Significance
It is accepted that the minimum gene set for nitrogen fixation includes nifH, nifD, and nifK for nitrogenase, and nifE, nifN, and nifB for FeMo-co biosynthesis. Conventionally, the NifDK homolog NifEN is essential for FeMo-co synthesis. However, this study shows that a thermophilic Roseiflexus bacterium can assemble FeMo-co without NifEN. In this bacterium, NifDK serves both as a catalyst for nitrogen fixation and as a maturase for its own cofactor. This suggests a simpler pathway for FeMo-co synthesis predating the current nifDK and nifEN genes. This finding could simplify genetic transfer of nitrogen fixation to crops, which could enable them to utilize atmospheric N2.
Keywords: nitrogen fixation evolution, metalloproteins, NifEN, Roseiflexus, FeMo-co
Abstract
The maturation and installation of the active site metal cluster (FeMo-co, Fe7S9CMo-R-homocitrate) in Mo-dependent nitrogenase requires the protein product of the nifB gene for production of the FeS cluster precursor (NifB-co, [Fe8S9C]) and the action of the maturase complex composed of the protein products from the nifE and nifN genes. However, some putative diazotrophic bacteria, like Roseiflexus sp. RS-1, lack the nifEN genes, suggesting an alternative pathway for maturation of FeMo-co that does not require NifEN. In this study, the Roseiflexus NifH, NifB, and apo-NifDK proteins produced in Escherichia coli are shown to be sufficient for FeMo-co maturation and insertion into the NifDK protein to achieve active nitrogenase. The E. coli expressed NifDKRS contained P-clusters but was devoid of FeMo-co (referred to as apo-NifDKRS). Apo-NifDKRS could be activated for N2 reduction by addition of preformed FeMo-co. Further, it was found that apo-NifDKRS plus E. coli produced NifBRS and NifHRS were sufficient to yield active NifDKRS when incubated with the necessary substrates (homocitrate, molybdate, and S-adenosylmethionine [SAM]), demonstrating that these proteins can replace the need for NifEN in maturation of Mo-nitrogenase. The E. coli produced NifHRS and NifBRS proteins were independently shown to be functional. The reconstituted NifDKRS demonstrated reduction of N2, protons, and acetylene in ratios observed for Azotobacter vinelandii NifDK. These findings reveal a distinct NifEN-independent pathway for nitrogenase activation involving NifHRS, NifBRS, and apo-NifDKRS.
Nitrogenases are enzyme complexes responsible for the reduction of inert N2 to bioavailable NH3 (1, 2). They are exclusively found in a selective group of microorganisms of the Bacteria and Archaea domains called diazotrophs that can use N2 as sole source of nitrogen (3, 4). All nitrogenases are composed of a dinitrogenase and a dinitrogenase reductase that contain FeS clusters necessary for N2 reduction. There are three types of nitrogenases that are genetically distinct and differ in the metal composition of their active site cofactors. The most common nitrogenase contains Fe and Mo, while alternative nitrogenases contain either V and Fe or Fe-only cofactors (5). The dinitrogenase component of the Mo nitrogenase is a NifDK heterotetramer of α2β2 composition while the dinitrogenase reductase is a NifH homodimer (6). NifH contains a site for ATP binding and hydrolysis in each subunit and a [Fe4S4] cluster that connects both subunits (7). NifDK contains a [Fe8S7] P-cluster at the interface of each αβ half and a [Fe7S9MoC(R)-homocitrate] FeMo-co at the active site of each α subunit (8–10). Electron transfer occurs during the association of both components, with transfer of an electron from the [Fe4S4] cluster of NifH to FeMo-co via the P-cluster, followed by the ATP hydrolysis and subsequent complex dissociation (11).
In addition to the structural nifH, nifD, and nifK genes, diazotrophs carry a variable number of nif genes, most of them involved in the biosynthesis of the nitrogenase metallo-cofactors (12). This variability in nif-gene composition is related to their physiology, metabolism, and ecological niche (13, 14). Furthermore, some nif functions might be performed by housekeeping counterparts encoded in non-nif genes. Both the physiological diversity and frequent functional overlap hinders the determination of the precise genetic requirements for N2 fixation in a specific diazotroph. The proposed minimal genetic requirement for Mo nitrogenases is associated with two major stages: the biosynthesis of FeMo-co, involving the genes nifB, nifH, nifE, and nifN, and the catalytic module formed by the nitrogenase structural genes nifH, nifD, and nifK (3, 12).
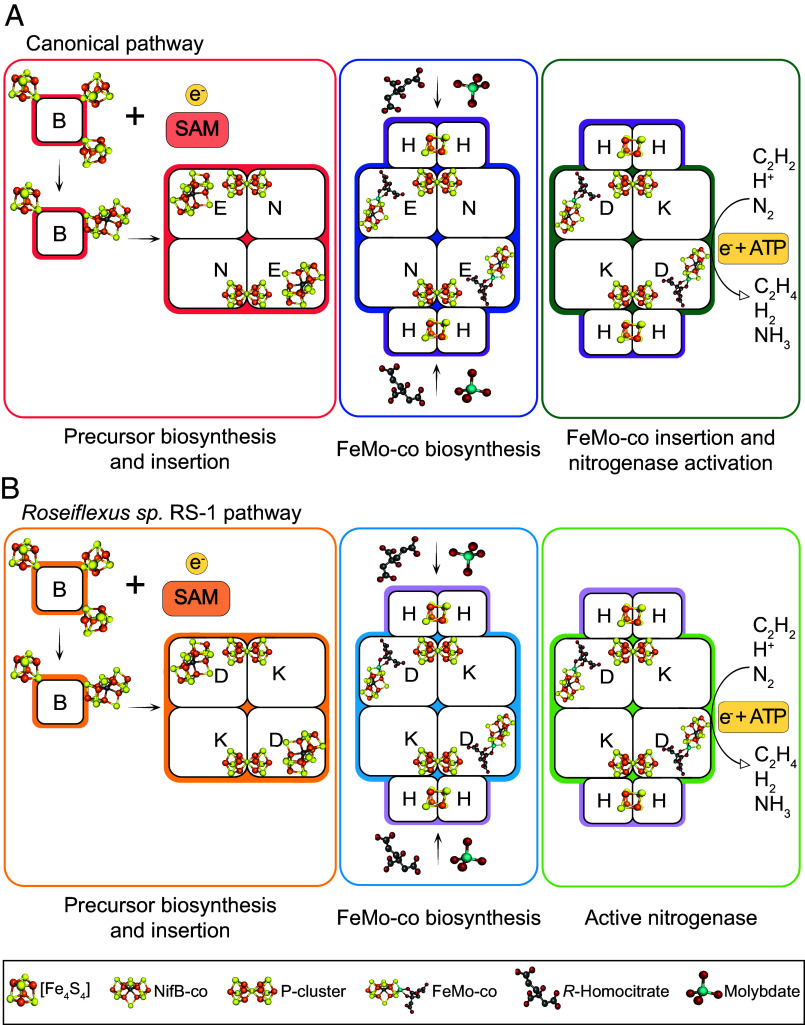
The biosynthesis of FeMo-co occurs outside of the NifDK protein. The first committed step in FeMo-co biosynthesis is the formation by NifB of a [Fe8S9C] cluster, referred to as NifB-co (15, 16), which is then transferred to the maturase complex, NifEN (17). Within NifEN and with the assistance of NifH, the precursor NifB-co is transformed into FeMo-co by the sequential addition of Mo (replacing an apical Fe atom) and homocitrate (ligated to the Mo atom) (18). Newly synthesized FeMo-co is finally transferred from NifEN to apo-NifDK (an immature form of the enzyme containing P-clusters but lacking FeMo-co) to obtain mature, active NifDK. Thus, within characterized diazotrophs, NifEN serves as irreplaceable scaffold for FeMo-co biosynthesis (Fig. 1) (12, 19).
Fig. 1.
Schemes of FeMo-co biosynthetic pathways. (A) Canonical NifEN-dependent pathway as studied in Azotobacter vinelandii. The S-adenosylmethionine [SAM]-radical enzyme NifB synthesizes the [Fe8S9C] cluster NifB-co, which is then inserted into NifEN. FeMo-co biosynthesis occurs at the NifEN scaffold by substituting Mo for an apical Fe atom and adding R-homocitrate. FeMo-co biosynthesis requires the interaction of NifH with NifEN. FeMo-co is then transferred to apo-NifDK (already containing mature P-clusters) to reconstitute active NifDK. (B) Putative NifEN-independent pathway of Roseiflexus. The SAM-radical enzyme NifBRS synthesizes the [Fe8S9C] cluster NifB-co, which is then inserted into apo-NifDKRS (already containing mature P-clusters or its precursors). FeMo-co biosynthesis occurs in situ in apo-NifDKRS and requires the interaction with NifHRS. After FeMo-co biosynthesis, mature NifDKRS is formed.
Since all known diazotrophs contain Mo nitrogenases, these six nif genes are regarded as the minimum set for biological nitrogen fixation and can be used as an in silico tool to identify new diazotrophs (3). Interestingly, certain potential diazotrophic thermophiles deviate from this rule due to the absence of nifE and/or nifN genes, suggesting the existence of alternative, more parsimonious pathways for FeMo-co biosynthesis (20). Although there is no direct evidence showing that thermophiles lacking both nifE and nifN can fix N2, or that their NifDK proteins carry the canonical FeMo-co, it has been proposed that these environments may have favored primitive lineages with FeMo-co biosynthetic pathways independent of NifEN.
Ancestral NifDK lineages at the most basal position of the nitrogenase phylogenetic tree have been classified as uncharacterized nitrogenases (4, 21–23). Although these proteins contain conserved motifs typical of Mo-dependent nitrogenases, they exhibit differences in the amino acid environments surrounding FeMo-co and the P-cluster that may confer distinct maturation or catalytic properties (22, 23). At least three species of the Chloroflexi phylum carry nifDK genes of the uncharacterized nitrogenase lineage. Since species in this lineage lack NifEN, it is possible that their putative nitrogenases evolved prior to the proposed duplication and divergence of the ancestor genes that led to the nifEN and nifDK genes (24–26). The hypothesis is that, for such nitrogenases, FeMo-co biosynthesis would start with NifB activity, and its NifB-co product would be matured to FeMo-co directly in an apo-NifDK variant (an immature form containing P-clusters but lacking FeMo-co) with both scaffolding and catalytic capabilities (Fig. 1). Roseiflexus sp. RS-1 is one of these potential diazotrophs, carrying a basic nifHBDK gene cluster predicted to encode a Mo nitrogenase but lacking nifEN. Interestingly, diazotrophic growth of Roseiflexus species has not been demonstrated (27, 28), raising the question of whether or not Mo nitrogenase assembly is possible in the absence of NifEN.
In this study, we show that the predicted nitrogenase from Roseiflexus sp. RS-1 (hereafter Roseiflexus) is indeed a Mo-dependent nitrogenase in which FeMo-co is synthesized by a simpler pathway that does not require the NifEN scaffold. To this end, the Roseiflexus NifH, NifB, and NifDK proteins were expressed and purified from Escherichia coli cells, their [FeS] clusters and activities were characterized, and a complete FeMo-co biosynthetic pathway was reconstituted in vitro using only the purified proteins and their necessary substrates.
Results
NifDKRS Amino Acid Sequence and Structure Correspond to a Mo Nitrogenase.
A comparative amino acid sequence analysis between NifDKRS and NifDKAv (A. vinelandii and Roseiflexus proteins are designated with Av and RS superscripts, respectively) indicates the conservation of the putative FeMo-co environment within NifD, including the C275 and H442 ligands to FeMo-co and S278, which is hydrogen-bonded to the S atom of C275 (SI Appendix, Table S1 and Fig. S1, using A. vinelandii amino acid numbering). These residues are strictly conserved in all known nitrogenases (22, 23). Other highly conserved residues found in NifDRS include G356 and G357, required to avoid steric interactions with FeMo-co; R96 and R359, known to stabilize FeMo-co by hydrogen bonding its S atoms; H195 involved in proton transfer reactions; G69 and V70 that control substrate access to FeMo-co; and Q191and E427 near homocitrate. In addition, all NifD and NifK residues serving as P-cluster ligands were conserved, as well as α-G87 and α-G185, which are important to avoid steric interactions with the P-cluster (SI Appendix, Table S1) (29). Furthermore, NifDRS conserves residues that are unique to Mo nitrogenases (SI Appendix, Fig. S1).
A structural model for NifDKRS was built with ProMod3 using the empirically solved NifDKAv structure (10) as template. The model included amino acid residues 3 to 459 for NifDRS (95% coverage) and 6 to 447 for NifKRS (93% coverage). The predicted structure of NifDKRS and the known structure of NifDKAv were very similar, overlapping 1,770 residues with an average RMSD of 0.368 Å. The NifD overlay showed no significant differences except for the α-helix formed by residues 4 to 19 in NifDAv, which is absent in NifDRS, and the loops formed by residues 101 to 108 and residues 208 to 214 in NifDAv (SI Appendix, Fig. S2A). The NifK subunits were also superimposable except for three NifKAv regions that are absent in the shorter NifKRS (SI Appendix, Fig. S2B). The model showed nearly identical 4 Å environments around FeMo-co (SI Appendix, Fig. S2C) and the P-cluster (SI Appendix, Fig. S2D). Thus, both the presence of amino acid residues characteristic of Mo nitrogenases and their 3D position in the modeled structure were consistent with NifDKRS being a FeMo-co containing nitrogenase.
Apo-NifDKRS Shows EPR Signals Characteristic of Immature P-clusters but Can Be Activated by the Simple Addition of FeMo-co.
To investigate the biochemical properties of NifDKRS, the streptagII-nifDRS and nifKRS genes were overexpressed together with nifHRS (to support the formation of P-clusters), and either the Escherichia coli isc gene cluster or the A. vinelandii nifUSAv genes to boost [FeS] cluster biosynthesis.
NifDKRS was purified by StrepTactin affinity chromatography under strict anaerobic conditions. Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) analysis of the purified protein revealed NifD and NifK bands with equal band intensities (SI Appendix, Fig. S3A). Size-exclusion chromatography estimated the molecular mass to be 220 kDa (SI Appendix, Fig. S3B), consistent with a theoretical α2β2 NifDKRS heterotetramer of 212 kDa, similar to all Mo nitrogenases described to date. NifDKRS preparations coexpressed with nifUSAv presented better protein solubility and higher purification yields (0.59 ± 0.24 mg NifDKRS · g−1 cell) than those coexpressed with the iscEc gene cluster (0.22 ± 0.06 mg NifDKRS · g−1 cell). Pure NifDKRS preparations had brown/greenish color characteristic of [FeS] clusters. The spectra of as isolated apo-NifDKRS showed a broad peak around 400 nm split into two peaks at around 320 and 420 nm when samples were exposed to air (SI Appendix, Fig. S4). This is consistent with the breakdown of [Fe4S4] clusters (or more complex clusters) into [Fe2S2] clusters, indicating their sensitivity to O2, as described for mature nitrogenase proteins (30, 31). As nifDKRS was coexpressed with nifUSAv (or iscEc genes) and nifHRS, a P-cluster containing but FeMo-co-deficient form of apo-NifDKRS containing 16 Fe atoms per tetramer would be expected if two pairs of [Fe4S4] clusters were correctly inserted and matured into two P-clusters. Indeed, purified apo-NifDKRS contained ca. 16 Fe atoms per tetramer (Table 1). Small differences were observed depending on the auxiliary genes used for [Fe4S4] cluster biosynthesis, with nifUSAv yielding slightly higher Fe content than the iscEc operon. Thus, coexpression with nifUSAv rather than iscEc was used for the remainder of the study.
Table 1.
Iron content and molecular size of Roseiflexus Nif proteins
| Nif protein | Fe atoms/molecule of protein | Molecular size (kDa) |
Quaternary structure |
|---|---|---|---|
| apo-NifDKRS | 212 | Heterotetramer | |
| with iscEc cluster | 14.2 ± 1.0 | ||
| with nifUSAv | 16.6 ± 0.8 | ||
| NifHRS (with iscEc cluster) | 3.7 ± 0.9 | 52 | Homodimer |
| NifBRS (with nifUSAv) | 8.2 ± 2.6 | 32 | Monomer |
Fe content data are the mean ± SD of independent purifications (n = 2 for apo-NifDKRS with iscEc and n = 4 for the rest).
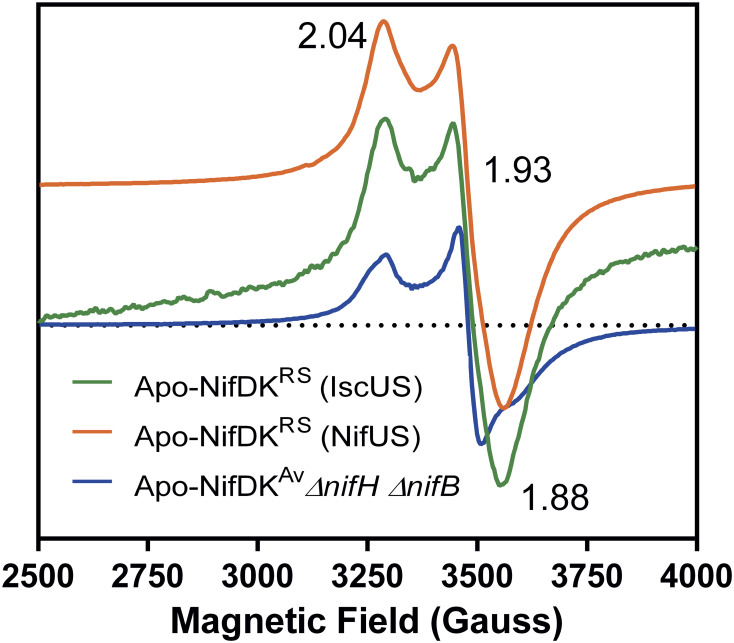
P-cluster maturation on NifDK proteins can be assessed by analyzing electron paramagnetic resonance (EPR) signals in perpendicular mode. While the immature P-clusters of a ΔnifBΔnifH apo-NifDKAv have an S = 1/2 EPR signal with g values at 2.04, 1.93, and 1.89, the dithionite (DTH)-reduced intact P-clusters are EPR silent (32–34). Apo-NifDKRS preparations coexpressed with nifHRS and nifUSAv (or iscEc genes) exhibited EPR signals similar to those of the of ΔnifBΔnifH apo-NifDKAv, likely originating from immature P-clusters in the samples (Fig. 2). Similar EPR signals have been observed in samples of the ΔnifB apo-VnfDK. These signals were proposed to correlate either with oxidized P-clusters (35) or with heterogeneous samples containing both mature and immature P-clusters (36).
Fig. 2.
X-band EPR spectra of apo-NifDK proteins. Perpendicular mode EPR spectra of apo-NifDKRS and apo-NifDKAv in the DTH reduced states. Apo-NifDKAv was purified from a ΔnifH ΔnifB A. vinelandii strain. Pure apo-NifDKRS preparations were obtained from E. coli cells coexpressing NifHRS and either NifUSAv or a plasmid-cloned iscEc gene cluster. Apo-NifDKRS (IscEc) spectrum was obtained from 10 scans; apo-NifDKRS (NifUSAv) and apo-NifDKAv spectra were obtained from 5 scans.
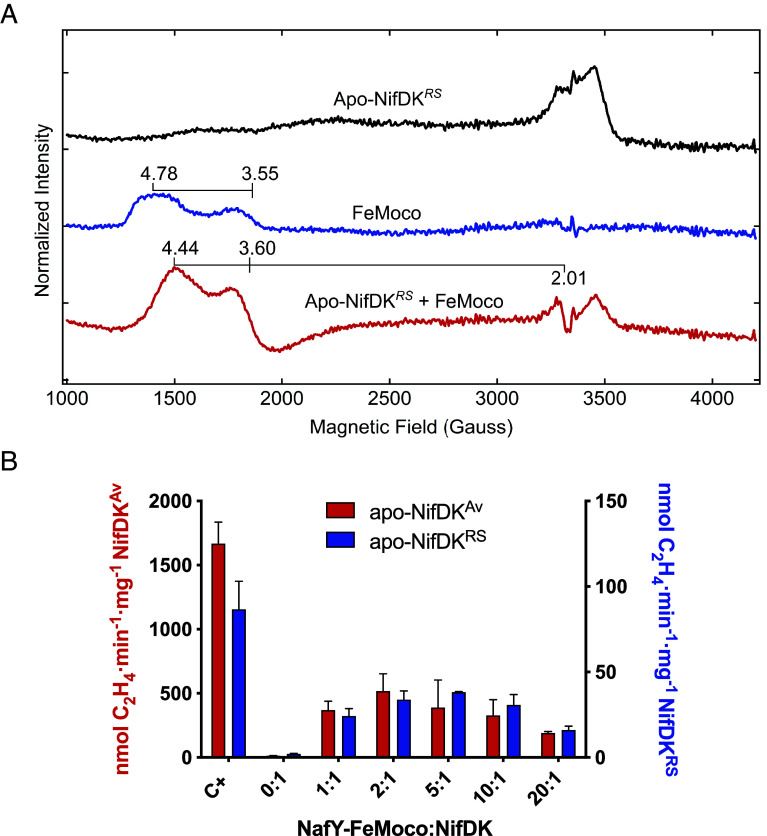
Apo-NifDK containing P-clusters but lacking FeMo-co (e.g., the form produced by a ΔnifB strain) can be reconstituted to an active form by the simple addition of purified FeMo-co in solution (9) or in complex with the A. vinelandii NafY carrier protein (37). Experiments of FeMo-co insertion into apo-NifDKRS were carried out by monitoring the C2H2-reducing activity of the reconstituted enzyme and the changes in EPR signals. FeMo-co in wild-type NifDKAv has a characteristic EPR signal with a distinct S = 3/2 signature at g = 4.3, 3.6, and 2.01. The EPR spectra of apo-NifDKRS were recorded before and after the addition of isolated FeMo-co. The emergence of a S = 3/2 FeMo-co EPR signal (g = 4.44 and 3.60) and the concomitant decrease in intensity of immature P-cluster EPR signal indicated the incorporation of FeMo-co into apo-NifDKRS (Fig. 3A and SI Appendix, Fig. S5).
Fig. 3.
Cofactor insertion into apo-NifDKRS. (A) Changes in EPR signals after incubation of apo-NifDKRS with purified FeMo-co in DTH-reducing conditions. Microwave power 1 mW; temperature 5 K. Spectra were obtained from 10 scans. (B) Titration of apo-NifDKAv (red bars, performed at 30 °C) and apo-NifDKRS (blue bars, performed at 48 °C) reconstitution with pure NafY–FeMo-co complex (ratios indicated in the x-axis). Control reactions consisted of assays with pure NifDKAv and NifHAv at 1:40 molar ratio (C+ red bar), or NifBRS-dependent FeMo-co synthesis and insertion into apo-NifDKRS (composition of complete reactions in Fig. 5A) (C+ blue bar).
Despite the apparent insertion of FeMo-co, the reconstituted NifDKRS protein did not show significant activity. Optimization of FeMo-co insertion into apo-NifDKRS at different temperatures was then tested. When FeMo-co insertion was carried out at 40 °C instead of 30 °C, in the presence of NifHRS, reconstituted NifDKRS produced 14.3 ± 0.3 nmol C2H4 · min−1 mg−1 NifDKRS (compared to 346 ± 42 nmol C2H4 · min−1·mg−1 produced by apo-NifDKAv reconstituted by FeMo-co at 30 °C in presence of NifHAv). To stabilize FeMo-co at higher temperatures, NafY–FeMo-co complexes were formed in vitro by incubation of NafY with FeMo-co and removal of excess FeMo-co. The highest activity of reconstituted NifDKRS (38 ± 0.4 nmol C2H4 · min−1 · mg−1 protein) was observed at 48 °C and 5-fold molar excess of the NafY–FeMo-co complex with respect to apo-NifDKRS (Fig. 3B), compared to the optimal 2:1 ratio of NafY to apo-NifDKAv (38). Thus, maximum activity, optimal temperature and optimal molar ratios of apo-NifDKRS to NafY–FeMo-co were different from those of the A. vinelandii NifDK. In conclusion, these experiments indicate sample heterogeneity, in which part of the apo-NifDKRS population could be activated by FeMo-co while another fraction contained immature P-clusters.
Purification and Characterization of NifHRS and NifBRS.
Once it was demonstrated that apo-NifDKRS could incorporate FeMo-co, it was necessary to investigate whether NifHRS, NifBRS, and apo-NifDKRS were sufficient to assemble the active-site cofactor and reconstitute nitrogenase activity. An in vitro FeMo-co synthesis and insertion assay involving only pure Roseiflexus Nif proteins and the appropriate reactants was chosen to achieve unambiguous results. Thus, in addition to apo-NifDKRS, the NifHRS and NifBRS proteins were independently purified and validated prior to developing this assay. Expression, purification, and characterization of NifHRS and NifBRS are described in SI Appendix, Figs. S6–S17, and Tables S2–S4. It was concluded that i) NifHRS is a [Fe4S4] cluster containing homodimer of 52 kDa (SI Appendix, Figs. S8 and S9) with the ability to participate in P-cluster formation, FeMo-co biosynthesis, and nitrogenase catalysis, both in vitro (SI Appendix, Fig. S10 and Table S3) and in vivo (SI Appendix, Fig. S11), similar to other characterized NifH proteins (39), although in this case NifHRSdid not require NifM for maturation; and ii) NifBRS is a 32-kDa monomer containing three [Fe4S4] clusters (SI Appendix, Figs. S14 and S15) that fulfills the known role of NifB proteins and its product, the [Fe8S9C] cluster NifB-co, in the biosynthesis of FeMo-co, both in vitro (SI Appendix, Fig. S16) and in vivo and (SI Appendix, Fig. S17) (15, 40).
In Vitro Cofactor Synthesis for the Roseiflexus Nitrogenase Requires Only the Proteins NifBRS, NifHRS, and apo-NifDKRS.
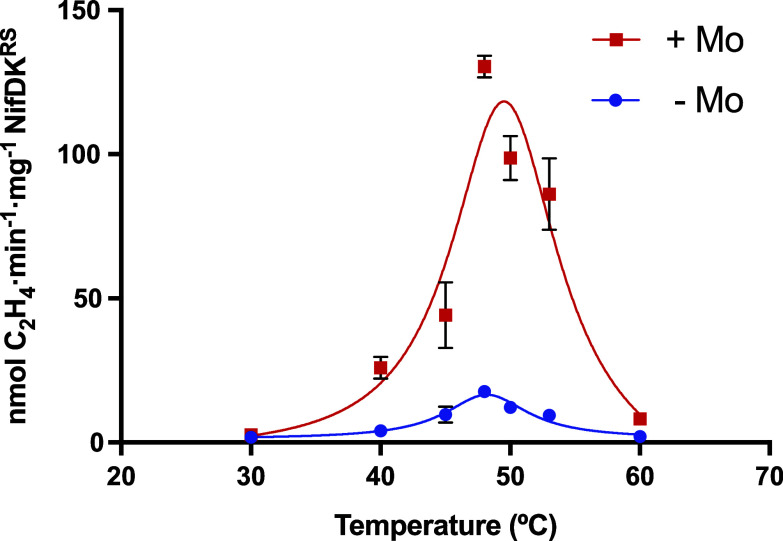
Conditions for NifBRS-dependent nitrogenase cofactor synthesis and NifDKRS reconstitution were established after temperature, reaction time, and component screening assays. The complete reaction mixtures contained NifHRS, NifBRS, apo-NifDKRS, and the necessary small molecules, homocitrate, SAM, ATP, and sources of Fe, S, and Mo, under DTH reducing conditions. The NifXAv protein was added to stabilize the NifB-co formed in vitro in the initial temperature and reaction-time screenings, but it was shown not to be essential in protein component screenings (see below). All reaction mixtures lacked NifEN, an essential component for FeMo-co synthesis in all known Mo nitrogenases. Temperature screenings were performed from 30 °C to 60 °C in the presence or absence of Mo. The activity of reconstituted NifDKRS was negligible at 30 °C, reached the maximum at 48 °C (130 ± 4 nmol C2H4 min−1 mg−1 NifDKRS), and was almost inactivated at 60 °C (8.1 ± 0.4 nmol C2H4 min−1 mg−1 NifDKRS) (Fig. 4). Optimal reaction time and NifHRS to NifDKRS ratio were found at 90 min and a molar ratio of 40, respectively (SI Appendix, Figs. S18 and S19). It is worth noting that, at any tested temperature, much higher activities were detected in the presence of Mo than in its absence. This acetylene-reducing activity could be the result of missincorporating NifB-co or FeFe-co like cofactors as previously shown for the A. vinelandii apo-NifDK (41), although the presence of Mo traces in the reaction assays cannot be completely excluded. The dependency and specificity of NifDKRS on Mo was also investigated using tungsten (W) as a competitor of Mo in cofactor synthesis. W has been shown to poison Mo nitrogenase activity, probably by impairing FeMo-co biosynthesis (42). In our competition assays, cofactor synthesis was carried out in the presence of 17.5 μM molybdate and increasing concentrations of tungstate up to 1.75 mM, a 100-fold excess compared to Mo. Inhibitory effects were observed in assays with at least 50-fold excess W, in which the sequence of metal addition was unimportant (SI Appendix, Table S5). NifDKRS and NifDKAv showed similar sensitivity to W. These results indicate that NifDKRS is in fact a Mo nitrogenase, showing strong preference for Mo and sensitivity to W.
Fig. 4.
Temperature dependence of cofactor synthesis and apo-NifDKRS reconstitution. Temperature screening was performed in the presence (red) or absence (blue) of molybdate. Reaction mixtures contained NifHRS, NifBRS, apo-NifDKRS, NifXAv, homocitrate, SAM, Fe, S, Mo (when indicated), and an ATP regenerating mixture (Materials and Methods). Negative control assays lacking NifBRS showed negligible activity. Data represent average activity ± SD (n = 2).
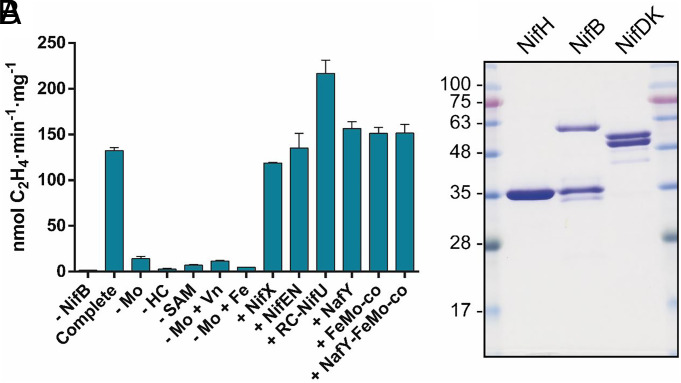
Protein and substrate requirements for cofactor synthesis and reconstitution of NifDKRS were determined by removing or adding individual components from a complete reaction mixture containing the NifHRS, NifBRS, apo-NifDKRS proteins, and SAM, Mo, and homocitrate as substrates (Fig. 5). External sources of Fe or S were not included because previous assays indicated that NifBRS [Fe4S4] clusters fulfilled the reaction requirements (SI Appendix, Fig. S20). In fact, addition to the reaction mixtures of reconstituted NifUAv (RC-NifUAv) loaded with [Fe4S4] clusters boosted ethylene production (217 ± 14 nmol C2H4 min−1 mg−1 NifDKRS). It had been previously shown that NifU was a biological source of [Fe4S4] clusters for NifB to synthesize NifB-co (43, 44). Homocitrate and SAM were essential to reconstitute NifDKRS. Homocitrate is present in the active-site cofactors of all nitrogenases (10, 45–48), and SAM is required for the synthesis of NifB-co by NifBRS (15, 16, 38, 49). Consistently, adding S-adenosylhomocysteine (SAH) instead of SAM inhibited NifB-co formation and did not reconstitute NifDKRS (SI Appendix, Fig. S20). Molybdate removal resulted in lower but measurable acetylene reduction activity (14 ± 2 nmol C2H4 min−1 mg−1 NifDKRS)(Fig. 5 and SI Appendix, Table S5). Replacement of molybdate by vanadate or ferrous iron in the reaction mixtures did not increase the acetylene reduction activity of reconstituted NifDKRS, pointing to Mo exclusivity as designated heterometal. Addition of NafY:FeMo-co complex, or FeMo-co alone, had not effect on NifDKRS reconstitution. Addition of NifXAv or NafYAv proteins had no effect on NifDKRS reconstitution. Since these proteins bind and stabilize NifB-co and FeMo-co, respectively, their dispensability suggests efficient transfer of intermediates between NifBRS and apo-NifDKRS. Importantly, the reconstitution of NifDKRS was completely independent of NifEN (Fig. 5).
Fig. 5.
Screening of minimal components required for cofactor synthesis and apo-NifDKRS reconstitution. (A) Complete reactions contained molybdate, SAM, homocitrate, NifHRS, NifBRS, and apo-NifDKRS. NifXAv, NafYAv, NifENAv or RC-NifUAv were added when indicated. RC-NifUAv refers to NifUAv loaded with [Fe4S4] clusters formed in vitro by incubation with Fe, cysteine, DTT, and NifS (SI Appendix, Materials and Methods). Reactions were performed at 48 °C. Negative control assays (- NifB) reached 1.60 ± 0.04 nmol C2H4 min−1 mg−1 NifDKRS. Data are average activities ± SD (n = 2). (B) SDS-PAGE analysis of purified Roseiflexus Nif proteins required for the in vitro synthesis of nitrogenase cofactors. NifBRS preparations contained two additional bands that were identified by mass spectrometry as GroEL (60 kDa) and C-terminal truncated NifBRS species (30 kDa).
NifDKRS Reduces N2 to NH3.
The reduction of C2H2, H+, and N2 by reconstituted NifDKRS was investigated and compared to that of the well-studied A. vinelandii NifDK. Reactions using NifDKAv were carried out under standard conditions using purified NifDKAv and NifHAv (30 °C and 1:40 component ratio). On the other hand, apo-NifDKRS required NifBRS-dependent cofactor synthesis to become active in substrate reduction. The conditions for NifDKRS reconstitution were established by the experiments described above and are detailed in Materials and Methods. Reconstitution mixtures contained NifBRS, RC-NifUAv, NifHRS, and apo-NifDKRS together with SAM, molybdate and homocitrate, and were incubated for 90 min at 48 °C. The substrate reducing activities of reconstituted NifDKRS were determined at 48 °C and with 40-fold molar excess of NifHRS. The data in Table 2 show that NifDKRS is indeed a nitrogenase as it is capable of reducing N2 to NH3. The inhibition of H+ reduction by N2 is consistent with the simultaneous reduction of N2 and H+, as occurs in the well-characterized A. vinelandii Mo nitrogenase (50). Although the activities for the reduction of different substrates by NifDKRS were lower than those of NifDKAv, the consistent ratios for NH3, C2H4, and H2 formation, and the almost identical selectivity for N2 reduction, as reflected by the NH3:H2 ratio under 1 atm N2, strongly suggest the presence of FeMo-co in NifDKRS and therefore its formation in the in vitro system independent of NifEN.
Table 2.
Ethylene, hydrogen, and ammonia production activities of A. vinelandii and Roseiflexus Mo nitrogenases
| Substrate | |||||
|---|---|---|---|---|---|
| N2 | Ar | C2H2 | |||
| Product | NH3 | H2 | NH3/H2 | H2 | C2H4 |
| NifDKAv | 849 ± 58 | 716 ± 103 | 1.19 | 2,123 ± 233 | 1,835 ± 38 |
| NifDKRS | 149 ± 30 | 122 ± 32 | 1.22 | 443 ± 50 | 391 ± 55 |
Activities are nmol produced per min and mg of the corresponding NifDK. H2 production was determined in Ar or N2 atmospheres. Data are the average of n = 4 independent reactions (± SD). Values from negative control reactions lacking NifDK components were subtracted. A. vinelandii reactions were carried out at 30 °C by mixing NifDKAv and NifHAv in molar ratio of 1:40. Roseiflexus reactions were carried out at 48 °C by mixing apo-NifDKRS and NifHRS in molar ratio of 1:40, together with NifBRS, RC-NifUAv, molybdate, homocitrate, and SAM.
Discussion
We have characterized a unique Mo nitrogenase that functions both as a maturase for the synthesis of its own active-site cofactor and as a functional nitrogenase. In vitro biochemical assays demonstrated that NifHRS, NifBRS, and apo-NifDKRS, along with the substrates homocitrate, molybdate, and SAM are sufficient to synthesize a NifDKRS that effectively reduces N2, C2H2, and H+. The ratios of substrate reduction by the Roseiflexus nitrogenase are characteristic of a Mo nitrogenase. Significant NH3 production, which accounted for one-third of C2H4 production, was determined. Additionally, nitrogenase substrate preference was assessed by observing differences in H2 production in the presence of inert Ar or in competing N2. The Roseiflexus Mo nitrogenase exhibits a clear thermophilic tendency, as indicated by the requirement for high temperatures in cofactor synthesis and activity assays, which aligns with the bacterium’s lifestyle (28).
This study provides evidence that the Roseiflexus nitrogenase utilizes FeMo-co. This is supported by several observations: i) Its synthesis is dependent on Mo and homocitrate, and it is inhibited by W but not activated by V. ii) The synthesis is initiated by the SAM-radical reaction of NifBRS. iii) The product of NifBRS must be the [Fe8S9C] cluster NifB-co because it restores the Nif+ phenotype of a K. oxytoca ΔnifB mutant, which only contains Mo nitrogenase genes. iv) Addition of pure FeMo-co to the P-cluster containing apo-NifDKRS reconstitutes its acetylene reduction activity. v) NifDKRS has amino acid residues that are only conserved in Mo nitrogenases. vi) The ratios for NH3, C2H4, and H2 formation, and the selectivity for N2 reduction, are consistent with Mo nitrogenases and not with hybrid NifDK nitrogenases containing alternative FeV-co or FeFe-co.
This study presents the first example of complete in vitro FeMo-co synthesis without the NifEN maturase. This introduces a new paradigm in nitrogenase biosynthesis and challenges the well-established principle of minimal gene requirement (nifHDKBEN) for a functional Mo nitrogenase (3, 38). The remarkable independence of NifEN confirms the existence of an alternative and simplified pathway for FeMo-co synthesis, which has been previously hypothesized for the uncharacterized nitrogenase clade (21, 22, 24, 26). This lineage of nitrogenase is found in the earliest branches of nitrogenase evolution and is currently present in Chloroflexi of the Roseiflexus genus. These organisms possess only the nifHBDK genes (22), but there is no evidence of their ability to grow diazotrophically (27, 28). It is important to mention that a recently identified class of nitrogenase-like homologs encoded by closely related deep-branching nitrogen fixation-like genes were linked to ethylene and methane reactions (51).
The inherent maturase and nitrogenase activities of NifDKRS provide insight into the characteristics of ancestral nitrogenases prior to the proposed duplication and divergence of the nifEN and nifDK ancestral genes, which occurred approximately 2.1 billion years ago (26). It appears that an ancient Mo nitrogenase, which existed approximately 3.2 billion years ago (52), may have performed N2 fixation prior to the evolution of the canonical FeMo-co biosynthetic pathway that includes NifEN. This is currently represented by the nitrogenase from Roseiflexus sp. RS-1.
An ongoing debate in nitrogenase evolution is whether maturases (NifEN) are evolutionarily derived from nitrogenases (NifDK) (26, 53, 54) or, conversely, whether extant nitrogenases are derived from a primitive maturase clade (25). A recent study based on sites that are enriched for positions contributing to the functional divergence between nitrogenases and maturases suggests that canonical NifDK sequences are derived from ancestral proteins more similar to extant NifEN maturases than nitrogenase homologs (25). The authors identified twenty-three sites conserved only in nitrogenases. All of them are present in the NifDKRS protein (SI Appendix, Table S1). A clear example is the presence of amino acid residue H442 (H422 in NifDRS) that binds to the Mo atom of FeMo-co. Other residues close to the active site cofactor show specificity for each nitrogenase class (Mo/V/Fe) (23). Many residues characteristic of Mo nitrogenases are conserved in NifDRS, consistent with our biochemical studies demonstrating the Mo dependence of NifDKRS reconstitution. For example, R96 is conserved in the NifD phylogeny and is also present in the uncharacterized nitrogenase group and in NifDRS as R85 (SI Appendix, Table S1). R96 is known to play a role in FeMo-co reactivity (55). In contrast, both AnfD and VnfD have a lysine residue at this specific position (23).
The biochemical results of this study, supported by in silico NifDKRS characterization, indicate that an ancient diazotrophic pathway with a NifDK component integrating FeMo-co maturation and nitrogenase activity is still present in Roseiflexus sp. RS-1, and probably other related species living in unique ecosystems where they may play relevant biological roles. Although these diazotrophs may seem insignificant when compared to the total diazotroph pool in the biosphere, they are not only important in their habitats but also relevant as a biotechnological tool to alleviate the nitrogen crisis that the planet is facing today. The Roseiflexus nitrogenase is encoded by an unusually simple nifHBDK operon, which would facilitate its genetic transfer to relevant crops, allowing them to use atmospheric N2.
Materials and Methods
Purification of His-NifHRS, Strep-Tagged-NifBRS, and Strep-Tagged-NifDKRS.
Purification procedures were conducted using an AKTA Prime FPLC apparatus (GE Healthcare) inside a glove box with <0.1 ppm of O2 (MBraun). The His-NifHRS purification protocol used cells resuspended in anaerobic buffer A (100 mM Tris-HCl pH 8, 100 mM NaCl, 10% glycerol, and 2 mM DTH). The resulting CFE was loaded at 2 mL/min onto a 5 mL IMAC HiTrap Co2+ column previously equilibrated with buffer A following the manufacturer’s instructions (GE Healthcare). The column was then washed by 5 column volumes (CV) of buffer A and 5 CV of buffer A supplemented with 10 mM imidazole (buffer B). Elution was performed using a gradient from 10 to 300 mM imidazole (buffer C) in a 100 mL volume (SI Appendix, Fig. S8). Pure protein fractions were selected based on SDS-PAGE analysis and were concentrated in 30-kDa centrifugal filter units (Millipore Sigma). Concentrated pure protein was desalted using PD-10 desalting columns (Cytiva Life Sciences) previously equilibrated in buffer A.
NifBRS and NifDKRS purifications were based on a common standard protocol. Cells were resuspended using anaerobic buffer A (100 mM Tris-HCl pH 8.3, 200 mM NaCl, 10% glycerol, and 2 mM DTH), although the NifBRS purification required additional supplementation with 5 mM β-mercaptoethanol. The CFE was loaded at a flow rate of 2 mL/min onto a previously equilibrated 5-mL StrepTactin-XT HiTrap column (IBA Life Sciences). The column was washed with 20 CV of washing buffer consisting of 100 mM Tris-HCl pH 8, 200 mM NaCl, 10% glycerol, and 2 mM DTH (buffer B). Proteins were eluted using 15 mL of buffer C consisting of 100 mM Tris-HCl pH 8, 200 mM NaCl, 10% glycerol, 2 mM DTH, and 50 mM D-Biotin. Pure NifBRS (SI Appendix, Fig. S3) and NifDKRS (SI Appendix, Fig. S14) were concentrated using 30-kDa and 100-kDa centrifugal filter units, respectively. Concentrated pure proteins were desalted using PD-10 desalting columns previously equilibrated in buffer B.
NifDKRS EPR Spectroscopy.
EPR analysis of NifDKRS was performed by continuous-wave X-band EPR spectra using a Bruker ESP-300 spectrometer with an EMX PremiumX microwave bridge and an EMXPLUS standard resonator in perpendicular mode. The system was equipped with an Oxford Instruments ESR900 continuous helium flow cryostat using VC40 flow controller for helium gas. Spectra were recorded in the 12 to 5 K temperature range, with a microwave frequency of around 9.38 GHz, microwave power of 20 mW (at 12 K) or 1 mW (at 5 K), modulation frequency of 100 kHz, modulation amplitude of 8.14 G, and time constant of 20.48 ms. If not specified, each spectrum is the sum of 10 scans and is presented after subtracting the cavity background signal recorded with an EPR tube with frozen 150 mM MOPS buffer at pH 7.3.
In Vitro FeMo-co Synthesis and NifDKRS Reconstitution.
Assays were performed in 100 μL reaction mixtures containing 20 μM NifBRS, 3.0 μM NifHRS, 0.6 μM apo-NifDKRS, 17.5 μM Na2MoO4, 175 μM R-homocitrate, 125 μM SAM, and ATP regenerating mixture (22 mM Tris-HCl buffer, pH 7.5, supplemented to 1.23 mM ATP, 18 mM phosphocreatine, 2.2 mM MgCl2, 3 mM DTH, 40 μg/mL creatine phosphokinase, and 1 mg/mL bovine serum albumin). Optimal assay conditions were established at 48 °C for 90 min. When indicated, the reaction mixtures were modified to include, remove, or replace some components, for example, 3.0 μM NifXAv, 1.5 μM apo-NifENAv (a form of NifEN containing two structural [Fe4S4] clusters but lacking bound FeMo-co precursors), 1.2 μM NafYAv, 1.2 μM FeMo-co, 80 μM RC-NifUAv, 500 μM SAH, 125 μM FeSO4, 125 μM Na2S, 1 mM NaVO3, and Na2WO4 up to 1.75 mM.
To determine the acetylene reduction activity of reconstituted NifDKRS, reaction mixtures were transferred to 9-mL sealed vials containing 24 μM of NifHRS (40-fold molar excess to apo-NifDKRS) in 500 μL of ATP regenerating mixture in 94% Ar / 6% C2H2 atmosphere. After 15 min incubation at 48 °C, reactions were stopped with 100 μL 8 M NaOH.
To measure H+ reduction activity of reconstituted NifDKRS, reaction mixtures were transferred to 9-mL sealed vials containing 24 μM of NifHRS in 500 μL of ATP regenerating mixture under 100% N2 or 100% Ar atmosphere. After 30 min incubation at 48 °C, reactions were stopped with 100 μL of 0.5 M ethylenediaminetetraacetic acid (EDTA).
To determine the N2 reduction activity of reconstituted NifDKRS, assays were carried out in 100 μL reaction mixes containing 10 μM NifBRS, 40 μM RC-NifUAv, 3.0 μM NifHRS, and 0.6 μM apo-NifDKRS, 125 μM SAM, 17.5 μM Na2MoO4, 175 μM R-homocitrate, and ATP-regenerating mixture in 100 mM MOPS pH 7.5. Samples were incubated for 90 min at 48 °C, and then transferred to 9-mL sealed vials containing 500 μL of ATP regenerating mixture supplemented with 24 μM of NifHRS in 100% N2 atmosphere. After 1 h incubation at 48 °C, reactions were stopped with 100 μL 0.5 M EDTA.
FeMo-co Insertion into apo-NifDKRS.
FeMo-co was isolated from pure NifDKAv using NMF as solvent (9), and the NafY–FeMo-co complex was generated as described in ref. 37. FeMo-co insertion assays were performed in 200 μL reaction mixtures by adding either 10 μM FeMo-co in NMF or different amounts of the NafY-FeMo-co complex (0 to 12 μM) to 0.6 μM apo-NifDKRS in 100 mM MOPS pH 7.5. Reactions were incubated for 20 min at 48 °C and then transferred to 9-mL sealed vials for acetylene reduction determinations as described above.
C2H4, NH3, and H2 Quantification.
The formation of C2H4 was quantified by gas chromatography in a Shimadzu GC-2014 equipped with a flame ionization detector (Shimadzu Corporation) coupled to a Porapak N 80/100 column (Agilent Technologies). H2 was quantified by gas chromatography in a Shimadzu GC-2014 equipped with a TCD detector and a 2-meter-long molecular sieve 5A 60/80 column (Agilent Technologies). Detector and column temperatures were 150 °C and 50 °C, respectively. Depending on the experiment, 25 mL/min N2 or Ar was used as carrier gas. Ammonia was quantified by fluorometry. Samples were filtered in 10-kDa centrifugal filter units, and 50-μL flow-through aliquots were mixed with 200 μL of Fluoraldehyde o-Phthalaldehyde reagent Solution (Thermo Scientific) and added to 96-well fluorescence detection plates. Samples were excited at 390 nm and emission was recorded at 472 nm using a VarioSkan Lux 96-well reader (Thermo Scientific). Background signal (initial fluorescence value at t = 0) was subtracted from each reaction as described (56).
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
Portions of the paper were developed from the thesis of L.P.-T. We thank Luis Fernández Pacios for NifDK structure models, Marcel Veldhuizen for help with E.coli fermentations, and Alvaro Salinero and Diana Coroian for technical support. This work was supported in part or in whole by the Bill & Melinda Gates Foundation (INV-005889) to L.M.R. Under the grant conditions of the Foundation, a Creative Commons Attribution 4.0 Generic License has already been assigned to the Author Accepted Manuscript version that might arise from this submission. This work was also supported by grant PID2021-128802OB-100 funded by MICIU/AEI/s1910.13039/501100011033 and by ERDF/EU. L.C.S. and Z.-Y.Y. were supported by US Department of Energy, Office of Science, Basic Energy Sciences (BES) award DE-SC0010687 to L.C.S. L.P.-T. was recipient of FPU16/02284 funded by Ministerio de Educación, Cultura y Deporte. N.M.-S. is a recipient of PRE2022-102872 funded by MICIU/AEI/10.13039/501100011033 and by ESF+. A.P.-G. is a recipient of RYC2021-031246-I funded by MICIU/AEI/10.13039/501100011033 and by European Union NextGenerationEU/PRTR.
Author contributions
L.P.-T., C.E.-E., A.P.-G., and L.M.R. designed research; L.P.-T., C.E.-E., N.M.-S., A.P.-G., Z.-Y.Y., and Y.G. performed research; L.P.-T., C.E.-E., N.M.-S., A.P.-G., Z.-Y.Y., Y.G., L.C.S., and L.M.R. analyzed data; and L.P.-T., C.E.-E., Z.-Y.Y., Y.G., L.C.S., and L.M.R. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Contributor Information
Carlos Echavarri-Erasun, Email: carlos.echavarri@upm.es.
Luis M. Rubio, Email: luis.rubio@csic.es.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix.
Supporting Information
References
- 1.Canfield D. E., Glazer A. N., Falkowski P. G., The evolution and future of earth’s nitrogen cycle. Science 330, 192–196 (2010). [DOI] [PubMed] [Google Scholar]
- 2.Seefeldt L. C., et al. , Reduction of substrates by nitrogenases. Chem. Rev. 120, 5082–5106 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dos Santos P. C., Fang Z., Mason S. W., Setubal J. C., Dixon R., Distribution of nitrogen fixation and nitrogenase-like sequences amongst microbial genomes. BMC Genomics 13, 1–12 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raymond J., Siefert J. L., Staples C. R., Blankenship R. E., The natural history of nitrogen fixation. Mol. Biol. Evol. 21, 541–554 (2004). [DOI] [PubMed] [Google Scholar]
- 5.Jasniewski A. J., Lee C. C., Ribbe M. W., Hu Y., Reactivity, mechanism, and assembly of the alternative nitrogenases. Chem. Rev. 120, 5107–5157 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bulen W. A., LeComte J. R., The nitrogenase system from Azotobacter: Two-enzyme requirement for N2 reduction, ATP-dependent H2 evolution, and ATP hydrolysis. Proc. Natl. Acad. Sci. U.S.A. 56, 979–986 (1966). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Georgiadis M. M., et al. , Crystallographic structure of the nitrogenase iron protein from Azotobacter vinelandii. Science 257, 1653–1659 (1992). [DOI] [PubMed] [Google Scholar]
- 8.Einsle O., Nitrogenase FeMo cofactor: An atomic structure in three simple steps. J. Biol. Inorg. Chem. 19, 737–745 (2014). [DOI] [PubMed] [Google Scholar]
- 9.Shah V. K., Brill W. J., Isolation of an iron-molybdenum cofactor from nitrogenase. Proc. Natl. Acad. Sci. U.S.A. 74, 3249–3253 (1977). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spatzal T., et al. , Evidence for interstitial carbon in nitrogenase FeMo cofactor. Science 334, 940–940 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seefeldt L. C., et al. , Energy transduction in nitrogenase. Acc. Chem. Res. 18, 2179–2186 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burén S., Jiménez-Vicente E., Echavarri-Erasun C., Rubio L. M., Biosynthesis of nitrogenase cofactors. Chem. Rev. 120, 4921–4968 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poudel S., et al. , Electron transfer to nitrogenase in different genomic and metabolic backgrounds. J. Bacteriol. 200, e00757 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang L., et al. , A minimal nitrogen fixation gene cluster from Paenibacillus sp. WLY78 enables expression of active nitrogenase in Escherichia coli. PLoS Genet. 9, 1–11 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curatti L., Ludden P. W., Rubio L. M., NifB-dependent in vitro synthesis of the iron-molybdenum cofactor of nitrogenase. Proc. Natl. Acad. Sci. U.S.A. 103, 5297–5301 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wiig J. A., Hu Y., Lee C. C., Ribbe M. W., Radical SAM-dependent carbon insertion into the nitrogenase M-cluster. Science 337, 1672–1675 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hernandez J. A., et al. , NifX and NifEN exchange NifB cofactor and the VK-cluster, a newly isolated intermediate of the iron-molybdenum cofactor biosynthetic pathway. Mol. Microbiol. 63, 177–192 (2007). [DOI] [PubMed] [Google Scholar]
- 18.Hernandez J. A., et al. , Metal trafficking for nitrogen fixation: NifQ donates molybdenum to NifEN/NifH for the biosynthesis of the nitrogenase FeMo-cofactor. Proc. Natl. Acad. Sci. U.S.A. 105, 11679–11684 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ribbe M. W., Hu Y., Hodgson K. O., Hedman B., Biosynthesis of nitrogenase metalloclusters. Chem. Rev. 114, 4063–4080 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dekas A. E., Poretsky R. S., Orphan V. J., Deep-Sea archaea fix and share nitrogen in methane-consuming microbial consortia. Science 326, 422–426 (2009). [DOI] [PubMed] [Google Scholar]
- 21.Boyd E. S., Hamilton T. L., Peters J. W., An alternative path for the evolution of biological nitrogen fixation. Front. Microbiol. 2, 1–11 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia A. K., McShea H., Kolaczkowski B., Kaçar B., Reconstructing the evolutionary history of nitrogenases: Evidence for ancestral molybdenum-cofactor utilization. Geobiology 18, 394–411 (2020). 10.1111/gbi.12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGlynn S. E., Boyd E. S., Peters J. W., Orphan V. J., Classifying the metal dependence of uncharacterized nitrogenases. Front. Microbiol. 3, 1–8 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boyd E. S., Garcia Costas A. M., Hamilton T. L., Mus F., Peters J. W., Evolution of molybdenum nitrogenase during the transition from anaerobic to aerobic metabolism. J. Bacteriol. 197, 1690–1699 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia A. K., Kolaczkowski B., Kacar B., Reconstruction of nitrogenase predecessors suggests origin from maturase-like proteins. Genome Biol. Evol. 14, evac031 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mus F., Colman D. R., Peters J. W., Boyd E. S., Geobiological feedbacks, oxygen, and the evolution of nitrogenase. Free Radic. Biol. Med. 140, 250–259 (2019). [DOI] [PubMed] [Google Scholar]
- 27.Hanada S., Takaichi S., Matsuura K., Nakamura K., Roseiflexus castenholzii gen. nov., sp. nov., a thermophilic, filamentous, photosynthetic bacterium that lacks chlorosomes. Int. J. Sys. Evol. Microbiol. 52, 187–193 (2002). [DOI] [PubMed] [Google Scholar]
- 28.Van Der Meer M. T. J., et al. , Cultivation and genomic, nutritional, and lipid biomarker characterization of Roseiflexus strains closely related to predominant in situ populations inhabiting yellowstone hot spring microbial mats. J. Bacteriol. 192, 3033–3042 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peters J. W., Fisher K., Dean D. R., Nitrogenase structure and function: A biochemical-genetic perspective. Annu. Rev. Microbiol. 49, 335–366 (1995). [DOI] [PubMed] [Google Scholar]
- 30.Shah V. K., Brill W. J., Nitrogenase I. V., Simple method of purification to homogeneity of nitrogenase components from Azotobacter vinelandii. Biochim. Biophys. Acta 305, 445–454 (1973). [DOI] [PubMed] [Google Scholar]
- 31.Eady R. R., Smith B. E., Cook K. A., Postgate J. R., Nitrogenase of Klebsiella pneumoniae. Purification and properties of the component proteins. Biochem. J. 128, 655–675 (1972). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Christiansen J., Goodwin P. J., Lanzilotta W. N., Seefeldt L. C., Dean D. R., Catalytic and biophysical properties of a nitrogenase apo-MoFe protein produced by a nifB-deletion mutant of Azotobacter vinelandii. Biochemistry 37, 12611–12623 (1998). [DOI] [PubMed] [Google Scholar]
- 33.Jimenez-Vicente E., et al. , Sequential and differential interaction of assembly factors during nitrogenase MoFe protein maturation. J. Biol. Chem. 293, 9812–9823 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee C. C., et al. , Stepwise formation of P-cluster in nitrogenase MoFe protein. Proc. Natl. Acad. Sci. U.S.A. 106, 18474–18478 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu Y., et al. , Nitrogenase reactivity with P-cluster variants. Proc. Natl. Acad. Sci. U.S.A. 102, 13825–13830 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang Z. Y., et al. , The electronic structure of FeV-cofactor in vanadium-dependent nitrogenase. Chem. Sci. 12, 6913–6922 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rubio L. M., Singer S. W., Ludden P. W., Purification and characterization of NafY (apodinitrogenase gamma subunit) from Azotobacter vinelandii. J. Biol. Chem. 279, 19739–19746 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Curatti L., et al. , In vitro synthesis of the iron-molybdenum cofactor of nitrogenase from iron, sulfur, molybdenum, and homocitrate using purified proteins. Proc. Natl. Acad. Sci. U.S.A. 104, 17626–17631 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rangaraj P., Shah V. K., Ludden P. W., ApoNifH functions in iron-molybdenum cofactor synthesis and apodinitrogenase maturation. Proc. Natl. Acad. Sci. U.S.A. 94, 11250–11255 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shah V. K., Allen J. R., Spangler N. J., Ludden P. W., In vitro synthesis of the iron-molybdenum cofactor of nitrogenase. Purification and characterization of NifB cofactor, the product of NIFB protein. J. Biol. Chem. 269, 1154–1158 (1994). [PubMed] [Google Scholar]
- 41.Soboh B., Boyd E. S., Zhao D., Peters J. W., Rubio L. M., Substrate specificity and evolutionary implications of a NifDK enzyme carrying NifB-co at its active site. FEBS Lett. 584, 1487–1492 (2010). [DOI] [PubMed] [Google Scholar]
- 42.Siemann S., Schneider K., Oley M., Müller A., Characterization of a Tungsten-substituted nitrogenase isolated from Rhodobacter capsulatus. Biochemistry 42, 3846–3857 (2003). [DOI] [PubMed] [Google Scholar]
- 43.Buren S., et al. , Biosynthesis of the nitrogenase active-site cofactor precursor NifB-co in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U.S.A. 116, 25078–25086 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao D., Curatti L., Rubio L. M., Evidence for nifU and nifS participation in the biosynthesis of the iron-molybdenum cofactor of nitrogenase. J. Biol. Chem. 282, 37016–37025 (2007). [DOI] [PubMed] [Google Scholar]
- 45.Hoover T. R., Imperial J., Ludden P. W., Shah V. K., Homocitrate is a component of the iron-molybdenum cofactor of nitrogenase. Biochemistry 28, 2768–2771 (1989). [DOI] [PubMed] [Google Scholar]
- 46.Imperial J., Hoover T. R., Madden M. S., Ludden P. W., Shah V. K., Substrate reduction properties of dinitrogenase activated in vitro are dependent upon the presence of homocitrate or its analogues during iron-molybdenum cofactor synthesis. Biochemistry 28, 7796–7799 (1989). [DOI] [PubMed] [Google Scholar]
- 47.Sippel D., Einsle O., The structure of vanadium nitrogenase reveals an unusual bridging ligand. Nat. Chem. Biol. 13, 956–960 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trncik C., Detemple F., Einsle O., Iron-only Fe-nitrogenase underscores common catalytic principles in biological nitrogen fixation. Nat. Catal. 6, 415–424 (2023). 10.1038/s41929-023-00952. [DOI] [Google Scholar]
- 49.Wiig J. A., Hu Y., Ribbe M. W., Refining the pathway of carbide insertion into the nitrogenase M-cluster. Nat. Commun. 6, 8034 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guth J. H., Burris R. H., Inhibition of nitrogenase-catalyzed NH3 formation by H2. Biochemistry 22, 5111–5122 (1983). [DOI] [PubMed] [Google Scholar]
- 51.North J. A., et al. , A nitrogenase-like enzyme system catalyzes methionine, ethylene, and methane biogenesis. Science 369, 1094–1098 (2020). [DOI] [PubMed] [Google Scholar]
- 52.Eva R. B. B. M. G., Stüeken E., Matthew C. K., Isotopic evidence for biological nitrogen fixation by molybdenum-nitrogenase from 3.2 Gyr. Nature 520, 666–669 (2015). [DOI] [PubMed] [Google Scholar]
- 53.Boyd E. S., Peters J. W., New insights into the evolutionary history of biological nitrogen fixation. Front. Microbiol. 4, 201 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fani R., Gallo R., Liò P., Molecular evolution of nitrogen fixation: The evolutionary history of the nifD, nifK, nifE, and nifN genes. J. Mol. Evol. 51, 1–11 (2000). [DOI] [PubMed] [Google Scholar]
- 55.Benton P. M., et al. , Interaction of acetylene and cyanide with the resting state of nitrogenase alpha-96-substituted MoFe proteins. Biochemistry 40, 13816–13825 (2001). [DOI] [PubMed] [Google Scholar]
- 56.Jiang X., et al. , Exploiting genetic diversity and gene synthesis to identify superior nitrogenase NifH protein variants to engineer N2-fixation in plants. Commun. Biol. 4, 1–11 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
All study data are included in the article and/or SI Appendix.