ABSTRACT
Arterial desaturation following surgical repair of tetralogy of Fallot (TOF) is rare. In most instances, it results from residual right ventricular outflow tract obstruction, causing right-to-left shunt across residual interatrial or interventricular communication. In this report, we present an unusual scenario of arterial desaturation due to a recanalized left cardinal vein in a child with repaired TOF. We also discuss stepwise evaluation that led to successful identification and occlusion of the abnormal venous channel by percutaneous device closure.
Keywords: One and half ventricle repair, postoperative cyanosis, venovenous collateral
INTRODUCTION
Tetralogy of Fallot (TOF) has characteristic cardiac morphology. Consequent to a large nonrestrictive ventricular septal defect (VSD) and right ventricular outflow tract obstruction (RVOTO), the right ventricle (RV) is almost always well-formed and hypertrophied. Nevertheless, coexisting tricuspid valve (TV) abnormalities rarely lead to RV hypoplasia, which warrants the construction of a bidirectional Glenn (BDG) shunt during TOF repair. Unlike one and half ventricle repair with a competent pulmonary valve, pulmonary regurgitation following TOF repair may interfere with the functioning of the cavopulmonary connection. While severe pulmonary regurgitation causes ineffective transpulmonary flow, the development of venovenous collateral can also result in right-to-left shunt and arterial desaturation. We report a child with repaired TOF with additional BDG who had recanalization of the left cardinal vein, causing severe arterial desaturation, emphasizing the role of careful assessment and planning for optimizing the management.
CASE REPORT
A 15-year-old boy presented three years following TOF repair (trans-RA Dacron patch VSD closure, infundibular resection, and transannular patch) with cyanosis (pulse oximeter saturation - 65%) and Ross class II dyspnea. He additionally underwent a superior cavopulmonary shunt with Danielson’s TV repair in view of mild RV hypoplasia and Ebsteinoid malformation of the TV with severe regurgitation. The patient had a complete heart block in the postoperative period, requiring epicardial pacemaker insertion. Neither residual/additional VSD nor RVOTO was noted on echocardiography. The patent foramen ovale (PFO) shunted left to right, and mild tricuspid regurgitation was present. Severe pulmonary regurgitation and pulsatile Glenn flow were noted without any gradient at the anastomotic sites. A saline contrast echocardiography performed from the left upper limb revealed bubbles in the left atrium, signifying patent left superior vena cava with unroofed coronary sinus or venovenous collateral. Contrast-enhanced computed tomography confirmed our suspicion of a venous channel connecting the brachiocephalic vein and the left atrium [Supplementary Image 1 (152.5KB, tif) ]. The channel also received the left intercostal vein and left upper pulmonary venous drainage. The postoperative cardiac anatomy and prevailing lesions are depicted in Figure 1.
Figure 1.
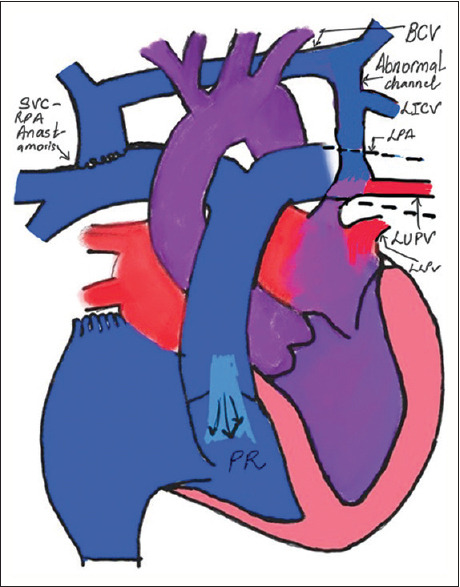
Schematic representation depicting the anatomical details and flow characteristics of the cardiac circuit (IV: Brachiocephalic vein, LUPV: Left upper pulmonary vein, LLPV: Left lower pulmonary vein, LICV: Left intercostal vein, PR: Pulmonary regurgitation)
Considering the risk associated with redo surgery, interventional management was preferred. Under conscious sedation, bilateral femoral venous access was obtained. Angiography was performed with a 6 Fr pigtail catheter position in the venous channel through the PFO [Figure 2]. Considering the diameter of the abnormal venous channel, a custom-made 30 mm × 28 mm Lifetech Duct Occluder™ (Lifetech Scientific, Shenzhen, PRC) was chosen. The device was successfully deployed at the junction of the venous channel and brachiocephalic vein through a 12 Fr Performer™ guiding sheath (Cook Medical LLC, Bloomington, USA) [Supplementary Video 1]. Postdeployment, the pulmonary artery (PA) pressures showed a mild rise up to 28/6/16 mm Hg (baseline 18/7/11). No residual shunt was seen on brachiocephalic vein angiography [Figure 3 and Supplementary Video 2]. Saturation improved to 96%. Pulmonary venous flow did not show any obstruction in the levophase of PA angiography [Figure 4 and Supplementary Video 3]. Despite the elevation in PA pressures, the child did not show any features suggestive of Glenn failure or SVC hypertension in the immediate postprocedure period or at follow-up. The child has significant functional class improvement and no desaturation on follow-up at 1 year.
Figure 2.
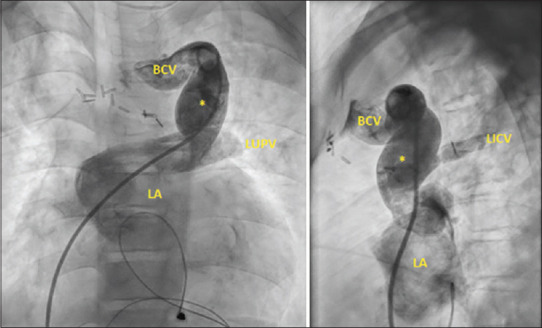
Angiogram in the anteroposterior (left) and lateral (right) view demonstrates the anomalous communication between the brachiocephalic vein and the left atrium. The drainage of the intercostal vein and left upper pulmonary vein is also profiled. (IV: Brachiocephalic vein, LICV: Left intercostal vein, LA: Left atrium, LUPV: Left upper pulmonary vein, *: Anomalous channel)
Figure 3.
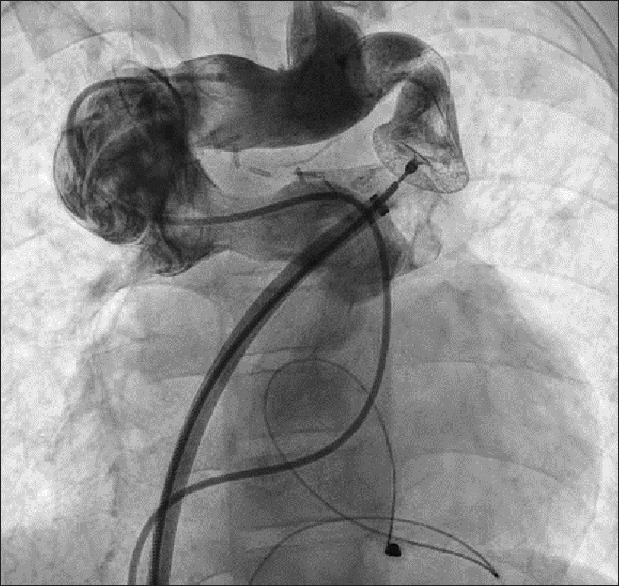
Angiogram performed in the brachiocephalic vein showing complete occlusion of the anomalous-brachiocephalic vein junction by the device
Figure 4.
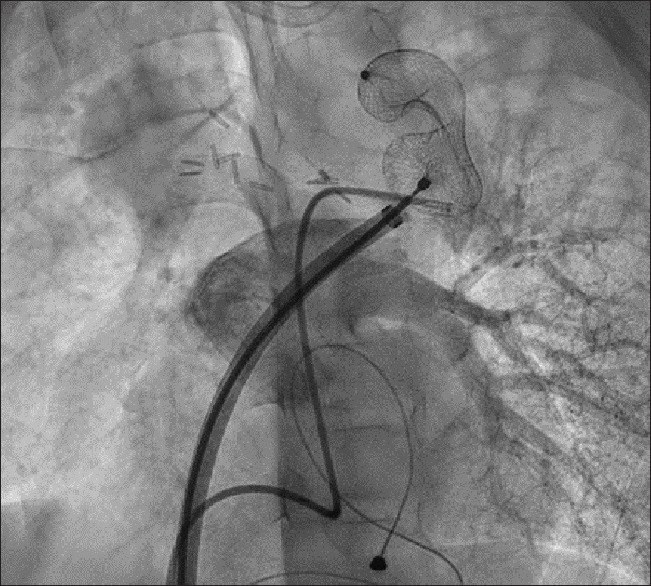
Left pulmonary artery angiogram demonstrating unobstructed flow through the pulmonary vein in levophase
DISCUSSION
Lesions characteristic of postoperative tetralogy include severe pulmonary insufficiency leading to RV dilatation with or without ventricular insufficiency. Less commonly encountered are residual lesions such as VSD, RVOTO, or branch PA abnormalities. The addition of Glenn physiology to this hemodynamics is rare; hence, effects on each other are unknown. Ensuing pulmonary regurgitation due to pulmonary valvotomy as a part of one and half ventricular repair may cause right heart failure due to the overwhelming volume overload of a hypoplastic RV. Second, it reduces the effective pulmonary blood flow. Third, it may lead to exaggerated pulsatility of the Glenn circuit akin to that observed in the systemic circulation of patients with severe aortic regurgitation. Superior vena cava hypertension is known to occur in nearly 40% of patients following one and half ventricular repair, while venovenous collateral formation is known in 14% of patients.[1,2] We believe the latter two factors led to the development of these communications in our patients.
The preoperative evaluation showed a good-sized right SVC and connecting cava. No sizeable left SVC was noticed during the preoperative assessment or surgery. Moreover, there was complete resolution of arterial desaturation following surgery and recurrence a few years later point against a sizeable left SVC. These points favor the argument that the channel may have recanalized over time. The exact anatomical nomenclature of this channel is debatable. The channel course anterior to the left PA and drainage of the left intercostal vein in its upper part favors the left superior vena cava. In contrast, its development over time and its drainage into the roof of the left atrium via the left upper pulmonary vein favors the levocardial vein. Similar instances of recanalization of the superior vena cave or development of venovenous communications after single ventricle palliation have been reported earlier.[3,4,5]
Occlusion of tubular communications is obtained by complete obliteration of flow by the bulk of the device. The largest available diameter of the most suitable devices in such lesion, namely Amplatzer vascular plugs, is 22 mm. The diameter of the channel was 24 mm. Thereby, occlusion with a single Amplatzer vascular plug was not feasible. Deploying two devices simultaneously would be uncontrolled, and the interplay of the devices with each other and the vessel would remain unpredictable. Interventional closure of similar communications has been reported earlier; however, none as large as seen in our case has been described.[6,7,8,9,10] The large diameter of the communication, absence of any constriction in the vertical segment, and restricted length of channel available due to the proximity of the pulmonary venous drainage in the lower part posed a unique technical challenge for occlusion. The tortuous, narrow upper end of the channel at the junction with the brachiocephalic vein was deemed suitable for anchoring an occluder device. We desired to position the wider distal (PA) end in the tortuous upper end, avoiding as little intrusion as possible in the low-flow brachiocephalic vein and Glenn circuit. The vertical segment of the channel was occluded with the waist of the device; however, encroachment of the pulmonary venous drainage in the lower part was avoided. In our opinion, a duct occluder most suitably achieves this waist-to-length ratio.
CONCLUSION
The case highlights the importance of knowledge and recognition of systemic-pulmonary venous communication in the evaluation of cyanosis following complex cardiac surgery. The approach emphasizes the role of saline contrast echocardiography as a valuable tool in identifying right to left shunts, especially extracardiac. Unusual anatomy and proximity of pulmonary venous drainage demanded a tailor-made interventional technique.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the legal guardian has given his consent for images and other clinical information to be reported in the journal. The guardian understands that names and initials will not be published and due efforts will be made to conceal patient identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Supplementary Videos available on: https://journals.lww.com/aopc
Volume rendered CT angiography image from the lateral aspect demonstrating the anomalous channel (*) and the drainage of the left upper pulmonary vein into the channel (arrow)
REFERENCES
- 1.Prasanna A, Tan CW, Anastasopulos A, Beroukhim RS, Emani SM. One and one-half ventricle repair: Role for restricting antegrade pulmonary blood flow. Ann Thorac Surg. 2022;114:176–83. doi: 10.1016/j.athoracsur.2021.04.058. [DOI] [PubMed] [Google Scholar]
- 2.Frommelt MA, Frommelt PC, Berger S, Pelech AN, Lewis DA, Tweddell JS, et al. Does an additional source of pulmonary blood flow alter outcome after a bidirectional cavopulmonary shunt? Circulation. 1995;92:I240–4. doi: 10.1161/01.cir.92.9.240. [DOI] [PubMed] [Google Scholar]
- 3.Marwah A, Khatri S, Shrivastava S, Iyer KS. Unusual systemic venous collateral channels to left atrium causing desaturation after Fontan operation closed percutaneously. Ann Pediatr Cardiol. 2013;6:191–3. doi: 10.4103/0974-2069.115284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McElhinney DB, Reddy VM, Hanley FL, Moore P. Systemic venous collateral channels causing desaturation after bidirectional cavopulmonary anastomosis: Evaluation and management. J Am Coll Cardiol. 1997;30:817–24. doi: 10.1016/s0735-1097(97)00223-4. [DOI] [PubMed] [Google Scholar]
- 5.Abdullah AF, Menahem S. Transcatheter closure of dilated left superior vena cava for resolution of late cyanosis following Fontan palliation. Heart Lung Circ. 2006;15:393–6. doi: 10.1016/j.hlc.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 6.Chowdhury UK, Airan B, Sharma R, Bhan A, Kothari SS, Saxena A, et al. One and a half ventricle repair with pulsatile bidirectional Glenn: Results and guidelines for patient selection. Ann Thorac Surg. 2001;71:1995–2002. doi: 10.1016/s0003-4975(01)02517-6. [DOI] [PubMed] [Google Scholar]
- 7.Recto MR, Elbl F, Austin E. Transcatheter closure of large persistent left superior vena cava causing cyanosis in two patients post-Fontan operation utilizing the Gianturco Grifka vascular occlusion device. Catheter Cardiovasc Interv. 2001;53:398–404. doi: 10.1002/ccd.1190. [DOI] [PubMed] [Google Scholar]
- 8.Troost E, Gewillig M, Budts W. Percutaneous closure of a persistent left superior vena cava connected to the left atrium. Int J Cardiol. 2006;106:365–6. doi: 10.1016/j.ijcard.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 9.Tomar M. Percutaneous device closure of persistent left superior vena cava connecting to the left atrium with intact coronary sinus: A rare entity. Images Paediatr Cardiol. 2017;19:1–8. [PMC free article] [PubMed] [Google Scholar]
- 10.Eddine AC, Kobayashi D. Persistent left superior vena cava draining into the left atrium: Review of associated venous anomalies and role of multimodality imaging. Prog Pediatr Cardiol. 2021;63:101377. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Volume rendered CT angiography image from the lateral aspect demonstrating the anomalous channel (*) and the drainage of the left upper pulmonary vein into the channel (arrow)


