Abstract
Background:
Gallbladder cancer (GBC) is a common gastrointestinal malignancy noted for its aggressive characteristics and poor prognosis, which is mostly caused by delayed detection. However, the scarcity of information regarding somatic mutations in Indian patients with GBC has hampered the development of efficient therapeutic options. In the present study, the authors attempted to bridge this gap by revealing the mutational profile of GBC.
Materials and methods:
To evaluate the somatic mutation profile, whole exome sequencing (WES) was performed on 66 tumor and matched blood samples from individuals with GBC. Somatic variant calling was performed using GATK pipeline. Variants were annotated at pathogenic and oncogenic levels, using ANNOVAR, VEP tools and the OncoKB database. Mutational signature analysis, oncogenic pathway analysis and cancer driver genes identification were performed at the functional level by using the maftools package.
Results:
Our findings focused on the eight most altered genes with pathogenic and oncogenic mutations: TP53, SMAD4, ERBB3, KRAS, ARID1A, PIK3CA, RB1, and AXIN1. Genes with pathogenic single nucleotide variations (SNVs) were enriched in oncogenic signaling pathways, particularly RTK-RAS, WNT, and TP53 pathways. Furthermore, our research related certain mutational signatures, such as cosmic 1, cosmic 6, and cosmic 18, 29, to known characteristics including patient age and tobacco smoking, providing important insights into disease etiology.
Conclusions:
Given the scarcity of exome-based sequencing studies focusing on the Indian population, this study represents a significant step forward in providing a framework for additional in-depth mutational analysis. Genes with substantial oncogenic and pathogenic mutations are promising candidates for developing targeted mutation panels, particularly for GBC detection.
Keywords: gallbladder cancer (GBC), genome analysis toolkit (GATK), OncoKB, whole exome sequencing (WES)
Introduction
Highlights
Provides a comprehensive analysis of the somatic mutational landscape of gallbladder cancer (GBC) using whole exome sequencing.
Findings spotlighted the eight most altered genes bearing pathogenic and oncogenic relevant mutations.
This study identified mutational signatures linked to age and tobacco addiction-that are prevalent among patients diagnosed at later stages.
Results underscore the intricate and heterogeneous nature of GBC within our cohort.
The discovered mutations have high potential for inclusion in the GBC diagnostic gene panels.
Gallbladder Cancer (GBC) is the most common biliary tract cancer1 (BTC), and is distinguished by its aggressive nature and nonspecific symptoms, with the majority of cases being found inadvertently at advanced clinical stages2. According to the GLOBOCON 2023 report, GBC ranks 22nd globally among all malignancies, with a reported incidence of 12,2491 cases. Heterogeneity is the primary reason for variance in the prevalence of GBC across geographical regions, with particularly high rates in South America and Asian countries3,4. In India, GBC is the most prevalent Gastrointestinal Tract cancers5, ranking 19th among all cancers, with 21,780 newly reported cases and 16,407 deaths. GBC incidence exhibits regional disparities within India, being higher in northeastern regions, and sex-wise occurrence is more susceptible in females than in males6. Owing to the multifactorial etiology of GBC, factors such as sex, age, chronic inflammation, gallbladder polyps, and lifestyle habits contributes significant level7. Gallstones are thought to be the main risk factor, although according to new research, only 1–3% of gallstone patients develop GBC. Furthermore, 10–15% of individuals have never had gallstones8,9. Numerous exome-based sequencing investigations have yielded significant information regarding the genetic makeup of GBC across a range of groups. The first Caucasian GBC exome study10, found a significant prevalence of TP53 (62.5%, 5/8) in the GBC cases analyzed. Subsequent another research11, comprising WES and targeted deep sequencing of GBC samples from a Chinese population, identified TP53 as the most frequently mutated gene (47.1%), followed by KRAS (7.8%), ERBB3 (11.8%), and ErbB signaling dysregulation at a significant level. A BTC spectra study identified ERBB and RB cell cycle as the prevalent pathways, along with TP53 mutations in GBC cases12. A follow-up exome study on GBC identified frequent ERBB2/3(9.8%,11.8%) mutation11,13. Further WES studies across different ethnicities corroborated these findings, with TP53 mutations recurring prominently in GBC, as highlighted in Japanese14, US15, and Chinese cohorts16,17 (29.3%, 27/92; 59%, 22 samples, respectively). Novel insights into GBC pathogenesis were obtained, including the significant role of ELF3 mutations in patients from diverse geographical regions, incorporated 64 patients of Indian origin18. Notably, among studies focusing on the Indian population19, somatic alterations implicating ERBB2 and KRAS genes have been unveiled. Another study20, performed WES (n=11), and found that TP53(6/10), ERBB2, and OBSCN (3/10) were the major mutated genes. One study21, analyzed the transcriptomic and genomic profiles (n=38) of patients with GBC and identified TP53(47%), ELF3(13%), and ARID1A (11%) as the major mutated genes along with alterations in TGF-β pathway. A comprehensive study22 (n=164) of GBC cases, found TP53 to be the most frequently mutated gene, with alterations in p53 and cell cycle pathways. Another study23 examined patients with GBC (n=7) and TP53 mutations were found to be prevalent, along with alterations in KM2TC, CDKN2A, and ARID1A in a subset of patients. Furthermore, a WES study24 on 11 patients with GBC, revealed CTNNB1 and ARID2 mutations in a significant proportion of cases, along with concurrent TP53 and ERBB3 alterations in a subset. Another exome-based study25, identified SYNE1 and TP53 as highly mutated driver genes in a GBC study.
Advancements in genomics have demonstrated the ability to deal with tailored therapies, which includes the use of mRNA vaccines and nanovaccines. mRNA vaccines in cancer attempt to trigger the immune system to recognize and target cancer cells, and nanovaccines provide a promising path for cancer therapy by improving vaccine distribution and efficacy. Their capacity to target specific antigens, protect and transport them efficiently, and strengthen the immune system makes them an effective tool in the battle against cancer26,27. Furthermore, advanced AI developed by OpenAI has the potential to play a key role in cancer research by being integrated into decision support systems, allowing researchers to quickly access evidence-based guidelines, treatment protocols, and diagnostic criteria28. Identification of biomarkers and broader research in these fields, may be applied to GBC and helps in the improvement of treatment outcomes. This study identified mutations linked to oncogenic and pathogenic genes along with mutational signatures. Moreover, this study provides insights into oncogenic pathways, offering valuable guidance for potential targeted therapeutic approaches in Indian patients with GBC.
Materials and methods
Study population
This study was approved by the ethical committees of the host institute. All samples were collected after obtaining written informed consent from patients. Fresh-frozen tumor tissues and matched peripheral blood (PB) samples were obtained during surgery and stored at −80°C for further processing as described previously29. A total of 66 paired samples were collected and histologically confirmed. Of these, 24 were male and 42 were female, with a median age of 53 years. According to the AJCC 8th edition, 17, 22, 22, and 5 patients had Stage I, II, III and IV disease, respectively. Patient data, primarily from those diagnosed with GBC were reviewed, and the associated clinical data are summarized in Table 1.
Table 1.
Clinical characteristics of 66 GBC samples.
| Sample_ID | Sex | Age | Gallstone | Jaundice | Addiction | Tumor stage |
|---|---|---|---|---|---|---|
| GBC_1 | Female | 49 | Yes | No | No | Stage I |
| GBC_2 | Female | 68 | No | No | No | Stage IIB |
| GBC_3 | Male | 31 | Yes | No | Tobacco chewing | Stage IIA |
| GBC_4 | Female | 59 | No | Yes | No | Stage IVB |
| GBC_5 | Female | 56 | No | No | No | Stage IIA |
| GBC_6 | Male | 63 | No | No | No | Stage IIIB |
| GBC_7 | Female | 64 | No | No | No | Stage IIB |
| GBC_8 | Male | 65 | No | Yes | No | Stage IIIB |
| GBC_9 | Female | 56 | No | No | No | Stage I |
| GBC_10 | Female | 56 | No | No | Tobacco chewing | Stage IIB |
| GBC_11 | Female | 40 | Yes | Yes | No | Stage IIIB |
| GBC_12 | Male | 43 | No | No | Smoking, Tobacco chewing | Stage IIA |
| GBC_13 | Male | 59 | No | Yes | Tobacco chewing, alcohol | Stage IIIB |
| GBC_14 | Female | 34 | No | Yes | No | Stage IIIA |
| GBC_15 | Female | 50 | Yes | No | Smoking | Stage I |
| GBC_16 | Female | 71 | No | No | No | Stage IIIB |
| GBC_17 | Male | 56 | No | No | Smoking, Tobacco chewing, alcohol | Stage IIIB |
| GBC_18 | Male | 30 | No | Yes | Tobacco chewing, alcohol | Stage IVB |
| GBC_19 | Male | 44 | Yes | Yes | Alcohol | Stage IVA |
| GBC_20 | Male | 63 | Yes | No | No | Stage IIIA |
| GBC_21 | Female | 48 | No | No | Smoking | Stage IIIA |
| GBC_23 | Female | 55 | No | No | No | Stage IIIB |
| GBC_24 | Female | 52 | No | No | No | Stage IIIB |
| GBC_25 | Female | 45 | No | No | No | Stage I |
| GBC_26 | Male | 34 | Yes | Yes | Tobacco chewing | Stage IIIA |
| GBC_27 | Male | 68 | No | No | Tobacco chewing | Stage IIB |
| GBC_28 | Male | 71 | Yes | No | No | Stage IIIB |
| GBC_29 | Female | 57 | No | No | Smoking | Stage IVB |
| GBC_30 | Female | 40 | Yes | Yes | Smoking | Stage I |
| GBC_31 | Female | 72 | Yes | Yes | Smoking | Stage IVB |
| GBC_32 | Female | 54 | Yes | Yes | No | Stage I |
| GBC_33 | Male | 77 | No | No | No | Stage IIIA |
| GBC_34 | Male | 50 | No | No | Tobacco chewing | Stage IIA |
| GBC_35 | Female | 63 | No | No | No | Stage I |
| GBC_36 | Female | 50 | No | Yes | No | Stage IIIA |
| GBC_86 | Female | 39 | No | Yes | No | Stage III |
| GBC_87 | Female | 35 | No | No | No | Stage IIB |
| GBC_103 | Female | 43 | No | No | Smoking, Tobacco chewing | Stage IIA |
| GBC_104 | Male | 27 | No | No | Tobacco chewing, alcohol | Stage I |
| GBC_113 | Male | 27 | No | No | No | Stage I |
| GBC_114 | Female | 51 | Yes | No | Smoking | Stage IIA |
| GBC_132 | Male | 57 | Yes | No | No | Stage I |
| GBC_133 | Female | 48 | Yes | No | Smoking | Stage IIIB |
| GBC_134 | Female | 27 | No | No | No | Stage IIB |
| GBC_135 | Female | 49 | Yes | No | No | Stage I |
| GBC_136 | Male | 65 | No | No | No | Stage IIA |
| GBC_137 | Female | 74 | No | No | N o | Stage IIB |
| GBC_138 | Male | 64 | No | No | No | Stage IIIA |
| GBC_139 | Female | 40 | Yes | No | No | Stage IIIB |
| GBC_140 | Female | 45 | Yes | No | No | Stage IIIB |
| GBC_141 | Female | 39 | Yes | No | No | Stage IIIB |
| GBC_142 | Male | 35 | No | No | No | Stage IIA |
| GBC_144 | Female | 25 | No | No | No | Stage I |
| GBC_145 | Female | 51 | Yes | No | Smoking | Stage IIA |
| GBC_146 | Female | 32 | No | No | No | Stage I |
| GBC_147 | Female | 65 | Yes | No | No | Stage I |
| GBC_148 | Female | 68 | Yes | No | No | Stage IIB |
| GBC_149 | Male | 45 | Yes | No | No | Stage IIIB |
| GBC_150 | Female | 40 | Yes | No | No | Stage I |
| GBC_151 | Female | 64 | No | No | Smoking | Stage I |
| GBC_152 | Female | 80 | Yes | No | No | Stage IIA |
| GBC_153 | Male | 34 | No | No | No | Stage I |
| GBC_154 | Female | 42 | Yes | No | Tobacco chewing | Stage IIB |
| GBC_155 | Female | 50 | Yes | Yes | Smoking | Stage IIB |
| GBC_156 | Male | 61 | Yes | No | No | Stage IIIA |
| GBC_157 | Male | 64 | Yes | No | No | Stage IIA |
DNA extraction, library preparation, and sequencing
Genomic DNA (gDNA) from the stored tumor tissue and matched blood was extracted using a Qiagen kit, in accordance with the manufacturer’s protocol. To ensure the integrity of gDNA, quantity and quality assessments were performed using a Qubit fluorimeter and agarose gel electrophoresis, respectively. Upon preliminary analysis, 200 ng of gDNA was used for library preparation using the Agilent SureSelectXT Target Enrichment System, according to the manufacturer’s instructions. Finally, an enriched indexed library was captured and assessed using a Bioanalyzer assay, followed by 150 bp paired-end sequencing using the Illumina NovaSeq 6000 system.
Preprocessing of raw reads
Quality assessment of raw data reads was conducted using FASTQC30, followed by adapter trimming using Trimmomatic31. High-quality trimmed reads were mapped to the UCSC human genome (GRCh38.p12) using Burrow-Wheeler Aligner BWA-MEM32. Further alignment processing, including quality control (QC) metrics, duplicate marking, and base quality score recalibration, was performed using the Genome Analysis Toolkit33 (GATK).
Panel of normals, somatic variant calling
Panel of Normals (PONs) is the collection of all germline samples in the vcf file format. PONs were created using Mutect, on individual normal samples and combining all normal variant calls including the criterion that specific germline variant sites should be present in at least two normal samples. GetPileupSummaries and Calculate Contamination tools of GATK were used for cross-sample contamination analysis, and identified somatic variants were filtered using FilterMutectCalls.
Somatic variants annotation with clinical and oncogenic relevance
We implemented SnpEff34, Annotate variation35 (ANNOVAR), and Variant Effect Predictor36 (VEP) for annotation and prioritization of variants based on their clinical significance. Initially, all variant files were annotated with dbSNP15537 and 1000 genomes38 databases using the SnpSift function of SnpEff. Variants with Minor allele frequency (MAF ≤0.01) and South Asian Allele frequency (SAS_AF ≤0.01) were selectively considered. Using ANNOVAR, mutations were prioritized and filtered through four deleterious predictors with associated scores: Sorting Intolerant from Tolerant39 (SIFT <0.05 as cutoff), Functional Analysis through Hidden Markov Model40 (FATHMM; D-deleterious, T-tolerated), Polymorphism Phenotyping v241 (PolyPhen-2; Polyphen-2<0.9 as probably damaging(D)), and likelihood ratio test42 (LRT; D-Deleterious, N-neutral, U-Unknown). Disease-specific variants were annotated using ClinVar43 (2022123139) and Catalog of Somatic Mutations In Cancer44,45 (COSMIC_95) databases were used for mapping with known somatic mutations. Clinical interpretation of the variants, such as - benign, variants of uncertain significance (VUS), Pathogenic, and Likely Pathogenic, was performed according to the American College of Medical Genetics (ACMG) and the Association for Molecular Pathology (AMP) guidelines, utilizing the InterVar database46.
Somatic mutations were meticulously annotated in our study based on diagnostic, therapeutic, and prognostic evidence levels, using the OncoKB database47. A cutoff of SIFT (<0.05) and Polyphen2 (<0.9) scores were applied to identify the deleterious effect of oncogenic mutations. Subsequently, we selectively considered variants annotated from the somatic mutation cancer database, which demonstrated a pathogenic or likely pathogenic effect with oncogenic activity. In addition to in-house shell scripts and R scripts, Maftools48 was used for summarizing, visualizing, and analyzing the maf files.
Cancer driver genes identification
MutSigCV49 was used to identify the genes associated with cancer driver activity in 66 GBC samples. Considering the approach used for identifying significant genes, the background mutation rate (BMR) was estimated using the observed count, coverage count of mutations per gene, and mutation category, using the pre-processed mutation data, coverage data, and covariate data. Genes that harbored a significant P (<0.01) and q value threshold of <0.1 were considered as significant cancer driver genes.
Somatic mutational signature analysis, tumor mutational burden analysis
Cancer progression leads to a characteristic mutational pattern that can reveal underlying mutagenic processes50,51. Implementing ‘de novo’ approach, involving frequency matrix generation and non-negative matrix factorization (NMF) decomposition to extract signatures and compared to reference signatures from COSMIC based on cosine similarity. Additionally, APOBEC enrichment was performed for individual tumor samples as described in a study52. We applied the ‘tcgaCompare’ function from the maftools package for tumor mutation burden (TMB) analysis and compare it to the 33 TCGA cancer types.
Oncogenic pathway analysis
Mutated genes were assigned to oncogenic signaling pathways based on data from previous TCGA based study53. We evaluated 10 canonical signaling pathways with frequent genetic alterations, by using the ‘Oncogenic Pathways’ module of maftools. Additionally, Pathwaymapper54 and the pathway template from TCGA PanCanAtlas53 were used to generate mutated signaling pathways.
Results
Mutational profile of GBC patients
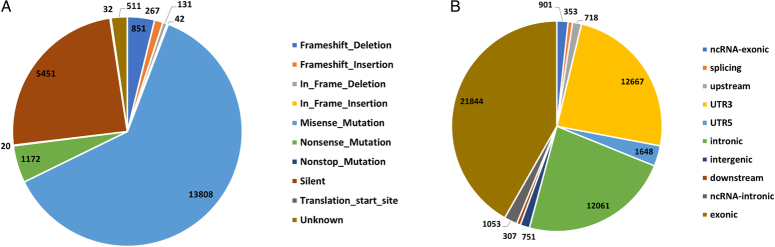
A total of 22,285 variants and 16,834 protein-altering-based somatic mutations were identified in our cohort. C>A substitutions were dominant (more than 50% of samples), followed by C>T substitutions (40% of samples) (Figure S1A, B; Supplemental Digital Content 1, http://links.lww.com/JS9/D293). The exonic region carried the highest number of mutations (n=21,844) (Fig. 1A, B). In our study, nonsense and silent mutations were the next most common variant type, after missense mutations (Table S1, Supplemental Digital Content 2, http://links.lww.com/JS9/D294). TP53 (47%), MUC16 (30%), SYNE1 (29%), CTNNB1 (27%), SMAD4 (26%), and OBSCN (24%) were the most frequently mutated genes in the study cohort (Figure S2; Supplemental Digital Content 1, http://links.lww.com/JS9/D293).
Figure 1.
Distribution of Somatic variants in 66 GBC Samples. (A) Types of mutations identified in GBC cohort. Highest occurence of Missense mutation is observed. (B) Classification of overall variants observed in GBC samples. Exonic variants are highest in number followed by the UTR3 regions and intronic regions.
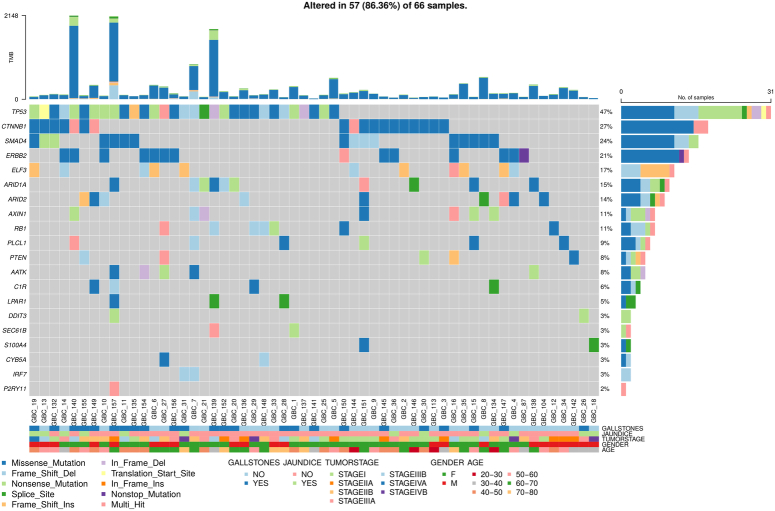
Cancer driver genes in GBC
We identified 11 genes with notable significance scores based on both p and q values (Table S2, Supplemental Digital Content 3, http://links.lww.com/JS9/D295). TP53 exhibited the highest mutation frequency (45%), followed by ELF3 (17%), CTNNB1 (27%), AXIN1 (11%), ERBB2 (21%), SMAD4 (24%), ARID1A (15%), RB1 (11%), ARID2 (14%), PTEN (8%), and ERBB2 (Fig. 2).
Figure 2.
Oncoplot illustrating the top genes with potential driver variants, correlated with sample, age, sex, tumor stage, gallstone presence, and jaundice status in 57 GBC samples. Each column represents a distinct GBC sample, while each row denotes a specific gene. Colored squares indicate altered genes, whereas grey squares signify non-mutated genes. Variants are color-coded according to their mutation types. Genes marked as "Multi_Hit" denote those with multiple mutations within the same sample. The barplot at the top shows the tumor mutation burden (TMB), with colors representing different mutation types.
Genes associated with pathogenic variants
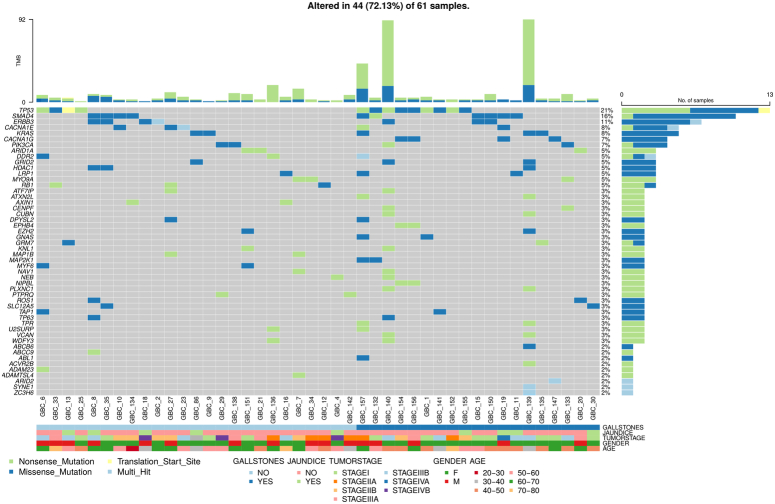
A total of 17,976 variants were categorized according to the ACMG and AMP guidelines. Among them, 12,499 were VUS variants and 4996 were likely benign variants. Additionally, 301 variants were pathogenic, and 180 were likely pathogenic (Figure S3A, B; Supplemental Digital Content 1, http://links.lww.com/JS9/D293). Missense, nonsense, stop-gain, and start-loss mutations (481 mutations) were predominant in 61 GBC samples. These pathogenic variants were distributed across 393 genes, including TP53 (21%), SMAD4 (16%), ERBB3 (11%), CACNA1E (8%), KRAS (8%), and PIK3CA (8%) (Fig. 3).
Figure 3.
Oncoplot showing pathogenic gene variants and their distribution among 44 out of 61 gallbladder cancer (GBC) samples. These genes were selected based on ACMG classification, highlighting pathogenic and likely pathogenic variants. The gene variants are correlated with clinical features, including age, sex, tumor stage, gallstone, and jaundice status. Each column represents a GBC sample, and each row corresponds to a specific gene. Colored squares indicate mutated genes, while grey squares represent non-mutated genes. Different mutation types are distinguished by various colors. Genes marked as "Multi_Hit" contain more than one mutation in the same sample. The barplot at the top displays tumor mutation burden (TMB), color-coded by mutation type, while the barplot on the right shows the number of patients with mutations in each gene.
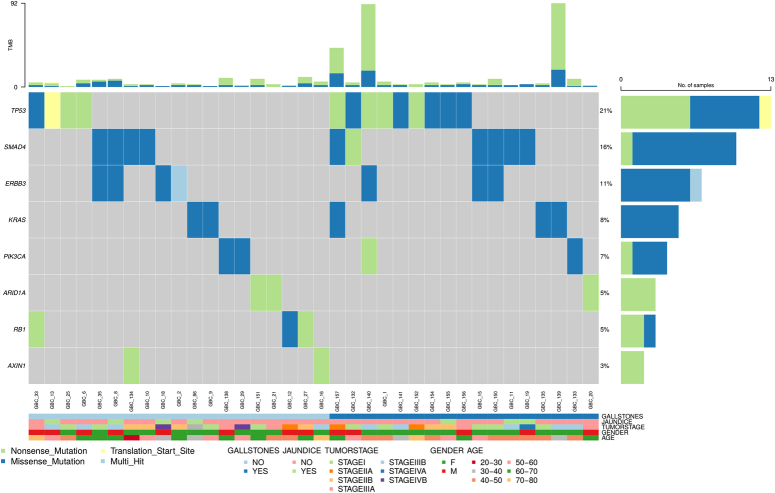
In addition to identifying mutations, these findings were annotated using the OncoKB database. Of these 481 variants, 433 were effectively annotated, revealing associations with 349 distinct genes. Among these, 39 genes were definitively linked to oncogenic activity, accounting for 43 of the initially 61 samples. Intriguingly, 12 samples exhibited deleterious scores, although the OncoKB classified the variation in activity as uncertain. Moreover, six samples failed to exhibit significant scores, and their oncogenic activity remained undetermined. Of the 39 genes associated with oncogenic activity, only eight genes-TP53(21%), SMAD4(16%), ERBB3(11%), KRAS (8%), ARID1A (5%), PIK3CA (7%), RB1(5%), and AXIN1(3%), were found to be mutated in at least two samples (Fig. 4). Further annotation of these oncogenic mutations revealed varying therapeutic implications, diagnostic relevance, and prognostic significance (Table S4; Supplemental Digital Content 4, http://links.lww.com/JS9/D296).
Figure 4.
Oncoplot depicting the distribution of eight prevalent genes—TP53, SMAD4, ERBB3, KRAS, PIK3CA, ARID1A, RB1, and AXIN1—within the GBC cohort. These genes, mutated in at least two GBC samples, are noted for their oncogenic mutations which have implications for therapy, diagnosis, and prognosis. Gene variants are correlated with sample, age, sex, tumor stage, and status of gallstones and jaundice. Each column represents a distinct GBC sample, and each row corresponds to a specific gene. Colored squares indicate altered genes, while grey squares denote non-mutated genes.
TP53 was the most frequently mutated gene in our study, with both pathogenic and likely pathogenic mutations. TP53 mutations were found in 13 samples, including 6 male and 7 female individuals, most aged over 50 years, and had been diagnosed with Stage II or III GBC. Interestingly, only half of the patients had cholelithiasis, with six variants as pathogenic and seven as likely pathogenic activity. All TP53 missense and nonsense mutations were prevalent at prognostically significant levels (Px1, Px3). The TP53 mutations found in GBC cohort included p.K132T (Level_Px1, Px3), p.M133T (Level_Px1), p.H179R (Level_Px1), p.E285K (Level_Px1), p.G245S, and p.S127F (Level_Px1) (Table 2). SMAD4 was the second most frequently mutated gene, with pathogenic mutations found in nine samples. Among them, six were female and three were female, with a median age of 50 years. Most patients presented with Stage II and III GBC, and five patients with SMAD4 mutations also had cholelithiasis. Mutations such as p.D537Y, p.D493N, p.E330K, p.W509R, p.R361H, and p.G352V were discovered to be related with SMAD4 in the GBC cohort (Table S5A-E; Supplemental Digital Content 2, http://links.lww.com/JS9/D294).
Table 2.
TP53 oncogenic mutations in GBC samples.
| Gene | Chr position | Variant | Oncogenic | Variant in OncoKB; level of evidence | Description |
|---|---|---|---|---|---|
| TP53 (chr17) | 7675217 7675214 7674894 7673767 7673537 7674230 7675232 7675119 7673740 7676097 7675076 |
p.K132T p.M133T p.R213* p.E285K p.Q331* p.G245S p.S127F p.Q165* p.E294* p.W91* p.H179R |
Likely Oncogenic Likely Oncogenic Likely Oncogenic Likely Oncogenic Likely Oncogenic Oncogenic Likely Oncogenic Likely Oncogenic Likely Oncogenic Likely Oncogenic Likely Oncogenic |
Yes; Level-Px1a Yes; Level-Px1 No; Level-Px1 Yes; Level-Px1 No; Level-Px1 Yes; Level_Px1 Yes; Level_Px1 No; Level_Px1 No; Level_Px1 No; Level_Px1 Yes; Level_Px1 |
Identified in glioblastoma58
Unregulated inflammatory response studied in breast cancer59 Truncating mutation and promote cancer cell proliferation62 Expression in yeast and human cancer cells is associated with loss of function61 Truncating mutation and its alterations are predicted to be inactivating and are associated with poor prognosis. This mutation in cell lines that lack TP53 expression did not enhance the TP53-mediated transcriptional activity62 Mutation is identified in xeroderma pigmentosum and is statically significant hotspot63 Truncating mutation and its alterations are predicted to be inactivating and are associated with poor prognosis63 Truncating mutation and its alterations are predicted to be inactivating and are associated with poor prognosis63 Expression of this mutation failed to induce expression of genes involved in apoptosis and cell cycle arrest60 |
FDA and /or professional guideline-recognized biomarker prognostic in this indication based on a well powered study/studies.
ERBB3 mutations were prevalent in seven samples, affecting five female and two male patients. The median age of these patients was 50 years, and they predominantly belonged to Stages III and IV. ERBB3 oncogenic mutations include p.G284R, p.V104L, p.P262H, and p.V104M. Additionally, p.E298G was also reported.
KRAS mutations were detected in four samples: three female and one male patient. Two of these patients had cholelithiasis, and their GBC stages ranged from I to III. KRAS has demonstrated a spectrum of therapeutic implications ranging from levels 2 to 4, along with resistance (level R1) and diagnostic relevance (level Dx2). The mutations identified were p.G12V, p.V14I, and p.G12D.
PIK3CA mutations were found in three samples, including two female and one male patient, all categorized as Stage III/IV GBC cases, with a median age of 40 years. PIK3CA mutations have been linked to Level 1 therapeutic implications. The p.R38H mutations, p.E545K, and p.E365K has been associated with PIK3CA.
ARID1A mutations were detected in three samples, two females and one male patient, two of whom had Stage I GBC and the remaining one had Stage III GBC. Mutations in ARID1A are indicative of Level 4 therapeutic implications. All ARID1A identified mutations (p.K1071*, p.R1461*, p.Q633*) were truncate nonsense oncogenic mutations. The retinoblastoma gene (Rb1) was found to be mutated in three male samples in our study corresponding to Stages II–IIIA GBC. RB1 Likely oncogenic truncating mutations (p.S567L, p.R556*, p.Y790*) result in loss of function of this gene. The AXIN1 gene was found to be mutated in two female patients (Stage IIB, Stage IIIB), with truncating mutations (p.E109* and p.Q678*).
Mutational signature analysis
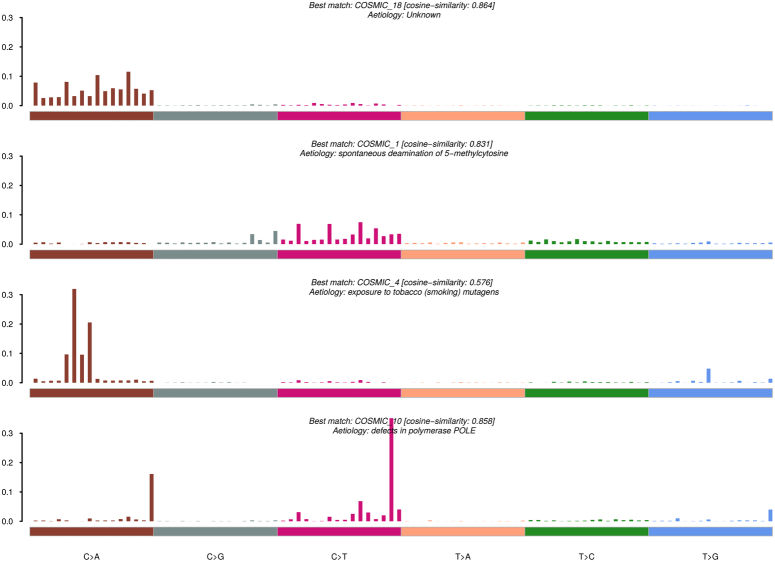
APOBEC-related mutations were enriched in 24% of all samples (APOBEC enrichment score > 2) (Figure S4; Supplemental Digital Content 1, http://links.lww.com/JS9/D293). De novo signatures included spontaneous deamination of 5-methylcytosine (COSMIC_1, cosine similarity-0.831), damage by reactive oxygen species (COSMIC_18 and COSMIC_29, cosine similarity-0.864), tobacco chewing habit, exposure to tobacco mutagens (COSMIC_4, cosine similarity-0.576), and defects in polymerase POLE, in hypermutated cases (COSMIC_10, cosine similarity-0.858) (Figure S5A; Supplemental Digital Content 1, http://links.lww.com/JS9/D293). Signature patterns were plotted and annotated using the COSMIC database (Fig. 5). SBS were also identified in each sample (Figure S5B, C; Supplemental Digital Content 1, http://links.lww.com/JS9/D293). Signature 1 (COSMIC_18, COSMIC_29, COSMIC_24, and COSMIC_4) occurred in 28.8% of GBC samples, whereas Signature 2 (COSMIC_6 and COSMIC_1) correlated with age at diagnosis in 54% of samples. Signature 3, associated with smoking, was found in 15% of patients, and Signature 4 was associated with hypermutations in one patient (Table 3).
Figure 5.
Mutational signatures identified in GBC cohort. The y-axis represents the exposure of 96 trinucleotide motifs to overall signatures. The plot information indicates the best match against validated COSMIC signatures, and cosine similarity value along with proposed aetiology.
Table 3.
Detailed summary of COSMIC matched denovo signatures in GBC cohort.
| GBC samples identified signatures | Match with COSMIC SBS signatures | Description |
|---|---|---|
| Signature 1 (C>A) (19 samples) |
Cosmic 18(0.8) Cosmic 29(0.8) Cosmic 4(0.7) Cosmic 24(0.7) |
Cosmic 18 -Mutational process underlying this signature is associated with damage by reactive oxygen species. Cosmic 29-Mutational process found in cancer samples from individuals with tobacco chewing habit. Cosmic 4- Associated with tobacco smoking. Cosmic 24- Mutational process found in cancer samples with known exposures to aflatoxin |
| Signature 2 (C>T) (36 samples) |
Cosmic 1(0.784) Cosmic 6(0.7) |
Cosmic 1- Mutation associated with associated by the enzymatic deamination of 5-methylcytosine and correlates with age of diagnosis. Cosmic 6- defective DNA mismatch repair |
| Signature 3 (10 samples) |
Cosmic 4 (0.576) |
Cosmic 4- Associated with tobacco smoking |
| Signature 4 (1 sample) |
Cosmic 10 (0.858) |
Cosmic10- Associated with generating large number of mutations and samples with these signatures have been termed as hypermutators |
Tumor mutational burden analysis
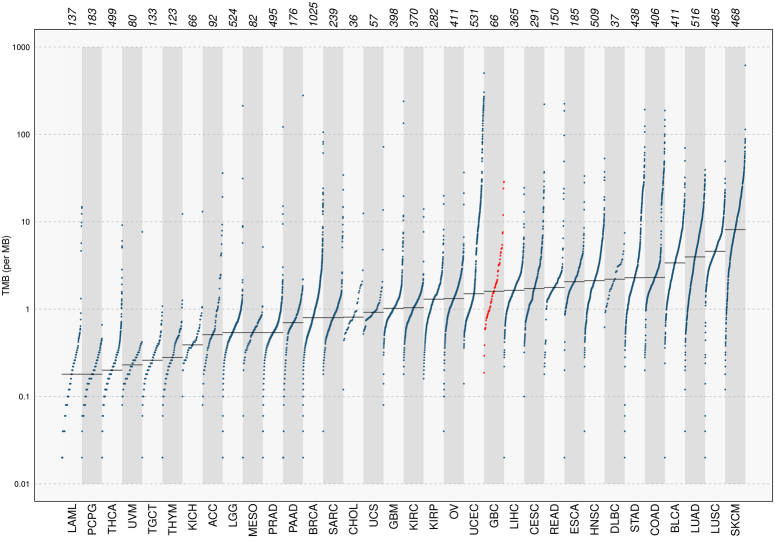
TMB, indicating mutations per million bases (muts/Mb), categorizes GBC samples into high (GBC_140, GBC_157, GBC_139), intermediate (seven samples), and low TMB (Figure S6; Supplemental Digital Content 1, http://links.lww.com/JS9/D293). Comparing GBC with 33 TCGA cohorts, GBC showed low TMB (1.6 muts/mb), similar to Hepatocellular carcinoma (LIHC) (t-test, P=0.79), and higher TMB than cholangiocarcinoma (CHOL) (Fig. 6).
Figure 6.
Prevalence of Somatic mutation burden in our population(GBC, n=66- highlighted in red color) compared to 33 TCGA cohorts. Each dot represents a single patient sample. The horizontal grey lines indicate the median number of mutations in each cancer category. Vertical axes (log scaled) showed the number of mutations per megabase.
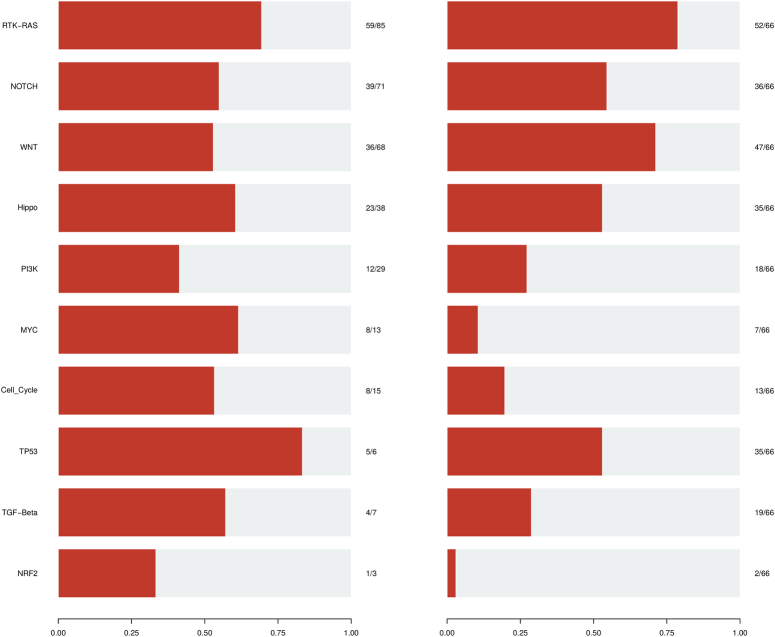
Mutated oncogenic signaling pathways
Genes associated with SNVs were explored across 10 key oncogenic pathways and their frequencies in affected samples are depicted (Fig. 7; Table S6; Supplemental Digital Content 2, http://links.lww.com/JS9/D294), also labeled on the signaling pathways (Fig. 8 (A-I)). The RTK-RAS pathway was enriched, with mutations detected in 52 samples (78.78%). Among these, 27 were oncogenes, while 4 exhibited tumor suppressor activity. The most frequently mutated genes were ERBB2(26.92%), ERBB3(23.07%), ERBB4(11.53%), and KRAS (13.46%) (Figure S7A; Supplemental Digital Content 1, http://links.lww.com/JS9/D293). WNT was the second most significantly altered pathway and was mutated in 71.2% of the samples. CTNNB1 (38.29%) was the central oncogene of this pathway. AXIN1 (14.89%) was altered in 14.89% of GBC patients with tumor suppressor activity (Figure S7B; Supplemental Digital Content 1, http://links.lww.com/JS9/D293).
Figure 7.
Overview of known altered oncogenic signaling pathways in GBC cohort. (Left) plot represents the fraction of oncogenic pathway affected and (Right) plot represents the fraction of GBC samples affected.
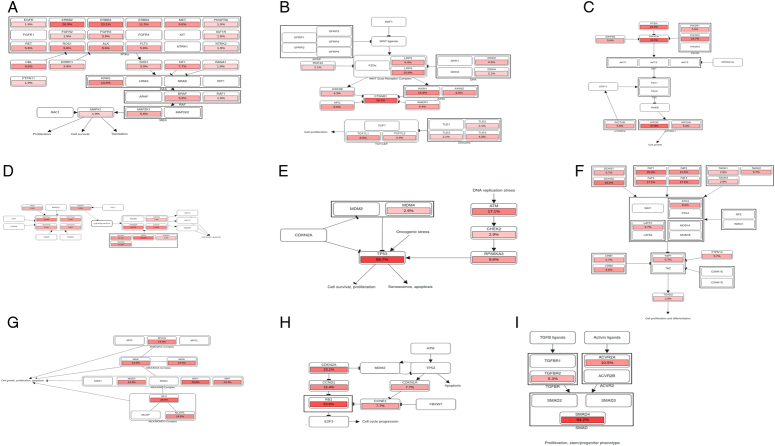
Figure 8.
(A) Alteration of RTK-RAS pathway in GBC cohort. (B) Alteration of WNT pathway in GBC cohort. (C) Alteration of PI3K pathway in GBC cohort. (D) Alteration of NOTCH pathway in GBC cohort. (E) Alteration of TP53 pathway in GBC cohort. (F) Alteration of Hippo pathway in GBC cohort. (G) Alteration of MYC pathway in GBC cohort. (H) Alteration of Cell_Cycle pathway in GBC cohort. (I) Alteration of TGF-Beta pathway in GBC cohort. The color intensity represented the alteration frequency of pathway members. An arrow indicated an activation; without an arrow represented the binding activity; a bar at the end of an edge indicated an inhibitory interaction.
The Notch signaling pathway (54.54%) predominantly involved SPEN (25%), CREBBP (19.4%), and EP300 (13.9%) as the main mutated tumor suppressor genes (Figure S7C; Supplemental Digital Content 1, http://links.lww.com/JS9/D293). Mutations in the TP53 pathway accounted for 53% of cases, including TP53 (86.11%), ATM (16.66%), and CHEK2 (Figure S7D; Supplemental Digital Content 1, http://links.lww.com/JS9/D293). TP53 and ATM act as tumor suppressors, whereas MDM4 and RPS6KA3 act as oncogenes. The Hippo signaling pathway included alterations in 53.03% of GBC samples, including 16 tumor suppressor genes. Notably, HMCN1 (34.3%), DCHS2 (20%), and FAT family genes, were represented. The oncogenes involved in this pathway include the TEAD (2,3,4) family, YAP1, and HIPK2 (Figure S7E; Supplemental Digital Content 1, http://links.lww.com/JS9/D293).
The Transforming Growth Factor-β (TGF-β) pathway was mutated in 28.78% of the samples and SMAD4 (84.2%) and ACVR2A (10.5%) were the main tumor suppressor genes (Figure S7F; Supplemental Digital Content 1, http://links.lww.com/JS9/D293). The Phosphatidylinositol 3-kinase (PI3K) pathway has a mutation in 27% of GBC samples, with PIK3CA (33.3%) and MTOR (27.8%) as the main oncogenes and PTEN (22.2%) as a tumor suppressor gene (Figure S7G; Supplemental Digital Content 1, http://links.lww.com/JS9/D293). The cell cycle pathway was mutated in 19.69% of the total samples, and RB1(53.9%) and CDKN2A (23.1%), were the main mutated tumor suppressor genes (Figure S7H; Supplemental Digital Content 1, http://links.lww.com/JS9/D293). The MYC pathway was altered in seven samples, with MAX-like protein (MLX) and MXI1 as the main mutated genes (Figure S7I; Supplemental Digital Content 1, http://links.lww.com/JS9/D293).
Discussion
In our study, we analyzed the somatic mutational landscape in 66 patients with GBC using WES, revealing a predominance of C>A and C>T mutations, which is consistent with previous GBC studies13,25. Missense mutations were found to be predominant in our GBC cohort. TP53, MUC16, SYNE1, SMAD4, OBSCN, ERBB2, and ERBB3 were found to be the most frequently mutated genes. Elaboration in the form of Functional analysis revealed TP53, CTNNB1, ELF3, SMAD4, ERBB2, ARID1A, AXIN1, and RB1 as potential driver genes in 86% of samples. Considering the genes with oncogenic and clinically significant effects in at least two patients, our study identified the major role of eight genes- TP53, SMAD4, ERBB3, KRAS, ARID1A, PIK3CA, RB1, and AXIN1.
Our analysis revealed significant TP53 alterations, which is consistent with prior WES studies on GBC. Previous studies have found TP53 mutations in 27–70% of gallbladder carcinomas, emphasizing the relevance of this gene in GBC pathology55. All TP53 missense and nonsense variants found in our investigation were related to loss of function and had a prognostic degree of significance (level Px1). TP53 plays a pivot role in tumor suppression and regulates various cellular processes such as – apoptosis, immune response modulation, and autophagy56. During DNA damage, wild-type p53 prevents defective cell proliferation and cancer development, whereas TP53 mutations promote DNA damage and disease progression57. In our GBC cohort, specific TP53 mutations such as p.K132T have been reported in glioblastoma58, p.M133T associated with an unregulated inflammatory response that has been studied in breast cancer59, p.H179R mutation related to downregulation of the genes associated with cell cycle arrest and apoptosis60, and p.E285K associated with loss of function61. The P.G245S mutation was found in cell lines that lacked TP53 expression and did not mediate transcriptional activity62. Another TP53 mutation p.S127F, has been identified as significant hotspot in xeroderma pigmentosum63. These alterations result in loss of tumor suppressor activity and aggravate the carcinogenic process in GBC patients64.
SMAD4 mutations have emerged prominently after TP53 alterations, indicating their sequential involvement in GBC progression. SMAD4 variants primarily disrupt the TGF-β signaling system, resulting in loss of function through missense and nonsense variants. Activated TGF receptors phosphorylate SMADs, which cause them to translocate to the nucleus and enhance transcriptional activity65. Initially, during normal pathway regulation, the associated TGF receptors have tumor suppressor activity but dysregulation by loss of function results in unregulated growth and progression of cancer66. Specific SMAD4 mutations in our study, such as p.D537Y, p.G352V reduce antiproliferative TGF-mediated transcriptional activity and have been reported in colorectal cancer67. Notably, our study identified the p.D493N mutation located in the MH2 domain, which has been previously reported in GBC11. Additionally, p.E330K, p.W509R, and p.R361H mutations were associated with decreased SMAD4-mediated transcription68, decreased BMP signaling69 and decreased antiproliferative mediated transcription70.
ERBB3 showed significant missense mutations associated with gain of function in the RTK-RAS pathway. ERBB3 forms dimers with ERBB2 to exert its effect. Although frequent ERBB2 mutations were observed in our study, their significance regarding cancer activity was uncertain. Previous studies have reported both ERBB2 and ERBB3 alterations in 12% of GBC cases11. The identified ERBB3 oncogenic mutations in GBC cohort include p.G284R, p.E298G reported in cholangiocarcinoma, breast, and colon cancer71, p.V104L found in bladder and colon cancer72, p.P262H, documented in colon and ovarian cancer73 and p.V104M mutations, identified in cholangiocarcinoma, breast cancer, and endometrial cancer74. KRAS mutations were significantly mutated from prognostic level 2 to 4 in the GBC cohort, with diagnostic relevance and missense mutations. In previous GBC studies, KRAS mutations were reported in 4–13% of GBC75. RTKs orchestrate essential signaling cascades involved in key cellular processes such as proliferation, differentiation, survival, and migration76. Alterations in RTKs are frequently observed as contributing factors in the progression of numerous cancers and uncontrolled cell proliferation77. The activation of RTKs by external ligands promotes downstream signaling mediated by RAS proteins. These proteins further relay signals for processes, such as anti-apoptosis and cell regulation. Specific KRAS mutations, such as p.G12V, p.G12D were found to be prevalent in lung and pancreatic cancer78, and p.V14I mutation is linked to Noon syndrome79.
Next, PIK3CA missense mutations were prevalent with level 1 therapeutic significance in our GBC cohort. PI3K signaling dysregulation has been investigated in previous GBC studies emphasizing cancer cell proliferation and metastasis with PIK3CA gain-of-function and loss of PTEN mutation80. Approximately 8 and 50% of human GBC cases are associated with dysregulated PI3K signaling in GBC pathogenesis81. Mutations such as p.R38H, p.E545K mutation has been reported in glioblastoma82, and p.E365K is linked to endometrial cancer83. ARID1A truncating mutations have level 4 implications underscoring their role in pathogenesis21,23. Previous studies have supported the role of ARID1A nonsense mutation in the proliferation of cancers, which is approximately prevalent among 13% of patients with GBC and has the worst prognosis correlation in BTC84,85.
RB1 truncating mutations lead to loss of function and truncation, without diagnostic or prognostic implications in the GBC cohort. RB1 mutations in the cell cycle pathway have been reported in a previous GBC study12. The pathway plays a major role in the progression and therapeutic response of different cancers86. AXIN1 associated truncating mutations had oncogenic activity but without any diagnostic or prognostic prevalence in the cohort. AXIN1 is a crucial component of the β-catenin destruction complex in the WNT pathway, and plays a pivotal role in regulating cell proliferation and differentiation. AXIN1 mutations have been detected in a various malignancy, including liver cancers87. Dysregulation of WNT system-associated genes promotes tumor growth and angiogenesis, and AXINI mutations linked to this pathway have been identified in a prior GBC study88.
Our analysis demonstrated a link between COSMIC signatures 1 and 6, which correlated with age at diagnosis and faulty DNA mismatch repair mechanisms, consistent with prior studies on GBC14,16,17. Furthermore, the COSMIC signatures of 18 and 29 mutations were detected in patients with a history of tobacco chewing, highlighting the clinical importance of addictive practices in the development of GBC.
Conclusion
Our findings revealed recurrent mutations, including possibly pathogenic and carcinogenic variations. The eight discovered genes – TP53, SMAD4, ERBB3, KRAS, ARID1A, PIK3CA, RB1, and AXIN1 – all exhibited important roles in driving GBC development – had a significant collective impact on GBC development in our cohort. These mutations have great potential for inclusion in the GBC diagnostic gene panels. Notably, TP53 and SMAD4 ERBB3 were identified as the most frequently altered genes, especially in advanced GBC cases. Furthermore, our research discovered mutational signatures associated with age and tobacco addiction, which are more common in patients diagnosed at a later stage. In our study, half of GBC patients had a smoking or tobacco addiction, indicating a clinical correlation with our findings. The RTK-RAS and WNT pathways were the most frequently altered oncogenic signaling pathways in our analysis, emphasizing GBC’s complexity and tumor heterogeneity. Our findings support the importance of personalized therapy based on individual genomic landscapes. However, further research is required to confirm our findings and determine their functional significance in tumor growth and progression. Integrating multiomics data with larger cohort studies has the potential to expand our understanding of disease biology, clinical management, and improving patient outcomes.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of All India Institute of Medical Sciences for a study involving humans. Ref No.-IEC-32/09.03.2018, OP-5/09.03.2018.
Consent
We received informed consent from all the cases involved in the study.
Source of funding
This work was supported by the Indian Council of Medical Research through grant 5/13/1/TF/GBC/AIIMS/2015/NCD-III.
Author contribution
P.T.: conceptualization, supervision, and writing – review and editing; S.A.: design, conceptualization, resources, WES experiments, data curation, data analysis, writing – original draft, and writing – review and editing; D.P.: conceptualization, design, review, and editing; Rahk, H.G.: writing – review and editing; N.R.: resource and initial WES experiment; N.R.D., S.K., and S.S.S.: resources; U.A., S.H., S.S.S., P.D., P.S., S.S., and R.R.: writing – review and editing; G.K.R., T.K., and R.S.D.: project administration, writing –review and editing. All authors have read and approved the final manuscript.
Conflicts of interest disclosure
The authors declare that they have no financial conflict of interest with regard to the content of this report.
Guarantor
Pranay Tanwar.
Data availability statement
The raw data generated during the current study is available in the NCBI repository with BioProject ID - PRJNA1058876 and PRJNA1049991. The data is not publicly available due to restrictions. Derived data supported the findings of this study are available from the corresponding author on request.
Presentation
None.
Supplementary Material
Acknowledgements
We acknowledge the overall guidance of Rita Mulherkar as chairperson of the review committee of Gallbladder Cancer TF under grant 5/13/1/TF/AIIMS/2015/NCD-III from the Indian Council of Medical Research. We are grateful to the patients for their consent and participation in this study. We also express our gratitude to the resident doctors of Surgical Oncology at B.R.A. Institute Rotary Cancer Hospital and the Department of Gastrointestinal Surgery, AIIMS, for their invaluable cooperation and coordination in sample collection.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal's website, www.lww.com/international-journal-of-surgery.
Published online 14 August 2024
Contributor Information
Supriya Awasthi, Email: supriya.awasthy@gmail.com.
Rahul Kumar, Email: rk10496mailm@gmail.com.
Dibyabhaba Pradhan, Email: dbpinfo@gmail.com.
Neetu Rawal, Email: neeturawal94@gmail.com.
Harsh Goel, Email: goel.harsh271@gmail.com.
Parameswar Sahu, Email: bio.parameswar@gmail.com.
Sandeep Sisodiya, Email: sandeepsisodiya99@gmail.com.
Rashmi Rana, Email: rashmi.rana@sgrh.com.
Sunil Kumar, Email: dr_sunilk@hotmail.com.
Nihar Ranjan Dash, Email: nagranjan@gmail.com.
Prasenjit Das, Email: prasenaiims@gmail.com.
GK Rath, Email: gkrath2006@gmail.com.
Tanvir Kaur, Email: doctanvirkaur@gmail.com.
RS Dhaliwal, Email: dhaliwalrs.hq@icmr.gov.in.
Showket Hussain, Email: hussainshowket@gmail.com.
Sundeep Singh Saluja, Email: sundeepsaluja@yahoo.co.in.
Pranay Tanwar, Email: pranaytanwar@gmail.com.
References
- 1. Ghidini M, Pizzo C, Botticelli A, et al. Biliary tract cancer: current challenges and future prospects. Cancer Manag Res 2019;11:379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273–1281. [DOI] [PubMed] [Google Scholar]
- 3. Koshiol J, Bellolio E, Vivallo C, et al. Distribution of dysplasia and cancer in the gallbladder: an analysis from a high cancer-risk population. Hum Pathol 2018;82:87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mondaca S, Schultz N, Roa JC, et al. Clinical and genomic characterization of ERBB2-altered gallbladder cancer. J Clin Oncol 2022;40:4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Butti AK, Yadav SK, Verma A, et al. Chronic calculus cholecystitis: is histopathology essential post-cholecystectomy? Indian J Cancer 2020;57:89–92. [DOI] [PubMed] [Google Scholar]
- 6. Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol 2014;6:99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shukla HS, Sirohi B, Behari A, et al. Indian Council of Medical Research consensus document for the management of gall bladder cancer. Indian J Med Paediatr Oncol 2015;36:79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lazcano-Ponce EC, Miquel JF, Muñoz N, et al. Epidemiology and molecular pathology of gallbladder cancer. CA Cancer J Clin 2001;51:349–364. [DOI] [PubMed] [Google Scholar]
- 9. Murthy NS, Rajaram D, Gautham MS, et al. Trends in incidence of gallbladder cancer – Indian scenario. GICTT 2011;1:1–9. [Google Scholar]
- 10. Jiao Y, Pawlik TM, Anders RA, et al. Exome sequencing identifies frequent inactivating mutations in BAP1, ARID1A and PBRM1 in intrahepatic cholangiocarcinomas. Nat Genet 2013;45:1470–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li M, Zhang Z, Li X, et al. Whole-exome and targeted gene sequencing of gallbladder carcinoma identifies recurrent mutations in the ErbB pathway. Nat Genet 2014;46:872–876. [DOI] [PubMed] [Google Scholar]
- 12. Nakamura H, Arai Y, Totoki Y, et al. Genomic spectra of biliary tract cancer. Nat Genet 2015;47:1003–1010. [DOI] [PubMed] [Google Scholar]
- 13. Li M, Liu F, Zhang F, et al. Genomic ERBB2/ERBB3 mutations promote PD-L1-mediated immune escape in gallbladder cancer: a whole-exome sequencing analysis. Gut 2019;68:1024–1033. [DOI] [PubMed] [Google Scholar]
- 14. Wardell CP, Fujita M, Yamada T, et al. Genomic characterization of biliary tract cancers identifies driver genes and predisposing mutations. J Hepatol 2018;68:959–969. [DOI] [PubMed] [Google Scholar]
- 15. Yang P, Javle M, Pang F, et al. Somatic genetic aberrations in gallbladder cancer: comparison between Chinese and US patients. Hepatobiliary Surg Nutr 2019;8:604–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nepal C, Zhu B, O’Rourke CJ, et al. Integrative molecular characterization of gallbladder cancer reveals microenvironment-associated subtypes.. J Hepatol 2021;74:1132–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu F, Li Y, Ying D, et al. Whole-exome mutational landscape of neuroendocrine carcinomas of the gallbladder. Sig Transduct Target Ther 2021;6:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pandey A, Stawiski EW, Durinck S, et al. Integrated genomic analysis reveals mutated ELF3 as a potential gallbladder cancer vaccine candidate. Nat Commun 2020;11:4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Iyer P, Shrikhande SV, Ranjan M, et al. ERBB2 and KRAS alterations mediate response to EGFR inhibitors in early stage gallbladder cancer. Int J Cancer 2019;144:2008–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yang D, Chen T, Zhan M, et al. Modulation of mTOR and epigenetic pathways as therapeutics in gallbladder cancer. Mol Ther Oncolytics 2020;20:59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ebata N, Fujita M, Sasagawa S, et al. Molecular classification and tumor microenvironment characterization of gallbladder cancer by comprehensive genomic and transcriptomic analysis. Cancers 2021;13:733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lin J, Cao Y, Yang X, et al. Mutational spectrum and precision oncology for biliary tract carcinoma. Theranostics 2021;11:4585–4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Feng F, Cheng Q, Li B, et al. Establishment and characterization of 38 novel patient-derived primary cancer cell lines using multi-region sampling revealing intra-tumor heterogeneity of gallbladder carcinoma. Hum Cell 2021;34:918–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lin J, Peng X, Dong K, et al. Genomic characterization of co-existing neoplasia and carcinoma lesions reveals distinct evolutionary paths of gallbladder cancer. Nat Commun 2021;12:4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang Y, Zuo C, Liu L, et al. Single-cell RNA-sequencing atlas reveals an MDK-dependent immunosuppressive environment in ErbB pathway-mutated gallbladder cancer. J Hepatol 2021;75:1128–1141. [DOI] [PubMed] [Google Scholar]
- 26. Priyanka, Chopra H, Choudhary OP. mRNA vaccines as an armor to combat the infectious diseases. Travel Med Infect Dis 2023;52:102550. [DOI] [PubMed] [Google Scholar]
- 27. Priyanka, Abusalah MAH, Chopra H, et al. Nanovaccines: a game changing approach in the fight against infectious diseases. Biomed Pharmacother 2023;167:115597. [DOI] [PubMed] [Google Scholar]
- 28. Choudhary OP, Saini J, Challana A. ChatGPT for veterinary anatomy education: an overview of the prospects and drawbacks. Int J Morphol 2023;41:1198–1202. [Google Scholar]
- 29. Kumar R, Kumar R, Goel H, et al. Whole exome sequencing identifies novel variants of PIK3CA and validation of hotspot mutation by droplet digital PCR in breast cancer among Indian population. Cancer Cell Int 2023;23:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ Babraham Bioinformatics - FastQC A Quality Control tool for High Throughput Sequence Data [Internet]. Accessed December 24, 2023.
- 31. Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 2014;30:2114–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li H, Durbin R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 2009;25:1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McKenna A, Hanna M, Banks E, et al. The genome analysis toolkit: a mapreduce framework for analyzing next-generation DNA sequencing data. Genome Res 2010;20:1297–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cingolani P, Platts A, Wang LL, et al. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff. Fly (Austin) 2012;6:80–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res 2010;38:e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McLaren W, Gil L, Hunt SE, et al. The ensembl variant effect predictor. Genome Biol 2016;17:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sherry ST, Ward M, Sirotkin K. dbSNP—Database for Single nucleotide polymorphisms and other classes of minor genetic variation. Genome Res 1999;9:677–679. [PubMed] [Google Scholar]
- 38. Devuyst O. The 1000 genomes project: welcome to a new world. Perit Dial Int 2015;35:676–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ng PC, Henikoff S. SIFT: predicting amino acid changes that affect protein function. Nucleic Acids Res 2003;31:3812–3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shihab HA, Gough J, Cooper DN, et al. Predicting the functional, molecular, and phenotypic consequences of amino acid substitutions using hidden Markov models. Hum Mutat 2013;34:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Adzhubei IA, Schmidt S, Peshkin L, et al. A method and server for predicting damaging missense mutations. Nat Methods 2010;7:248–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liu X, Jian X, Boerwinkle E. dbNSFP: a lightweight database of human nonsynonymous SNPs and their functional predictions. Hum Mutat 2011;32:894–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Landrum MJ, Chitipiralla S, Brown GR, et al. ClinVar: improvements to accessing data. Nucleic Acids Res 2020;48(D1):D835–D844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tate JG, Bamford S, Jubb HC, et al. COSMIC: the Catalogue Of Somatic Mutations In Cancer. Nucleic Acids Res 2019;47(Database issue):D941–D947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sondka, Dhir Z, Carvalho-Silva NB, et al. COSMIC: a curated database of somatic variants and clinical data for cancer. Nucleic Acids Res 2024;52(D1):D1210–D1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li Q, Wang K. InterVar: clinical interpretation of genetic variants by the 2015 ACMG-AMP guidelines. Am J Hum Genet 2017;100:267–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chakravarty D, Gao J, Phillips SM, et al. OncoKB: a precision oncology knowledge base. JCO Precis Oncol 2017;2017:PO.17.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mayakonda A, Lin DC, Assenov Y, et al. Maftools: efficient and comprehensive analysis of somatic variants in cancer. Genome Res 2018;28:1747–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lawrence MS, Stojanov P, Polak P, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 2013;499:214–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Alexandrov LB, Nik-Zainal S, Wedge DC, et al. Deciphering signatures of mutational processes operative in human cancer. Cell Reports 2013;3:246–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Alexandrov LB, Kim J, Haradhvala NJ, et al. The repertoire of mutational signatures in human cancer. Nature 2020;578:94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Roberts SA, Lawrence MS, Klimczak LJ, et al. An APOBEC cytidine deaminase mutagenesis pattern is widespread in human cancers. Nat Genet 2013;45:970–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sanchez-Vega F, Mina M, Armenia J, et al. Oncogenic signaling pathways in the cancer genome atlas. Cell 2018;173:321–337.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bahceci I, Dogrusoz U, La KC, et al. PathwayMapper: a collaborative visual web editor for cancer pathways and genomic data. Bioinformatics 2017;33:2238–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Quan ZW, Wu K, Wang J, et al. Association of P53, P16, and vascular endothelial growth factor protein expressions with the prognosis and metastasis of gallbladder cancer. J Am Coll Surg 2001;193:380. [DOI] [PubMed] [Google Scholar]
- 56. Hafner A, Bulyk ML, Jambhekar A, et al. The multiple mechanisms that regulate p53 activity and cell fate. Nat Rev Mol Cell Biol 2019;20:199–210. [DOI] [PubMed] [Google Scholar]
- 57. Marei HE, Althani A, Afifi N, et al. p53 signaling in cancer progression and therapy. Cancer Cell Int 2021;21:703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Simmons ML, Lamborn KR, Takahashi M, et al. Analysis of complex relationships between age, p53, epidermal growth factor receptor, and survival in glioblastoma patients. Cancer Res 2001;61:1122–1128. [PubMed] [Google Scholar]
- 59. Herbert BS, Chanoux RA, Liu Y, et al. A molecular signature of normal breast epithelial and stromal cells from Li-Fraumeni syndrome mutation carriers. Oncotarget 2010;1:405–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ashur-Fabian O, Adamsky K, Trakhtenbrot L, et al. Apaf1 in chronic myelogenous leukemia (CML) progression: reduced Apaf1 expression is correlated with a H179R p53 mutation during clinical blast crisis. Cell Cycle 2007;6:589–594. [DOI] [PubMed] [Google Scholar]
- 61. Jordan JJ, Inga A, Conway K, et al. Altered-Function p53 missense mutations identified in breast cancers can have subtle effects on transactivation. Mol Cancer Res 2010;8:701–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Baroni TE, Wang T, Qian H, et al. A global suppressor motif for p53 cancer mutants. Proc Natnl Acad Sci 2004;101:4930–4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bouaoun L, Sonkin D, Ardin M, et al. TP53 variations in human cancers: new lessons from the IARC TP53 database and genomics data. Hum Mutat 2016;37:865–876. [DOI] [PubMed] [Google Scholar]
- 64. Nigam P, Misra U, Negi TS, et al. Alterations of p53 gene in gallbladder cancer patients of North India. Trop Gastroenterol 2010;31:96–100. [PubMed] [Google Scholar]
- 65. Holcombe RF, Xiu J, Pishvaian MJ, et al. Tumor profiling of biliary tract carcinomas to reveal distinct molecular alterations and potential therapeutic targets. JCO 2015;33(3_suppl):285 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sicklick JK, Fanta PT, Shimabukuro K, et al. Genomics of gallbladder cancer: the case for biomarker-driven clinical trial design. Cancer Metastasis Rev 2016;35:263–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Fleming NI, Jorissen RN, Mouradov D, et al. SMAD2, SMAD3 and SMAD4 mutations in colorectal cancer. Cancer Res 2013;73:725–735. [DOI] [PubMed] [Google Scholar]
- 68. Yoshioka Y, Togashi Y, Chikugo T, et al. Clinicopathological and genetic differences between low-grade and high-grade colorectal mucinous adenocarcinomas. Cancer 2015;121:4359–4368. [DOI] [PubMed] [Google Scholar]
- 69. Carr JC, Dahdaleh FS, Wang D, et al. Germline mutations in SMAD4 disrupt bone morphogenetic protein signaling. J Surg Res 2012;174:211–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wu JW, Hu M, Chai J, et al. Crystal structure of a phosphorylated Smad2: recognition of phosphoserine by the MH2 domain and insights on smad function in TGF-β signaling. Mol Cell 2001;8:1277–1289. [DOI] [PubMed] [Google Scholar]
- 71. Seshagiri S, Stawiski EW, Durinck S, et al. Recurrent R-spondin fusions in colon cancer. Nature 2012;488:660–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Chang MT, Asthana S, Gao SP, et al. Identifying recurrent mutations in cancer reveals widespread lineage diversity and mutational specificity. Nat Biotechnol 2016;34:155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bertotti A, Papp E, Jones S, et al. The genomic landscape of response to EGFR blockade in colorectal cancer. Nature 2015;526:263–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Schneeweiss A, Park-Simon TW, Albanell J, et al. Phase Ib study evaluating safety and clinical activity of the anti-HER3 antibody lumretuzumab combined with the anti-HER2 antibody pertuzumab and paclitaxel in HER3-positive, HER2-low metastatic breast cancer. Invest New Drugs 2018;36:848–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ross JS, Wang K, Catenacci DVT, et al. Comprehensive genomic profiling of biliary tract cancers to reveal tumor-specific differences and genomic alterations. JCO 2015;33(3_suppl):231–231. [Google Scholar]
- 76. Lemmon MA, Schlessinger J. Cell signaling by receptor-tyrosine kinases. Cell 2010;141:1117–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Stephen AG, Esposito D, Bagni RK, et al. Dragging ras back in the ring. Cancer Cell 2014;25:272–281. [DOI] [PubMed] [Google Scholar]
- 78. AACR Project GENIE Consortium . AACR Project GENIE: powering precision medicine through an international consortium. Cancer Discov 2017;7:818–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Gremer L, Merbitz-Zahradnik T, Dvorsky R, et al. Germline KRAS mutations cause aberrant biochemical and physical properties leading to developmental disorders. Hum Mutat 2011;32:33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ligresti G, Militello L, Steelman LS, et al. PIK3CA mutations in human solid tumors. Cell Cycle 2009;8:1352–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Liu DC, Yang ZL. Overexpression of EZH2 and loss of expression of PTEN is associated with invasion, metastasis, and poor progression of gallbladder adenocarcinoma. Pathol Res Pract 2011;207:472–478. [DOI] [PubMed] [Google Scholar]
- 82. Yu K, Lin CCJ, Hatcher A, et al. PIK3CA variants selectively initiate brain hyperactivity during gliomagenesis. Nature 2020;578:166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Rudd ML, Price JC, Fogoros S, et al. A unique spectrum of somatic PIK3CA (p110α) mutations within primary endometrial carcinomas. Clin Cancer Res 2011;17:1331–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Conci S, Ruzzenente A, Simbolo M, et al. Multigene mutational profiling of biliary tract cancer is related to the pattern of recurrence in surgically resected patients. Updates Surg 2020;72:119–128. [DOI] [PubMed] [Google Scholar]
- 85. Jones S, Li M, Parsons DW, et al. Somatic mutations in the chromatin remodeling gene ARID1A occur in several tumor types. Hum Mutat 2012;33:100–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Knudsen ES, Pruitt SC, Hershberger PA, et al. Cell cycle and beyond: exploiting new RB1 controlled mechanisms for cancer therapy. Trends Cancer 2019;5:308–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zhang R, Li S, Schippers K, et al. Analysis of tumor-associated AXIN1 missense mutations identifies variants that activate β-catenin signaling. Cancer Res 2024;84:1443–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Yang J, Zhang W, Evans PM, et al. Adenomatous polyposis coli (APC) differentially regulates beta-catenin phosphorylation and ubiquitination in colon cancer cells. J Biol Chem 2006;281:17751–17757. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data generated during the current study is available in the NCBI repository with BioProject ID - PRJNA1058876 and PRJNA1049991. The data is not publicly available due to restrictions. Derived data supported the findings of this study are available from the corresponding author on request.