Abstract
Introduction:
Sacrococcygeal teratoma (SCT) is a rare congenital tumour. The risk of malignancy and recurrence is not well defined. Previous studies are small and report differing conclusions about the timing of surgery and the duration of follow-up. The authors studied the risk of malignant transformation and SCT recurrence after surgery to address these gaps.
Methods:
This was a global retrospective cohort study. Data of consecutive SCT patients was obtained from 145 institutes in 62 countries. Malignant transformation, defined as malignancy at initial resection, malignant recurrence or death due to malignancy, and its risk factors were analysed.
Results:
Of the 3612 included patients, 3407 entered analysis. The risk of malignant transformation of the initial tumour was 3.3, 5.1, 10.1, and 32.9% at age 3 months, 6 months, 1 year, and 2 years, respectively. After 6 years, the censored risk of malignancy (64%) did not further increase. Recurrent SCT was diagnosed in 349 (10.2%) children with 126 (36.1%) malignant recurrences. Risk factors for recurrence were Altman type II [odds ratio (OR): 1.6, 95% confidence interval (CI): 1.2–2.2], Altman type III (OR: 1.6, 95% CI: 1.2–2.3), initial immature histology (OR: 1.9, 95% CI: 1.4–2.6), and initial malignant histology (OR: 4.0, 95% CI: 2.9–5.4).
Conclusion:
The risk of malignancy at initial resection in SCT increases with age reaching a plateau at 6 years of age. Recurrence after resection occurred in 10% of patients and 36% of these were malignant at that time. Altman type II or type III, and immature or malignant histology were associated with recurrence.
Level of evidence:
Level III.
Keywords: malignant transformation, recurrence, sacrococcygeal teratoma
Introduction
Highlights
This global retrospective cohort study includes 3612 sacrococcygeal teratoma patients from 62 countries worldwide.
The risk of malignant transformation increases with age.
The risk of recurrent sacrococcygeal teratoma after initial resection is 10%.
If possible, complete resection of sacrococcygeal teratoma is recommended before age 1 month.
Postoperatively, 6 years of follow-up is advised to detect possible recurrence.
Sacrococcygeal teratoma (SCT) is the most common neonatal tumour with a reported incidence of one per 14 000–35 000 live births1. The preferred treatment for SCT is complete resection at a young age. However, there is no generally accepted age at which resection should be done. Some clinicians advocate early surgery to minimise the risk of malignant tumour transformation as malignancy rates up to 70% have been reported if SCT is resected at age one year or older2. Others claim that surgery at a young age may lead to more operative and postoperative complications3.
Tumour recurrence after surgery occurs in two to 33% of patients and decreases the overall 10-year survival ranging from 60 to 92%2,4,5. Recurrent SCT is much more often malignant than SCT at initial resection with yolk sac tumour (YST) found in 22–56% of recurrences1,6–8. Definitive risk factors for tumour recurrence are relatively unknown, although tumour histology and completeness of resection, including coccygectomy, are reported as potential risk factors for recurrence2,5. However, most studied series are relatively small due to the rarity of the disease and the results are often contradictory. Furthermore, the duration of follow-up varies in the existing literature, which likely impacts the reported recurrence rate; although most recurrences present within 3 years after initial SCT resection, late recurrences up to 15 years have been described1,5. From a clinical perspective, there is no consensus regarding the appropriate duration of oncological follow-up of SCT patients.
The vast majority of the reported series of patients with SCT are from high-income countries (HICs) in which SCT patients are operated on within a few weeks after birth. The number of publications about SCT from low-income countries (LICs) and lower-middle-income countries (LMICs) is very limited9,10. Recently, unacceptable differences in mortality for congenital gastrointestinal anomalies have been shown in LICs, compared to upper middle-income countries (UMICs), and high-income countries (HIC’s)11. It is unknown if these differences are also present for SCT.
Given these uncertainties, we aimed to estimate the risk of malignant transformation of untreated SCT at different ages and, hence, the optimal time for resection. Furthermore, we aimed to identify factors associated with recurrent SCT and finally compare the treatment and outcome of SCT patients from different income countries.
Methods
Study design and participants
We did a global retrospective cohort study of SCT patients: ‘The SCT-study’ STROBE (The Strengthening the Reporting of Observational Studies in Epidemiology) and strengthening the reporting of cohort, cross-sectional, and case–control studies in surgery (STROCSS) (Supplemental Digital Content 1, http://links.lww.com/JS9/D406) 2021 guidelines were followed12,13.
We recruited as many patients from participating hospitals as possible regardless of geography. Paediatric surgeons and paediatric oncologists were invited to participate in the study through personal communication, the European Paediatric Surgeons’ Association (EUPSA) Network Office, PubMed publications, and a network of national and international study leads. Study information was provided in English, Spanish, French, and Russian. Participation was voluntary; no payment was made for data collection. Centres, which could include ten or more patients were invited to participate. An exception was made for centres from LICs and LMICs. For these centres, the minimum number of inclusions was set at five. The Supplementary Appendix (Supplemental Digital Content 2, http://links.lww.com/JS9/D407) provides an overview of the participating countries.
Due to the rarity of the condition, no sample size calculation was done and all eligible patients were included. Consecutive patients treated for SCT between 1982 and 2020 or a shorter time period in this era could be included into the study. Exclusion criteria were (a) born before 1982, (b) born after 2020, or (c) SCT as part of Currarino Syndrome (CS) as the risk of malignant transformation in (CS) may be reduced compared to ‘ordinary’ SCT14,15. The exact period of time for inclusions was determined by each individual participating centre as long as all consecutive patients in the chosen period were included.
All participating centres obtained local approval to participate in the study following their own legal and ethical regulations. Data transfer agreements were used to guarantee safe data use and storage.
Charts were reviewed by the individual local investigator. Data were validated with warning messages about possible errors when entered in Castor Electronic Data Capture (EDC). Furthermore, Castor files were structured so that out-of-range values could not be entered. Dependency fields were used for data and included initial resection, recurrence, death and follow-up. Furthermore, overall data distribution and frequencies in SPSS were checked to detect invalid entered data.
Procedures
Data was anonymized by the local investigator to transform individual patient data into general anonymous information. Transformed patient data were uploaded with a personal link in Castor EDC and encrypted16. Every participating centre had only access to its own transformed patient data.
Included data was carefully selected by a group of experienced paediatric surgeons with interest in SCT treatment and was chosen based on main outcome variables used in previously published studies.
Collected data included generic and condition-specific variables. Generic variables included: country, sex (male/female/unknown), age at diagnosis (days), preoperative imaging modalities (none/ultrasound/computed tomography/MRI/unknown), initial tumour resection at the participating centre (yes/no/unknown), age at initial resection (days), outcome (survival/deceased/unknown), age at follow-up (days), age at death (days), and cause of death. Condition-specific variables were Altman classification (I/II/III/IV/unknown)17, CS (yes/no/unknown), initial SCT treatment (chemotherapy/surgery/no treatment/unknown), pathology (mature/immature/malignant/unknown), recurrence (yes/no/unknown), period between birth and recurrence (days), detection of recurrence (clinical examination/imaging/AFP/unknown), serum AFP-level at recurrence (μg/l), recurrent SCT pathology (mature/immature/malignant/unknown), and treatment of recurrent SCT (chemotherapy/surgery/no treatment/unknown).
Cause of death was collected as a free-text category. Participating country was used to categorise the country’s income status into LICs, LMICs, UMIC, and HICs according to the World Bank criteria18.
Statistical analysis
Data are presented as mean with standard deviation (SD) if normally distributed and median with interquartile range (IQRs) if skewed; count data are presented as numbers and percentages. To assess malignant transformation of initial SCT and risk of recurrence over time, Kaplan-Meier curves were used.
Identification of factors associated with recurrent SCT was done by univariable and multivariable logistic regression analysis. All variables included in multivariable logistic regression analysis had maximum of 6.8% missing data. Sensitivity analysis was performed to analyse the effect of data imputation on the results of the multivariable logistic regression analysis.
Forward Wald selection was used to select variables significantly related to recurrent SCT. We report variables with the odds ratio (OR) and accompanying 95% confidence interval (CI).
Differences in patient demographics between country income were analysed with Fisher exact test for categorical variables, one-way ANOVA for normally distributed continuous variables, and Kruskal–Wallis test for non-normal continuous variables.
Statistical analyses were performed using SPSS for Windows version 25.0 software (SPSS) and Graph Pad Prism 8 (Graph Pad Software, Inc.). P<0.05 was considered statistically significant.
Definitions
SCTs were classified according to the criteria proposed by the Surgical Section of the American Academy of Paediatrics17. Recurrence was defined as relapse of SCT at least 3 months after initial resection15. Recurrent SCT before 3 months is unlikely and is probably due to incomplete resection, therefore, these recurrences were excluded from data analysis. Malignancy-free survival was defined as time from birth to malignancy or death due to malignancy. Patients were censored at the number of days from birth to resection. In case of malignant recurrence or death due to malignancy, the number of days from birth to recurrence detection or death was used.
Variables investigated for recurrent SCT, with their respective categories, were Altman classification (I, II, III, and IV), histology (mature, immature, and malignant), age at diagnosis (days), income of the country (LIC, LMIC, UMIC, and HIC), and age at initial resection (days).
Results
Patient characteristics
In total, 145 centres from 62 countries treated 3612 patients for SCT. In 205 patients, SCT was associated with CS. These patients were excluded from the analysis. In total, 3407 patients were included in the study. Patient characteristics for the total population are described in Table 1.
Table 1.
Patient characteristics for the total population and stratified per income countries.
| Total (n=3407) | High-income countries (n=2296) | Upper-middle income countries (n=677) | Lower-middle income countries (n=377) | Low-income countries (n=57) | P | |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Male | 844 (24.8%) | 571 (24.9%) | 161 (23.8%) | 103 (27.3%) | 9 (15.8%) | 0.067 |
| Female | 2500 (73.4%) | 1722 (75.0%) | 491 (72.5%) | 239 (63.4%) | 48 (84.2%) | |
| Missing | 63 (1.8%) | 3 (0.1%) | 25 (3.7%) | 35 (9.3%) | ||
| Median age at diagnosis, days | 0 (0–54) | 0 (0–17.5) | 1 (0–108.5) | 8 (0–150) | 30 (2.5–556.5) | <0.001 |
| Altman classification | ||||||
| I | 1036 (30.4%) | 669 (29.1%) | 227 (33.5%) | 125 (33.2%) | 15 (26.3%) | <0.001 |
| II | 1118 (32.8%) | 753 (32.8%) | 201 (29.7%) | 137 (36.3%) | 27 (47.4%) | |
| III | 609 (17.9%) | 407 (17.7%) | 130 (19.2%) | 63 (16.7%) | 9 (15.8%) | |
| IV | 548 (16.1%) | 432 (18.8%) | 851 (12.6%) | 25 (6.6%) | 6 (10.5%) | |
| Missing | 96 (2.8%) | 35 (1.5%) | 34 (5.0%) | 27 (7.2%) | – | |
| Median age at resection, days | 13 (4–134.3) | 9 (3–99) | 17 (6–187.3) | 40 (14–271.5) | 52 (14.0–570) | <0.001 |
| Pathology | ||||||
| Mature | 2168 (63.7%) | 1504 (65.5%) | 442 (65.3%) | 184 (48.8%) | 38 (66.7%) | 0.004 |
| Immature | 625 (18.3%) | 469 (20.4%) | 91 (13.4%) | 54 (14.3%) | 11 (19.3%) | |
| Malignant | 366 (10.7%) | 245 (10.7%) | 91 (13.4%) | 27 (7.2%) | 3 (5.3%) | |
| Missing | 248 (7.3%) | 78 (3.4%) | 53 (7.8%) | 112 (29.7%) | 5 (8.8%) | |
| Recurrence | ||||||
| Yes | 349 (10.2%) | 254 (11.1%) | 56 (8.3%) | 33 (8.8%) | 6 (12.3%) | 0.604 |
| No | 2829 (83.0%) | 1967 (85.7%) | 536 (79.2%) | 276 (73.2%) | 50 (87.7%) | |
| Missing | 229 (6.8%) | 75 (3.3%) | 85 (12.6%) | 68 (18.0%) | 1 (1.8%) | |
| Median time between primary resection and recurrence, days | 348 (196–666) | 348 (200.5–679) | 358 (182.5–673.8) | 357 (195.5–668) | 311.5 (131.5–654.5) | 0.948 |
| Recurrence pathology | ||||||
| Mature | 117 (33.5%) | 96 (37.8%) | 14 (25.0%) | 7 (21.2%) | – | 0.001 |
| Immature | 34 (9.7%) | 23 (9.1%) | 4 (7.1%) | 7 (21.2%) | – | |
| Malignant | 126 (36.1%) | 91 (35.8%) | 22 (39.3%) | 7 (21.2%) | 6 (100%) | |
| Missing | 72 (20.6%) | 44 (17.3%) | 16 (28.6%) | 12 (36.4%) | – | |
| Outcome | ||||||
| Alive | 2876 (84.4%) | 2073 (90.3%) | 534 (78.9%) | 219 (58.1%) | 50 (87.7%) | 0.059 |
| Death | 140 (4.1%) | 93 (4.1%) | 25 (3.7%) | 20 (5.3%) | 2 (3.5%) | |
| Missing | 391 (11.5%) | 130 (5.7%) | 118 (17.4%) | 138 (36.6%) | 5 (8.8%) | |
Data are n (%) or median (IQR).
P values represent univariable testing between country income strata.
Preoperative imaging was done with ultrasound in 595 (17.5%), CT in 280 (8.2%), and MRI in 526 (15.4%) patients, respectively. A combination of imaging modalities was performed in 1400 (41.1%) patients. In 606 children (17.8%), no imaging was performed or was unknown.
Surgery was the initial treatment in 2947 (86.5%) children. Surgery and chemotherapy were applied in 388 (11.4%) patients. Sixteen (0.4%) children received only chemotherapy. Furthermore, 13 (0.4%) children received no treatment and died immediately after birth due to bleeding or respiratory distress. In 43 (1.3%) children, treatment was unknown.
Overall for the entire cohort, the median follow-up was 5.1 years (IQR 2.3–9.2 years) after resection. In total, 140 (4.1%) patients died at a median age of 10.2 months (IQR 3 days–3.2 years). Ten years after the initial resection, 505 patients remained in follow-up. The 5-year survival was 95.4% and 10-year survival was 94.8%.
Malignant transformation
Histological diagnosis after initial resection was mature teratoma in 2168 patients (63.7%), immature teratoma in 625 (18.3%), and malignant teratoma in 366 (10.7%). In 248 patients (7.3%), the histological diagnosis was unknown. The proportion of patients with malignant SCT increased with age. The probability of malignant transformation diagnosed at initial resection starts to increase directly after birth and increases further with age. The risk was 3.3, 5.1, 10.3, and 32.9% at 3 months, 6 months, 1 year, and 2 years, respectively (Fig. 1). After 6 years of age, the probability of initial malignant SCT did not further increase (64.2%). After 6 years, only one patient presented with an initial malignant SCT at the age of 8 years.
Figure 1.
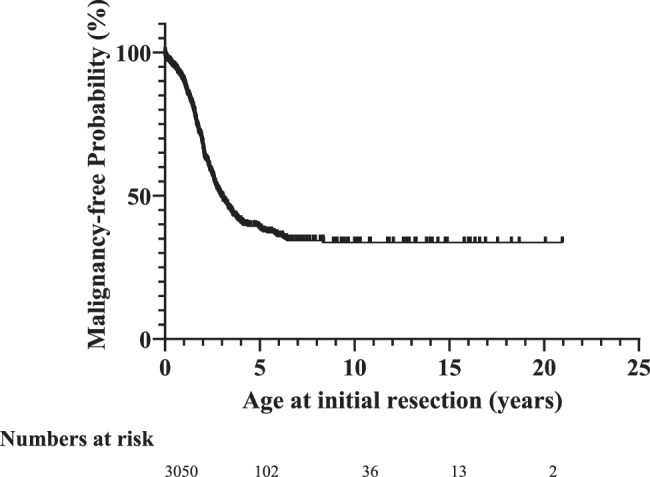
Malignancy-free probability at initial SCT resection.
Malignancy-free survival including initial malignancies, malignant recurrences and deaths due to malignant disease, was 94.7% at age 1 year and 88.2% at 2 years (Fig. 2). Probability of overall malignancy-free survival remained relatively stable after the age of 6 years at 80.2%. After this period, four late malignancies were found; one initial malignancy, one malignant recurrence and two deaths due to malignancy. Late malignancies up to 15 years of age were found with an overall malignancy-free survival of 79.1%.
Figure 2.
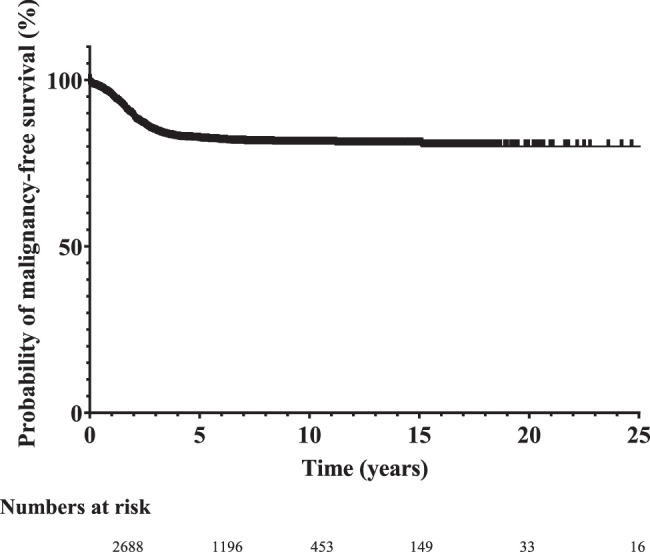
Probability of malignancy-free survival.
Recurrent sacrococcygeal teratoma
Three hundred forty-nine children (10.2%) developed recurrences at a median period of 11.4 months (IQR 6.4 months–1.8 years) after surgery. Ninety-six per cent of recurrences presented within the first 5 years after initial resection with a probability of recurrence-free survival after 5 years of 85.9% (Fig. 3). Late recurrences occurred up to 22.1 years after initial resection. Forty children died after recurrence at a median age of 15.6 months (IQR 6.2 months–2.3 years) after recurrence detection. Thirty died due to tumour progression or complications of chemotherapy. In 10 patients the cause of death was unknown. The other 284 survived; in 25 patients the outcome after recurrence was unknown.
Figure 3.
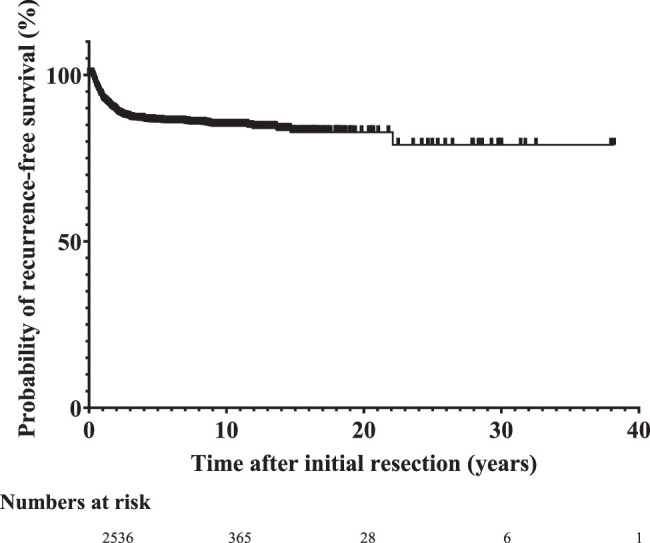
Probability of recurrence-free survival.
Recurrent SCT was resected in 131 children, in 157 resections was combined with chemotherapy, and 33 children were treated with chemotherapy only. Four children received no treatment and treatment was unknown in 24 children.
The histology of recurrent SCT was mature teratoma in 117, immature teratoma in 34, and malignant teratoma in 126 children. The histology of recurrent SCT was unknown in 72 children. In 65 tumours (18.6%), there was a shift towards immaturity or malignancy.
Income country
In this global study, 62 countries participated. Table 1 shows the main patient characteristics per income group. SCT diagnosis and resection were later in LICs compared to LMICs, UMICs and HICs. The median age at diagnosis was 30 days (IQR 2.5–556.5) in LICs, 8 (0–150) in LMICs, 1 (0–108.5) UMICs, and 0 (0–17.5) in HICs (P<0.001). The median age at resection was older: 52 days (IQR 14.0–570) in LICs compared to 40 (14–271.5), 17 (6–187.3), and 9 (3–99) days in LMICs, UMICs, and HICs, respectively (P<0.001). Despite the earlier diagnosis and resection, a higher proportion of malignant SCTs at initial treatment was found in HICs (n=245, 10.7%) and UMICs (n=91, 13.4%) compared to LMICs (n=27, 7.2%) and LICs (n=3, 5.3%) (P=0.004). The recurrence rates in all income groups were equivalent (P=0.604).
Recurrence histology differed between groups with a malignancy percentage of 100% in LIC compared to 21.2, 39.3, and 35.8% in LMICs, UMICs, and HICs, respectively (P=0.001). However, only six (12.2%) patients in LICs presented with recurrent SCT.
Factors associated with recurrent SCT
Logistic regression analysis with forward selection using the Wald statistics was used to determine factors associated with recurrent SCT. Sensitivity analysis with multiple imputation showed no effect of the imputed data on the multivariable outcomes. Therefore, missing data imputation was not done. The final models showed a good fit according to the Hosmer and Lemeshow test with a value of 0.993 and a Concordance Index of 0.655 with the following variables being associated with recurrent SCT: Altman type II (OR 1.62, 95% CI: 1.18–2.23), Altman type III (OR 1.63, 95% CI: 1.23–2.35), immature pathology of the initial tumour (OR 1.91, 95% CI: 1.43–2.56), and malignant pathology of the initial tumour (OR 4.0, 95% CI: 2.91–5.41). Income of countries (P=0.760), age at diagnosis (P=0.254), and age at resection (P=0.073) were not associated with recurrence (Table 2).
Table 2.
Risk factors for recurrent SCT.
| Univariable | Multivariable | ||||
|---|---|---|---|---|---|
| Recurrence rate | Odds ratio | P | Odds ratio | P | |
| Altman classification | |||||
| I | 73 of 1036 | 1 | |||
| II | 122 of 1118 | 1.631 (1.203–2.211) | 0.002* | 1.624 (1.180–2.234) | 0.003* |
| III | 76 of 609 | 1.900 (1.353–2.668) | <0.001* | 1.627 (1.227–2.348) | 0.009 |
| IV | 63 of 548 | 1.696 (1.189–2.419) | 0.004 | 1.423 (0.967–2.093) | 0.073 |
| Income country | |||||
| Low | 6 of 57 | 1 | |||
| Lower-middle | 33 of 377 | 0.996 (0.397–2.502) | 0.994 | ||
| Higher-middle | 56 of 677 | 0.871 (0.357–2.121) | 0.760 | ||
| High | 254 of 2296 | 1.076 (0.457–2.535) | 0.867 | ||
| Primary histology | |||||
| Mature | 155 of 2168 | 1 | |||
| Immature | 80 of 625 | 1.863 (1.3980–2.481) | <0.001* | 1.911 (1.427–2.558) | <0.001* |
| Malignant | 91 of 366 | 4.337 (3.245–5.795) | <0.001* | 3.965 (2.906–5.411) | <0.001* |
| Median age at diagnosis (days) | |||||
| Yes | 1 (0–248.3) | ||||
| No | 0 (0-40) | 0.254 | |||
| Median age at resection (days) | |||||
| Yes | 17 (4–318) | 0.073 | |||
| No | 13 (4–120) | ||||
Discussion
This global, retrospective study provides information about the treatment and outcome of 3407 patients with SCT in 62 countries. We found that the risk of initial malignant transformation increases with age, with a malignancy rate of ~30% after 2 years. After 6 years, the risk of initial malignant transformation stabilized at 64%. Furthermore, due to the increasing risk of malignant transformation, initial resection is preferably done at a young age. However, young neonates and infants are at higher risk of operative and postoperative complications3. In the current study, information about gestational age at birth or operative and postoperative complications has not been collected, which could influence the optimal time for resection.
Overall malignancy-free survival was 94.7% at age 1 year and 88.2% at 2 years. This was higher than reported by others with 80 and 58% of malignancy-free survival at age 1 and 2 years, respectively15. However, the latter study defined malignancy-free survival as time from birth to malignancy or death. In the present study, only deaths due to malignant disease were included in the survival analysis. Malignancy-free survival stabilized after the age of 6 years at 80%.
In this study, 349 (10.2%) children developed recurrence after a median period of 11.4 months after initial resection with late recurrences up to 22 years after initial resection. Others have also described occasional late recurrences up to 15 years after surgery1. There is no consensus about the duration of follow-up after SCT resection and recommendation varies from 3 to 6 years after resection5,19. In the current study, 96% of the recurrences were found within 5 years after initial resection including all malignant recurrences.
Factors associated with SCT recurrence were Altman type II or type III and initial immature or malignant histology, which has been previously documented5. Other risk factors for recurrence described by others included incomplete resection, no coccyx removal, and tumour spillage5,20. In the current study, data of these risk factors was not collected.
Income countries
Age at diagnosis and initial SCT resection were older in patients from LICs and LMICs compared to UMICs and HICs. This was also found in a recent study in children with retinoblastoma. This is probably due to late recognition and limited access to care21. In LIC Uganda only, it is estimated that only 15.2% of the Ugandan children with SCT presents in the hospital22. Moreover, the lack of paediatric intensive care facilities may force surgeons to postpone the SCT operation to an age with more physiological reserve.
In our study, the overall patient survival of children for all income countries was equivalent, which shows that SCT surgery can be done well and safely in LICs and LMICs. This is in contrast with others who found far better surgical outcomes for infants with other congenital disorders in HICs than in LICs or LMICs and found the income of the country as the greatest risk of mortality11.
The difference with our study results may be explained by the very small and selected proportion of SCT patients who present for surgery in LICs and LMICs22. In many LICs and LMICs, patients with large tumours might have died without reaching the hospital due to cultural believes or inability to access paediatric surgical care. Evidence for this presumption comes from the fact that many centres invited to participate in the study declined because they never resected a SCT. Furthermore, the proportion of LICs and LMICs with a traditionally good healthcare system such as Syria, was relatively high in our study.
Many SCT patients who present in hospitals in LICs and LMICs do not receive components of neonatal surgical care that are considered essential in HICs. It is estimated that only half of the hospitals, with paediatric surgery in West African countries have neonatal ICU and many countries have fewer than one paediatric surgeon per million children9,11,23–25.
Limitations
This study has several limitations such as its retrospective study design and long inclusion period. The incidence of SCT is very low, therefore, it would take a very long time before a similar prospective dataset could be obtained. Secondly, the number of collected variables was limited. This may have facilitated participation. Most variables could be obtained from electronic data files or patient letters. The percentage of missing data used in the analyses was, therefore, small, with a maximum percentage of 6.8%. Thirdly, the use of anonymized data made data validation not possible. On the other hand, this also may have reduced the risk of selection bias by not sharing data with an unfavourable outcome. Finally, there is bias because an unknown proportion of patients has not been included in the study. In an unknown but probably a relatively small number of patients in Western countries, the pregnancy of SCT patients is discontinued. Many SCT patients in LICs and LMICs do not receive any treatment.
Recommendations
We recommend complete resection of SCT before 1 month of age to minimise the risk of malignant transformation as long as it is safe and feasible. Furthermore, we advise to follow-up to 6 years of age since the vast majority of recurrences present within this timeframe. After this period, the chance of malignant transformation is probably small.
Conclusion
The United Nations encourages nations to create networks of experts for rare diseases and to increase support for research, by strengthening international collaboration and coordination of research efforts and the sharing of data, while respecting its protection and privacy26. We have shown that it is feasible to set up a large global study for a rare disease. The comprehensive study design herein and the large patient cohort enabled the identification of risk factors for recurrent SCT and essential information regarding malignant SCT transformation. With large, shared patient data sets, it is possible to answer important clinical questions that cannot be answered otherwise in order to improve the quality of care in every part of the world.
The SCT-study consortium
Siffredi Juan Ignacio (Department of Pediatric Surgery, Hospital Garrahan Buenos Aires, Argentina); Pablo Lobos (Hospital Italiano de Buenos Aires, Argentina); Holger Till (Head of the Dept. of Paediatric and Adolescent Surgery, Medical University of Graz, Austria); Ashrarur Rahman Mitul (Bangladesh Shishu Hospital & Institute, Dhaka, Bangladesh); Olga Govorukhina (Center of Pediatric Surgery of Belarus); Natalya Prokopenya (Center of Pediatric Surgery of Belarus); Antoine De Backer (Universitair Ziekenhuis Brussel and Saffier Network for Rare Diseases in Pediatric Surgery, Belgium); Helena Reusens (Department of Pediatric Surgery, Hôpital des Enfants Reine Fabiola, Université Libre de Bruxelles); Simone de Campos Vieira Abib (Pediatric Oncology Institutute - GRAACC - Federal University of São Paulo, Brazil); Penka Peneva Stefanova-Peeva (Department of Pediatric surgery, University Hospital "St. George" and Medical University, Plovdiv, Bulgaria); Nadezhda Tolekova (Pediatric Surgery department of UMHATEM "N. I. Pirogov" Sofia, Bulgaria); Mouafo Tambo Faustin (Yaounde Gynaeco-Obstetric and Pediatric Hospital-faculty of medecine and biomedical sciences- University of Yaounde Cameroon); Jean-Martin Laberge (McGill University, Montreal Children’s Hospital – Shriners Hospital for Children Canada); Augusto Zani (Division of General and Thoracic Surgery, The Hospital for Sick Children, Toronto, ON, Canada); Richard J.B. Walker (Division of General and Thoracic Surgery, The Hospital for Sick Children, Toronto, Canada; Division of General Surgery, Department of Surgery, University of Toronto, Toronto, Canada); Maricarmen Olivos Pérez (Hospital de Niños Dr. Roberto del Rio. Santiago, Chile); Marco Andrés Valenzuela (Hospital de Niños Dr. Roberto del Río. Santiago, Chile); Yuanchao Shen (Department of Pediatric Surgery, Guangzhou Women and Children’s Medical Center, Guangzhou Medical University, China); Yan Zou (Department of Pediatric Surgery, Guangzhou Women and Children's Medical Center, Guangzhou Medical University, Guangzhou,510623, China); Stanko Ćavar (Division pediatric surgery, University Hospital centre Zagreb, Croatia); Zenon Pogorelić (Department of Pediatric Surgery, University Hospital of Split and Department of Surgery, University of Split, School of Medicine, Croatia); Lucie Pos (Department of Paediatric Surgery, 2nd Faculty of Medicine, Charles University and University Hospital Motol, V Uvalu 84, Prague 5, 150 06, Prague, Czech Republic); Richard Skaba (Department of Paediatric surgery, 2nd Faculty of Medicine, Prague, Czech Republic); Peter Hjorth Jørgensen (Department of Pediatric Surgery, Rigshospitalet Copenhagen University Hospital, Copenhagen, Denmark); Amr Abdelhamid AbouZeid (Faculty of medicine, Department of Pediatric Surgery; Ain Shams University, Cairo, Egypt); Mahmoud Elfiky (Kasr Al Ainy Faculty of Medicine, Cairo University); Heba Taher (Pediatric Surgery Cairo University, Egypt); Matis Märtson (Tallinn Children's Hospital, Head of the Surgical Clinic, Tallinn, Estonia); Miliard Derbew (Medical Education Partnership Initiative Junior Faculty Project, School of Medicine, College of Health Sciences, Addis Ababa University, Ethiopia); Workye Molla Tigabie (St.Peter Specialized Hospital, Addis Ababa, Ethiopia); Antti Koivusalo (New Children´s Hospital, (Section of Pediatric Surgery, University of Helsinki), Helsinki, Finland); Mikko Pakarinen (Section of Pediatric Surgery, Pediatric Liver and Gut Research Group, New Children’s Hospital, University of Helsinki and Helsinki University Hospital, Helsinki, Finland, and Department of Women’s and Children’s Health, Karolinska Institute, Stockholm, Sweden); Olivier Abbo (Surgical Department, Hôpital des Enfants de Toulouse, France); Alexis Pierre Emmanuel Arnaud (Department of pediatric surgery, CHU Rennes, Univ Rennes, Rennes, France); Quentin Ballouhey (Department of Pediatric Surgery, Limoges University Hospital Center, Limoges, France); Francois Bastard (University Hospital, Angers, France); Anne Dariel (Pediatric surgery department, Hospital for sick children La Timone, Assistance publique des hôpitaux de Marseille, Marseille, France); Sabine Irtan (Sorbonne Université, Armand Trousseau Hopsital –Assistance Publique Hôpitaux de Paris, Paris, France); Jean Francois Lecompte (Pediatric Surgery Department, Hopitaux Pédiatriques de Nice CHU-Lenval, Nice, France); Guillaume Levard (Pediatric Surgery Department, University Hospital, Poitiers); Sabine Sarnacki (Department of Pediatric Surgery, Hôpital Necker-Enfants Malades - APHP GH Centre and Université de Paris Cité, Paris, France); Rony Sfeir (Pediatric Surgery Unit, University Hospital of Lille Jeanne de Flandre, Lille, France); Nicolas Vinit (Department of Pediatric Surgery and Urology, Necker-Enfants Malades Hospital, APHPand Université Paris Cité, Paris, France); Anne-Sophie Holler (Department of Pediatric Surgery, Dr. von Hauner Children's Hospital, University Hospital, LMU Munich); Marietta Jank (Department of Pediatric Surgery, University Medical Center Mannheim, Heidelberg University, Mannheim, Germany); Martin Lacher (Head of Department of Pediatric Surgery, University of Leipzig, Leipizig, Germany; Oliver J. Muensterer (Department of Pediatric Surgery, Dr. von Hauner Children's Hospital, University Hospital, LMU Munich, Germany); Karin Rothe (Department of Pediatric Surgery, Charité Universitätsmedizin Berlin, corporate member of Freie Universität Berlin and Humboldt Universität zu Berlin, Germany); Udo Rolle (University Hospital Frankfurt/M., Department of Paediatric Surgery and Paediatric Urology); Zoi Lamprinou (P. & A. Kyriakou Children’s Hospital, Athens, Greece); Zsuzsanna Jakab (Hungarian Childhood Cancer Registry, 2nd Department of Pediatrics, Semmelweis University, Budapest, Hungary); Peter Vajda (Division of Paediatric Surgery, Medical School, University of Pécs, Hungary); Agnes Vojcek (Division of Pediatric Hematology and Oncology, Department of Pediatrics, University of Pécs Medical School, Pécs, Hungary); Kenneth KY Wong (Division of Paediatric Surgery, Department of Surgery, Li Ka Shing Faculty of Medicine, University of Hong Kong, Queen Mary Hospital, 102 Pokfulam Road, Hong Kong, Hong Kong); Mohan K. Abraham (Department of Pediatric surgery and Pediatric urology, Amrita Institute Of medical sciences, Kochi, Kerala, India); Kirtikumar J Rathod (Department of Pediatric Surgery, All India Institute of Medical Sciences, Jodhpur, India); Shilpa Sharma (Department of Pediatric Surgery, All India Institute of Medical Sciences, New Delhi); Gunadi (Pediatric Surgery Division, Department of Surgery, Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada, Dr. Sardjito Hospital, Yogyakarta, Indonesia); Mehdi Sarafi (Pediatric Surgery Research Center, Research Institute for Children’s Health, Shahid Beheshti University of Medical Sciences, Teheran, Iran); Ahmad Khaleghnejad Tabari (Pediatric Surgery Research Center, Research Institute for Children's Health, Shahid Beheshti University of Medical Sciences, Tehran, Iran); Muataz Al Ani (Ninevah college of Medicine, Alkhansaa teaching hospital, Mosul pediatric surgery centre, Iraq); Gavin Kane (Department of Paediatric Surgery, Children's Health Ireland at Crumlin, Dublin, Ireland); Igor Sukhotnik (Department Pediatric Surgery, Tel Aviv Sourasky Medical Center, Tel Aviv University, Israel); Stefano Avanzini (Pediatric Surgery Department, IRCCS Istituto Giannina Gaslini, Genoa, Italy); Pietro Bagolan (Area of Fetal, Neonatal, and Cardiological Sciences, Children's Hospital Bambino Gesù-Research Institute, Rome, Italy; Department of Systems Medicine, University of Rome "Tor Vergata", Rome, Italy); Piergiorgio Gamba (Pediatric Surgery, Department of Women's and Children's Health, University of Padua, Padua, Italy); Riccardo Guanà (Pediatric and Neonatal Surgery Unit, Regina Margherita Children’s Hospital, Turin, Italy); Alessandro Inserra (Tor Vergata University of Rome, Director of the graduate school in Pediatric Surgery; Academic Director of Pediatric Surgery, Head of U.O.C.General and Thoracic Surgery, Bambino Gesu' Pediatric Hospital); Mario Lima (Pediatric Surgery Sant'Orsola Hospital, IRCSS, University of Bologna, Bologna, Italy); Antonino Morabito (Meyer Children's Hospital IRCCS Florence, University of Florence, Italy); Alessandro Raffaele (Department of Pediatric Surgery, Fondazione IRCCS Policlinico San Matteo, Pavia, Lombardia, Italy); Giovanna Riccipetitoni (Fondazione IRCCS Policlinico San Matteo, Pavia, Italy); Calogero Virgone (Pediatric Surgery, Department of Women's and Children's Health, University of Padua, Padua, Italy); Yapi Landry Ake (Cocody Teaching hospital at Abidjan in Ivory Coast ; Department of Mother and Child health, pediatric surgery); Yoshiaki Hirohata (Department of Pediatric Surgery, Kyoto Prefectural University of Medicine, Kyoto, Japan); Maho Inoue (Department of Surgery, Saitama Children's Medical Center, Saitama, Japan); Yutaka Kanamori (Department of General Surgery, National Center for Child Health and Development, Tokyo, Japan); Naonori Kawakubo (Department of Pediatric Surgery, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan); Takashi Sasaki (Department of Pediatric Surgery, Osaka City General Hospital, Osaka, Japan); Tomoaki Taguchi (Department of Pediatric Surgery, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan); Tatsuro Tajiri (Department of Pediatric Surgery, Kyoto Prefectural University of Medicine, Kyoto, Japan); Hirofumi Tomita (Department of Surgery, Tokyo Metropolitan Children's Medical Center, Tokyo, Japan); Noriaki Usui (Department of Pediatric Surgery, Osaka Women's and Children's Hospital, Izumi, Japan); Mohit Kakar (Department of Pediatric Surgery, Riga Stradins University & Children's Clinical University Hospital, Riga, Latvia); Vidmantas Barauskas (Vaikų chirurgijos klinika, Lietuvos sveikatos mokslų universiteto ligoninė Kauno klinikos, Eivenių g. 2 LT-50161, Kaunas); Toni Risteski (University Clinic for Pediatric Surgery, Faculty of Medicine, Ss. Cyril and Methodius, University in Skopje, N Macedonia); Dayang Anita Abdul Aziz (Paediatric Surgery Unit, Department of Surgery, Faculty of Medicine, Universiti Kebangsaan Malaysia, Kuala Lumpur, Malaysia); Mohd Yusran Othman (Hospital Tunku Azizah, Kuala Lumpur Women's and Children's Hospital, Malaysia); Jose Martin Palacios Acosta (Servicio de Cirugía Oncológica, Instituto Nacional de Pediatría, Mexico City, Mexico); Marija Kolinovic (Pediatric Surgeon, Institute for children's diseases, Clinical Centre of Montenegro, Podgorica, Montenegro); Mohammed Oulad Saiad (Department of general pediatric surgery, Mother and child unit, university hospital Mohamed VI, Cadi Ayyad University, Marrakesh, Morocco); Robertine van Baren (Department of Surgery and Pediatric Surgery, University Medical Center Groningen, Groningen, Netherlands); Ivo de Blaauw (Department of Pediatric Surgery, Radboud University Medical Center, Amalia Children's Hospital, Nijmegen, The Netherlands); Wim G. van Gemert (Department of Pediatric Surgery, University Medical Centre Maastricht, Maastricht, the Netherlands); Ramon Gorter (Emma Children’s Hospital, Amsterdam UMC, location University of Amsterdam, Paediatric Surgery, Amsterdam, The Netherlands); Marijke E.B. Kremer (Department of Pediatric Surgery, University Medical Centre Maastricht, Maastricht, the Netherlands); Cornelius E.J. Sloots (Department of Pediatric Surgery, Erasmus MC Sophia Children’s Hospital, Rotterdam, The Netherlands); Marc Wijnen (Department of Pediatric Surgery, Radboud University Medical Center, Amalia Children's Hospital, Nijmegen, The Netherlands & Prinses Maxima MC); Rene M.H. Wijnen (Department of Pediatric Surgery and Intensive Care, Erasmus MC-Sophia Children's Hospital, Rotterdam, Netherlands); Marieke J. Witvliet (Department of Pediatric Surgery, University of Utrecht, Wilhelmina Children's Hospital, UMC Utrecht, Utrecht, The Netherlands); Adesoji Ademuyiwa (Paediatric Surgery Unit, Department of Surgery, Faculty of Clinical Sciences, College of Medicine, University of Lagos. Idi Araba, Lagos, Nigeria; Paediatric Surgery Unit, Department of Surgery, Lagos University Teaching Hospital. Idi Araba, Lagos, Nigeria); Chris Bode (Paediatric Surgery Unit, Department of Surgery, Faculty of Clinical Sciences, College of Medicine, University of Lagos. Idi Araba, Lagos, Nigeria; Paediatric Surgery Unit, Department of Surgery, Lagos University Teaching Hospital. Idi Araba, Lagos, Nigeria); Okechukwu Hyginus Ekwunife (Nnamdi Azikiwe University Teaching Hospital Nnewi, Nigeria); Justina Seyi-Olajide (Paediatric Surgery Unit, Department of Surgery, Lagos University Teaching Hospital. Idi Araba, Lagos, Nigeria); Aminu Muhammed Umar (Abubakar Tafawa Balewa University Teaching Hospital, Bauchi, Nigeria); Kristin Bjornland (Department of pediatric surgery, Oslo University Hospital, postboks 4950 Oslo, Norway and University of Oslo); Muhammad Arshad (Professor of Pediatric surgery, Liaquat National Hospital and Aga khan university, Karachi Pakistan); Mohammad Ajad Chaudhry (Department of pediatric surgery, Children Hospital, Shaheed Zulfiqar Ali Bhutto Medical University, Islamabad, Pakistan); Muhammad Bilal Mirza (University of Child Health Sciences Lahore Pakistan); Beda Espineda (Philippine Children's Medical Center as a Senior Consultant in Department of Pediatric Surgery); Maria Celine A. Villegas (Chief, Division of Pediatric Surgery, Department of Surgery, University of the Philippines - Philippine General Hospital; Associate Professor, University of the Philippines College of Medicine, University of the Philippines Manila); Weronika Jaron (Department of Pathology, The Children's Memorial Health Institute, Warsaw, Poland); Piotr Kalicinski (Department of Pediatric Surgery and Organ Transplantation, Children's Memorial Health Institute, 04-730 Warsaw, Poland); Maciej Murawski (Department of Pediatric Surgery and Urology, Medical University of Gdansk, Poland); Rui Alves (Pediatric Surgery Department, Hospital Dona Estefania, Lisboa, Portugal), Maria Carolina Sobral (Hospital Dona Estefânia - Centro Hospitalar Universitário Lisboa Central); Vlad-Laurentu David (Department of Pediatric Surgery and Orthopedics, "Victor Babes" University of Medicine and Pharmacy Timisoara, Romania); Alexey A. Gusev (FSAI "NMRC of Сhildren Health" MHRF, Moscow, Russian Federation; RUDN University, Moscow, Russian Federation); Khvorostov I.N. (Volgograd State Medical University); Yury Kozlov (Irkutsk Regional Children’s Hospital, Director of Regional Center for Pediatric Minimally Invasive Surgery and Pediatric Robotics, Irkutsk, Russia; Department of Pediatric Surgery, Irkutsk State Medical Academy of Postgraduate Education, Russia; Department of Pediatric Surgery and Pediatrics, Irkutsk State Medical University, Russia); Minaev Sergey Viktorovich (Department of pediatric surgery of Stavropol State Medical University, Russia); Maja Milickovic (Department of Abdominal Surgery, Institute for Mother and Child Healthcare of Serbia "Dr Vukan Cupic", Belgrade, Serbia); Sanja Sindjic-Antunovic (University Children’s Hospital, Center for Pediatric Surgery and Medical Faculty University of Belgrade, Serbia); York Tien Lee (Senior Consultant, Department of Paediatric Surgery, KK Women’s and Children’s Hospital, Singapore; Clinical Assistant Professor, DUKE-NUS Medical School, Singapore); Rebeka Pechanová (National Institute of Children´s Diseases, Bratislava, Slovakia); Jože Maučec (Department for Pediatric Surgery, University Medical centre Ljubljana, Slovenia); Hyun-Young Kim (Department of Pediatric Surgery, Seoul National University Hospital, 101 Daehak-ro, Jongno-gu, Seoul, 03080, Republic of Korea; Department of Pediatric Surgery, Seoul National University College of Medicine, Seoul, Republic of Korea); Seong Chul Kim (Department of Pediatric Surgery, University of Ulsan College of Medicine and Asan Medical Center, Seoul, Korea); Sohyun Nam (Division of Pediatric surgery, Department of Surgery, Inje University Busan Paik hospital, Busan, South Korea); Gabriela Guillén (Department of Pediatric Surgery, University Hospital Vall d´Hebron, Barcelona, Spain); Leopoldo Martinez (General-Oncologic Pediatric Surgery, Children's Hospital La Paz, Spain); Maria Molina (Virgen del Rocio Children's Hospital, Department of Pediatric Surgery, Sevilla, Spain); Fernando Vázquez Rueda ( Pediatric Oncologic Surgery, Pediatric Surgery Service, Hospital Universitario Reina Sofía. Córdoba, Spain); Oscar Girón Vallejo (Pediatric Surgery Department. Pediatric Surgical Oncology, Virgen de la Arrixaca University Clinical Hospital, Murcia, Spain); Maria Bordallo Vazquez (Pediatric Surgery Department. Pediatric Surgical Oncology Unit. La Fe University and Polytechnic Hospital, Valencia, Spain); Naveen Wijekoon (Department of Surgery, University of Colombo, Consultant Paediatric Surgeon - Lady Ridgeway Hospital, Sri Lanka); Mette Hambraeus (Department of Pediatric surgery, Skane University Hospital Lund, Lund, Sweden); Helene Engstrand Lilja (Department of Women’s and Children’s Health | Karolinska Institutet, Unit of Pediatric Surgery | Karolinska University Hospital); Par-Johan Svensson (Consultant Paediatric Surgeon Department of Paediatric Surgery, Astrid Lindgren´s Childrens Hospital, Karolinska Hospital and Karolinska Institute, Stockholm, Sweden); Tomas Wester (Karolinska University Hospital, Stockholm, Sweden); Husam Dalati (Department of Pediatric Surgery, Children Hospital, Damascus, Syria); Houssain Al Halabi (Department of Pediatric Surgery, Children Hospital, Damascus, Syria); Qusai Mashlah (Department of Pediatric Surgery, Children Hospital, Damascus, Syria); Shih-Hsiang Chen (Division of Hematology/Oncology, Department of Pediatrics, Chang Gung Memorial Hospital, Chang Gung University College of Medicine, Taoyuan, Taiwan); Montinee Supchatura (Department of Surgery, Queen Sirikit National Institute of Child Health, Bangkok, Thailand); Saloua Ammar (Département of Pediatric surgery, Hedi Chaker Hospital University of medecine of Sfax, University of Sfax, Tunisia); Salma Mani (Département of Pediatric surgery, Fattouma Bourguiba Hospital, University of medecine of Monastir, Tunisia); Orkan Ergün (Ege University Department of Pediatric Surgery, Izmir, Türkiye); Cigdem Ulukaya Durakbasa (Department of Pediatric Surgery, Istanbul Medeniyet University Faculty of Medicine); Samir Hasan (Ege University Department of Pediatric Surgery, Izmir, Türkiye); Ayşe Karaman (University of Health Sciences Turkey, Dr Sami Ulus Maternity and Children Health and Research Application Center, Department of Pediatric Surgery); Ibrahim Karaman (University of Health Sciences Turkey, Dr Sami Ulus Maternity and Children Health and Research Application Center, Department of Pediatric Surgery); Tezer Kutluk (Department of Pediatric Oncology, Hacettepe University, Faculty of Medicine & Cancer Institute Ankara, Turkey); Yevhen Rudenko (Department of Pediatric Surgery, Bogomolets National Medical University, Neonatal Surgery unit, National Specialized Children Hospital “Ohmatdyt“, Kyiv, Ukraine); Sarah Braungart (Department of Paediatric Surgery, Leeds Teaching Hospitals, UK); Deepika Bhojwani (Department of paediatric surgery, Cambridge University Hospitals NHS Foundation Trust); Alison Morag Campbell (Department of paediatric surgery, Great North Children's Hospital, Newcastle); Manal Dhaiban(FRCS England, MS Paediatric surgery, Speciality Registrar Paediatric surgery in Birmingham Children hospital UK); Paul Farrelly (Department of Paediatric Surgery, Royal Manchester Children's Hospital, Manchester, UK); Florian Friedmacher (Department of Pediatric Surgery, The Royal London Hospital, London, United Kingdom; Department of Pediatric Surgery, University Hospital Frankfurt, Goethe University Frankfurt, Germany); Robin Garrett-Cox (Consultant Paediatric Surgeon at Bristol Royal Children's Hospital, Bristol UK); Stefano Guiliani (Developmental Biology and Cancer Programme, UCL Great Ormond Street Institute of Child Health, London, UK; Department of Specialist Neonatal and Paediatric Surgery, NHS Foundation Trust, Great Ormond Street Hospital for Children, London, UK); Paul Losty (Professor of Paediatric Surgery, Institute Of Life Course And Medical Sciences, University Of Liverpool, UK); Jonathan Neville (Department of Paediatric Surgery, University Surgery Unit, University Hospitals Southampton); Kathryn O'Shea (Royal Manchester Children's Hospital, Manchester, UK); Rebecca A Roberts (Department of Paediatric Surgery and Urology, Bristol Royal Hospital for Children, Bristol); Mohamed Shalaby (Department of Pediatric Surgery, Royal Hospital for Sick Children, Edinburgh, United Kingdom); G. Suren Arul (Consultant paediatric surgeon, Birmingham Children’s Hospital, Birmingham, UK); Zachary Kastenberg (Division of Pediatric Surgery, Department of Surgery, University of Utah School of Medicine, Salt Lake City, USA); Maria E. Knaus (Department of Surgery, University of Tennessee Health Science Center); Bethany J. Slater (Department of Surgery, University of Chicago, Chicago, IL); Bruce Bvulani (Department of Paediatric Surgery, University Teaching Hospital of Lusaka, Lusaka, Zambia)
Ethical approval
The Medical Ethical Board of Amsterdam University Medical Centre (Amsterdam UMC), determined that Medical Research Involving Human Subject Act (WMO) does not apply to the study and that official approval of the committee was not required (reference number W19_329 # 19.388).
Consent
Individual patient written consent was not obtained since the study included patients with intrauterine and neonatal death. Patient data collection was fully anonymous and cannot be traced back to the individual patient. The absence of written consent was discussed with the Medical Ethical Board.
Source of funding
This study received funding from Kika Children Cancer-free Foundation. Kika Children Cancer-free Foundation had no involvement in collection, analysis and interpretation of data or in writing and decision to submit the manuscript for publication.
Author contribution
L.W.E.v.H.: gained study funding, wrote the study protocol, and established the SCT-study consortium; L.J.v.H.: designed the data collection forms, coordinated the data collection and validation, undertook the data analysis, and wrote and revised the manuscript; the writing committee (J.H.A., M.M.B., L.B.C., J.P.M.D., S.F., N.J.H., A.H., L.J.v.H., L.W.E.v.H., T.S., S.StP., J.T., and T.Y.) contributed to the data interpretation, manuscript content, and revisions; L.J.v.H. and J.T.: statistical analysis; L.W.E.v.H., L.J.v.H., J.P.M.D., and statistician (J.T.) had full access to all the study data; L.J.v.H. and statistician (J.T.) validated the data. All authors (SCT-study group) approved the manuscript and had final responsibility for the decision to submit for publication. All authors were local investigators. Local investigators gained local study approval, used the protocol to identify eligible patients, collected data, entered data in Castor EDC, and checked the data to prevent duplicate entries and ensure accuracy. All authors have read and approved the final manuscript.
Conflicts of interest disclosure
All authors declare no conflicts of interest
Research registration unique identifying number (UIN)
ISRCTN Registry ISRCTN17493302 Malignant transformation and tumour recurrence in sacrococcygeal teratoma, a congenital disorder in newborns. Retrospectively registered on 28 April 2024.
Guarantor
L.W.E. van Heurn, J.P.M. Derikx, and L.J. van Heurn are guarantors for this study.
Data availability statement
Following publication of the study results, the full, anonymous de-identified patient dataset will be made available. Data requestors will need to sign a data access agreement.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Supplementary Material
Acknowledgements
The authors thank the EUPSA Network Office for their help in raising awareness for the study and contacting centres for possible participation. We would like to thank Mikhail Povarov for helping with the Russian translation of the study documentation.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal's website, www.lww.com/international-journal-of-surgery.
Published online 6 September 2024
Contributor Information
Lieke J. van Heurn, Email: l.j.vanheurn@amsterdamumc.nl.
Joep P.M. Derikx, Email: j.derikx@amsterdamumc.nl.
Nigel Hall, Email: n.j.hall@soton.ac.uk.
Jennifer H. Aldrink, Email: Jennifer.Aldrink@nationwidechildrens.org.
Maria M. Bailez, Email: mariamarcelabailez4@gmail.com.
Lohfa B. Chirdan, Email: lohfab@yahoo.com.
Shigehisa Fumino, Email: fumin@koto.kpu-m.ac.jp.
Afua Hesse, Email: afua.hesse@acm.edu.gh.
Tuktu Soyer, Email: soyer.tutku@gmail.com.
Shawn StPeter, Email: sspeter@cmh.edu.
Jos Twisk, Email: jwr.twisk@amsterdamumc.nl.
Tianyou Yang, Email: mdtianyouyang@hotmail.com.
Ernst L.W. van Heurn, Email: e.vanheurn@amsterdamumc.nl.
Collaborators: Siffredi Juan Ignacio, Pablo Lobos, Holger Till, Ashrarur Rahman Mitul, Olga Govorukhina, Natalya Prokopenya, Antoine De Backer, Helena Reusens, Simone de Campos Vieira Abib, Penka Peneva Stefanova-Peeva, Nadezhda Tolekova, Mouafo Tambo Faustin, Jean-Martin Laberge, Augusto Zani, Richard J.B. Walker, Maricarmen Olivos Pérez, Marco Andrés Valenzuela, Yuanchao Shen, Yan Zou, Stanko Ćavar, Zenon Pogorelić, Lucie Pos, Richard Skaba, Peter Hjorth Jørgensen, Amr Abdelhamid AbouZeid, Mahmoud Elfiky, Heba Taher, Matis Märtson, Miliard Derbew, Workye Molla Tigabie, Antti Koivusalo, Mikko Pakarinen, Olivier Abbo, Alexis Pierre Emmanuel Arnaud, Quentin Ballouhey, Francois Bastard, Anne Dariel, Sabine Irtan, Jean Francois Lecompte, Guillaume Levard, Sabine Sarnacki, Rony Sfeir, Nicolas Vinit, Anne-Sophie Holler, Marietta Jank, Martin Lacher, Oliver J. Muensterer, Karin Rothe, Udo Rolle, Zoi Lamprinou, Zsuzsanna Jakab, Peter Vajda, Agnes Vojcek, Kenneth KY Wong, Mohan K. Abraham, Kirtikumar J Rathod, Shilpa Sharma, Gunadi, Mehdi Sarafi, Ahmad Khaleghnejad Tabari, Muataz Al Ani, Gavin Kane, Igor Sukhotnik, Stefano Avanzini, Pietro Bagolan, Piergiorgio Gamba, Riccardo Guanà, Alessandro Inserra, Mario Lima, Antonino Morabito, Alessandro Raffaele, Giovanna Riccipetitoni, Calogero Virgone, Yapi Landry Ake, Yoshiaki Hirohata, Maho Inoue, Yutaka Kanamori, Naonori Kawakubo, Takashi Sasaki, Tomoaki Taguchi, Tatsuro Tajiri, Hirofumi Tomita, Noriaki Usui, Mohit Kakar, Vidmantas Barauskas, Toni Risteski, Dayang Anita Abdul Aziz, Mohd Yusran Othman, Jose Martin Palacios Acosta, Marija Kolinovic, Mohammed Oulad Saiad, Robertine van Baren, Ivo de Blaauw, Wim G. van Gemert, Ramon Gorter, Marijke E.B. Kremer, Cornelius E.J. Sloots, Marc Wijnen, Rene M.H. Wijnen, Marieke J. Witvliet, Adesoji Ademuyiwa, Chris Bode, Okechukwu Hyginus Ekwunife, Justina Seyi-Olajide, Aminu Muhammed Umar, Kristin Bjornland, Muhammad Arshad, Mohammad Ajad Chaudhry, Muhammad Bilal Mirza, Beda Espineda, Maria Celine A. Villegas, Weronika Jaron, Piotr Kalicinski, Maciej Murawski, Rui Alves, Vlad-Laurentu David, Alexey A. Gusev, I.N. Khvorostov, Yury Kozlov, Minaev Sergey Viktorovich, Maja Milickovic, Sanja Sindjic-Antunovic, York Tien Lee, Rebeka Pechanová, Jože Maučec, Hyun-Young Kim, Seong Chul Kim, Sohyun Nam, Gabriela Guillén, Leopoldo Martinez, Maria Molina, Fernando Vázquez Rueda, Oscar Girón Vallejo, Maria Bordallo Vazquez, Naveen Wijekoon, Mette Hambraeus, Helene Engstrand Lilja, Par-Johan Svensson, Tomas Wester, Husam Dalati, Houssain Al Halabi, Qusai Mashlah, Shih-Hsiang Chen, Montinee Supchatura, Saloua Ammar, Salma Mani, Orkan Ergün, Cigdem Ulukaya Durakbasa, Samir Hasan, Ayşe Karaman, Ibrahim Karaman, Tezer Kutluk, Yevhen Rudenko, Sarah Braungart, Deepika Bhojwani, Alison Morag Campbell, Manal Dhaiban, Paul Farrelly, Florian Friedmacher, Robin Garrett-Cox, Stefano Guiliani, Paul Losty, Jonathan Neville, Kathryn O'Shea, Rebecca A Roberts, Mohamed Shalaby, G. Suren Arul, Zachary Kastenberg, Maria E. Knaus, Bethany J. Slater, and Bruce Bvulani
References
- 1. Padilla BE, Vu L, Lee H, et al. Sacrococcygeal teratoma: late recurrence warrants long-term surveillance. Pediatr Surg Int 2017;33:1189–1194. [DOI] [PubMed] [Google Scholar]
- 2. De Backer A, Madern GC, Hakvoort-Cammel FG, et al. Study of the factors associated with recurrence in children with sacrococcygeal teratoma. J Pediatr Surg 2006;41:173–181; discussion 173-81. [DOI] [PubMed] [Google Scholar]
- 3. Michelet D, Brasher C, Kaddour HB, et al. Postoperative complications following neonatal and infant surgery: common events and predictive factors. Anaesth Crit Care Pain Med 2017;36:163–169. [DOI] [PubMed] [Google Scholar]
- 4. De CF, Sarnacki S, Patte C, et al. Prognosis of malignant sacrococcygeal germ cell tumours according to their natural history and surgical management. Surg Oncol 2012;21:e31–e37. [DOI] [PubMed] [Google Scholar]
- 5. Derikx JP, De BA, van de Schoot L, et al. Factors associated with recurrence and metastasis in sacrococcygeal teratoma. Br J Surg 2006;93:1543–1548. [DOI] [PubMed] [Google Scholar]
- 6. Wang Y, Wu Y, Wang L, et al. Analysis of recurrent sacrococcygeal teratoma in children: clinical features, relapse risks, and anorectal functional sequelae. Med Sci Monit 2017;23:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pauniaho SL, Tatti O, Lahdenne P, et al. Tumor markers AFP, CA 125, and CA 19-9 in the long-term follow-up of sacrococcygeal teratomas in infancy and childhood. Tumour Biol 2010;31:261–265. [DOI] [PubMed] [Google Scholar]
- 8. Yao W, Li K, Zheng S, et al. Analysis of recurrence risks for sacrococcygeal teratoma in children. J Pediatr Surg 2014;49:1839–1842. [DOI] [PubMed] [Google Scholar]
- 9. Chirdan LB, Uba AF, Pam SD, et al. Sacrococcygeal teratoma: clinical characteristics and long-term outcome in Nigerian children. Ann Afr Med 2009;8:105–109. [DOI] [PubMed] [Google Scholar]
- 10. Abubakar AM, Nggada HA, Chinda JY. Sacrococcygeal teratoma in Northeastern Nigeria: 18-years experience. Pediatr Surg Int 2005;21:645–648. [DOI] [PubMed] [Google Scholar]
- 11. Global PaedSurg Research Collaboration . Mortality from gastrointestinal congenital anomalies at 264 hospitals in 74 low-income, middle-income, and high-income countries: a multicentre, international, prospective cohort study. Lancet 2021;398:325–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008;61:344–349. [DOI] [PubMed] [Google Scholar]
- 13. Mathew G, Agha R, Albrecht J, et al. STROCSS 2021: strengthening the reporting of cohort, cross-sectional and case–control studies in surgery. Int J Surg 2021;96:106165. [DOI] [PubMed] [Google Scholar]
- 14. Currarino G, Coln D, Votteler T. Triad of anorectal, sacral, and presacral anomalies. Am J Roentgenol 1981;137:395–398. [DOI] [PubMed] [Google Scholar]
- 15. Dirix M, van Becelaere T, Berkenbosch L, et al. Malignant transformation in sacrococcygeal teratoma and in presacral teratoma associated with Currarino syndrome: a comparative study. J Pediatr Surg 2015;50:462–464. [DOI] [PubMed] [Google Scholar]
- 16. Castor Electronic Data Capture . Castor Electronic Data Capture 2019 [27 August 2019], https://castoredc.com
- 17. Altman RP, Randolph JG, Lilly JR. Sacrococcygeal teratoma: American Academy of Pediatrics Surgical Section Survey-1973. J Pediatr Surg 1974;9:389–398. [DOI] [PubMed] [Google Scholar]
- 18. Hamadeh NRv, C. Metreau E. New World Bank country classifications by income level: 2022-2023; 2022. https://blogs.worldbank.org/opendata/new-world-bank-country-classifications-income-level-2022-2023
- 19. Kops AL, Hulsker CC, Fiocco M, et al. Malignant recurrence after mature Sacrococcygeal teratoma: a meta-analysis and review of the literature. Crit Rev Oncol Hematol 2020;156:103140. [DOI] [PubMed] [Google Scholar]
- 20. De BA, Madern GC, Hakvoort-Cammel FG, et al. Study of the factors associated with recurrence in children with sacrococcygeal teratoma. J Pediatr Surg 2006;41:173–181. [DOI] [PubMed] [Google Scholar]
- 21. Fabian ID, Abdallah E, Abdullahi SU. Global retinoblastoma presentation and analysis by national income level. JAMA Oncol 2020;6:685–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Badrinath R, Kakembo N, Kisa P, et al. Outcomes and unmet need for neonatal surgery in a resource-limited environment: estimates of global health disparities from Kampala, Uganda. J Pediatr Surg 2014;49:1825–1830. [DOI] [PubMed] [Google Scholar]
- 23. Chirdan LB, Ameh EA, Abantanga FA, et al. Challenges of training and delivery of pediatric surgical services in Africa. J Pediatr Surg 2010;45:610–618. [DOI] [PubMed] [Google Scholar]
- 24. Okoye MT, Ameh EA, Kushner AL, et al. A pilot survey of pediatric surgical capacity in West Africa. World J Surg 2015;39:669–676. [DOI] [PubMed] [Google Scholar]
- 25. Sitkin NA, Ozgediz D, Donkor P, et al. Congenital anomalies in low- and middle-income countries: the unborn child of global surgery. World J Surg 2015;39:36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. United Nations . Addressing the challenges of persons living with a rare disease and their families - Resolution adopted by the General Assembly on 16 December 2021; 2022. https://www.rarediseasesinternational.org/wp-content/uploads/2022/01/Final-UN-Text-UN-Resolution-on-Persons-Living-with-a-Rare-Disease-and-their-Families.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Following publication of the study results, the full, anonymous de-identified patient dataset will be made available. Data requestors will need to sign a data access agreement.


