Abstract
BACKGROUND:
Transcatheter aortic valve replacement (TAVR) is an alternative to surgery in patients with severe aortic stenosis, but data are limited on younger, low-risk patients. This analysis compares outcomes in low-surgical-risk patients aged <75 years receiving TAVR versus surgery.
METHODS:
The Evolut Low Risk Trial randomized 1414 low-risk patients to treatment with a supra-annular, self-expanding TAVR or surgery. We compared rates of all-cause mortality or disabling stroke, associated clinical outcomes, and bioprosthetic valve performance at 3 years between TAVR and surgery patients aged <75 years.
RESULTS:
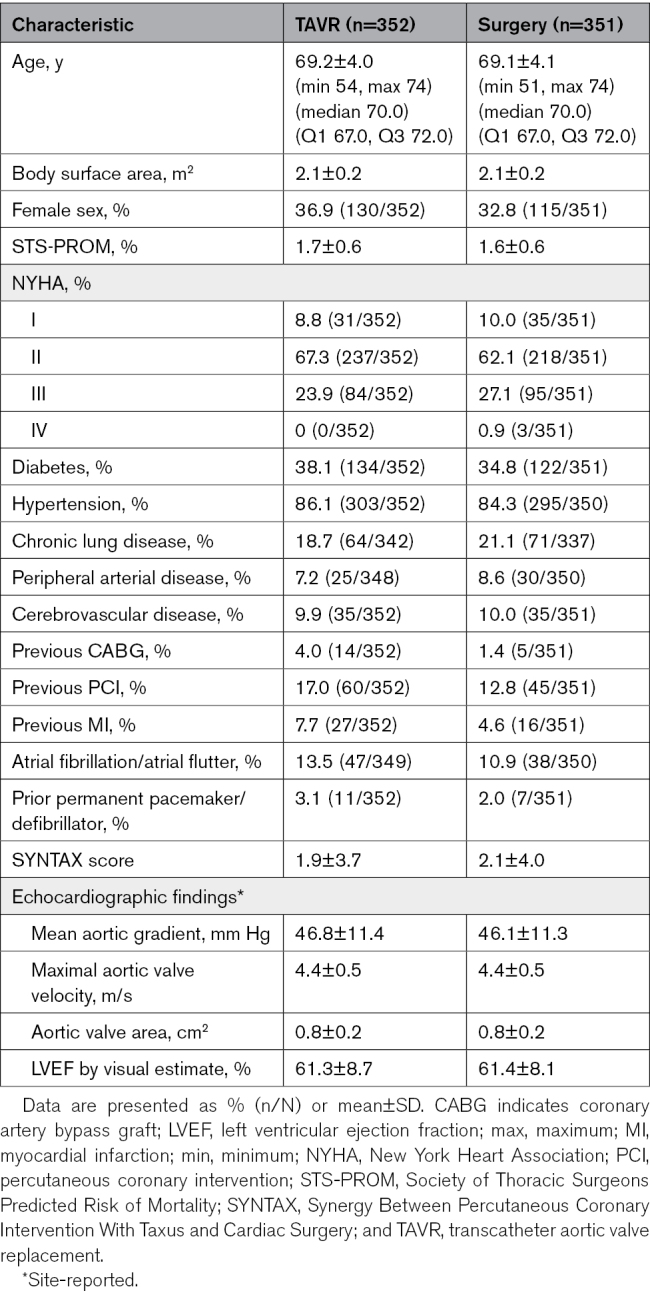
In patients <75 years, 352 were randomized to TAVR and 351 to surgery. Mean age was 69.1±4.0 years (minimum 51 and maximum 74); Society of Thoracic Surgeons Predicted Risk of Mortality was 1.7±0.6%. At 3 years, all-cause mortality or disabling stroke for TAVR was 5.7% and 8.0% for surgery (P=0.241). Although there was no difference between TAVR and surgery in all-cause mortality, the incidence of disabling stroke was lower with TAVR (0.6%) than surgery (2.9%; P=0.019), while surgery was associated with a lower incidence of pacemaker implantation (7.1%) compared with TAVR (21.0%; P<0.001). Valve reintervention rates (TAVR 1.5%, surgery 1.5%, P=0.962) were low in both groups. Valve performance was significantly better with TAVR than surgery with lower mean aortic gradients (P<0.001) and lower rates of severe prosthesis-patient mismatch (P<0.001). Rates of valve thrombosis and endocarditis were similar between groups. There were no significant differences in rates of residual ≥moderate paravalvular regurgitation.
CONCLUSIONS:
Low-risk patients <75 years treated with supra-annular, self-expanding TAVR had comparable 3-year all-cause mortality and lower disabling stroke compared with patients treated with surgery. There was significantly better valve performance in patients treated with TAVR.
REGISTRATION:
URL: https://clinicaltrials.gov; Unique identifier: NCT02701283.
Keywords: aortic valve stenosis, humans, incidence, stroke, transcatheter aortic valve replacement
WHAT IS KNOWN
Randomized trials have supported the use of transcatheter aortic valve replacement without risk as a standalone criterion.
Age is still listed in the decision process and differs between the United States and European guidelines.
WHAT THE STUDY ADDS
This study explores the age gap between the United States and the European guidelines for outcomes in a low-risk population.
Transcatheter aortic valve replacement (TAVR) has been established as an alternative to surgery in patients with severe, symptomatic aortic stenosis at all levels of surgical risk.1–4 Two recent randomized clinical trials in low-surgical-risk aortic stenosis patients4,5 have shown comparable outcomes with TAVR and surgery at 46 and 5 years,7 with an average patient age of ≈74 years. The American College of Cardiology/American Heart Association has recommended shared decision-making for TAVR and surgery in patients aged 65 to 80 years,8 whereas the European consensus guidelines recommend that surgery is performed in patients 75 years and younger and that TAVR is recommended in patients over 75 years of age.9 Less is known about longer-term outcomes in younger patients who may be considered for less invasive therapies.10
Early results in TAVR in the low-risk trials were excellent, and the primary concerns for TAVR in younger patients relate to lifetime management,11,12 including concerns about transcatheter bioprosthetic valve durability,10 higher rates of residual aortic regurgitation, higher postprocedural pacemaker rates requirements,10 potential difficulty with coronary access,10,13 rates, and treatment options for late bioprosthetic valve failures.10 While transcatheter bioprosthetic valve design and operator techniques have improved substantially over the past several years, longer-term outcomes in younger low-risk patients treated with contemporary technology and techniques have not been reported.
The objective of this study was to evaluate the 3-year clinical outcomes of patients <75 years of age who were enrolled in the Evolut Low Risk Trial and randomized to treatment with TAVR or surgery. We also sought to compare indices of bioprosthetic valve performance in younger patients over 3 years in these patients.
Methods
Trial Design
The Evolut Low Risk trial (NCT02701283) was a prospective, international, multi-center, randomized clinical trial performed at 86 centers with the purpose of evaluating the safety and efficacy of the Evolut supra-annular, self-expanding transcatheter bioprosthesis in patients with symptomatic, severe aortic stenosis at low risk for surgery.4 A detailed description of the trial design, trial oversight, and randomization process have been previously reported.4 In brief, patients who met eligibility criteria were randomized 1:1 between March 2016 and May 2019 to undergo TAVR with a self-expanding bioprosthesis or surgical aortic valve replacement. Notably, patients who were deemed candidates for mechanical aortic valves were excluded from the trial. The trial was conducted according to Good Clinical Practice principles and conforms to the Declaration of Helsinki requirements for patient rights. The trial protocol was approved by the institutional review board at each site, and all patients provided informed written consent. The data, analytical methods, and study materials are owned by the sponsor and, therefore, will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Patients
Patients with symptomatic, severe aortic stenosis who met clinical and anatomic suitability criteria for the Evolut Low Surgical Risk Trial were randomized 1:1 to TAVR with the self-expanding, supra-annular Medtronic valve (CoreValve, Evolut R, or Evolut PRO) or to surgery with the bioprosthetic valve chosen by the surgeon to provide the best outcome for the patients. For this post hoc analysis, only patients younger than 75 years were included (703 patients).
Trial End Points
The primary end point for the Evolut Low Risk trial was a composite of all-cause mortality or disabling stroke at 2 years evaluated using a Bayesian group sequential design in the as-treated population and showed noninferiority of TAVR to surgery.4 The end points evaluated at 3 years in this analysis were all-cause mortality or disabling stroke, valve performance assessed using serial Doppler echocardiography, and functional status as determined by quality of life measured by the Kansas City Cardiomyopathy Questionnaire (KCCQ)14 and the New York Heart Association functional classification. Safety events assessed included aortic valve rehospitalization, prosthetic valve endocarditis, prosthetic valve thrombosis, new permanent pacemaker implantation, and valve reinterventions. Time-to-first-event analyses were undertaken to assess the composite of all-cause mortality, disabling stroke, and aortic valve hospitalization (with stroke defined and adjudicated as previously reported).4
Other prespecified end points were compared using Valve Academic Research Consortium-2 criteria.15 Clinical end point definitions are available in Table S1. Trial end points were adjudicated by an independent Clinical Events Committee except for permanent pacemaker implantation, atrial fibrillation, and aortic valve hospitalizations, which were site-reported. All echocardiograms were evaluated by an Echocardiography Core Laboratory (Mayo Clinic, Rochester, MN).
Statistical Analysis
Continuous variables are reported as mean±SD or median (first quartile [Q1], third quartile [Q3]). Comparisons were based on Student t test. Categorical variables are summarized as counts (frequencies) and percentages with comparisons based on the χ2 test or Fisher exact test for nominal variables and Cochran-Mantel-Haenszel tests for ordinal variables. Clinical outcomes to 30 days, 1 year, and 3 years are reported as Kaplan-Meier estimates (%) and are compared between the 2 treatment arms using the log-rank test and reported with hazard ratios and 95% CIs. Absolute differences in Kaplan-Meier survival rates for TAVR and surgery patients were reported at yearly intervals for the all-cause mortality or disabling stroke. Cox proportional hazards regression with a treatment-by-subgroup interaction was used to examine the treatment effect across subgroups. P values for change from baseline for KCCQ and valve performance were based on the Student t test. No statistical technique was used to impute missing data. No adjustments were made for multiplicity. Statistical analyses were performed using SAS, version 9.4 (SAS Institute, Cary, NC).
Results
Baseline Demographics
There were 703 patients younger than 75 years of age with severe aortic stenosis and low surgical risk in this analysis; of these, 352 underwent attempted treatment with TAVR and 351 with surgery. Baseline characteristics were similar in the 2 treatment groups (Table 1). The mean patient age was 69.1±4.0 years (minimum 51 and maximum 74), 34.9% were female sex, and patients were at low surgical risk, as evaluated by the heart team and through the Society for Thoracic Surgery Predicted Risk of Mortality assessment (1.7±0.6%). For the TAVR group, the median duration of follow-up was 48.7 months; for the surgery group, it was 48.1 months. Between the time of implant and 3 years, 6 patients in the TAVR group (all withdrawals) and 27 patients in the surgery group (24 withdrawals and 3 lost to follow-up) exited the trial (Figure S1).
Table 1.
Baseline Patient Characteristics
Thirty-Day Clinical Outcomes
The 30-day clinical outcomes are found in Table S2. There was no difference in the rate of all-cause mortality or disabling stroke between the treatment groups. The rates of new-onset atrial fibrillation, acute kidney injury, and life-threatening/disabling bleeding were lower with TAVR than surgery, while the need for a new pacemaker due to conduction abnormalities was higher with TAVR than surgery. Other procedural findings for TAVR (Table S3) and surgery (Table S4) found shorter overall procedure times with TAVR.
One- and Three-Year Clinical Outcomes
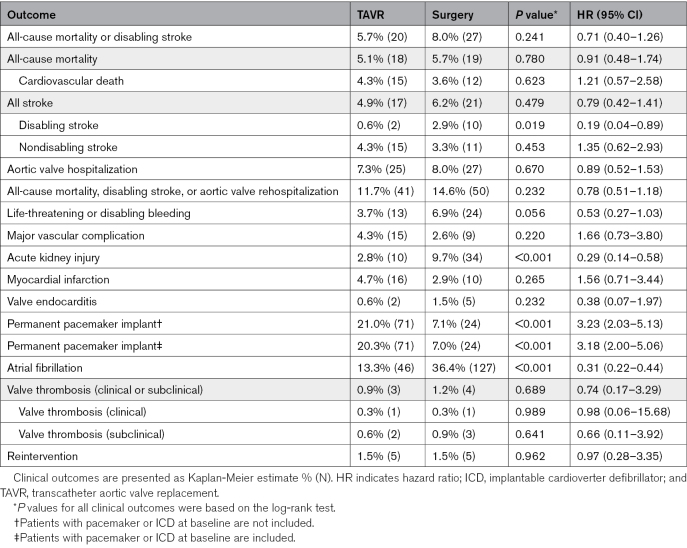
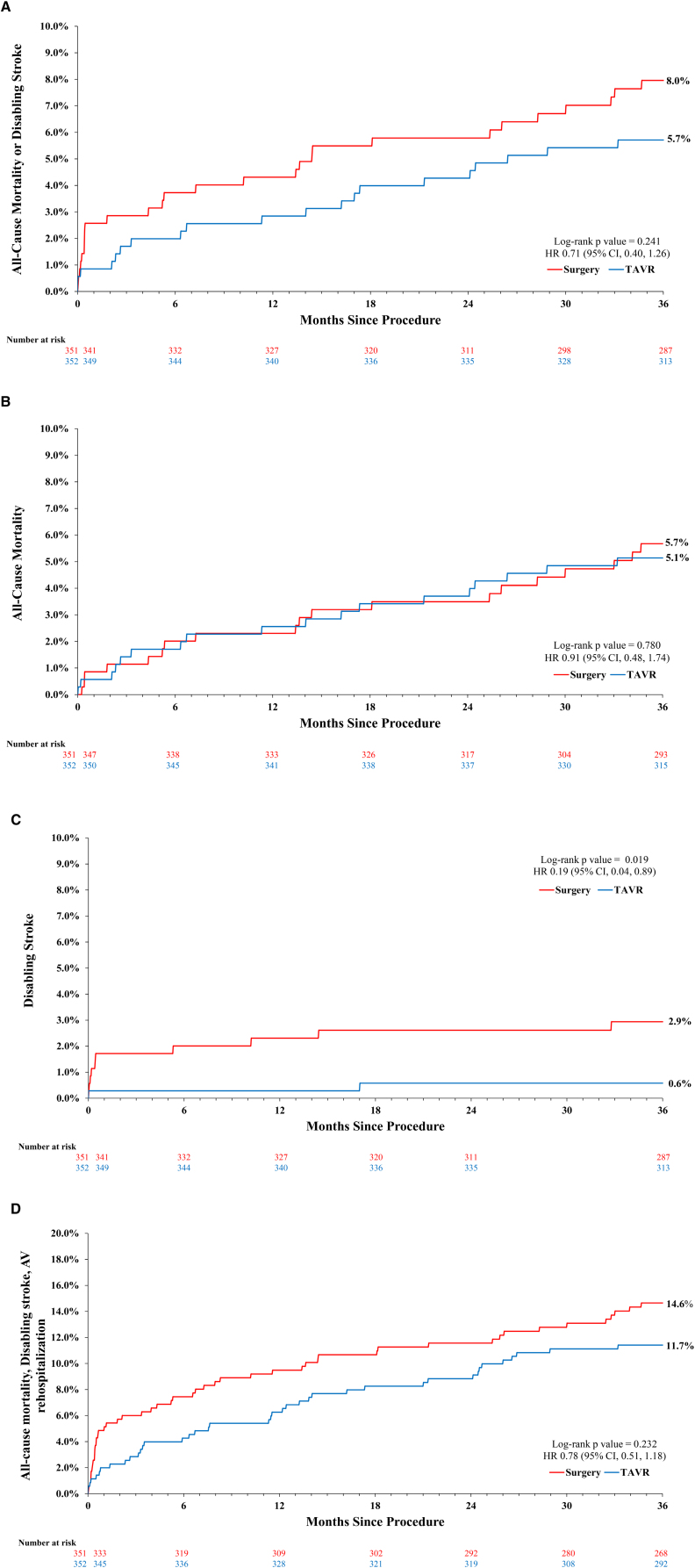
One-year clinical outcomes are found in Table S5, and 3-year clinical outcomes are found in Table 2 and Figure 1. All-cause mortality or disabling stroke at 3 years was not significantly different between the 2 treatment groups (TAVR, 5.7%; surgery, 8.0%; P=0.241; Table 2; Figure 1A). The 3-year rate of all-cause mortality was not significantly different (TAVR, 5.1%; surgery, 5.7%; P=0.780; Figure 1B), although TAVR was associated with a significantly lower rate of disabling stroke (0.6% versus 2.9%; P=0.019; Figure 1C). The composite end point of all-cause mortality, disabling stroke, or aortic valve rehospitalization was 11.7% in the TAVR group and 14.6% in the surgery group (P=0.232; Figure 1D). Subgroup analysis of key baseline demographics did not reveal any significant interactions in the treatment effect for all-cause mortality or disabling stroke (Figure S2).
Table 2.
Three-Year Clinical Outcomes
Figure 1.
Clinical outcomes in young patients undergoing transcatheter aortic valve replacement (TAVR) or surgery. Kaplan-Meier time-to-event curves and log-rank P values through 3 years for clinical outcomes in TAVR and surgery patients. A, Three-year all-cause mortality or disabling stroke in patients <75 years undergoing TAVR or surgery. B, Three-year all-cause mortality in patients <75 years undergoing TAVR or surgery. C, Three-year disabling stroke in patients <75 years undergoing TAVR or surgery. D, Three-year all-cause mortality, disabling stroke, or aortic valve hospitalization in patients <75 years undergoing TAVR or surgery. AV indicates aortic valve; and HR, hazard ratio.
The rate of atrial fibrillation was lower with TAVR than with surgery (13.3% versus 36.4%; P<0.001), but TAVR was associated with a higher rate of pacemaker implantation (21.0% versus 7.1%; P<0.001). Valve reintervention rates were low in both groups (TAVR, 1.5%; surgery, 1.5%; P=0.962). Rates of clinical (0.3% versus 0.3%; P=0.989) and subclinical (0.6% versus 0.9%; P=0.641) valve thrombosis at 3 years were low for both groups (Table 2). A total of 10 patients had a repeat aortic valve replacement out to 3 years (5 TAVR, 5 surgery). All 5 reinterventions in the TAVR patients were for surgical aortic valve replacement (3 due to leaflet tears, 1 due to undersized TAVR, and 1 due to endocarditis), and for the surgical patients, 1 of the reinterventions (due to stenosis) was percutaneous, while the remaining 4 were surgical (1 due to thrombosis and 4 due to endocarditis).
Bioprosthetic Valve Performance
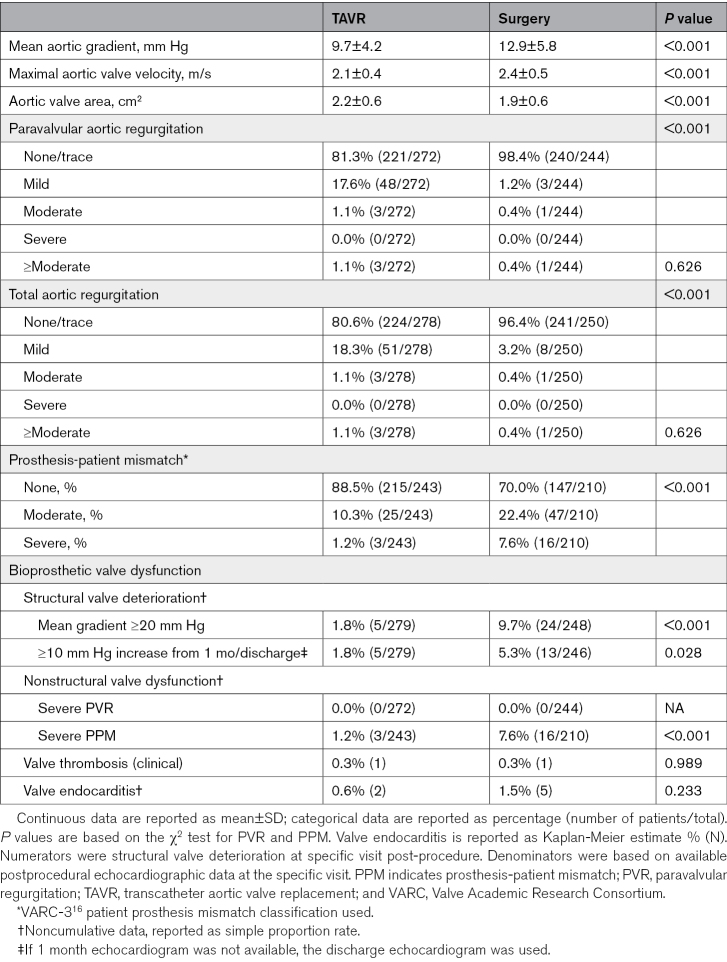
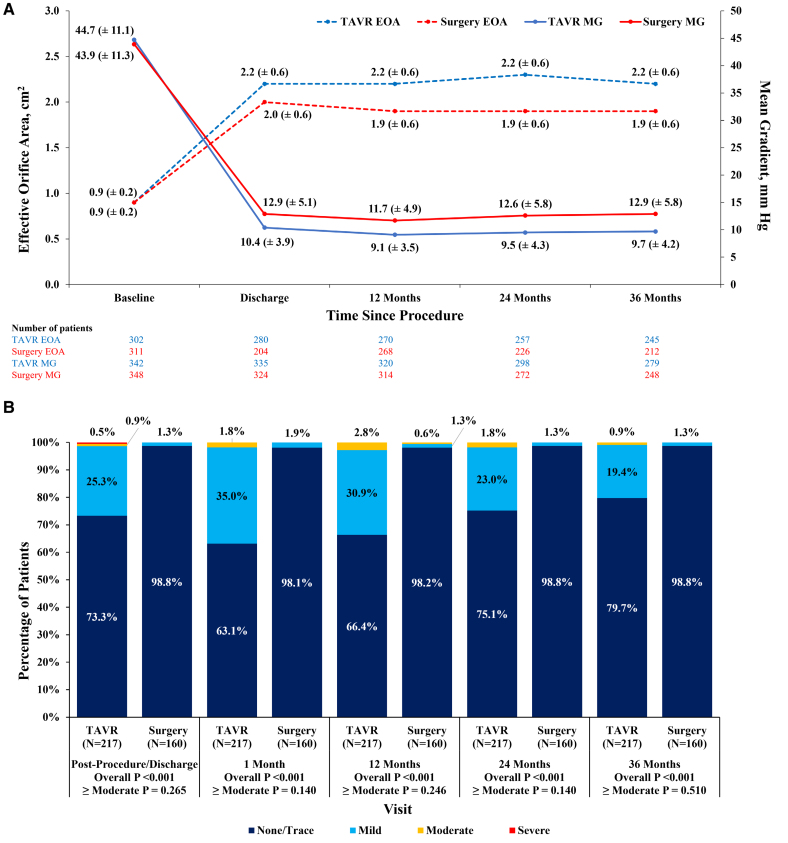
Echocardiographic findings are summarized in Table 316 and Table S6. Mean aortic gradients were consistently and significantly lower in TAVR patients compared with surgery patients (at 3 years: 9.7 mm Hg for TAVR versus 12.9 mm Hg for surgery; P<0.001; Figure 2A), and effective orifice areas were significantly larger (2.2 versus 1.9 cm2; P<0.001; Figure 2A). Moderate or greater paravalvular regurgitation at 3 years was numerically higher for TAVR than surgery but not significantly different (TAVR, 1.1%; surgery, 0.4%; P=0.626; Figure 2B; Figure S3) with paravalvular regurgitation rates overall being higher in TAVR patients. Patients treated with TAVR showed lower rates of severe prosthesis-patient mismatch (Table 3) than surgery patients (1.2% versus 7.6%; P<0.001).
Table 3.
Three-Year Bioprosthetic Valve Performance
Figure 2.
Hemodynamic valve performance. Three-year echocardiography core laboratory findings. Mean±SDs are reported for each time point. A, Effective orifice area and mean gradients in patients <75 years undergoing transcatheter aortic valve replacement (TAVR) or surgery. B, The rates of residual paravalvular regurgitation in patients <75 years undergoing TAVR or surgery. EOA indicates effective orifice area; and MG, mean gradient.
Quality of Life
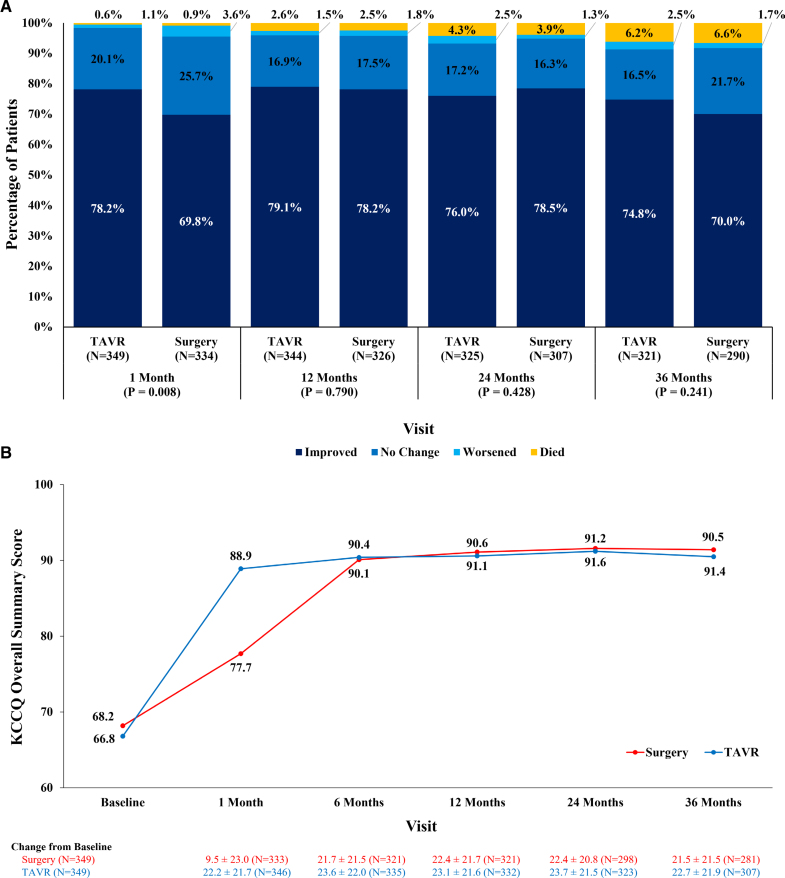
The New York Heart Association score improved from baseline to 3 years by at least 1 functional class in 74.8% of TAVR and 70.0% of surgery patients (Figure 3A). From the overall KCCQ summary score, TAVR patients manifested improved quality of life earlier than surgery patients (88.9 versus 77.7; P<0.001) at 1 month with both groups maintaining improvement up to 3 years (Figure 3B). An approximate 22-point increase from baseline KCCQ was observed for both groups at 3 years, confirming clinically significant improvement in the patients’ quality of life, with this improvement occurring within the first 30 days after TAVR and by 6 months after surgery.
Figure 3.
Three-year functional status in patients treated with transcatheter aortic valve replacement (TAVR) or surgery. Ordinal variables are presented as frequencies and continuous variables are expressed as mean±SDs. A, Change in the New York Heart Association class for symptoms of heart failure in patients <75 years undergoing TAVR or surgery. B, Kansas City Cardiomyopathy Questionnaire (KCCQ) summary score over time in patients <75 years undergoing TAVR or surgery.
Discussion
Our post hoc analysis of the Evolut Low Risk clinical trial in patients under 75 years of age found that that the 3-year all-cause mortality or disabling stroke was not statistically significant in patients treated with self-expanding, supra-annular TAVR versus surgery. While there was no difference in 3-year all-cause mortality between the 2 groups, the occurrence of disabling stroke was lower in patients treated with TAVR compared with surgery, largely attributable to the early periprocedural difference in stroke rates. Bioprosthetic valve performance was also better in patients treated with TAVR than surgery at 3 years, and although there was less improvement in mild paravalvular regurgitation in TAVR patients, overall reintervention rates were low in this population under 75 years. Functional status was better at 1 month with TAVR compared with surgery, but there were no differences at 3 years. It is plausible that the lower incidence of disabling stroke and the rapid functional recovery experienced by younger patients at 1 month are key reasons that patients prefer TAVR since it allows a rapid return to everyday activities and work. While longer-term follow-up to 10 years is underway, these intermediate-term results support the use of TAVR in low-risk patients under 75 years.
Societal Guidelines
Societal guidelines differ with respect to the appropriate age to consider TAVR, as the American College of Cardiology/American Heart Association recommends surgery for patients under 65 years of age,8 while the European consensus guidelines recommend surgery for low-risk patients 75 years and younger.9 Concerns for TAVR in younger patients relate to transcatheter bioprosthetic valve durability,10 the impact of higher rates of residual aortic regurgitation and postprocedural pacemaker requirements, potential difficulty with coronary access,10,13,17 and treatment options for late bioprosthetic valve failures.10
Prior TAVR Studies in Younger Patients
Interpretation of prior studies in younger patients has been confounded by the fact that many of these patients were often deemed higher risk for surgery.18 Substantial comorbidities were documented in younger patients, including previous coronary artery bypass grafting, porcelain aorta, severe left ventricular systolic dysfunction, prior chest radiation, severe lung disease, hemodynamic instability, advanced liver disease, higher proportion of previous stroke, peripheral vascular disease, diabetes, corticosteroids, and active cancer.18,19
Our study is the largest post hoc analysis to date of a randomized trial of low-risk patients under 75 years of age who were assigned to treatment with supra-annular, self-expanding TAVR, or surgery. Patients were deemed low-risk for surgery and did not have the comorbidities that were present in prior studies.18,20,21 Clinical outcomes were balanced between the 2 groups, with higher rates of atrial fibrillation and acute kidney injury in surgery patients, and higher rates of new permanent pacemakers and residual paravalvular regurgitation in the TAVR patients. Importantly, functional status, as assessed by the New York Heart Association Class and KCCQ improvements, was similar at 3 years in the 2 groups.
These data support the use of self-expanding supra-annular TAVR in low-risk patients in the age group <75 years. There have since been improvements in both the bioprosthetic valve design (eg, Evolut FX) and operator technique (eg, cusp overlap technique) to improve both residual paravalvular regurgitation and the need for permanent pacemaker placement. The Optimize PRO study demonstrates how using a more modern technique (cusp overlap technique) contributes to lower rates of new permanent pacemaker implantation (9.8%) when valves of the same product family are used.22 Commissural alignment with newer generation valve design and operator technique has improved coronary access.17 Algorithms for management of late failure of transcatheter aortic valves have been developed.10 Similarly, there have been newer generation surgical valve designs and a growing recognition of the importance of aortic root enlargement procedures and other techniques to optimize valve replacement sizing to optimize hemodynamics.
Bioprosthetic Valve Performance
Bioprosthetic valve performance has been a central tenant after surgical aortic valve placement.23 Bioprosthetic valve dysfunction includes the occurrence of structural valve deterioration and nonstructural valve dysfunction and includes prothesis patient mismatch and residual paravalvular regurgitation, thrombosis, and infectious endocarditis. These factors are associated with a worse prognosis.11,24 Our study found more favorable initial hemodynamics and less severe prosthesis-patient mismatch in patients treated with TAVR than surgery and similar rates of valve thrombosis and endocarditis. Longer follow-up in this cohort will provide additional needed information.
Limitations
Our study has several important limitations. It is a post hoc analysis of a randomized trial of low-surgical-risk TAVR patients. Dedicated randomized clinical trials may be needed. The small number of deaths and disabling strokes (only 47 in total) limited the power/ability to demonstrate statistical significance. The follow-up is limited to 3 years, and a complete assessment of valve durability may require a 10-year follow-up, which is ongoing in the Evolut Low Risk trial. The surgical arm included 14 different types of surgical valves. We did not account for patients lost to follow-up in this analysis; however, a sensitivity analysis performed for the primary analysis indicated that there was no impact of missing data on the primary end point at 2 years.4 The reason for the lower disabling stroke rate in patients undergoing TAVR has not been ascertained but may relate to less intracardiac manipulation and lower rates of periprocedural atrial fibrillation.
Conclusions
Our study supports the use of supra-annular, self-expanding TAVR in aortic stenosis patients younger than 75 years who are low-risk for surgery with similar all-cause mortality and lower rates of disabling stroke at 3 years. Bioprosthetic valve performance is significantly better in patients treated with TAVR compared with surgery. Longer-term studies are needed to provide insight into the long-term durability of these 2 approaches to patients with severe aortic stenosis.
ARTICLE INFORMATION
Acknowledgments
Panagiota Kopsiafti, BSc (Hons), MSc, MPH, and Matt Brown, PhD, CMPP, employees of Medtronic, provided medical writing support under the direction of the lead authors.
Sources of Funding
The Evolut Low Risk Trial is funded by Medtronic (Minneapolis, MN).
Disclosures
Dr Modine is a consultant and Senior Advisory Board member for Medtronic. Dr Tchétché is a consultant for Medtronic. Dr Van Mieghem receives grants from Medtronic during the conduct of the trial and grants from Abbott Vascular, Edwards Lifesciences, Boston Scientific, Abiomed, PulseCath BV, and Daiichi Sankyo outside the submitted work. Dr Chetcuti receives personal fees from Medtronic; grants from Edwards, Boston Scientific, and Jena (paid to institution) during conduct of trial; and on an advisory board for Biotrace and Jena valve without remuneration. Dr Yakubov receives grants from Boston Scientific and Medtronic (paid to institution) and personal fees from Medtronic during the conduct of the trial. Dr Sorajja is a consultant to Boston Scientific and Medtronic. Dr Gada receives personal fees from Medtronic, Abbott Vascular, Becton Dickenson, and Boston Scientific outside the submitted work. Dr Mumtaz is a consultant and has received honoraria and research grants from Medtronic, Edwards, Atricure, Teleflex, Foldax, Japanese Organization for Medical Device Development, and Abbott. Dr Ramlawi receives grants, personal fees, and nonfinancial support from Medtronic, Liva Nova, and AtriCure. Dr Bajwa is a consultant for Medtronic. Dr Teirstein receives research grant and honoraria from Abbott, Boston Scientific, Cordis, and Medtronic, and serves on an advisory board for Boston Scientific and Medtronic. Dr Kleiman receives clinical trial reimbursement to his institution (Houston Methodist DeBakey Heart and Vascular Center) during the conduct of the trial. Dr Bagur serves as a consultant for Medtronic. Dr Chu receives Speakers’ honoraria from Medtronic, Edwards Lifesciences, Terumo Aortic and Artivion. Dr Huang is an employee and shareholder of Medtronic, plc. Dr Popma is a full-time employee and shareholder for Medtronic. Dr Forrest receives grant support/research contracts and consultant fees/honoraria/Speakers Bureau fees from Edwards Lifesciences and Medtronic. Dr Reardon receives grants from Medtronic (paid to institution) during conduct of trial, and consulting fees from Abbott, Boston Scientific, and Gore Medical (paid to institution) outside of the submitted work. The other authors report no conflicts.
Supplemental Material
Tables S1–S6
Figures S1–S3
Supplementary Material
Nonstandard Abbreviations and Acronyms
- KCCQ
- Kansas City Cardiomyopathy Questionnaire
- TAVR
- transcatheter aortic valve replacement
This manuscript was sent to Michael H. Sketch, Jr, Senior Guest Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCINTERVENTIONS.124.014018.
For Sources of Funding and Disclosures, see page 1038.
Contributor Information
Thomas Modine, Email: thomasmodine@gmail.com.
Nicolas M. Van Mieghem, Email: n.vanmieghem@erasmusmc.nl.
G. Michael Deeb, Email: mdeeb@med.umich.edu.
Stanley J. Chetcuti, Email: chetcuti@umich.edu.
Steven J. Yakubov, Email: steven.yakubov@ohiohealth.com.
Paul Sorajja, Email: paul.sorajja@allina.com.
Hemal Gada, Email: hgada@pinnaclehealth.org.
Mubashir Mumtaz, Email: Mumtazma@upmc.edu.
Basel Ramlawi, Email: RamlawiB@mlhs.org.
Tanvir Bajwa, Email: tanvir.bajwa@aah.org.
John Crouch, Email: publishing161@aurora.org.
Paul S. Teirstein, Email: Teirstein.paul@scrippshealth.org.
Neal S. Kleiman, Email: nkleiman@houstonmethodist.org.
Ayman Iskander, Email: ayman.iskander@gmail.com.
Rodrigo Bagur, Email: rodrigobagur@yahoo.com.
Michael W.A. Chu, Email: michael.chu@lhsc.on.ca.
Pierre Berthoumieu, Email: pberthoum@clinique-pasteur.com.
Arnaud Sudre, Email: a.sudre@yahoo.fr.
Rik Adrichem, Email: r.adrichem@erasmusmc.nl.
Saki Ito, Email: ito.saki@mayo.edu.
Jian Huang, Email: jian.huang@medtronic.com.
John K. Forrest, Email: john.k.forrest@yale.edu.
References
- 1.Popma JJ, Adams DH, Reardon MJ, Yakubov SJ, Kleiman NS, Heimansohn D, Hermiller J, Jr, Hughes GC, Harrison JK, Coselli J, et al. ; CoreValve United States Clinical Investigators. Transcatheter aortic valve replacement using a self-expanding bioprosthesis in patients with severe aortic stenosis at extreme risk for surgery. J Am Coll Cardiol. 2014;63:1972–1981. doi: 10.1016/j.jacc.2014.02.556 [DOI] [PubMed] [Google Scholar]
- 2.Adams DH, Popma JJ, Reardon MJ, Yakubov SJ, Coselli JS, Deeb GM, Gleason TG, Buchbinder M, Hermiller J, Jr, Kleiman NS, et al. ; U.S. CoreValve Clinical Investigators. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;370:1790–1798. doi: 10.1056/NEJMoa1400590 [DOI] [PubMed] [Google Scholar]
- 3.Reardon MJ, Van Mieghem NM, Popma JJ, Kleiman NS, Søndergaard L, Mumtaz M, Adams DH, Deeb GM, Maini B, Gada H, et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2017;376:1321–1331. doi: 10.1056/NEJMoa1514616 [DOI] [PubMed] [Google Scholar]
- 4.Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O’Hair D, Bajwa T, Heiser JC, Merhi W, Kleiman NS, et al. ; Evolut Low Risk Trial Investigators. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. 2019;380:1706–1715. doi: 10.1056/NEJMoa1816885 [DOI] [PubMed] [Google Scholar]
- 5.Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, Kapadia SR, Malaisrie SC, Cohen DJ, Pibarot P, et al. ; PARTNER 3 Investigators. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380:1695–1705. doi: 10.1056/NEJMoa1814052 [DOI] [PubMed] [Google Scholar]
- 6.Forrest JK, Deeb GM, Yakubov SJ, Gada H, Mumtaz MA, Ramlawi B, Bajwa T, Teirstein PS, Tchétché D, Huang J, et al. ; Evolut Low Risk Trial Investigators. 4-year outcomes of patients with aortic stenosis in the evolut low risk trial. J Am Coll Cardiol. 2023;82:2163–2165. doi: 10.1016/j.jacc.2023.09.813 [DOI] [PubMed] [Google Scholar]
- 7.Mack MJ, Leon MB, Thourani VH, Pibarot P, Hahn RT, Genereux P, Kodali SK, Kapadia SR, Cohen DJ, Pocock SJ, et al. ; PARTNER 3 Investigators. Transcatheter aortic-valve replacement in low-risk patients at five years. N Engl J Med. 2023;389:1949–1960. doi: 10.1056/NEJMoa2307447 [DOI] [PubMed] [Google Scholar]
- 8.Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP, 3rd, Gentile F, Jneid H, Krieger EV, Mack M, McLeod C, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143:e35–e71. doi: 10.1161/CIR.0000000000000932 [DOI] [PubMed] [Google Scholar]
- 9.Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, Capodanno D, Conradi L, De Bonis M, De Paulis R, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. EuroIntervention. 2022;17:e1126–e1196. doi: 10.4244/EIJ-E-21-00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yerasi C, Rogers T, Forrestal BJ, Case BC, Khan JM, Ben-Dor I, Satler LF, Garcia-Garcia HM, Cohen JE, Kitahara H, et al. Transcatheter versus surgical aortic valve replacement in young, low-risk patients with severe aortic stenosis. JACC Cardiovasc Interv. 2021;14:1169–1180. doi: 10.1016/j.jcin.2021.03.058 [DOI] [PubMed] [Google Scholar]
- 11.Flameng W, Herregods MC, Vercalsteren M, Herijgers P, Bogaerts K, Meuris B. Prosthesis-patient mismatch predicts structural valve degeneration in bioprosthetic heart valves. Circulation. 2010;121:2123–2129. doi: 10.1161/CIRCULATIONAHA.109.901272 [DOI] [PubMed] [Google Scholar]
- 12.Hanzel GS, Gersh BJ. Transcatheter aortic valve replacement in low-risk, young patients: natural expansion or cause for concern? Circulation. 2020;142:1317–1319. doi: 10.1161/CIRCULATIONAHA.120.047874 [DOI] [PubMed] [Google Scholar]
- 13.Rogers T, Greenspun BC, Weissman G, Torguson R, Craig P, Shults C, Gordon P, Ehsan A, Wilson SR, Goncalves J, et al. Feasibility of coronary access and aortic valve reintervention in low-risk TAVR patients. JACC Cardiovasc Interv. 2020;13:726–735. doi: 10.1016/j.jcin.2020.01.202 [DOI] [PubMed] [Google Scholar]
- 14.Spertus JA, Jones PG, Sandhu AT, Arnold SV. Interpreting the Kansas City cardiomyopathy questionnaire in clinical trials and clinical care: JACC state-of-the-art review. J Am Coll Cardiol. 2020;76:2379–2390. doi: 10.1016/j.jacc.2020.09.542 [DOI] [PubMed] [Google Scholar]
- 15.Kappetein AP, Head SJ, Genereux P, Piazza N, van Mieghem NM, Blackstone EH, Brott TG, Cohen DJ, Cutlip DE, van Es GA, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the valve academic research consortium-2 consensus document. J Am Coll Cardiol. 2012;60:1438–1454. doi: 10.1093/ejcts/ezs533 [DOI] [PubMed] [Google Scholar]
- 16.Généreux P, Piazza N, Alu MC, Nazif T, Hahn RT, Pibarot P, Bax JJ, Leipsic JA, Blanke P, Blackstone EH, et al. ; VARC-3 WRITING COMMITTEE. Valve academic research consortium 3: updated endpoint definitions for aortic valve clinical research. J Am Coll Cardiol. 2021;77:2717–2746. doi: 10.1016/j.jacc.2021.02.038 [DOI] [PubMed] [Google Scholar]
- 17.Tarantini G, Nai Fovino L, Scotti A, Massussi M, Cardaioli F, Rodino G, Benedetti A, Boiago M, Matsuda Y, Continisio S, et al. Coronary access after transcatheter aortic valve replacement with commissural alignment: the ALIGN-ACCESS study. Circ Cardiovasc Interv. 2022;15:e011045. doi: 10.1161/CIRCINTERVENTIONS.121.011045 [DOI] [PubMed] [Google Scholar]
- 18.Ancona MB, Toscano E, Moroni F, Ferri LA, Russo F, Bellini B, Sorropago A, Mula C, Festorazzi C, Gamardella M, et al. Patients younger than 70 undergoing transcatheter aortic valve implantation: procedural outcomes and mid-term survival. Int J Cardiol Heart Vasc. 2021;34:100817. doi: 10.1016/j.ijcha.2021.100817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Regev E, Finkelstein A, Assali A, Barbash I, Fefer P, Ben-Shoshan J, Orvin K, Konigstein M, Guetta V, Kornowski R, et al. Comparison of outcome of transcatheter aortic valve implantation for severe aortic stenosis in 3 age groups (</=70; 71 to 80, and >/=81 years). Am J Cardiol. 2017;120:1607–1611. doi: 10.1016/j.amjcard.2017.07.060 [DOI] [PubMed] [Google Scholar]
- 20.Mach M, Poschner T, Hasan W, Kerbel T, Szalkiewicz P, Hasimbegovic E, Andreas M, Gross C, Strouhal A, Delle-Karth G, et al. Transcatheter versus isolated surgical aortic valve replacement in young high-risk patients: a propensity score-matched analysis. J Clin Med. 2021;10:3447. doi: 10.3390/jcm10153447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fraccaro C, Testa L, Schiavo A, Brambilla N, Napodano M, Azzolina D, Bedogni F, Tarantini G. Transcatheter aortic valve implantation in patients younger than 75 years: guidelines-based patients selection and clinical outcome. Int J Cardiol. 2018;272:273–278. doi: 10.1016/j.ijcard.2018.08.021 [DOI] [PubMed] [Google Scholar]
- 22.Grubb KJ, Gada H, Mittal S, Nazif T, Rodes-Cabau J, Fraser DGW, Lin L, Rovin JD, Khalil R, Sultan I, et al. Clinical impact of standardized TAVR technique and care pathway: insights from the optimize PRO study. JACC Cardiovasc Interv. 2023;16:558–570. doi: 10.1016/j.jcin.2023.01.016 [DOI] [PubMed] [Google Scholar]
- 23.Johnston DR, Soltesz EG, Vakil N, Rajeswaran J, Roselli EE, Sabik JF, 3rd, Smedira NG, Svensson LG, Lytle BW, Blackstone EH. Long-term durability of bioprosthetic aortic valves: implications from 12,569 implants. Ann Thorac Surg. 2015;99:1239–1247. doi: 10.1016/j.athoracsur.2014.10.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Hair D, Yakubov SJ, Grubb KJ, Oh JK, Ito S, Deeb GM, Van Mieghem NM, Adams DH, Bajwa T, Kleiman NS, et al. Structural valve deterioration after self-expanding transcatheter or surgical aortic valve implantation in patients at intermediate or high risk. JAMA Cardiol. 2023;8:111–119. doi: 10.1001/jamacardio.2022.4627 [DOI] [PMC free article] [PubMed] [Google Scholar]