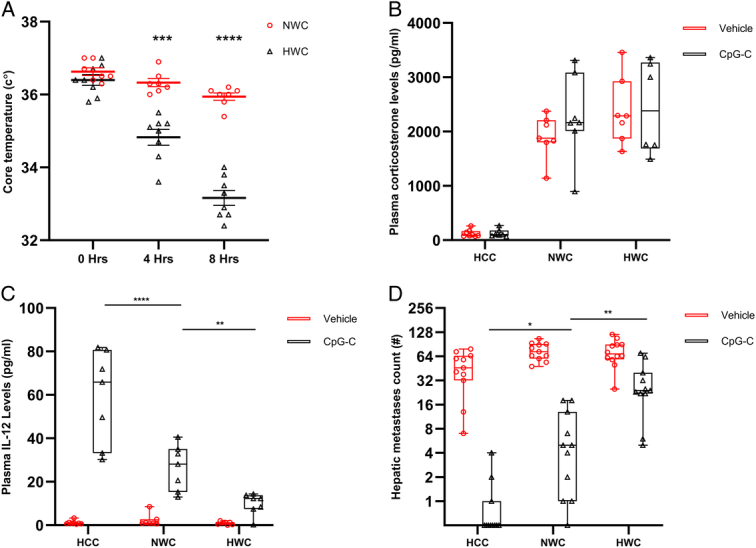
Comparison between the hypothermic and normothermic stress paradigms. (A) Mice were subjected to hypothermic (HWC) or normothermic (NWC) wet-cage stress, and core temperatures were measured at 0, 4, and 8 h following stress initiation. (B–D) Mice were subjected to 8 h of HWC, NWC, or served as HCC, and were administered with CpG-C or vehicle 2 h after the onset of stress. Visible hepatic metastases were counted 20 days later for males and 25 days later for females. (A) HWC significantly reduced core body temperatures at 4 h (n=15, n=7–8 per group, P=0.003) and at 8 h (P<0.0001) compared to NWC. (B) Both HWC and NWC significantly elevated plasma corticosterone levels to a similar degree (main effect – n=43, n=7–8 per group, P<0.0001, Tukey – P=0.6024, P=0.9935). (C) CpG-C significantly increased plasma IL-12 levels (main effect – n=43, n=7–8 per group, P<0.0001), and in animals administered with CpG-C, NWC decreased plasma IL-12 levels compared to HCC (P<0.0001), and HWC decreased it significantly more (P=0.0087). Similarly, CpG-C significantly reduced the number of hepatic metastases (D) (main effect – n=67, n=11–12 per group, P<0.0001), and in animals administered with CpG-C NWC increased the number of hepatic metastases compared to HCC (P=0.0126), and HWC elevated this number significantly more (P=0.0036) (*P<0.05; **P<0.001, ***P<0.001; ****P<0.0001). (A) Graphs represent mean±SEM. (B–D) Boxes represent the second and third quartiles, and whiskers show min and max values.
Figure 6.
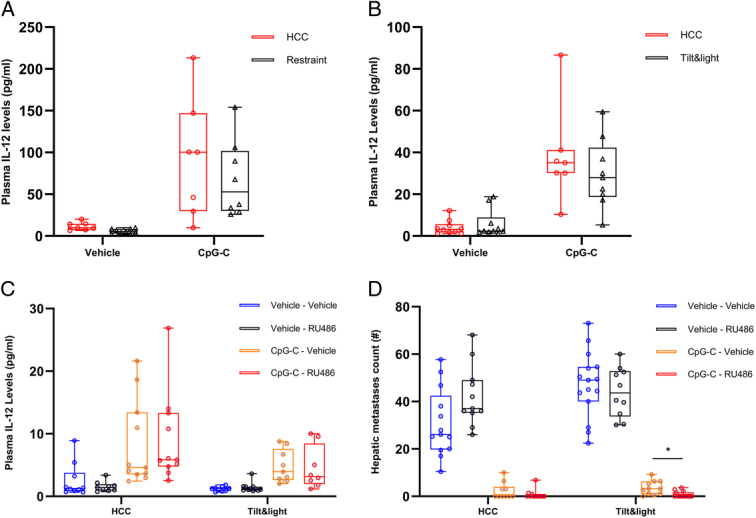
In four different experiments, mice were subjected to 8 h of restraint stress (A) or 12 h of tilt&light (B–D) stress. In experiments C and D, mice were administered with RU486 or vehicle 30 min prior to the onset of stress. In all experiments (A–D) mice were administered with CpG-C or vehicle 2 h after the onset of stress. (A–C) Plasma IL-12 levels were assessed at the end of each stress paradigm. (D) Visible hepatic metastases were counted 20 days later. (A, B) Restraint (n=31, n=7–9 per group) and tilt&light (n=36, n=7–10 per group) stress paradigms did not interrupt the CpG-C-induced elevation in plasma IL-12 levels. (C) Tilt&light stress significantly decreased plasma IL-12 levels (main effect, n=78, n=9–11 per group, P=0.0149) and (D) increased numbers of CT26 metastases (main effect, n=97, n=10–15 per group, P=0.0149), but did not prevent the beneficial effects of CpG-C on these indices: (C) increased IL-12 levels (main effect, P<0.0001, and (D) decreased CT26 metastases, main effect, P<0.0001). RU486 (C) did not negate the deleterious effects of stress on IL-12 levels, but (D) did improve the antimetastatic effects of CpG-C under stress conditions (P=0.0104) (*P<0.05). Boxes represent the second and third quartiles, and whiskers show min and max values.
Results
Hypothermic wet-cage stress abolished CpG-C-induced increase in plasma IL-12 levels
We first tested whether the hypothermic wet-cage (HWC – see Methods) stress paradigm affects CpG-C-induced increase in plasma IL-12 levels, when administered in different doses and routes of administration in two separate experiments. C57BL/6J mice were subjected to 8 h of HWC or served as home cage control (HCC). Two hours after the onset of stress, in the first experiment, mice were administered with 100 µg CpG-C, 200 µg CpG-C, or with vehicle (Fig. 1A, n=30); and in the second experiment, were administered with 100 µg CpG-C or with the vehicle either s.c. or i.p. (Fig. 1B, n=44). Plasma IL-12 levels were assessed at the end of stress.
Figure 1.
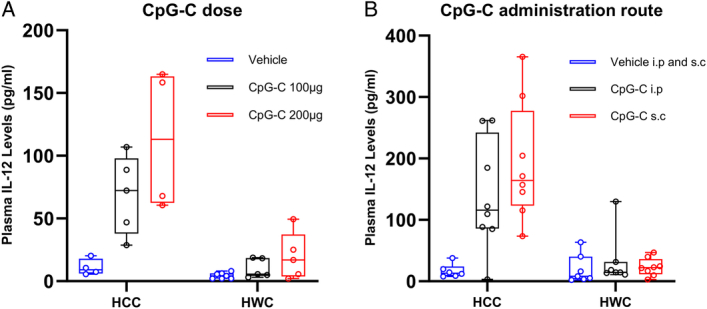
Mice were subjected or not to 8 h of wet-cage stress and were administered with CpG-C or vehicle 2 h after the onset of stress. Plasma IL-12 was assessed at the end of stress. CpG-C elevated IL-12 levels in both experiments (A, n=30, n=4–7 per group, P=0.0002; B, n=44, n=6–8 per group, P=0.011), and hypothermic wet-cage (HWC) stress abolished the CpG-C-induced increase in IL-12 (A, n=30, P<0.0001; B, n=44, P<0.0001). Boxes represent the second and third quartiles, and whiskers show min and max values.
In both experiments, CpG-C administration significantly increased plasma IL-12 levels (main effects, Fig. 1A, F(2,24)=12.91, P=0.0002; Fig. 1B, F(2,38)=8.193, P=0.011) and HWC significantly reduced this effect (A) (main effects, F(1,24)=31.65, P<0.0001), (B) (F(1,38)=24.29, P<0.0001). These findings indicate that CpG-C was significantly less effective in elevating IL-12 levels under hypothermic stress conditions at all doses and administration routes studied.
Beta-adrenergic, glucocorticoid, or opioid signaling did not mediate the effects of hypothermic wet-cage stress on CpG-C-induced increase in plasma IL-12 levels
As HWC abolished CpG-C-induced IL-12 plasma levels, we tested whether beta-adrenergic, opioid, or glucocorticoid signaling are involved in mediating the effects of HWC through blockade of each receptor system (Fig. 2A–C) or by performing adrenalectomy (ADX – Fig. 2D, see Methods). To this end, in four different experiments, C57BL/6J mice were subjected to 8 h of HWC, or served as HCC. In experiments A–C, 30 min prior to the onset of stress, mice were administered with propranolol (Fig. 2A, 5 mg/kg, s.c., n=57); naltrexone (Fig. 2B, 2 mg/kg, s.c., n=30); or with RU486 (Fig. 2C, 25 mg/kg, s.c., n=75), or injected with their respective vehicles. In experiment D, mice underwent ADX or sham surgery 4 weeks prior to stress (Fig. 2D, n=91). In all four experiments, 2 h after the onset of stress, mice were administered with CpG-C (100 µg, i.p.) or a vehicle. Plasma IL-12 (Fig. 2) levels were assessed at the end of stress.
Figure 2.
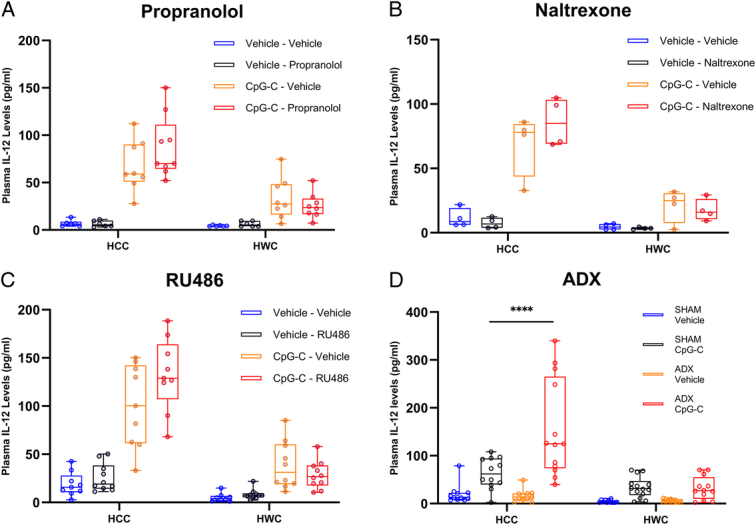
In experiments A–C, 30 min prior to the onset of stress, mice were administered with propranolol (A), RU486 (B), or naltrexone (C), or with vehicle. In experiment D, mice were adrenalectomized (ADX) or underwent sham surgery 4 weeks before stress. In all four experiments, mice were subjected not to 8 h of hypothermic wet-cage (HWC) stress and administered with CpG-C or vehicle 2 h after the onset of stress. IL-12 plasma levels were assessed at the end of stress. CpG-C significantly elevated plasma IL-12 levels (A, n=57, n=6–9 per group; B, n=32, n=4 per group; C, n=75, n=8–10 per group, P<0.0001; D, n=91, n=11–14, P<0.0001) and wet-cage markedly attenuated CpG-C-induced increase in plasma IL-12 (A, P<0.0001; B, P<0.0001; C, P<0.0001; and D, P<0.0001). Adrenalectomy improved CpG-C-induced IL-12 levels, but only under HCC conditions (****P<0.0001). Boxes represent the second and third quartiles, and whiskers show min and max values.
In all four experiments, CpG-C significantly increased plasma IL-12 levels (main effects: Fig. 2A, F(1,49)=83.31, P<0.0001; Fig. 2B, F(1,24)=87.89, P<0.0001; Fig. 2C, F(1,67)=123.4, P<0.0001; and Fig. 2D, F(1,83)=59.75, P<0.0001) and HWC markedly decreased plasma IL-12 levels (main effects: Fig. 2A, F(1,49)=22.53, P<0.0001; Fig. 2B, F(1,24)=51.00, P<0.0001; Fig. 2C F(1,67)=81.73, P<0.0001; and Fig. 2D F(1,83)=35.08, P<0.0001). None of the antagonists significantly altered plasma levels of IL-12, including under HWC conditions. Interestingly, ADX significantly modulated plasma IL-12 levels compared to sham operation, but only in nonstressed animals administered with CpG-C (P<0.0001). Thus, it appears that none of these three neuroendocrine mechanisms mediate the IL-12-reducing effects of hypothermic stress.
Hypothermic and normothermic wet-cage stressors differentially affected core body temperatures, plasma IL-12 levels, and CT26 metastasis, but not corticosterone levels
To start elucidating the specific impact of hypothermia in HWC, we developed a normothermic counterpart variation (NWC – see Methods) and conducted three experiments in which we compared the effects of the two paradigms on the efficacy of CpG-C.
In experiment 1 (Fig. 3A, n=45), BALB/c mice were subjected to 8 h of HWC or NWC. Mice core temperatures were measured at the onset of stress and 4 and 8 h after its initiation. HWC decreased mice core body temperatures to nearly 33°C (main effect, Fig. 3A, F(1,13)=201.328, P<0.0001). Additionally, HWC significantly decreased core temperatures at 4 h (P=0.0003) and 8 h (P<0.0001) compared to NWC, which did not affect core body temperatures.
In experiment 2 (n=43), we compared the impact of the two paradigms on plasma corticosterone (Fig. 3B) and IL-12 (Fig. 3C) levels. In experiment 3 (n=67), we compared the impact of the two paradigms on CT26 metastasis (Fig. 3D). In both experiments, 2 h after the onset of stress, BALB/c mice were administered with CpG-C (100 µg, i.p.) or vehicle. In experiment 2, plasma IL-12 and corticosterone levels were assessed at the end of stress. In experiment 3, mice underwent CT26 tumor cell inoculation (see Methods) at stress cessation, and 20 days later hepatic metastases were enumerated. Both HWC and NWC significantly elevated corticosterone levels (main effect, Fig. 3B, F(2,36)=73.09, P<0.0001) in a similar manner (no significant difference between the two paradigms P=0.6042) and CpG-C did not affect corticosterone levels. CpG-C administration significantly (i) increased IL-12 levels (Fig. 3C, F(1,37)=100.1, P<0.0001) and (ii) markedly reduced the number of hepatic metastases (Fig. 3D, F(1,61)=135.1, P<0.0001). The stress paradigms significantly (i) decreased plasma IL-12 levels (Fig. 3C, F(2,37)=23.02, P<0.0001) and (ii) increased the number of metastases (Fig. 3D, F(2,61)=13.44, P<0.0001). Most importantly, in mice that were administered with CpG-C, NWC markedly (i) decreased IL-12 levels (Fig. 3C, Wt(8.681)=3.592, P<0.0001) and (ii) significantly increased the number of metastases (Fig. 3D, Wt(10.75)=2.989, P=0.0126) compared to HCC, and HWC further reduced plasma IL-12 levels (Fig. 3C, Wt(11.81)=3.138, P=0.0087) and further increased metastases (Fig. 3D, Wt (12.06)=3.599, P=0.0036) compared to NWC. Overall, while the HWC and NWC stress paradigms caused a very similar corticosterone response, the HWC paradigm had significantly more deleterious effects on IL-12 and metastasis than the NWC paradigm.
Hypothermic and normothermic wet-cage stress paradigms differentially affected CpG-C-induced liver NK cell maturation and cytotoxicity
Our previous studies indicated that CpG-C reduces the development of CT26 metastases through increasing hepatic NK cell numbers and cytotoxicity49. Therefore, we compared HWC to NWC with respect to their impact on hepatic NK cells. To this end, BALB/c mice (n=31) were subjected to 8 h of HWC or NWC, or served as HCC. Two hours after the onset of stress, mice were administered with CpG-C (100 µg, i.p.) or vehicle. Hepatic leukocytes harvested (see Methods) at the end of stress, and studied for maturation and numbers using flow cytometry and assessed for NK cells cytotoxicity (see Methods).
CpG-C administration markedly (i) elevated NK cells cytotoxicity (main effect, Fig. 4B, F(1,25)=32.16, P<0.0001); (ii) increased NK cell numbers (main effect, Fig. 4C, F(1,24)=11.17, P=0.0027); and (iii) increased percentages of mature NK cells (CD11b+ CD27−) of the entire NK cell population (main effect, Fig. 4D, F(1,24)=12.36, P=0.0018). Both stress paradigms significantly (i) reduced NK cells cytotoxicity (main effect, Fig. 4B, F(2,25)=3.954, P=0.0322); (ii) reduced NK cell numbers (main effect, Fig. 4C, F(2,24)=3.468, P=0.0475) and (ii) decreased the percentage of mature NK cells (main effect, Fig. 4D, F(2,24)=6.736, P=0.0048). Most importantly, in animals that were administered with CpG-C, HWC but not NWC significantly reduced NK cells cytotoxicity (Fig. 4B, Tukey, P=0.033) and numbers (Fig. 4C, Wt(6.608)=2.753, P=0.03) compared to HCC. Additionally, both HWC and NWC significantly reduced the percentage of mature NK cells compared to HCC (Fig. 4D, Wt(5.006)=2.963, P=0.031; Wt(3.64)=3.557, P=0.027).
In-vitro PBMCs subjected to 33°C exhibited a significantly attenuated CpG-C-induced IL-12 production compared to 37°C
To assess the direct effects of hypothermic conditions on PBMCs in vitro (see Methods), PBMCs were incubated with CpG-C or not, for 10 or 20 h at 33°C or 37°C (Fig. 5). Supernatants were collected in duplicates from each well at the end of each incubation period. Hypothermic conditions significantly decreased (F(1,8)=580.367, P<0.0001) the production of IL-12 by PBMCs, and CpG-C elevated it (F(1,8)=3470.541, P<0.0001). Additionally, hypothermic conditions attenuated CpG-C-induced elevation of IL-12 at 10 h (P<0.0001) and at 20 h (P<0.0001). These results indicate that in-vitro IL-12 production by leukocytes, with or without CpG-C is modulated by temperature. Additionally, these findings suggest that the in-vivo effects of hypothermic stress we found in the previous experiments are at least partially mediated by direct effects of temperature on leukocyte activities.
Figure 5.
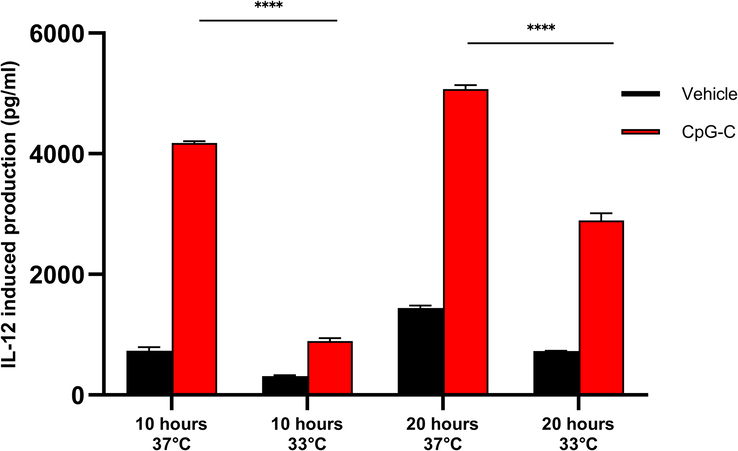
Comparison between hypothermic and normothermic conditions on CpG-C-induced IL-12 production. PBMCs were incubated for 10 or 20 h, at 33°C or 37°C. The study was conducted in duplicates, and supernatants were collected in duplicates from each well at the end of each incubation period. Hypothermic conditions significantly decreased (main effect, P<0.0001) the production of IL-12 by PBMCs, and CpG-C elevated it (main effect, P<0.0001). Hypothermic conditions attenuated CpG-C-induced elevation of IL-12 at 10 h (P<0.0001) and at 20 h (P<0.0001) (****P<0.0001). The graph represents mean±SEM.
Restraint and tilt&light normothermic stress paradigms did not affect CpG-C-induced elevation of plasma IL-12 levels
To further study the unique impact of hypothermic stress, we employed two additional normothermic stress paradigms in C57BL/6J mice, which were subjected to 8 h of the restraint stress paradigm (Fig. 6A, n=31, see Methods) or 12 h of the tilt&light stress paradigm (Fig. 6B, n=36, see Methods), each compared to HCC condition. Two hours after the onset of the stress paradigms, mice were administered with CpG-C (50 µg, i.p.) or vehicle. Plasma IL-12 and corticosterone levels were assessed at the end of stress.
CpG-C significantly increased plasma IL-12 levels under both stress conditions (Fig. 6A, F(1,27)=23.27, P<0.0001; Fig. 6B, F(1,32)=40.48, P<0.0001). As opposed to the HWC paradigms (Figs 1–4), restraint and tilt&light paradigms did not significantly interfere with CpG-C-induced elevation in plasma IL-12 levels.
Tilt&light stress reduced IL-12 levels and increased the number of CT26 metastases but did not prevent the beneficial effects of CpG-C: limited mediation by glucocorticoids
Previous studies indicated that glucocorticoids, but not catecholamines or opioids, modulate plasma IL-12 levels. Thus, in the context of tilt&light stress, we tested whether attenuation of glucocorticoid signaling affects plasma IL-12 levels and CT26 metastasis and whether it interacts with CpG-C-induced immune stimulation. To this end we conducted two studies with the same design in BALB/c mice, one assessing IL-12 levels (n=78, Fig. 6C) and the second studying CT26 metastases (n=97, Fig. 6D). Mice were subjected to the tilt&light stress paradigm for 12 h or served as HCC, and were injected with RU486 or vehicle 30 min before stress initiation. Two hours after the onset of the stress, mice were administered with CpG-C (50 µg, i.p.) or vehicle. Plasma IL-12 levels were assessed at the end of stress (experiment 1, Fig. 6C), or mice were inoculated with CT26 tumor cells at the end of stress, and 20 days later, the number of hepatic metastases was assessed (experiment 2, Fig. 6D).
Tilt&light stress significantly decreased plasma IL-12 levels (main effect – Fig. 6C, F(1,70)=6.237, P=0.0149), and increased numbers of CT26 metastases (main effect – Fig. 6D, F(1,89)=6.170, P=0.0149). CpG-C administration significantly elevated plasma IL-12 levels (main effect – Fig. 6C, F(1,70)=28.40, P<0.0001) and markedly reduced numbers of CT26 metastasis (main effect – Fig. 6D, F(1,89)=403.9, P<0.0001). RU486 did not negate the deleterious effects of stress on IL-12 levels but did improve the antimetastatic effects of CpG-C under stress conditions (Wt(12.94)=2.993, P=0.0104).
Discussion
In this study, we explored how hypothermic and normothermic stress conditions modulate (i) immune responses and resistance to cancer metastasis, (ii) the beneficial effects of perioperative CpG-C immunotherapy on these indices, and (iii) the involvement of specific neuroendocrine mechanisms in these modulations. The results indicate that perioperative hypothermic stress can decrease (i) both baseline IL-12 levels and their elevation following CpG-C treatment, (ii) hepatic NK cell cytotoxicity, and (iii) resistance to CT26 hepatic metastasis. Additionally, blockade of glucocorticoids, β-adrenergic, or opioid receptors, or adrenalectomy did not overcome these deleterious effects of hypothermic stress.
These results emphasize the potential detrimental effects of hypothermic stress, and to a lesser extent of normothermic stress, in the clinical context of oncological surgery. Operated cancer patients often experience psychological and physiological stress during the IPP, along with inflammatory responses, all of which may inhibit antimetastatic immunity and immune-based therapies and promote metastatic properties of MRD2,3. These stressors are mainly characterized by excessive release of CAs and PGs, which were shown to specifically promote immunosuppression and the progression of MRD. Preclinical studies have demonstrated that CAs and PGs can facilitate suppression of antitumor immunity by reducing the number and activity of CD8+ and CD4+ effector T cells53, impairing checkpoint inhibitor therapy53, enhancing the activity of regulatory T cells and myeloid-derived suppressor cells54–56, diminishing natural killer (NK) cell cytotoxicity50,57, promoting M2 macrophage polarization58,59, and shifting the TH1/TH2 balance toward TH2 dominance. Notably, clinical studies suggest that cancer patients may experience both preoperative and postoperative perturbations of inflammatory and immune-suppressive factors. Stress and inflammatory responses are promoting each other31, and in cancer patients, preoperative psychological stress was reported to elevate systemic inflammatory status, and various tumors are known to secrete prostaglandins. For example, patients with breast or ovarian cancer have exhibited elevated plasma levels of cortisol, IL-6, and CRP prior to surgery28,60. Additionally, decreased levels of interferon-gamma (IFN-γ) and IL-12 production were reported in breast cancer patients the morning following surgery28. In lung cancer patients, postoperative increases in PD-1/PD-L1 expression on CD4+ and CD8+ T cells and NK cells, along with reduced T and NK cell counts, have been reported61. Our results indicate that while the beneficial effects of CpG-C were significantly attenuated by hypothermic stress, CpG-C demonstrated resilience under normothermic stress conditions, maintaining its efficacy despite various stressors, including surgery. These and additional findings point to CpG-C as a promising candidate for IPP treatment to reduce cancer metastasis under normothermic stress conditions.
Perioperative hypothermia is a common complication of anesthesia and surgery. Mild hypothermia (36–32°C), as in the current study, is known to occur in ~70% of patients undergoing surgery62–64. In a prospective study of patients, Sari et al.63 indicated that when the operation was longer than 2 h, all patients suffered some degree of hypothermia, and 24.4% of the patients exhibited a core temperature below 35°C. The great majority of these patients were subjected to passive intraoperative warming, as opposed to active warming. Active warming was shown to be more effective in reducing intraoperative hypothermia, although hypothermia may still persist even with active warming65. Additionally, cancer patients may be subjected to cold stress irrespective of surgery (elaborated in66,67), and such conditions in awake animals were shown to dampen anticancer immunity and promote tumorgenesis68. Specifically, Repasky et al. have shown that the common vivarium condition (22°C) subject animals to chronic cold stress, promoting beta-adrenergic signaling53, which in turn can lead to: increased resistance to cytotoxic therapies69; enlarged primary tumors70; decreased numbers of antigen-specific CD8+ T lymphocytes and active CD8+ T cells in the tumor microenvironment53,70; elevated numbers of regulatory T cells and MDSCs53,70; and decreased non-plasmacytoid DC activation71.
In this study, we sought to determine the unique impact of perioperative hypothermic stress vs. normothermic stress on cancer metastasis, and mediating immune mechanisms. The hypothermic wet-cage stress paradigm gradually decreased core temperatures to ~33°C within 8 h, whereas the normothermic variation of this paradigm maintained body temperature above 36°C. Notably, the two paradigms induced a similar elevation in glucocorticoid release. In the context of CpG-C immune stimulation, the hypothermic stress paradigm reduced plasma IL-12 and hepatic NK cell maturation and cytotoxicity levels, and increased metastasis, significantly and markedly more than the normothermic paradigm. Additionally, in vitro, hypothermic conditions (33°C) significantly reduced IL-12 production by PBMCs, compared to normothermic (37°C) conditions, with or without CpG-C. Last, we employed two additional stress paradigms that do not involve hypothermia – the restraint and the tilt&light paradigms. Neither paradigm attenuated CpG-C-induced IL-12 secretion nor its beneficial effects on CT26 metastases. NK cell cytotoxicity is known to be potentiated by IL-1224, and our previous studies showed that the reduction in CT26 metastases induced by CpG-C administration is mediated by elevated NK cell cytotoxicity49. Thus, we suggest that in this study the deleterious impact of hypothermia is mediated, at least partly, through reduced IL-12 and hepatic NK maturation and cytotoxicity levels (observed herein), which in turn jeopardize the host capacity to eliminate liver metastases.
When stress conditions do not involve hypothermia, the efficacy of CpG-C may be further enhanced if specific stress responses are restricted. In our previous studies, we found that endogenous levels of glucocorticoids and prostaglandins-E2, but not catecholamines or opioids, mediate the effects of stress in reducing plasma IL-12 levels48. Additionally, in-vivo pharmacological administration of catecholamines or opioids, as may also occur clinically, was shown to decrease IL-12 levels through endogenous glucocorticoid response rather than directly48. Thus, it appears that glucocorticoids are the main modulator of IL-12 secretion48,72. Additionally, high levels of glucocorticoids have been shown to directly induce pDC apoptosis, suppress IFNα production, suppress pDCs induction of high plasma IL-12 levels, and downregulate co-stimulatory molecules73–75, all associated with reduced antimetastatic immunity. Considering the above, we assessed the efficacy of CpG-C treatment and a glucocorticoid antagonist on IL-12 levels and CT26 metastasis in the context of tilt&light normothermic stress. Stress alone significantly elevated the numbers of hepatic CT26 metastases but did not dampen the beneficial effects of CpG-C on IL-12 levels and the number of metastases. Importantly, under stress conditions, the combined treatment of CpG-C and glucocorticoid blockade decreased the number of CT26 metastases compared to CpG-C treatment alone, suggesting a synergistic effect of CpG-C and attenuation of glucocorticoid signaling in the context of non-hypothermic stress.
While preclinical studies of immunotherapy show great potential in the treatment of cancer metastasis, clinical outcomes are clearly less promising2,76,77. Herein, we suggest that one important difference that may contribute to such discrepancies is the prevalence of physiological and psychological stressors in cancer patients31, including hypothermic conditions62, which are prevalent clinically but are rarely simulated in preclinical studies. We herein simulated hypothermic and normothermic stress conditions and studied their effects on TLR-9 immune activation during the IPP. While the wet-cage stress paradigm enabled us to specifically isolate the effects of hypothermia by comparing it to its normothermic counterpart, the robustness of this paradigm may mask more subtle stress responses that could occur clinically in cancer patients31. Additionally, despite their significant potential in reducing metastatic burden in preclinical studies, TLR-9 agonists have rarely demonstrated similar success in clinical research78,79. Such inefficacies may be due to the delayed administration of these agents in the clinical setting80, which typically begins at least a month postoperatively. This timing may reduce the effectiveness of these agents due to surgery-induced and/or chemotherapy-induced immunosuppression, in contrast to the perioperative use of CpG-C explored in this study. Additionally, clinical inefficacy may also be attributed to a different distribution of TLR-9 on immunocytes in humans compared to rodents81,82. The difference in the distribution of TLR-9 may lead to an altered immune response in humans, potentially indicating CpG-C therapy to be less effective clinically. Lastly, herein we employed an experimental metastasis model (CT26), which did not involve the removal of a primary tumor, and CpG-C was administered before CT26 was introduced to the mice, thus distancing the model from clinical context. This further warrants the testing of CpG-C in tumor-bearing animals, under various perioperative stress conditions. Notably, we have previously shown the efficacy of CpG-C in tumor-bearing animals but not in the broader clinical perioperative context6.
Considering the above, we suggest several approaches to promote the use of immunotherapy during the IPP, and specifically CpG-C, to reduce metastatic development in cancer patients. First, the use or the development of new psychological stress paradigms that better mimic stress responses of cancer patients during the IPP will enable better simulation of the clinical context, and potentially reduce the efficacy gap between preclinical and clinical studies regarding immunotherapies. Additionally, although tumor cell inoculation in the CT26 model was conducted in the context of a surgical procedure, future studies should employ several types of cancer models, including models of spontaneous metastasis, to promote the generalization of the impact of CpG-Cs and the simulation of the clinical oncological context. Additionally, translational research of CpG-C is warranted in models that better mimic human TLR-9 distribution, such as TLR-9 partial knockouts or humanized mouse models and human tissues. Herein, plasma IL-12 and hepatic NK cytotoxicity and maturation levels were shown to at least partially mediate the detrimental effects of hypothermia on hepatic metastasis. Future studies should evaluate the predictive value and causal effects of IL-12 and of NK cytotoxicity and maturation levels under both hypothermic and normothermic stress conditions during the IPP in various metastasis models and organs. If found to predict long-term cancer outcomes, perioperative levels of IL-12 could be used as a clinical biomarker for perioperative NK activity and hepatic metastases risk, and IL-12 replacement therapy may be tested.
Conclusions
This study indicates that perioperative stressors, hypothermic and normothermic, can modulate perioperative immune function, dampen resistance to experimental metastasis, and jeopardize the efficacy of CpG-C immunotherapy. Mild hypothermia, which is prevalent clinically, can lead to potent suppression of immunity and reduced resistance to cancer metastasis, which herein was not mediated by common neuroendocrine stress responses but apparently through direct effects of low temperature on immunocytes. These results emphasize the importance of routine thermoregulation monitoring and maintenance in cancer patients throughout the IPP. Patients undergoing surgery with general anesthesia are typically warmed (actively or passively) only during the surgical procedure itself, but not during critical time points in the IPP, including induction of anesthesia and postoperative awakening and recovery periods. Notably, current practices of thermoregulation were found insufficient to prevent hypothermia even during the procedure itself65,83. The current study also suggests potential strategies to enhance the beneficial effects of CpG-C immunotherapy under non-hypothermic conditions. Pharmacological reduction of stress responses or behavioral stress management may effectively complement various immunotherapies during the IPP, thereby reducing metastasis and improving long-term cancer outcomes2,3. These findings have significant clinical implications for oncological surgery, including the potential implementation of immune-stimulating approaches in cancer patients undergoing surgery and more effective and comprehensive prevention of hypothermia throughout the IPP.
Ethical approval
Housing conditions are regularly monitored by the Institutional Animal Care and Use Committee of Tel Aviv University, which also approved all studies described herein. P-12-003, 31/12/2016; 10-18-005, 09/07/2018; 10-18-008, 06/12/2018.
Consent
Not applicable.
Source of funding
NIH, NCI, 1R01CA172138-01A1; Israeli Science Foundation (ISF), 1758/19; Emerson Collective Cancer Research RFA, 20191112081945; Israel Cancer Research Fund (ICRF), PG-20-106.
Author contribution
E.S.: conceptualization, data curation, formal analysis, investigation, methodology, project administration, validation, visualization, writing – original draft, and writing – review and editing; P.M.: conceptualization, investigation, and validation; A.E.: investigation, methodology, validation, and writing – review and editing; L.S.: investigation and writing – review and editing; E.R.: investigation; I.N.: writing – review and editing; S.B.-E.: conceptualization, formal analysis, funding acquisition, methodology, resources, supervision, and writing – review and editing.
Conflicts of interest disclosure
The authors have no conflicts of interest.
Research registration unique identifying number (UIN)
Not applicable.
Guarantor
Professor Shamgar Ben-Eliyahu.
Data availability statement
The data generated during this study will be available upon request.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Acknowledgements
Assistance with the study: none.
Presentation: presented in PNIRS 2019 and PNIRS 2023.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Published online 14 August 2024
Contributor Information
Elad Sandbank, Email: eladsandbank@mail.tau.ac.il.
Pini Matzner, Email: matzner.pini@gmail.com.
Anabel Eckerling, Email: anabele@mail.tau.ac.il.
Liat Sorski, Email: liatsors@tauex.tau.ac.il.
Ella Rossene, Email: ellar@post.tau.ac.il.
Ido Nachmani, Email: ido.nachmany@sheba.health.gov.il.
Shamgar Ben-Eliyahu, Email: shamgar@tauex.tau.ac.il.
References
- 1. Horowitz M, Neeman E, Sharon E, et al. Exploiting the critical perioperative period to improve long-term cancer outcomes. Nat Rev Clin Oncol 2015;12:213–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Matzner P, Sandbank E, Neeman E, et al. Harnessing cancer immunotherapy during the unexploited immediate perioperative period. Nat Rev Clin Oncol 2020;17:313–326. [DOI] [PubMed] [Google Scholar]
- 3. Sandbank E, Eckerling A, Margalit A, et al. Immunotherapy during the immediate perioperative period: a promising approach against metastatic disease. Curr Oncol 2023;30:7450–7477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vallejo R, Hord ED, Barna SA, et al. Perioperative immunosuppression in cancer patients. J Environ Pathol Toxicol Oncol 2003;22:139–146. [DOI] [PubMed] [Google Scholar]
- 5. Hogan BV, Peter MB, Shenoy HG, et al. Surgery induced immunosuppression. Surgeon 2011;9:38–43. [DOI] [PubMed] [Google Scholar]
- 6. Benbenishty A, Gadrich M, Cottarelli A, et al. Prophylactic TLR9 stimulation reduces brain metastasis through microglia activation. PLoS Biol 2019;17:e2006859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Matzner P, Sorski L, Shaashua L, et al. Perioperative treatment with the new synthetic TLR-4 agonist GLA-SE reduces cancer metastasis without adverse effects. Int J Cancer 2016;138:1754–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Park CG, Hartl CA, Schmid D, et al. Extended release of perioperative immunotherapy prevents tumor recurrence and eliminates metastases. Sci Transl Med 2018;10:eaar1916. [DOI] [PubMed] [Google Scholar]
- 9. Sorski L, Melamed R, Levi B, et al. Prevention of liver metastases through perioperative acute CpG-C immune stimulation. Cancer Immunol Immun 2020;69:2021–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tai LH, de Souza CT, Bélanger S, et al. Preventing postoperative metastatic disease by inhibiting surgery-induced dysfunction in natural killer cells. Cancer Res 2013;73:97–107. [DOI] [PubMed] [Google Scholar]
- 11. Gengenbacher N, Singhal M, Augustin HG. Preclinical mouse solid tumour models: status quo, challenges and perspectives. Nat Rev Cancer 2017;17:751–765. [DOI] [PubMed] [Google Scholar]
- 12. Klatte T, Ittenson A, Röhl FW, et al. Perioperative immunomodulation with interleukin-2 in patients with renal cell carcinoma: results of a controlled phase II trial. Br J Cancer 2006;95:1167–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brivio F, Fumagalli L, Lissoni P, et al. Pre-operative immunoprophylaxis with interleukin-2 may improve prognosis in radical surgery for colorectal cancer stage B-C. Anticancer Res 2006;26(1B):599–603. [PubMed] [Google Scholar]
- 14. Caprotti R, Brivi F, Fumagalli L, et al. Free-from-progression period and overall short preoperative immunotherapy with IL-2 increases the survival of pancreatic cancer patients treated with macroscopically radical surgery. Anticancer Res 2008;28(3B):1951–1954. [PubMed] [Google Scholar]
- 15. Huang AC, Orlowski RJ, Xu XW, et al. A single dose of neoadjuvant PD-1 blockade predicts clinical outcomes in resectable melanoma. Nat Med 2019;25:454–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chiocca EA, Gelb AB, Chen CC, et al. Combined immunotherapy with controlled interleukin-12 gene therapy and immune checkpoint blockade in recurrent glioblastoma: an open-label, multi-institutional phase I trial. Neuro Oncol 2022;24:951–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rolfo C, Giovannetti E, Martinez P, et al. Applications and clinical trial landscape using Toll-like receptor agonists to reduce the toll of cancer. NPJ Precis Oncol 2023;7:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dongye Z, Li J, Wu Y. Toll-like receptor 9 agonists and combination therapies: strategies to modulate the tumour immune microenvironment for systemic anti-tumour immunity. Br J Cancer 2022;127:1584–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. von Meyenn F, Schaefer M, Weighardt H, et al. Toll-like receptor 9 contributes to recognition of Mycobacterium bovis Bacillus Calmette–Guerin by Flt3-ligand generated dendritic cells. Immunobiology 2006;211:557–565. [DOI] [PubMed] [Google Scholar]
- 20. Gilkeson GS, Conover J, Halpern M, et al. Effects of bacterial DNA on cytokine production by (NZB/NZW)F1 mice. J Immunol 1998;161:3890–3895. [PubMed] [Google Scholar]
- 21. Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol 2002;20:709–760. [DOI] [PubMed] [Google Scholar]
- 22. Goldfarb Y, Sorski L, Benish M, et al. Improving postoperative immune status and resistance to cancer metastasis: a combined perioperative approach of immunostimulation and prevention of excessive surgical stress responses. Ann Surg 2011;253:798–810. [DOI] [PubMed] [Google Scholar]
- 23. Goldfarb Y, Benish M, Rosenne E, et al. CpG-C oligodeoxynucleotides limit the deleterious effects of beta-adrenoceptor stimulation on NK cytotoxicity and metastatic dissemination. J Immunother 2009;32:280–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Trinchieri G. Interleukin-12 – a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol 1995;13:251–276. [DOI] [PubMed] [Google Scholar]
- 25. Ben-Eliyahu S, Page GG, Yirmiya R, et al. Evidence that stress and surgical interventions promote tumor development by suppressing natural killer cell activity. Int J Cancer 1999;80:880–888. [DOI] [PubMed] [Google Scholar]
- 26. Brittenden J, Heys SD, Ross J, et al. Natural killer cells and cancer. Cancer 1996;77:1226–1243. [DOI] [PubMed] [Google Scholar]
- 27. Nersesian S, Schwartz SL, Grantham SR, et al. NK cell infiltration is associated with improved overall survival in solid cancers: a systematic review and meta-analysis. Transl Oncol 2021;14:100930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shaashua L, Shabat-Simon M, Haldar R, et al. Perioperative COX-2 and beta-adrenergic blockade improves metastatic biomarkers in breast cancer patients in a phase-II randomized trial. Clin Cancer Res 2017;23:4651–4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Greenfeld K, Avraham R, Benish M, et al. Immune suppression while awaiting surgery and following it: dissociations between plasma cytokine levels, their induced production, and NK cell cytotoxicity. Brain Behav Immun 2007;21:503–513. [DOI] [PubMed] [Google Scholar]
- 30. Antoni MH, Dhabhar FS. The impact of psychosocial stress and stress management on immune responses in patients with cancer. Cancer 2019;125:1417–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Eckerling A, Ricon-Becker I, Sorski L, et al. Stress and cancer: mechanisms, significance and future directions. Nat Rev Cancer 2021;21:767–785. [DOI] [PubMed] [Google Scholar]
- 32. Dubowitz JA, Sloan EK, Riedel BJ. Implicating anaesthesia and the perioperative period in cancer recurrence and metastasis. Clin Exp Metastas 2018;35:347–358. [DOI] [PubMed] [Google Scholar]
- 33. Kim R. Effects of surgery and anesthetic choice on immunosuppression and cancer recurrence. J Transl Med 2018;16:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Beilin B, Shavit Y, Razumovsky J, et al. Effects of mild perioperative hypothermia on cellular immune responses. Anesthesiology 1998;89:1133–1140. [DOI] [PubMed] [Google Scholar]
- 35. Wenisch C, Narzt E, Sessler DI, et al. Mild intraoperative hypothermia reduces production of reactive oxygen intermediates by polymorphonuclear leukocytes. Anesth Analg 1996;82:810–816. [DOI] [PubMed] [Google Scholar]
- 36. Nielsen HJ. Detrimental effects of perioperative blood-transfusion. Br J Surg 1995;82:582–587. [DOI] [PubMed] [Google Scholar]
- 37. Hiller JG, Perry NJ, Poulogiannis G, et al. Perioperative events influence cancer recurrence risk after surgery. Nat Rev Clin Oncol 2018;15:205–218. [DOI] [PubMed] [Google Scholar]
- 38. Desborough JP. The stress response to trauma and surgery. Br J Anaesth 2000;85:109–117. [DOI] [PubMed] [Google Scholar]
- 39. Angka L, Martel AB, Kilgour M, et al. Natural killer cell IFNγ secretion is profoundly suppressed following colorectal cancer surgery. Ann Surg Oncol 2018;25:3747–3754. [DOI] [PubMed] [Google Scholar]
- 40. Mehta OH, Barclay KL. Perioperative hypothermia in patients undergoing major colorectal surgery. ANZ J Surg 2014;84:550–555. [DOI] [PubMed] [Google Scholar]
- 41. Melling AC, Ali B, Scott EM, et al. Effects of preoperative warming on the incidence of wound infection after clean surgery: a randomised controlled trial. Lancet 2001;358:876–880. [DOI] [PubMed] [Google Scholar]
- 42. Rajagopalan S, Mascha E, Na J, et al. The effects of mild perioperative hypothermia on blood loss and transfusion requirement. Anesthesiology 2008;108:71–77. [DOI] [PubMed] [Google Scholar]
- 43. Frank SM, Fleisher LA, Breslow MJ, et al. Perioperative maintenance of normothermia reduces the incidence of morbid cardiac events. A randomized clinical trial. JAMA 1997;277:1127–1134. [PubMed] [Google Scholar]
- 44. Lenhardt R, Marker E, Goll V, et al. Mild intraoperative hypothermia prolongs postanesthetic recovery. Anesthesiology 1997;87:1318–1323. [DOI] [PubMed] [Google Scholar]
- 45. Kilkenny C, Browne WJ, Cuthill IC, et al. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 2010;8:e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ben-Eliyahu S, Yirmiya R, Shavit Y, et al. Stress-induced suppression of natural killer cell cytotoxicity in the rat: a naltrexone-insensitive paradigm. Behav Neurosci 1990;104:235–238. [DOI] [PubMed] [Google Scholar]
- 47. Corbett TH, Griswold DP, Jr., Roberts BJ, et al. Tumor induction relationships in development of transplantable cancers of the colon in mice for chemotherapy assays, with a note on carcinogen structure. Cancer Res 1975;35:2434–2439. [PubMed] [Google Scholar]
- 48. Shaashua L, Rosenne E, Neeman E, et al. Plasma IL-12 levels are suppressed in vivo by stress and surgery through endogenous release of glucocorticoids and prostaglandins but not catecholamines or opioids. Psychoneuroendocrinology 2014;42:11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liat S, Rivka M, Pini M, et al. Reducing liver metastases of colon cancer in the context of extensive and minor surgeries through beta-adrenoceptors blockade and COX2 inhibition. Brain Behav Immun 2016;58:91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Melamed R, Rosenne E, Shakhar K, et al. Marginating pulmonary-NK activity and resistance to experimental tumor metastasis: suppression by surgery and the prophylactic use of a beta-adrenergic antagonist and a prostaglandin synthesis inhibitor. Brain Behav Immun 2005;19:114–126. [DOI] [PubMed] [Google Scholar]
- 51. Melamed R, Rosenne E, Benish M, et al. The marginating-pulmonary immune compartment in rats: characteristics of continuous inflammation and activated NK cells. J Immunother 2010;33:16–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Faul F, Erdfelder E, Lang AG, et al. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007;39:175–191. [DOI] [PubMed] [Google Scholar]
- 53. Bucsek MJ, Qiao GX, MacDonald CR, et al. β-adrenergic signaling in mice housed at standard temperatures suppresses an effector phenotype in CD8 T cells and undermines checkpoint inhibitor therapy. Cancer Res 2017;77:5639–5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Saul AN, Oberyszyn TM, Daugherty C, et al. Chronic stress and susceptibility to skin cancer. J Natl Cancer Inst 2005;97:1760–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Xu PB, He H, Gu YC, et al. Surgical trauma contributes to progression of colon cancer by downregulating CXCL4 and recruiting MDSCs. Exp Cell Res 2018;370:692–698. [DOI] [PubMed] [Google Scholar]
- 56. Mohammadpour H, MacDonald CR, Qiao GX, et al. β2 adrenergic receptor-mediated signaling regulates the immunosuppressive potential of myeloid-derived suppressor cells. J Clin Invest 2019;129:5537–5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Inbar S, Neeman E, Avraham R, et al. Do stress responses promote leukemia progression? An animal study suggesting a role for epinephrine and prostaglandin-E through reduced NK activity. PLoS One 2011;6:e19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lamkin DM, Ho HY, Ong TH, et al. β-Adrenergic-stimulated macrophages: comprehensive localization in the M1–M2 spectrum. Brain Behav Immun 2016;57:338–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Armaiz-Pena GN, Gonzalez-Villasana V, Nagaraja AS, et al. Adrenergic regulation of monocyte chemotactic protein 1 leads to enhanced macrophage recruitment and ovarian carcinoma growth. Oncotarget 2015;6:4266–4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schrepf A, Clevenger L, Christensen D, et al. Cortisol and inflammatory processes in ovarian cancer patients following primary treatment: relationships with depression, fatigue, and disability. Brain Behav Immun 2013;30:S126–S134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Xu PB, Zhang P, Sun ZR, et al. Surgical trauma induces postoperative T-cell dysfunction in lung cancer patients through the programmed death-1 pathway. Cancer Immunol Immun 2015;64:1383–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Giuliano KK, Hendricks J. Inadvertent perioperative hypothermia: current nursing knowledge. Assoc Oper Room Nurs 2017;105:453–463. [DOI] [PubMed] [Google Scholar]
- 63. Sari S, Aksoy SM, But A. The incidence of inadvertent perioperative hypothermia in patients undergoing general anesthesia and an examination of risk factors. Int J Clin Pract 2021;75:e14103. [DOI] [PubMed] [Google Scholar]
- 64. Rauch S, Miller C, Brauer A, et al. Perioperative hypothermia - a narrative review. Int J Env Res Pub Health 2021;18:8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Shaw CA, Steelman VM, DeBerg J, et al. Effectiveness of active and passive warming for the prevention of inadvertent hypothermia in patients receiving neuraxial anesthesia: a systematic review and meta-analysis of randomized controlled trials. J Clin Anesth 2017;38:93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bandyopadhayaya S, Ford B, Mandal CC. Cold-hearted: a case for cold stress in cancer risk. J Therm Biol 2020;91:102608. [DOI] [PubMed] [Google Scholar]
- 67. Kokolus KM, Hong CC, Repasky EA. Feeling too hot or cold after breast cancer: is it just a nuisance or a potentially important prognostic factor? Int J Hyperther 2010;26:662–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hylander BL, Gordon CJ, Repasky EA. Manipulation of ambient housing temperature to study the impact of chronic stress on immunity and cancer in mice. J Immunol 2019;202:631–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Eng JWL, Reed CB, Kokolus KM, et al. Housing temperature-induced stress drives therapeutic resistance in murine tumour models through beta(2)-adrenergic receptor activation. Nat Commun 2015;6:6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kokolus KM, Capitano ML, Lee CT, et al. Baseline tumor growth and immune control in laboratory mice are significantly influenced by subthermoneutral housing temperature. Proc Natl Acad Sci USA 2013;110:20176–20181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kokolus KM, Spangler HM, Povinelli BJ, et al. Stressful presentations: mild cold stress in laboratory mice influences phenotype of dendritic cells in naive and tumor-bearing mice. Front Immunol 2014;5:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Shaashua L, Sominsky L, Levi B, et al. In vivo suppression of plasma IL-12 levels by acute and chronic stress paradigms: Potential mediating mechanisms and sex differences. Brain Behav Immun 2012;26:996–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Schulz O, Edwards AD, Schito M, et al. CD40 triggering of heterodimeric IL-12 p70 production by dendritic cells in vivo requires a microbial priming signal. Immunity 2000;13:453–462. [DOI] [PubMed] [Google Scholar]
- 74. Krug A, Towarowski A, Britsch S, et al. Toll-like receptor expression reveals CpG DNA as a unique microbial stimulus for plasmacytoid dendritic cells which synergizes with CD40 ligand to induce high amounts of IL-12. Eur J Immunol 2001;31:3026–3037. [DOI] [PubMed] [Google Scholar]
- 75. Boor P, Metselaar HJ, Mancham S, et al. Prednisolone suppresses the function and promotes apoptosis of plasmacytoid dendritic cells. Am J Transplant 2006;6:2332–2341. [DOI] [PubMed] [Google Scholar]
- 76. Hegde PS, Chen DS. Top 10 challenges in cancer immunotherapy. Immunity 2020;52:17–35. [DOI] [PubMed] [Google Scholar]
- 77. Gould SE, Junttila MR, de Sauvage FJ. Translational value of mouse models in oncology drug development. Nat Med 2015;21:431–439. [DOI] [PubMed] [Google Scholar]
- 78. Mak IWY, Evaniew N, Ghert M. Lost in translation: animal models and clinical trials in cancer treatment. Am J Transl Res 2014;6:114–118. [PMC free article] [PubMed] [Google Scholar]
- 79. Krieg AM. CpG still rocks! Update on an accidental drug. Nucleic Acid Ther 2012;22:77–89. [DOI] [PubMed] [Google Scholar]
- 80. Cunningham D, Salazar R, Sobrero A, et al. Lefitolimod vs standard of care (SOC) for patients with metastatic colorectal cancer (mCRC) responding to first-line standard treatment: results from the randomized phase III IMPALA trial. Ann Oncol 2019;30:868–869. [Google Scholar]
- 81. Hornung V, Rothenfusser S, Britsch S, et al. Quantitative expression of Toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol 2002;168:4531–4537. [DOI] [PubMed] [Google Scholar]
- 82. Dalpke AH, Heeg K. CpG-DNA as immune response modifier. Int J Med Microbiol 2004;294:345–354. [DOI] [PubMed] [Google Scholar]
- 83. Yoo JH, Ok SY, Kim SH, et al. Efficacy of active forced air warming during induction of anesthesia to prevent inadvertent perioperative hypothermia in intraoperative warming patients: comparison with passive warming, a randomized controlled trial. Medicine 2021;100:e25235. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated during this study will be available upon request.