Significance
Bacteroidota are key members of the human gut microbiota that influence human health by degrading polysaccharides. This degradation is achieved by a suite of lipoproteins, a class of membrane protein characterized by lipidation. Lipoprotein synthesis in Bacteroidota is understudied. Here, we used a genetic screen to identify gene(s) responsible for N-acylation, the last step in lipoprotein biosynthesis. Our screen identified the lipoprotein N-acyltransferase in Bacteroides (Lnb) that performs this step. We show that deletion of Lnb negatively affects cellular growth and ability to degrade polysaccharides, deepening our understanding of Bacteroidota and lipoproteins.
Keywords: lipoproteins, Bacteroides, acyltransferase, cell surface
Abstract
Members of the Bacteroidota compose a large portion of the human gut microbiota, contributing to overall gut health via the degradation of various polysaccharides. This process is facilitated by lipoproteins, globular proteins anchored to the cell surface by a lipidated N-terminal cysteine. Despite their importance, lipoprotein synthesis by these bacteria is understudied. In Escherichia coli, the α-amino-linked lipid of lipoproteins is added by the lipoprotein N-acyltransferase Lnt. Herein, we have identified a protein distinct from Lnt responsible for the same process in Bacteroides, named lipoprotein N-acyltransferase in Bacteroides (Lnb). Deletion of Lnb yields cells that synthesize diacylated lipoproteins, with impacts on cell viability and morphology, growth on polysaccharides, and protein composition of membranes and outer membrane vesicles (OMVs). Our results not only challenge the accepted paradigms of lipoprotein biosynthesis in gram-negative bacteria but also suggest the existence of a new family of lipoprotein N-acyltransferases.
Bacteroidota account for nearly 50% of the human gut microbiota and dramatically influence host health and disease (1). These bacteria degrade a wide variety of structurally diverse glycans provided via diet or derived from the host (e.g., intestinal mucin), a metabolic attribute that is key to their survival and persistence in the mammalian gut (2). Breakdown of glycans provides nutrients for fellow gut inhabitants, in turn releasing short-chain fatty acids that are beneficial to the host (3). Bacteroides spp. devote ~20% of their genomes to encoding the genes necessary for glycan breakdown in distinct polysaccharide utilization loci (4). A typical PUL consists of an outer membrane ß-barrel and one or more associated cell-surface lipoproteins that facilitate capture and breakdown of polysaccharides (5). The most well-studied PUL is the starch uptake system (Sus), composed of the ß-barrel SusC and four lipoproteins SusDEFG (5). The ability of Bacteroides to degrade polysaccharides is intricately linked with lipoprotein biosynthesis and subsequent localization to the cell surface. Many of these lipoproteins are also preferentially packaged onto outer membrane vesicles (OMVs) that have far-reaching impacts on cell and host physiology (6–12). Alongside polysaccharide utilization, lipoproteins are involved in numerous other essential processes, including nutrient uptake, immunomodulation, cell envelope integrity, pilus production, and more (13–16).
Lipoproteins are a class of membrane protein characterized by the posttranslational attachment of lipids to a conserved N-terminal cysteine residue. Lipoprotein precursors are initially translated with an N-terminal signal peptide that directs their insertion into the cytoplasmic membrane by the Secretory (Sec) or twin-arginine translocation pathways. This signal peptide also encodes a conserved sequence motif, called a lipobox, immediately before the invariant cysteine that marks the precursor for posttranslational modification (17). First, a diacylglycerol moiety sourced from a membrane phospholipid is attached to the cysteine thiol by lipoprotein diacylglycerol transferase (Lgt) (18). Then, lipoprotein signal peptidase (Lsp) cleaves the signal peptide at the α-amino group of the now-lipidated cysteine (19). In model gram-negative bacteria such as Escherichia coli, lipoprotein N-acyltransferase (Lnt) transfers an additional lipid from a second phospholipid donor to form an amide linkage at the cysteine, yielding the mature triacylated lipoprotein (20). N-acylated lipoproteins are preferred substrates for the localization of lipoprotein (Lol) exporter system, which sorts and localizes lipoproteins to the inner or outer membrane based on the amino acids directly following the lipidated cysteine (21–23). Lnt/lipoprotein N-acylation is widely regarded as essential to gram-negative bacteria as a prerequisite to proper lipoprotein localization (22). There are some known exceptions to this paradigm, however, as Lnt is dispensable in Francisella tularensis (24), Neisseria spp. (24, 25), Acinetobacter spp. (26), and Helicobacter pylori (27). These bacteria all encode an alternate Lol system component called LolF, which is hypothesized to transport both N-acylated and non-N-acylated lipoproteins alike to the outer membrane.
While Lgt and Lsp are conserved across all bacteria, Lnt is less so, even among bacteria known to make triacylated lipoproteins (28, 29). Two such species, Staphylococcus aureus and S. epidermidis, instead employ the two-component system LnsAB for lipoprotein N-acylation (30). Enterococcus faecalis and Bacillus cereus synthesize lyso-form lipoproteins, with N-acylation catalyzed by the lipoprotein intramolecular transacylase Lit (31, 32). Both LnsAB and Lit represent enzyme families unique in sequence and structure to E. coli Lnt (30, 33, 34). In fact, all four of these bacterial species were previously presumed to make diacylated lipoproteins based on the absence of an lnt ortholog in their genomes. The recent discoveries of Lit and LnsAB demonstrate that assumption of lipoprotein N-terminal structure cannot be made based on the genomic absence of a known lipoprotein-modifying enzyme such as Lnt. Furthermore, they also support the possibility of additional lipoprotein biosynthesis pathways that have yet to be identified. Overall, experimental validation of lipoprotein N-terminal structure in various bacteria as well as their corresponding lipoprotein-modifying enzymes is necessary to fully understand lipoproteins.
To our knowledge, only one study has empirically characterized lipoprotein N-terminal structure in Bacteroides: Hashimoto et al. found that Bacteroides fragilis NCTC 9343 synthesizes triacylated lipoproteins (14). However, further investigation into the B. fragilis genome by our group reveals no identifiable orthologs of lnt. Thus we hypothesized that B. fragilis employs distinct enzyme machinery from Lnt to generate triacylated lipoproteins. To identify functional Lnt orthologs in B. fragilis, we used a growth rescue strategy involving the E. coli conditional lethal lnt strain that previously identified Lit as an alternate N-acyltransferase (31). With this approach, a library of B. fragilis genomic DNA was screened for the ability to functionally rescue Lnt-depleted E. coli cells. Herein, we present the results of our experiment, which revealed a single gene responsible for lipoprotein N-acylation that is conserved among Bacteroides spp. Deletion of this gene negatively affects cell viability and ability to grow on certain polysaccharides, alters cell morphology, and impacts lipoprotein localization and OMV production. Our findings not only demonstrate the importance of lipoprotein N-acylation to Bacteroides physiology but also suggest the existence of a new family of lipoprotein N-acyltransferases.
Results
Identification of a Bacteroides Gene that Complements Lnt-Depleted E. coli.
B. fragilis NCTC 9343 produces triacylated lipoproteins (14) despite lacking an ortholog to E. coli lnt. In search of candidate Lnt enzymes from B. fragilis, we constructed and transformed a library of B. fragilis genomic DNA into the E. coli conditional-lethal lnt strain KA349. In KA349, lnt is under the control of the arabinose-inducible promoter PBAD, thus it requires arabinose for cell survival (31). Transformants were plated onto solid media containing glucose, which represses PBAD-driven expression of lnt. Colonies that grew indicated phenotypic rescue of the lethal depletion of Lnt. Indeed, plasmids encoding two distinct B. fragilis genomic inserts were recovered, referred herein as p1 and p31 (Fig. 1A). Both inserts include the open reading frame BF9343_0945, a predicted transmembrane protein containing the domain of unknown function (DUF) 4105. Plasmid p31 also encodes downstream gene BF9343_0944, annotated as an alkaline phosphatase, and the start of BF9343_0943, a component of the Sec translocon. Along with BF9343_0945 and the start of BF9343_0944, p1 encodes the pantothenate kinase BF9343_0946, putative outer membrane protein BF9343_0947, and the start of tetratricopeptide repeat protein BF9343_0948.
Fig. 1.
Identification of BF9343_0945 as the lipoprotein triacylating enzyme in Lnt-depleted E. coli. (A) DNA fragments recovered from viable Lnt-depleted cells were mapped to the B. fragilis genome. The two distinct inserts overlap on the single open reading frame BF9343_0945 (boxed and shaded). (B) Lnt-depleted KA349 was transformed with the indicated plasmids and spread on three arabinose plates and three glucose plates each. Colonies were enumerated and represented as a weighted ratio of glucose-grown to arabinose-grown colonies. (C) Whole-cell lysates of KA349 (PBAD-lnt) grown under inducing (+) and noninducing (−) conditions, and Δlnt strains with plasmids p1, p31, and pBF9343_0945 were separated by SDS-PAGE and immunoblotted against Lpp(K58A)-Strep. Lipoprotein having three acyl chains (arrowhead marked “3”) and two acyl chains (arrowhead marked “2”) are indicated. (D) Trypsinized lipopeptides of Lpp purified from the Δlnt strain expressing BF9343_0945 were eluted from nitrocellulose and analyzed by MALDI-TOF MS. The prominent peak at m/z 1396 corresponds to the triacylated N-terminal peptide possessing acyl chains totaling 48:1 (with 48 and 1 referring to the total number of carbons and a double bond, respectively), and m/z 1424 is the same peptide with acyl chains totaling 50:1.
To narrow down which gene(s) are responsible for rescue, p1, p31, and plasmids encoding their respective individual genes were tested for the ability to rescue KA349. Lnt-depleted KA349 cells were transformed with each plasmid and spread onto three agar plates containing arabinose and three containing glucose. Regardless of plasmid, all transformations yielded colonies in the presence of arabinose. On glucose, no colonies grew when KA349 was transformed with pBF9343_0944, pBF9343_0946, and pBF9343_0947 (Fig. 1B). In contrast, when KA349 was transformed with p31 or pBF9343_0945, respectively, 76% and 43% of colonies also grew on glucose compared to arabinose. From these results, we conclude that BF9343_0945 is sufficient for rescue of KA349, asserting it as our main candidate as the Bacteroides lipoprotein N-acyltransferase. Despite p1 also encoding BF9343_0945, no colonies grew on glucose when KA349 was transformed with p1. This lack of rescue by p1 may be due to the addition of vancomycin to reduce background growth, which was not included in the original rescue experiment.
BF9343_0945 is Sufficient for Lipoprotein Triacylation in E. coli.
To demonstrate that the observed rescue cannot be attributed to leaky expression of PBAD-lnt or residual Lnt enzyme, we sought to determine whether lnt could be fully deleted in these cells. The marked lnt::specR allele from TXM1036, a strain previously used to generate diacylated lipoproteins (31), was packaged into P1vir and transduced into Δlpp cells containing p31 and pBF9343_0945. We also tested p1 because it rescued KA349 in our initial library screen (Fig. 1A). The lnt::specR allele was successfully transduced into cells harboring each of the three plasmids, indicating that lnt can be deleted when they are present.
We then introduced a plasmid encoding the Strep-tagged lipoprotein Lpp(K58A) into the resulting Δlnt strains and assessed for triacylation by immunoblot. Previously used as a reporter for acylation, the triacylated and diacylated forms of Lpp(K58A)-Strep (a difference of 238.24 Da for palmitate (C16), for example) can be separated by sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE) using a 16.5% Tris-tricine gel (31, 35). The triacyl and diacyl controls are derived from KA349 cells grown under Lnt-inducing and Lnt-depleting conditions, respectively. Seen in Fig. 1C, triacylated Lpp was observed in lnt::specR cells containing p1, p31, and pBF9343_0945. This result not only indicates rescue by lipoprotein triacylation but also that BF9343_0945 is sufficient for triacylation in E. coli.
To further confirm lipoprotein N-acylation, we purified Lpp(K58A)-Strep from the Δlnt strain carrying pBF9343_0945 and analyzed its N-terminal tryptic peptide by matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS). As a control, Lpp(K58A)-Strep purified from wildtype (WT) lnt cells was also analyzed, whereby a prominent peak appeared at m/z 1396 that is consistent with the triacylated-CSSNAK tryptic peptide (SI Appendix, Fig. S1A), as previously observed (31). The same peak was detected in the Δlnt strain expressing BF9343_0945 (Fig. 1D). Notably, no peak was observed on either spectrum at m/z 1157, the mass corresponding to diacylated-CSSNAK (33). Triacylation was further confirmed in the MS/MS analyses of parent peak m/z 1396, revealing the characteristic fragment ions m/z 813 and 845 indicative of N-acylation (SI Appendix, Fig. S1 B and C) (31).
Deletion of the Candidate Lnt-Like Protein from Bacteroides Results in Diacylated Lipoproteins.
We validated our Lnt-like protein in Bacteroides thetaiotaomicron (B. theta), a close relative of B. fragilis that is genetically tractable and a model organism for studies in our lab. The B. theta homolog BT_4364 is 66% identical (77% similar) to BF9343_0945 and shares the same genomic neighborhood. Despite the essentiality of Lnt in E. coli (22), BT_4364 was deleted from the B. theta genome, albeit with several impacts to cell physiology (described below).
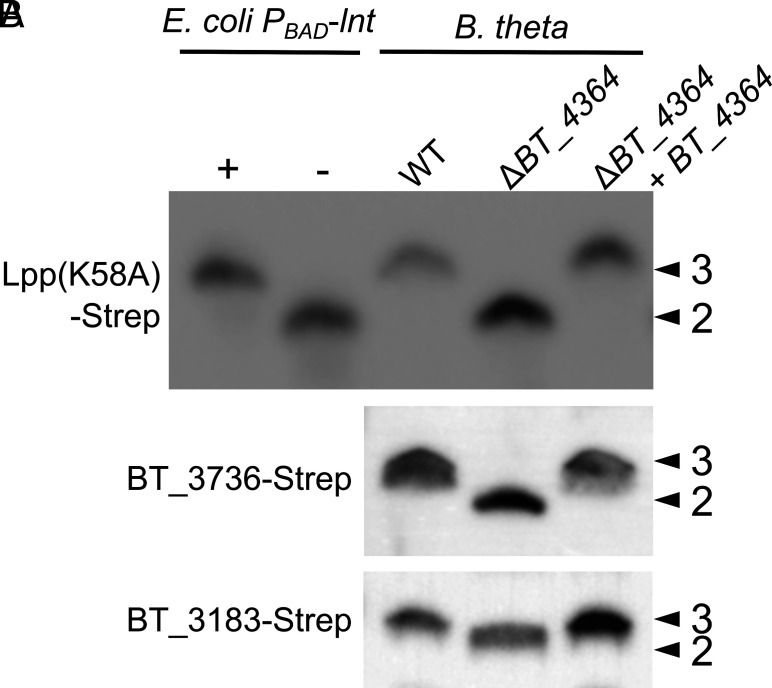
We sought to demonstrate that BT_4364 functions as a lipoprotein N-acyltransferase in its native cell background. First, we performed an immunoblot using the same E. coli Lpp(K58A)-Strep reporter lipoprotein as in Fig. 1C, recombinantly expressed in B. theta from plasmid pWW1376 integrated at an att site (36). Lpp(K58A)-Strep from WT B. theta cells is consistent in size with the triacylated control from E. coli, while Lpp(K58A) from ΔBT_4364 cells demonstrated a shift in size consistent with the loss of a single acyl chain (Fig. 2A). We then analyzed endogenous B. theta lipoproteins BT_3736 and BT_3183 (Strep-tagged; chosen for their small size and compatibility with the assay) and observed a similar band shift in ΔBT_4364 cells (Fig. 2B). In each case, complementation of the ΔBT_4364 strain with BT_4364 under the constitutive promoter us1311 was sufficient to restore triacylation. Taken together, we conclude that BT_4364 is indeed the lipoprotein N-acylation enzyme in B. theta, and, as such, we have annotated it as lipoprotein N-acyltransferase in Bacteroides (Lnb).
Fig. 2.
Evidence of lipoprotein N-acyltransferase activity by BT_4364 in B. theta Immunoblots against Lpp(K58A)-Strep (A), and BT_3736-Strep and BT_3183-Strep (B) using whole-cell lysates of B. theta WT, ΔBT_4364, and the complemented strain. Lysates from E. coli KA349 (PBAD-lnt) grown under inducing (+) and noninducing (−) conditions were included as triacyl and diacyl controls for Lpp(K58A)-Strep. Lipoproteins having three acyl chains (arrowheads marked 3) and two acyl chains (arrowheads marked 2) are indicated.
Lnb Shares Structural Homology with NlpC/P60 Superfamily Enzymes.
A BLAST search of Lnb identifies orthologs (>55% amino acid identity) in 487 Bacteroides/Parabacteroides species, and orthologs (>33% amino acid identity) in 103 reference genomes in RefSeq (37, 38). Despite performing the same N-acylation activity, Lnb differs considerably from the well-studied Lnt of gram-negative E. coli and Pseudomonas aeruginosa. The canonical Lnt protein (512 amino acids in E. coli) contains eight transmembrane helices and a large carbon-nitrogen hydrolase domain located in the periplasm (Fig. 3 A, Right) (39–41). At 398 amino acids, Lnb is predicted to have five transmembrane helices and a periplasmic domain containing a DUF4105 (Fig. 3 A, Left). A DALI search of the Lnb periplasmic domain shares limited homology to the top result TseH (PDB 6v98, Z-score=11.6, 12% identity) (Fig. 3B), an NlpC/P60-family cysteine protease, part of a type VI secretion system in Vibrio cholerae (42). Interestingly, one of the two proteins required for lipoprotein N-acylation in S. aureus, LnsA, is also an NlpC/P60 superfamily enzyme (30). While S. aureus LnsA and the periplasmic domain of Lnb have no sequence homology, alignment of their AlphaFold-predicted structures shows significant similarities (Fig. 3B).
Fig. 3.
Structural comparisons and active site residues of Lnb. (A) Side-by-side comparison of the AlphaFold-predicted structure of Lnb (Left) to the solved structure of E. coli Lnt (PDB 5xhq; Right), colored rainbow from blue (N terminus) to red (C terminus). (B) An overlay of the predicted periplasmic domain of Lnb (blue), with the AlphaFold-predicted structure of S. aureus LnsA (purple) and the solved structure of V. cholerae TseH (PDB 6v98; white). (C) Close-up of the active site of TseH (white) overlaid with Lnb (blue). (D) Whole-cell lysates of indicated B. theta strains were separated by SDS-PAGE and immunoblotted against Lpp(K58A)-Strep. Lipoprotein having three acyl chains (arrowhead marked 3) and two acyl chains (arrowhead marked 2) are indicated.
Based on the overlay with the catalytic triad Glu81-His64-Cys172 of TseH (Fig. 3C), we predicted that C148 or C198 could be a catalytic residue of Lnb. We mutated both residues to serine and tested the resulting mutants’ ability to N-acylate the reporter Lpp(K58A)-Strep in B. theta. Only Lnb(C198S) could restore N-acylation activity to Δlnb cells, suggesting that C148 is essential for activity of Lnb (Fig. 3D). Notably, E. coli Lnt functions by forming a thioester intermediate between its catalytic cysteine (C387) and acyl chain substrate (43). A multisequence alignment of Lnb homologs from 30 common type strains of various gut Bacteroidota revealed that the putative catalytic triad Asn66-His49-Cys148 from the Lnb model overlay with TseH is completely conserved (SI Appendix, Fig. S2).
Cells Lacking Lnb Exhibit Altered Morphology and are Outcompeted by WT Cells.
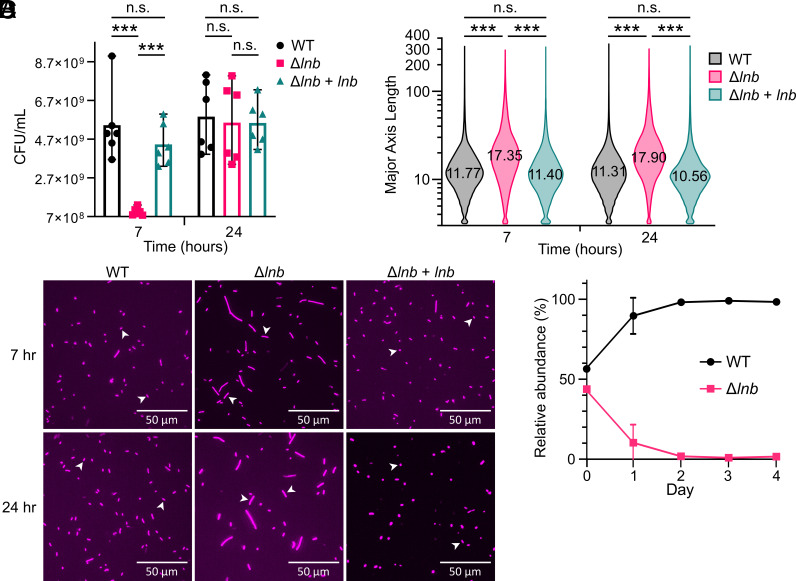
Lipoproteins are involved in many important cellular processes, including cell envelope integrity and division. As such, deletion of lipoprotein-modifying enzymes from gram-negative bacteria is usually lethal or can result in altered cell morphology (26, 44, 45). Thus, we asked whether the loss of lipoprotein triacylation affects cell viability or morphology of B. theta. To assess cell viability, WT, Δlnb, and complemented strains were grown in rich media (TYG) for 7 and 24 h, representing exponential and late stationary growth phases, respectively; then, OD-normalized cells were plated on solid media. All three strains yielded comparable colony-forming units (CFU) from the 24 h cultures; however, modestly less CFU (~7-fold, <1 log) were counted from the 7 h culture of Δlnb cells than WT and complemented (Fig. 4A). To assess cell morphology, WT, Δlnb, and complemented strains grown in rich media for 7 and 24 h were visualized by confocal microscopy. Δlnb cells showed increased filamentation and are on average approximately 64% longer than WT and complemented cells (Fig. 4 B and C). Taken together, these data suggest that Δlnb cells are less fit than WT cells. Indeed, when cocultured 1:1 with WT cells, Δlnb cells significantly decreased in percentage by day 1 and are near undetectable by day 2 (Fig. 4D).
Fig. 4.
Viability, morphology, and competition of WT versus Δlnb cells. (A) The CFU/mL of WT, Δlnb, and complemented strains when grown in rich media for 7 and 24 h. Results are from two biological replicates with three technical replicates each. (B) Representative images of WT, Δlnb, and complemented cells grown in rich media for 7 and 24 h. Cells were stained with CellMask Deep Red, with images taken at 40x magnification. White arrows indicate representative cell sizes. (C) Violin plot quantifying the major axis length of WT, Δlnb, and complemented strains grown in rich media for 7 and 24 h (n = 36,692 to 81,618 objects). The average length is indicated. (D) In vitro competitions of barcoded B. theta WT and Δlnb strains in rich media. Relative abundance was calculated as the percent composition of a strain’s DNA relative to the total DNA in the sample. Data shown are representative of two biological replicates with three technical replicates each. ***P < 0.005 by the paired t test. n.s., not significant.
Cells Lacking Lnb Exhibit Growth Defects that Vary by Media Composition and Carbon Source.
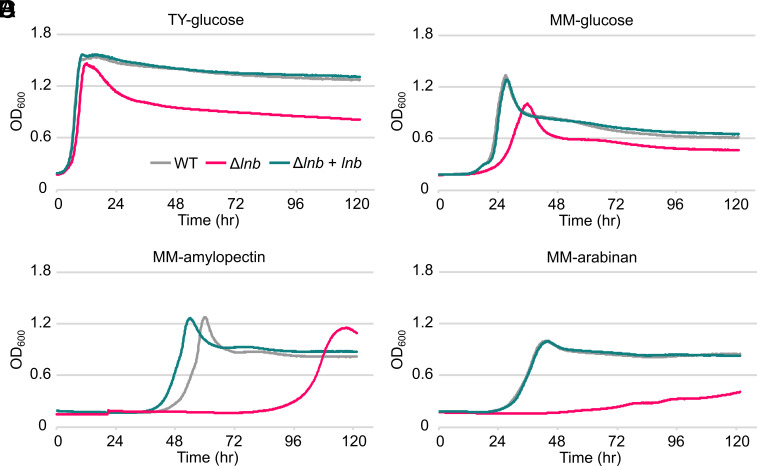
Bacteroides spp. employ distinct lipoproteins involved in uptake, degradation, and import of polysaccharides as carbon sources (2). To examine how loss of lipoprotein triacylation affects growth of B. theta, we measured the growth of our strains in rich (tryptone-yeast extract; TY) and minimal media (MM) supplemented with representative polysaccharides that B. theta is known to efficiently degrade based on previous work and their cognate monosaccharides (4, 46). When grown in TY-glucose, a monosaccharide whose uptake and utilization do not rely on lipoproteins, the Δlnb strain grows similar to WT (Fig. 5A). When grown in MM-glucose, however, the Δlnb strain exhibits a lag in growth and does not achieve the same final optical density (OD) as WT (Fig. 5B). Of the monosaccharides we tested, the same trend was observed for fructose, arabinose, and mannose, though not for galactose (SI Appendix, Fig. S3 A–D). This phenotype is not due to a general inability of the Δlnb strain to grow on monosaccharides, as Δlnb cells grow akin to WT in TY plus monosaccharide (SI Appendix, Fig. S3 E–H). Taken together, these results suggest that the absence of Lnb negatively impacts growth in MM. It is likely that general physiological defects attributed to the loss of lipoprotein N-acylation may be exacerbated by growth in MM.
Fig. 5.
Growth of B. theta strains in rich or MM with various carbon sources. The OD at 600 nm (OD600) of B. theta WT, Δlnb, and complemented strains on TY-glucose (A), MM-glucose (B), MM-amylopectin (C), and MM-arabinan (D) over time. Curves are the average of three technical replicates. An outlier well for Δlnb cells grown in MM-amylopectin was omitted from the dataset. The graphs shown are representative of three biological replicates.
In testing growth on more complex polysaccharides in MM, we observed two distinct growth phenotypes of the Δlnb strain that appear dependent on the provided polysaccharide. The first phenotype consists of an initial lag in growth, but similar kinetics to that of WT once the Δlnb strain begins growing. Of the polysaccharides we tested, this was seen with the starch polysaccharide amylopectin (Fig. 4C) and levan (SI Appendix, Fig. S4A). The lag was not observed with TY-levan, though a small lag persisted in TY-amylopectin (SI Appendix, Fig. S4 B and C).
The second phenotype is characterized by altered growth kinetics to that of WT, which was observed for Δlnb cells grown in MM-arabinan (Fig. 5D), yeast α-mannan, and mucin O-glycans (MOG) (SI Appendix, Fig. S4 D and E). Interestingly, the defect of Δlnb cells was not rescued by growing in TY with the same polysaccharides (SI Appendix, Fig. S4 F–H).
Deletion of Lnb Reduces Cell Surface Localization of Sus Lipoproteins.
Canonically, triacylation is prerequisite to lipoprotein OM localization performed by the Lol system (47–49). Thus, we hypothesized that the growth defects observed when Lnb is deleted may be due to mislocalization of diacylated lipoproteins. To investigate this, we immunostained intact WT, Δlnb, and complemented cells for SusE and SusG, two lipoproteins that normally localize to the cell surface during growth on maltosides or starch (e.g., maltose and amylopectin) (5). When fluorescent intensity was quantified, we observed a significant decrease in signal intensity for both lipoproteins on the surface of Δlnb cells compared to WT (an average decrease of approximately 66% for SusE and 38% for SusG; Fig. 6 and SI Appendix, Fig. 5A). ΔsusE and ΔsusG cells were also imaged as negative controls (SI Appendix, Fig. S5B). In the Lnb complemented strain, we see restoration of SusE surface abundance though SusG surface abundance remains significantly lower than WT cells. A Western blot against normalized whole-cell lysates confirms that this result is not due to lower expression or protein abundance of SusG (SI Appendix, Fig. S6). We cannot conclude from these data where mislocalized SusE or SusG are (i.e., retained in the inner membrane or facing the periplasm from the inner leaflet of the outer membrane) or what step of localization is being perturbed by diacylation (i.e., recognition by LolCDE, surface exposure, etc.).
Fig. 6.
Surface localization of Sus lipoproteins in B. theta. (A) Representative microscopy images of B. theta WT, Δlnb, and complemented cells when immunostained for SusG. Alexa Fluor 488 and CellMask Deep Red images were taken in the green and red channels, respectively, with equal contrast across all images within the same channel. White arrows indicate representative cells. Quantification of the integrated fluorescent intensity is graphed as a violin plot for SusG (B) and SusE (C). Averages are indicated. ***P < 0.005 by the paired t test. n.s., not significant.
Deletion of Lnb Globally Alters Membrane and OMV Protein Composition.
Considering our immunostaining data (Fig. 6), we sought a higher throughput and unbiased measurement of lipoprotein localization. To begin, we grew B. theta WT and Δlnb strains in MM supplemented with starch and then isolated both membranes and OMVs from each. We chose to include OMVs in our analysis because they are highly decorated with polysaccharide-degrading lipoproteins and can influence cell growth (11, 12). SDS-PAGE analysis of the fractions shows that while the total membrane (TM) protein profiles appear similar between the two strains, the OMV protein profiles from Δlnb differed from that of WT (Fig. 7A).
Fig. 7.
Lnb mutant shows altered TM and OMV protein profiling in B. theta. (A) Coomassie blue staining after SDS-PAGE of B. theta WT and Δlnb TM and vesicle (OMV) fractions. Equivalent amounts of protein were loaded in each lane (10 μg). (B) Cartoon representation of lipoprotein comportment in B. theta WT (Left) versus Δlnb (Right) in TM and OMVs. Each shape represents approximately 3% of the total lipoproteins detected. (C) Parts-of-a-whole diagram of the same data, following the same legend as in panel B. The white wedge represents unclassified lipoproteins.
To further analyze membrane and vesicle composition, we performed comparative proteomic analyses of these samples (Dataset S1). Principal component analysis showed that the four proteomes clustered separately (SI Appendix, Fig. S7), revealing major differences between the groups. Functional analysis of proteins enriched in the TM of WT versus those of Δlnb showed decreased number of proteins associated with energy production and conversion, amino acids and coenzyme transport and metabolism, as well as translation, ribosomal structure, and biogenesis (SI Appendix, Table S1). These results suggest a global decrease in metabolism when Lnb is deleted and may partially account for the growth defects observed even when grown in rich media (SI Appendix, Fig. S4). Additionally, more proteins associated with cell wall/membrane/envelope biogenesis were found in the TM of Δlnb than WT (SI Appendix, Table S1), potentially suggesting a cell envelope stress response.
In analyzing lipoproteins specifically, we observed a significant reduction in the quantity of lipoproteins detected in Δlnb cells compared to WT. Of the 421 total lipoproteins identified in the proteomics, approximately 30% (124/421) were absent from Δlnb cells (Fig. 7 B and C; full dataset in Dataset S1). Differences in lipoprotein sorting between TM and OMVs were also observed between WT and mutant. Of 86 lipoproteins showing altered sorting, 20 lipoproteins were retained in the TM of WT but were “leaky” to the OMVs of Δlnb (5%), while 66 lipoproteins enriched in the OMVs from WT (16%) were not enriched in OMVs from the mutant. Surprisingly, a minor portion of lipoproteins was present only in Δlnb (3%, 11/421). Despite these differences, a large portion of lipoproteins were unaltered between the two strains (42%, 177/421). As analysis was performed on TM, these data do not indicate protein localization in the inner versus outer membrane. It is currently unclear what factor(s) determine which lipoproteins are mislocalized when Lnb is deleted.
Discussion
Lipoproteins are ubiquitous components of bacterial cell envelopes and carry out an array of functions essential to survival. Bacteroidota in particular deploy cell-surface lipoproteins that capture and degrade various polysaccharides, contributing to their persistence in the human gut and influence on host health and disease. Canonically in gram-negative bacteria, lipoproteins are sequentially modified by the diacylglycerol transferase Lgt and signal peptidase II Lsp and then triacylated by the N-acyltransferase Lnt. Overall, however, empirical validation of lipoprotein acylation and biosynthetic pathways across bacteria is lacking. Indeed, studies on various gram-positive species revealed more lipoprotein forms and lipoprotein-modifying enzymes than previously thought existed (30–32). While empirical evidence supports N-acylation of lipoproteins in B. fragilis (14), no prior study described how N-acylation occurs in this species. Herein, we show that lipoproteins in Bacteroides spp. are not N-acylated by Lnt, but rather by a previously undescribed protein that we named Lnb.
Lipoprotein N-acylation is widely regarded as essential in gram-negative bacteria, being crucial to proper recognition by the LolCDE exporter and subsequent localization to the OM (21). Nevertheless, we were able to delete Lnb from B. theta, resulting in diacylated lipoproteins (Fig. 2). In E. coli, lethality resulting from the loss of Lnt is largely attributed to mislocalization of diacylated Lpp to the inner membrane (22). Deletion of Lpp or mutation to its peptidoglycan-cross-linking amino acid (K58) can partially restore growth when Lnt is depleted in these genetic backgrounds (50, 51). Bacteroides spp., however, do not encode an identifiable ortholog to Lpp (52), possibly allowing for deletion of Lnb. Moreover, Lnt can be successfully deleted in some gram-negative species that encode an alternate Lol system component (LolF) that exports diacylated lipoproteins to the OM (24–27), though it is currently unclear whether Bacteroides encodes LolF. We cannot yet fully exclude the possibility of a second functional ortholog of Lnt.
While our immunostaining (Fig. 6) and proteomics (Fig. 7) show differences between WT and Δlnb cells, overall some portion of lipoproteins are still localized to the cell surface when Lnb is absent. This suggests that lipoprotein N-acylation is not solely for proper OM localization, as further evidenced by the existence of N-acylated lipoproteins among gram-positive bacteria lacking an OM, such as some Staphylococcus spp. (29). Instead, lipoprotein N-modification has been proposed to be a “postedit” to the conserved Lgt-Lsp pathway, with different bacterial species evolving their own methods for N-modification (30, 31, 53). That Lnb appears phylogenetically confined to Bacteroides spp. supports this theory. The selective pressure(s) driving lipoprotein N-modification are uncertain, though a role in resistance to copper may be one factor (53, 54). Structural similarities between Lnb and LnsA of Staphylococcus spp. (Fig. 3B), both NlpC/P60 superfamily enzymes, suggest the existence of a new family of lipoprotein N-acyltransferases and warrant further research into other species encoding such genes. How lipoproteins are cell-surface localized in Bacteroides spp. and the role of acylation in this process also remains to be seen, as Bacteroidota are missing several known systems for lipoprotein surface exposure. These include the Type 2 Secretion System (T2SS), the “autotransporter” T5SS, and the surface lipoprotein assembly modulator of Neisseria spp. (55, 56). Recently, it was shown that Borrelia burgdorferi uses a distant homolog of the lipopolysaccharide transporter LptD for lipoprotein surface exposure (57), but it is currently unknown whether Bacteroides spp. do the same. At least in the case of the pullulanase lipoprotein in Klebsiella oxytoca, acylation is not required for secretion through T2SS (58).
Although Lnb can be deleted from B. theta, its deletion negatively impacts cell physiology. Δlnb cells may be less viable and are outcompeted by their WT counterparts and display altered cell morphology (Fig. 4). It is likely that there are other phenotypes resulting from the deletion of Lnb that we did not explore in this study, such as capsule and peptidoglycan formation, and outer membrane stability and permeability. Notably, Δlnb cells grow relatively well on some polysaccharides but not on others, even in rich media (Fig. 5, SI Appendix, Figs. S3 and S4). The complexity of the polysaccharide and the suite of lipoproteins involved in its utilization are likely factors to the observed growth phenotypes. As a portion of lipoproteins indeed reach the cell surface in Δlnb cells (Fig. 6), cell growth may be slow until enough polysaccharide is degraded to allow more robust growth. It is also possible that Δlnb cells are more prone to lysis due to membrane defects, in turn releasing polysaccharide-degrading proteins that feed intact cells. More studies are needed to explore these hypotheses.
Relatedly, altered OMV composition likely also influences growth. When grown in MM with starch, Δlnb cells produce OMVs; however, their lipoprotein cargo differs significantly from those produced by WT (Fig. 7). Similar results are expected for Δlnb cells grown in rich media or MM supplemented with other carbon sources, though the individual proteins identified would vary, as observed for B. theta WT by Sartorio et al. (12). In our dataset, an astounding 30% of lipoproteins were absent from Δlnb cells. This absence may be the result of degradation, or by cleavage in such a way that the protein domain is soluble in the periplasm, and thus not included in our sample preparation. Another 20% of lipoproteins showed altered sorting between TM and OMV, and yet still nearly half of lipoproteins were unchanged between WT and Δlnb. These results may indicate that N-acylation is required for certain lipoproteins but is less important for others. An alternate pathway(s) for lipoprotein transport is also possible, as has been suggested in E. coli (59). Nevertheless, our data indicate widespread alterations in lipoprotein abundance and sorting, including proteins beyond those necessary for polysaccharide utilization, highlighting the fundamental importance of Lnb.
Lipoprotein biosynthesis has been suggested as a target for novel antibiotics (60–62). However, some bacteria tolerate losing their lipoprotein biosynthetic enzymes, especially among gram-positive organisms (60, 63, 64). Indeed, this is also true for B. theta, albeit with negative outcomes for the cell, notably the inability to grow on certain polysaccharides such as MOG (Fig. 4, SI Appendix, Fig. S4). Bacteroides is one of the main genera implicated in intestinal mucus degradation that can lead to colitis (65, 66). Thus, development of an antibiotic against Lnb would target mucin-degrading Bacteroides spp. in a highly specific manner, since Lnb is phylogenetically confined to Bacteroides. Further studies are required to characterize Lnb at the enzymatic level and within other Bacteroides strains.
Methods
Bacterial Strains and Growth Conditions.
The strains in this study are listed in SI Appendix, Table S2. E. coli strains were derived from reference strain BW25113 and grown aerobically in Luria Bertani (LB) medium at 37 °C with agitation. Cultures were supplemented with 0.2% (wt/vol) L-arabinose or 0.2% (wt/vol) D-glucose where indicated. Antibiotic markers were selected with carbenicillin (100 µg/mL), kanamycin (25 µg/mL), and spectinomycin (50 µg/mL).
B. theta strains were derived from VPI-5482 Δtdk (herein referred to as WT) and were routinely cultured in a Coy anaerobic chamber at 37 °C with an atmosphere of 10% H2, 5% CO2, 85% N2. Strains were grown in either Brain heart infusion medium supplemented with 5 μg/mL hemin and 1 μg/mL vitamin K3, or TYG medium (67). When applicable, strains were grown in Bacteroides MM containing 100 mM KH2PO4 (pH 7.2), 15 mM NaCl, 8.5 mM (NH4)2SO4, 4 mM L-cysteine, 1.9 mM hematin/200 mM L-histidine (prepared together as a 1,000x solution), 100 mM MgCl2, 1.4 mM FeSO4.7H2O, 50 mM CaCl2, 1 μg/mL vitamin K3, and 5 ng/mL vitamin B12. Carbohydrates were supplemented to a concentration of 5 mg/mL, except MOG (10 mg/mL). When applicable, gentamicin was used at 200 μg/mL, erythromycin at 25 μg/mL, and tetracycline at 3 μg/mL.
For growth experiments in a plate reader, overnight cultures were washed thrice in phosphate-buffered saline (PBS) and normalized to a starting OD600 of 0.05 in MM plus the experimental carbohydrate. Growth experiments were performed in triplicate at 37 °C in 96-well plates and the OD600 was recorded every 10 min. The averages are reported in each figure. Three biological replicates were performed.
Construction of Plasmids, E. coli Δlnt Strain, and Bacteroides Strains.
All plasmids (except the genomic library, described below) were constructed via FastCloning (68) or with the In-Fusion HD Cloning Kit (Takara). Primers used in this study are listed in SI Appendix, Table S3. The lnt::specR allele was transduced from strain TXM1036 (31) (a gift from Dr. Timothy Meredith) into recipient strains using P1vir (a gift from Dr. Lydia Freddolino) when appropriate using standard protocols (69). Deletions in B. theta were created using the Δtdk allelic exchange method (70). Overexpression of Lpp(K58A)-Strep and BT_3736-Strep were driven by the strong promoter of pWW1376, a gift from Dr. Justin Sonnenburg (36), integrated at an att site. For complementation of BT_4364 to the deletion strain, the constitutive promoter us1311 was engineered into pWW1376 for dual or single expression.
Construction of B. fragilis Genomic Library and Complementation Method.
Following the protocol of Cho 2019 (71), genomic DNA from Bacteroides fragilis NCTC 9343 was digested with Sau3AI at 20 °C for an optimized time of 40 min. After separation by gel electrophoresis, DNA fragments ranging from 3 to 5 kb were isolated. Fragments were then ligated with T4 ligase into pUC19 vector that had been linearized with BamHI and dephosphorylated with shrimp alkaline phosphatase (rSAP; NEB). The resulting plasmids were transformed into Stellar Competent E. coli cells (Takara Bio) and plated on LB agar plus carbenicillin. Transformants were scraped from the agar surface into 1 mL PBS, with the final library then isolated from 100 µL of these cells.
The library was transformed into the conditionally lethal E. coli lnt mutant KA349 (31) and plated on LB agar containing carbenicillin and glucose. A portion of the transformed cells was also plated on LB agar containing arabinose as a positive control. Transformants that grew in the absence of arabinose were substruck to confirm phenotypic rescue. Plasmids isolated from the positive colonies were sequenced to identify the B. fragilis gDNA insert.
Colony Formation by Lnt-Depleted KA349 Transformed with Experimental Plasmids.
KA349 cells grown in LB plus kanamycin and arabinose were washed thrice in PBS and then seeded 1:1,000 into fresh LB plus kanamycin and glucose. At OD600 ~0.5 to 0.6, cells were made competent and transformed via heat shock with 50 ng of plasmid. After 1 h outgrowth with no arabinose or glucose, 100 µL each was spread onto three LB agar plates containing arabinose and three containing glucose. Plates also contained carbenicillin for transformant selection and 10 µg/mL vancomycin to reduce background growth (31). CFU were enumerated the following day. Three biological replicates were performed.
Immunoblotting.
Whole-cell lysates were separated by SDS-PAGE with a 16.5% Tris-tricine gel (72) for Lpp(K58A)-Strep, BT_3736-Strep, and BT_3183-Strep, and a 10% Tris-glycine gel for SusG. Proteins were transferred to polyvinylidene difluoride membrane (0.2 µm). For Lpp(K58A)-Strep, BT_3736-Strep, and BT_3183-Strep, the membrane was incubated with a 1:5,000 dilution of StrepTactin-horseradish peroxidase (HRP) conjugate (Bio-Rad), and 1:500 dilution of custom polyclonal antibodies (Cocalico Biologicals) for SusG. Goat anti-rabbit IgG HRP conjugate (Bio-Rad) was used as a secondary at 1:5,000 dilution when necessary. Signals were detected by enhanced chemiluminescence.
Affinity Purification of Tagged Lipoproteins.
Lpp(K58A)-Strep was purified from E. coli as previously described (31), with cells lysed by sonication.
MALDI-TOF MS and MS/MS.
Preparation of lipoproteins for N-terminal structural characterization by MALDI-TOF MS was described previously (73). Briefly, purified Lpp(K58A)-Strep was separated by SDS-PAGE (15% Tris-glycine), transferred to nitrocellulose membrane, then stained with Ponceau S. Bands were excised, washed with water, and proteolyzed with trypsin at 37 °C overnight. The nitrocellulose was then sequentially washed with 0.1% trifluoroacetic acid (TFA), 10% acetonitrile, and 20% acetonitrile. A final incubation with 10 mg/mL α-cyano-4-hydroxycinnamic acid in 2:1 chloroform-methanol eluted the final tryptic N-terminal lipopeptides. MS spectra were collected on an UltrafleXtreme MALDI-TOF (Bruker Daltonics) mass spectrometer in positive reflectron mode. MS/MS spectra were acquired on the same instrument in LIFT mode.
Viable Cell Counting.
Overnight cultures of B. theta were inoculated into fresh TYG to an OD600 of 0.05. After 7 and 24 h of growth, 1 mL of cells were removed, pelleted, and the OD600 normalized to 1.9 in PBS. Cells were serially diluted, and the 10−5, 10−6, and 10−7 dilutions were used to quantify CFU via drip plating method. Briefly, 20 µL of each dilution was spotted onto TYG-agar and then the plate rotated 90° vertically to allow spots to run down the agar surface. Colonies were counted after ~36 h incubation, and the CFU/mL was calculated based on the dilution factor. Two biological replicates with three technical replicates each were performed.
Cell Competition and qPCR.
pNBU2-based plasmids encoding isogenic barcode 1 or 14 (gifts from Dr. Eric C. Martens) (74) were integrated into B. theta WT and the Δlnb mutant, respectively. Strains were grown overnight in TYG, normalized to an OD600 of 0.5, and then mixed in a 1:1 ratio. One mL of the mix was reserved while the rest was diluted to an OD600 of 0.05 into fresh TYG and divided into replicates of 2 mL each. At 24 h intervals for 5 d, cells were passaged 1:100 into fresh TYG, with an aliquot from each passage collected and frozen. Genomic DNA was isolated from these aliquots using the Qiagen DNeasy Blood and Tissue kit, quantified via Nanodrop (Thermo Scientific), and normalized to 10 ng/µL. Quantitative PCR (qPCR) was carried out in a CFX Connect Real-Time System (Bio-Rad) using a homemade SYBR mastermix for 40 cycles of 95 °C for 3 s, 55 °C for 20 s, 72 °C for 20 s, followed by a melting step to determine amplicon purity. Forward primers were specific to barcodes 1 or 14 and were paired with a universal reverse primer. Samples were normalized to a DNA standard curve of genomic DNA from each respective strain. Relative abundance was calculated as the percent composition of a strain’s DNA relative to the total DNA in the sample.
Preparation of Cells for Microscopy.
Overnight cultures of B. theta WT, Δlnb, and complemented cells were passaged into fresh TYG to an OD600 of 0.05. Aliquots were removed at 7 and 24 h, washed twice with PBS, and resuspended in 250 µL PBS. Cells were then incubated for 1.5 h at room temperature with 750 µL of 6% formalin. Cells were again washed twice in PBS, then resuspended in 50 µL of a 1:5,000 dilution of CellMask Deep Red (Thermo Fisher Scientific), and incubated for 30 min in the dark. Cells were washed thrice in PBS and resuspended to 1 mL. Three µL of a 1:100 dilution of the cells were deposited onto slides for imaging.
Immunostaining.
Overnight cultures of B. theta WT, Δlnb, and complemented cells were diluted into MM with maltose to an OD600 of 0.05. Cells were removed once they reached an OD600 of 0.7, washed twice with PBS, fixed in formalin as above, and then washed twice again. Cells were resuspended in 500 µL blocking solution (2% goat serum and 0.02% NaN3 in PBS) and rocked overnight at 4 °C. After washing, cells were incubated with a 1:500 dilution of anti-SusG and 1:5,000 dilution of anti-SusE antibodies in blocking solution for 2 h at room temperature. Following two washes in PBS for 10 min each, cells were rocked in 0.4 mL of a 1:500 dilution of goat anti-rabbit IgG Alexa Fluor 488 secondary antibody (Invitrogen) in blocking solution for 1 h in the dark. Cells were again washed in PBS for 10 min each, stained in 50 µL of CellMask Deep Red as above, and washed a final three times before deposition onto slides for imaging.
Image Acquisition and Analysis.
Cells were imaged using a CellVoyager CQ1 Benchtop High-Content Analysis System (Yokogawa) with a 40× lens. Alexa Fluor 488 and CellMask Deep Red images were taken in the green and red channels with exciting wavelengths of 488 nm and 659 nm, respectively, with spinning disk confocal and 500 ms exposure times (75). Laser power was adjusted to yield an optimal signal-to-noise ratio for each channel. Laser autofocus was used and 100 fields per slide were imaged. Maximum intensity projection images were collected from 25 confocal planes with a 2.5 µm step size. Bacterial cells were segmented using Otsu two-class thresholding with CellProfiler using the CellMask Deep Red channel and intensity and size/shape features were tabulated for the Alexa Fluor 488 and CellMask Deep Red channels (76). Cell-level data were preprocessed and analyzed in the open source Knime analytics platform. Data analysis was completed using Microsoft Excel and GraphPad Prism. A paired Student t test was performed to determine the significant differences in Alexa Fluor 488 intensity for each strain.
Subcellular Fractionation.
OMVs were purified by ultracentrifugation of filtered spent media. Briefly, 50 mL of B. theta cultures from early stationary phase were centrifuged at 6,500 rpm at 4 °C for 10 min. Supernatants were filtered using a 0.22-μm-pore membrane (Millipore) to remove residual cells. The filtrate was ultracentrifuged at 200,000 × g for 2 h (Optima L-100 XP ultracentrifuge; Beckman Coulter). Supernatants were discarded, and the pellet resuspended in 50 mM HEPES. Purified OMV preparations were lyophilized for MS analysis.
TM preparations were performed by cell lysis and ultracentrifugation. Cultures from early stationary phase were harvested by centrifugation at 6,500 rpm at 4 °C for 10 min. The pellets were gently resuspended in 50 mM HEPES containing complete EDTA-free protease inhibitor mixture (Roche Applied Science) followed by cell disruption. Centrifugation at 6,500 rpm at 4 °C for 5 min was performed to remove unbroken cells. TM were collected from the resulting supernatant by ultracentrifugation at 200,000 xg for 1 h at 4 °C. TM fractions were lyophilized for MS analysis. Membrane and vesicle fractions were analyzed by standard 10% Tris-glycine SDS-PAGE. Protein concentrations were determined using a DC Protein Assay kit (Bio-Rad).
Sample Preparation for Proteomic Analyses.
As described previously (77), lyophilized protein samples were solubilized in 4% SDS, 100 mM HEPES by boiling for 10 min at 95 °C. Protein concentrations were determined by the bicinchoninic acid protein assay (Thermo Fisher Scientific), and 200 μg of each biological replicate was prepared for digestion using Micro S-traps (Protifi, USA) according to the manufacturer’s instructions. Briefly, samples were reduced with 10 mM DTT for 10 min at 95 °C and then alkylated with 40 mM iodoacetamide in the dark for 1 h. Samples were acidified to 1.2% with phosphoric acid and diluted with seven volumes of S-trap wash buffer (90% methanol and 100 mM tetraethylammonium bromide pH 7.1) before loading onto S-traps and washing three times with 400 μL of S-trap wash buffer. Samples were then digested with 2 μg of trypsin (a 1:100 protease/protein ratio) in 100 mM tetraethylammonium bromide overnight at 37 °C. The digests were collected by centrifugation and then sequentially washed with 100 mM tetraethylammonium bromide, 0.2% formic acid, and 0.2% formic acid/50% acetonitrile. Samples were dried down and further cleaned using C18 Stage (78, 79) tips to ensure the removal of any particulate matter.
Reverse Phase Liquid Chromatography–Mass Spectrometry.
As described previously (77), prepared C18-purified peptides from each sample were resuspended in Buffer A* (2% acetonitrile, 0.01% TFA) and separated using a two-column chromatography setup composed of a PepMap100 C18 20-mm by 75-μm trap and a PepMap C18 500-mm by 75-μm analytical column (Thermo Fisher Scientific). Samples were concentrated onto the trap column at 5 μL/min for 5 min with Buffer A (0.1% formic acid, 2% DMSO) and then infused into an Orbitrap Fusion Lumos equipped with a FAIMS Pro interface at 300 nL/min via the analytical columns using a Dionex Ultimate 3000 UPLCs (Thermo Fisher Scientific). 125-min analytical runs were undertaken by altering the buffer composition from 2% Buffer B (0.1% formic acid, 77.9% acetonitrile, and 2% DMSO) to 22% B over 95 min, then from 22% B to 40% B over 10 min, and then from 40% B to 80% B over 5 min. The composition was held at 80% B for 5 min and then dropped to 2% B over 2 min before being held at 2% B for another 8 min. The Fusion Lumos Mass Spectrometer was operated in a stepped FAIMS data-dependent mode at two different FAIMS CVs -40 and -60. For each FAIMS CV a single Orbitrap MS scan (300 to 1,600 m/z and a resolution of 60 k) was acquired every 1.5 s followed by Orbitrap MS/MS HCD scans of precursors (fixed NCE 35%, maximal injection time of 55 ms and a resolution of 15 k).
Proteomic Data Analysis.
Identification and LFQ analysis were accomplished using MaxQuant (v2.0.2.0) (80) with the B. theta VPI-5482 proteome (UniProt: UP000001414) allowing for oxidation on methionine. Prior to MaxQuant analysis, data files were separated into individual FAIMS fractions using the FAIMS MzXML Generator (81). The LFQ and “Match Between Run” options were enabled to allow comparison between samples. The resulting data files were processed using Perseus (v1.4.0.6) (82) to compare the growth conditions using Student t tests as well as Pearson correlation analyses. Classification of proteins identified by MS was performed using UniProt (83), PSORT (84), SignalP (85), and PULDB (86). Functional analysis was performed using eggNOG-mapper (PMID: 30418610).
Supplementary Material
Appendix 01 (PDF)
Dataset S01 (XLSX)
Acknowledgments
This work was supported by NIH grants R01DK125445 (to N.M.K.) and R21AI151873 and R21AI168719 (to M.F.F.). K.M.A. is partially supported by the Michigan Life Sciences Fellows program. N.E.S. is supported by an Australian Research Council Future Fellowship (FT200100270) and an ARC Discovery Project Grant (DP210100362). We thank the Melbourne Mass Spectrometry and Proteomics Facility of the Bio21 Molecular Science and Biotechnology Institute, as well as the Mass Spectrometry and Metabolomics Core at Michigan State University, for access to MS instrumentation.
Author contributions
K.M.A., J.J., M.G.S., and N.M.K. designed research; K.M.A., J.J., M.G.S., N.E.S., J.M.P., and J.Z.S. performed research; K.M.A., J.J., M.G.S., N.E.S., J.Z.S., M.F.F., and N.M.K. analyzed data; and K.M.A., J.J., M.G.S., and N.M.K. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
The mass spectrometry proteomics data have been deposited in the Proteome Xchange Consortium via the PRIDE partner repository with the dataset identifier PXD043219 (87). All plasmids, proteins, bacterial strains, and other reagents generated for this work will be made freely available to researchers using them for noncommercial reasons.
Supporting Information
References
- 1.Méndez-Salazar E. O., et al. , Altered gut microbiota and compositional changes in firmicutes and proteobacteria in mexican undernourished and obese children. Front. Microbiol. 9, 2693 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koropatkin N. M., et al. , How glycan metabolism shapes the human gut microbiota. Nat. Rev. Microbiol. 10, 323–335 (2012), 10.1038/nrmicro2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ríos-Covián D., et al. , Intestinal short chain fatty acids and their link with diet and human health. Front. Microbiol. 7, 185 (2016), 10.3389/fmicb.2016.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martens E. C., et al. , Recognition and degradation of plant cell wall polysaccharides by two human gut symbionts. PLoS Biol. 9, e1001221 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foley M. H., et al. , The Sus operon: A model system for starch uptake by the human gut Bacteroidetes. Cell. Mol. Life Sci. 73, 2603–2617 (2016), 10.1007/s00018-016-2242-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hickey C. A., et al. , Colitogenic Bacteroides thetaiotaomicron antigens access host immune cells in a sulfatase-dependent manner via outer membrane vesicles. Cell Host Microbe 17, 672 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen Y., et al. , Outer membrane vesicles of a human commensal mediate immune regulation and disease protection. Cell Host Microbe 12, 509 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mazmanian S. K., et al. , An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 122, 107–118 (2005). [DOI] [PubMed] [Google Scholar]
- 9.Stentz R., et al. , Cephalosporinases associated with outer membrane vesicles released by Bacteroides spp. protect gut pathogens and commensals against β-lactam antibiotics. J. Antimicrob. Chemother. 70, 701 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elhenawy W., et al. , Preferential packing of acidic glycosidases and proteases into Bacteroides outer membrane vesicles. mBio 5, e00909-14 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valguarnera E., Scott N. E., Azimzadeh P., Feldman M. F., Surface exposure and packing of lipoproteins into outer membrane vesicles are coupled processes in Bacteroides. mSphere 3, 1–14 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sartorio M. G., Pardue E. J., Scott N. E., Feldman M. F., Human gut bacteria tailor extracellular vesicle cargo for the breakdown of diet- and host-derived glycans. Proc. Natl. Acad. Sci. U.S.A. 120, e2306314120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wexler A. G., et al. , Human gut bacteroides capture vitamin B12 via cell surface-exposed lipoproteins. eLife 7, e37138 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hashimoto M., Eguchi H., Tawaratsumida K., Kirikae T., Suda Y., Identification of a TLR2-stimulating lipoprotein in Bacteroides fragilis JCM 11019 (NCTC 9343). Innate Immun. 19, 132–139 (2012). [DOI] [PubMed] [Google Scholar]
- 15.Porter N. T., et al. , Phase-variable capsular polysaccharides and lipoproteins modify bacteriophage susceptibility in Bacteroides thetaiotaomicron. Nat. Microbiol. 5, 1170-1181 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu Q., et al. , A distinct type of pilus from the human microbiome. Cell 165, 690-703 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Juncker A. S., et al. , Prediction of lipoprotein signal peptides in Gram-negative bacteria. Protein Sci. 12, 1652–1662 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sankaran K., Wu H. C., Lipid modification of bacterial prolipoprotein. Transfer of diacylglyceryl moiety from phosphatidylglycerol. J. Biol. Chem. 269, 19701–19706 (1994). [PubMed] [Google Scholar]
- 19.Hussain M., Ichihara S., Mizushima S., Mechanism of signal peptide cleavage in the biosynthesis of the major lipoprotein of the Escherichia coli outer membrane. J. Biol. Chem. 257, 5177–5182 (1982). [PubMed] [Google Scholar]
- 20.Gupta S. D., Wu H. C., Identification and subcellular localization of apolipoprotein N-acyltransferase in Escherichia coli. FEMS Microbiol. Lett. 62, 37–41 (1991). [DOI] [PubMed] [Google Scholar]
- 21.Fukuda A., et al. , Aminoacylation of the N-terminal cysteine is essential for Lol-dependent release of lipoproteins from membranes but does not depend on lipoprotein sorting signals. J. Biol. Chem. 277, 43512–43518 (2002). [DOI] [PubMed] [Google Scholar]
- 22.Robichon C., Vidal-Ingigliardi D., Pugsley A. P., Depletion of apolipoprotein N-acyltransferase causes mislocalization of outer membrane lipoproteins in Escherichia coli. J. Biol. Chem. 280, 974–983 (2005). [DOI] [PubMed] [Google Scholar]
- 23.Yamaguchi K., Yu F., Inouye M., A single amino acid determinant of the membrane localization of lipoproteins in E. coli. Cell 53, 423–432 (1988). [DOI] [PubMed] [Google Scholar]
- 24.LoVullo E. D., Wright L. F., Isabella V., Huntley J. F., Pavelka M. S., Revisiting the gram-negative lipoprotein paradigm. J. Bacteriol. 197, 1705–1715 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.da Silva R. A. G., et al. , The role of apolipoprotein N-acyl transferase, Lnt, in the lipidation of factor H binding protein of Neisseria meningitidis strain MC58 and its potential as a drug target. Br. J. Pharmacol. 174, 2247–2260 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gwin C. M., et al. , The apolipoprotein N-acyl transferase Lnt is dispensable for growth in Acinetobacter species. Microbiology (N Y) 164, 1547–1556 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McClain M. S., Voss B. J., Cover T. L., Lipoprotein processing and sorting in Helicobacter pylori. mBio 11, e00911-20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sutcliffe I. C., Harrington D. J., Hutchings M. I., A phylum level analysis reveals lipoprotein biosynthesis to be a fundamental property of bacteria. Protein Cell 3, 163–170 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakayama H., Kurokawa K., Lee B. L., Lipoproteins in bacteria: Structures and biosynthetic pathways. FEBS J. 279, 4247–4268 (2012). [DOI] [PubMed] [Google Scholar]
- 30.Gardiner J., et al. , Lipoprotein N-acylation in Staphylococcus aureus is catalyzed by a two component acyl transferase system. mBio 11, e01619-20 (2020), 10.1128/mBio.01619-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Armbruster K. M., Meredith T. C., Identification of the lyso-form N-acyl intramolecular transferase in low-GC Firmicutes. J. Bacteriol. 199, e00099-17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kurokawa K., et al. , Novel bacterial lipoprotein structures conserved in low-GC content Gram-positive bacteria are recognized by Toll-like receptor 2. J. Biol. Chem. 287, 13170–13181 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Armbruster K. M., Komazin G., Meredith T. C., Bacterial lyso-form lipoproteins are synthesized via an intramolecular acyl chain migration. J. Biol. Chem. 295, 10195–10211 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olatunji S., et al. Structural basis of the membrane intramolecular transacylase reaction responsible for lyso-form lipoprotein synthesis. Nat. Commun. 12, 4254 (2021), 10.1038/s41467-021-24475-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vidal-Ingigliardi D., Lewenza S., Buddelmeijer N., Identification of essential residues in apolipoprotein N-acyl transferase, a member of the CN hydrolase family. J. Bacteriol. 189, 4456–4464 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whitaker W. R., Shepherd E. S., Sonnenburg J. L., Tunable expression tools enable single-cell strain distinction in the gut microbiome. Cell 169, 538–546.e12 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Leary N. A., et al. , Reference sequence (RefSeq) database at NCBI: Current status, taxonomic expansion, and functional annotation. Nucleic Acids Res. 44 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Altschul S. F., et al. , Basic local alignment search tool. J. Mol. Biol. 215, 403–410 (1990). [DOI] [PubMed] [Google Scholar]
- 39.Zhai Y., et al. , Crystal structure of E. coli apolipoprotein N-acyl transferase. Nat. Commun. 8, 15948 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu M., et al. , Structural insights into lipoprotein N-acylation by Escherichia coli apolipoprotein N-acyltransferase. Proc. Natl. Acad. Sci. U.S.A. 114, E6044–E6053 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wiktor M., et al. , Structural insights into the mechanism of the membrane integral N-acyltransferase step in bacterial lipoprotein synthesis. Nat. Commun. 8, 15952 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hersch S. J., et al. , Envelope stress responses defend against type six secretion system attacks independently of immunity proteins. Nat. Microbiol. 5, 706–714 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buddelmeijer N., Young R., The essential Escherichia coli apolipoprotein N-acyltransferase (Lnt) exists as an extracytoplasmic thioester acyl-enzyme intermediate. Biochemistry 49, 341–346 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Legood S., et al. , A defect in lipoprotein modification by Lgt leads to abnormal morphology and cell death in Escherichia coli that is independent of major lipoprotein Lpp. J. Bacteriol. 204, e0016422 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Diao J., et al. , Inhibition of Escherichia coli lipoprotein diacylglyceryl transferase is insensitive to resistance caused by deletion of Braun’s lipoprotein. J. Bacteriol. 203, e0014921 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sonnenburg J. L., et al. , Glycan foraging in vivo by an intestine-adapted bacterial symbiont. Science 307, 1955–1959 (2005). [DOI] [PubMed] [Google Scholar]
- 47.Sharma S., et al. , Mechanism of LolCDE as a molecular extruder of bacterial triacylated lipoproteins. Nat. Commun. 12, 1–11 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Masuda K., Matsuyama S. I., Tokuda H., Elucidation of the function of lipoprotein-sorting signals that determine membrane localization. Proc. Natl. Acad. Sci. U.S.A. 99, 7390–7395 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hara T., Matsuyama S. I., Tokuda H., Mechanism underlying the inner membrane retention of Escherichia coli lipoproteins caused by Lol avoidance signals. J. Biol. Chem. 278, 40408–40414 (2003). [DOI] [PubMed] [Google Scholar]
- 50.Yakushi T., et al. , Lethality of the covalent linkage between mislocalized major outer membrane lipoprotein and the peptidoglycan of Escherichia coli. J. Bacteriol. 179, 2857–2862 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Braun V., Rotering H., Ohms J.-P., Haganmaier H., Conformational studies on murein-lipoprotein from the outer membrane of Escherichia coli. Eur. J. Biochem. 70, 601–610 (1976). [DOI] [PubMed] [Google Scholar]
- 52.Egan A. J. F., Bacterial outer membrane constriction. Mol. Microbiol. 107, 676–687 (2018). [DOI] [PubMed] [Google Scholar]
- 53.Armbruster K. M., Komazin G., Meredith T. C., Copper-induced expression of a transmissible lipoprotein intramolecular transacylase alters lipoprotein acylation and the toll-like receptor 2 response to Listeria monocytogenes. J. Bacteriol. 201, e00195 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rogers S. D., Bhave M. R., Mercer J. F., Camakaris J., Lee B. T., Cloning and characterization of cutE, a gene involved in copper transport in Escherichia coli. J. Bacteriol. 173, 6742–6748 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilson M. M., Bernstein H. D., Surface-exposed lipoproteins: An emerging secretion phenomenon in Gram-negative bacteria. Trends Microbiol. 24, 198–208 (2016), 10.1016/j.tim.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hooda Y., Lai C. C. L., Moraes T. F., Identification of a large family of Slam-dependent surface lipoproteins in Gram-negative bacteria. Front. Cell Infect. Microbiol. 7, 207 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.He H., et al. , A Borrelia burgdorferi LptD homolog is required for flipping of surface lipoproteins through the spirochetal outer membrane. Mol. Microbiol. 119, 752-767 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Francetić O., Pugsley A. P., Towards the identification of type II secretion signals in a nonacylated variant of pullulanase from Klebsiella oxytoca. J. Bacteriol. 187, 7045-55 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grabowicz M., Silhavy T. J., Redefining the essential trafficking pathway for outer membrane lipoproteins. Proc. Natl. Acad. Sci. U.S.A. 114, 4769–4774 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Narita S., Tokuda H., Bacterial lipoproteins; biogenesis, sorting and quality control. Biochim Biophys Acta Mol. Cell Biol. Lipids 1862, 5–14 (2016). [DOI] [PubMed] [Google Scholar]
- 61.Legood S., Boneca I. G., Buddelmeijer N., Mode of action of lipoprotein modification enzymes—Novel antibacterial targets. Mol. Microbiol. 115, 356–365 (2021), 10.1111/mmi.14610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.El Arnaout T., Soulimane T., Targeting lipoprotein biogenesis: Considerations towards antimicrobials. Trends Biochem. Sci. 44, 701–715 (2019), 10.1016/j.tibs.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 63.Buddelmeijer N., The molecular mechanism of bacterial lipoprotein modification-how, when and why? FEMS Microbiol Rev 39, 246–261 (2015), 10.1093/femsre/fuu006. [DOI] [PubMed] [Google Scholar]
- 64.Nguyen M. T., Götz F., Lipoproteins of gram-positive bacteria: Key players in the immune response and virulence. Microbiol. Mol. Biol. Rev. 80, 891–903 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fang J., et al. , Slimy partners: The mucus barrier and gut microbiome in ulcerative colitis. Exp. Mol. Med. 53, 772–787 (2021), 10.1038/s12276-021-00617-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pei L. Y., et al. , Role of colonic microbiota in the pathogenesis of ulcerative colitis. BMC Gastroenterol. 19, 10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Holdeman L. V., CEMW. Anaerobe Laboratory Manual (V.P.I. Anaerobe 691 Laboratory, Virginia Polytechnic Institute and State University, Blacksburg, VA, 4th ed., 1977). [Google Scholar]
- 68.Li C., et al. , FastCloning: A highly simplified, purification-free, sequence- and ligation-independent PCR cloning method. BMC Biotechnol. 11, 1–10 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thomason L. C., et al. , E. coli genome manipulation by P1 transduction Curr. Protoc. Mol. Biol. 79, 1.17.1–1.17.8 (2007). [DOI] [PubMed] [Google Scholar]
- 70.Koropatkin N. M., Martens E. C., Gordon J. I., Smith T. J., Starch catabolism by a prominent human gut symbiont is directed by the recognition of amylose helices. Structure 16, 1105–1115 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cho H., Construction of a multicopy genomic DNA library and its application for suppression analysis. J. Microbiol. 57, 1041–1047 (2019). [DOI] [PubMed] [Google Scholar]
- 72.Schägger H., Tricine-SDS-PAGE. Nat. Protoc. 1, 16–22 (2006). [DOI] [PubMed] [Google Scholar]
- 73.Armbruster K. M., Meredith T. C., Enrichment of bacterial lipoproteins and preparation of N-terminal lipopeptides for structural determination by mass spectrometry. J. Vis. Exp., e56842 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hoces D., et al. , Fitness advantage of Bacteroides thetaiotaomicron capsular polysaccharide in the mouse gut depends on the resident microbiota. eLife 12, e81212 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mirabelli C., et al. , Morphological cell profiling of SARS-CoV-2 infection identifies drug repurposing candidates for COVID-19. Proc. Natl. Acad. Sci. U.S.A. 118, e2105815118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stirling D. R., et al. , Cell Profiler 4: Improvements in speed, utility and usability. BMC Bioinformatics 22, 433 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kaur A., et al. , Identification of levoglucosan degradation pathways in bacteria and sequence similarity network analysis. Arch. Microbiol. 205, 155 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rappsilber J., Ishihama Y., Mann M., Stop and go extraction tips for matrix-assisted laser desorption/ionization, nanoelectrospray, and LC/MS sample pretreatment in proteomics. Anal. Chem. 75, 663–670 (2003). [DOI] [PubMed] [Google Scholar]
- 79.Rappsilber J., Mann M., Ishihama Y., Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nat. Protoc. 2, 1896–1906 (2007). [DOI] [PubMed] [Google Scholar]
- 80.Cox J., Mann M., MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 26, 1367–1372 (2008). [DOI] [PubMed] [Google Scholar]
- 81.Hebert A. S., et al. , Comprehensive single-shot proteomics with FAIMS on a hybrid orbitrap mass spectrometer. Anal. Chem. 90, 9529–9537 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tyanova S., et al. , The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 13, 731–740 (2016). [DOI] [PubMed] [Google Scholar]
- 83.T. U. Consortium, UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Res. 49, D480–D489 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yu N. Y., et al. , PSORTb 3.0: Improved protein subcellular localization prediction with refined localization subcategories and predictive capabilities for all prokaryotes. Bioinformatics 26, 1608–1615 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Almagro J. J., et al. , SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat. Biotechnol 37, 420–425 (2019). [DOI] [PubMed] [Google Scholar]
- 86.Terrapon N., et al. , PULDB: The expanded database of polysaccharide utilization loci. Nucleic Acids Res. 46, D677–D683 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Scott N. E., Bacteroides thetaiotaomicron OMV proteomics. PRIDE. https://www.ebi.ac.uk/pride/archive/projects/PXD043219. Deposited 23 June 2023.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Dataset S01 (XLSX)
Data Availability Statement
The mass spectrometry proteomics data have been deposited in the Proteome Xchange Consortium via the PRIDE partner repository with the dataset identifier PXD043219 (87). All plasmids, proteins, bacterial strains, and other reagents generated for this work will be made freely available to researchers using them for noncommercial reasons.