Abstract
Purpose
OBI-888 is a humanized, monoclonal IgG1 antibody specific to the tumor-associated carbohydrate antigen Globo H. We conducted a phase I-II study of OBI-888 in patients with advanced cancer.
Methods
Patients were treated with OBI-888 5, 10, or 20 mg/kg IV weekly in Part A (“3 + 3” design) and 20 mg/kg IV weekly in Part B (Simon’s 2-stage design) (1 cycle = 28 days).
Results
Overall, 54 patients were treated (Part A, n = 14; Part B, n = 40). OBI-888 was safe and well tolerated across the doses studied, with a low incidence of OBI-888-related treatment emergent adverse events. The maximum tolerated dose of OBI-888 was not reached. No dose-limiting toxicities were noted up to the 20 mg/kg dose level (recommended phase 2 dose). Stable disease (SD) was noted in 28.6% and 20% of Parts A and B, respectively, including three patients with SD for 6+, 7+, and 9 months. Antibody-dependent cellular cytotoxicity (ADCC) was induced after each OBI-888 treatment (average increase, 3.8-fold and 4.7-fold in Parts A and B, respectively), suggesting that ADCC induction is a potential mechanism of action of OBI-888.
Conclusions
OBI-888 was well tolerated. Prolonged SD was noted in three patients. ADCC was induced after each OBI-888 treatment.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00280-024-04714-z.
Keywords: OBI-888, IgG1 antibody, Globo H, Advanced cancer
Introduction
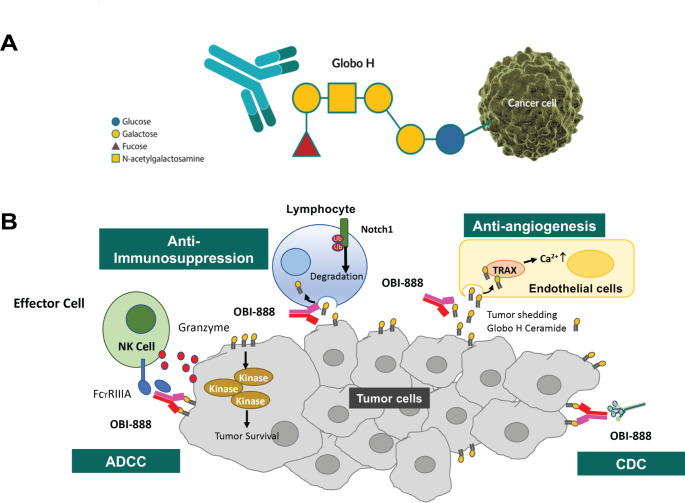
Globo H, a hexasaccharide (Fucα1–2Galβ1–3GalNAcβ1–3Galα1–4Galβ1–4Glc), is a Globo series glycosphingolipid originally isolated from the human breast cancer cell line MCF-7 [1] and is highly overexpressed in multiple epithelial cancer cells [2–4]. Globo H plays an essential role in tumor survival by promoting immunosuppression and tumor survival signaling, triggering endothelial cell migration, tube formation, and intracellular calcium ion mobilization to promote angiogenesis (Fig. 1A) [5]. It may also act as an immune checkpoint molecule, facilitating cancer cell evasion from immunosurveillance [5, 6]. In normal tissues, Globo H expression is limited to the secretory borders of apical epithelial cells, which makes it difficult to access by the immune system [7, 8]. Therefore, Globo H is a potential target for anticancer therapeutics, such as monoclonal antibodies and antibody-drug conjugates (ADCs) [2].
Fig. 1.
Potential mechanisms of action of OBI-888. (A) OBI-888 may block immunosuppression caused by the Globo H antigen recruitment of kinases to promote tumor cell survival, prevent angiogenesis by blocking Globo H–ceramide shed into the tumor microenvironment, activate antibody-dependent cell-mediated cytotoxicity (ADCC) through natural killer (NK) cell activation, and activate complement-dependent cytotoxicity (CDC) through the generation of anti–Globo H immunoglobulin G and immunoglobulin M antibodies. Ca2+, calcium ions; TRAX, translin-associated factor X. (B) OBI-888 Globo H–targeting monoclonal antibody (mAb). OBI-888 is a monoclonal antibody (mAb) that targets the Globo series carbohydrate antigen Globo H that is expressed on the cell surface of many solid tumors
OBI-888 is a humanized monoclonal immunoglobulin G (IgG)1 antibody that targets Globo H (Fig. 1B). In in vitro studies, OBI-888 triggered complement-dependent cytotoxicity (CDC), antibody-dependent cell-mediated cytotoxicity (ADCC), and antibody-dependent phagocytosis mechanisms, induced tumor lysis and inhibited Globo H-ceramide-induced T-cell suppression [9]. In xenograft tumor models, OBI-888 induced tumor growth inhibition, decreased M2 macrophage populations, and reversed the immunosuppressive effect of Globo H-ceramide, suggesting that immune modulation is a potential mechanism of action (Fig. 1B) [10]. OBI-888 is highly specific to Globo H and does not bind to the other Globo series antigens, SSEA-3 and SSEA-4. Competitive enzyme-linked immunosorbent assay (ELISA) showed that OBI-888 does not bind to truncated Globo H, requiring a full Globo H structure for complete binding. OBI-888 inhibited tumor growth by 40–85% in breast, pancreatic, lung, and colon cancer xenograft animal models and was well tolerated at repeated doses of up to 100 mg/kg [Data on File, OBI Pharma Inc.]. Evaluations of cardiovascular effects by electrocardiography and central nervous system (CNS) effects by functional observational battery testing were incorporated into the 28-day repeated-dose study in cynomolgus monkeys and Sprague Dawley rats, respectively. No adverse events (AEs) were noted at dose levels of up to 200 mg/kg, nor were there undesired effects on cardiovascular or CNS function [Data on File, OBI Pharma Inc]. As a glycosphingolipid, the structure of Globo H does not differ across species, and thus the binding efficacy of OBI-888 is expected to remain consistent in humans. The pharmacokinetic (PK) profile of OBI-888 was evaluated in mice (T1/2 8.5 days), rats (T1/2 2.0 to 2.8 days), and monkeys (T1/2 1.6 days). Biodistribution and single-photon emission computed tomography (SPECT) imaging of MCF-7 xenograft mice were used to demonstrate the tumor specificity of OBI-888. OBI-888 was preferentially localized to the tumor site (Supplementary Fig. 1) [10]. The results of 7-day single-dose and 28-day repeat-dose toxicology studies in rats and monkeys were unremarkable [Data on File, OBI Pharma Inc.]. The potential risk of an OBI-888–induced cytokine release syndrome was evaluated using a human peripheral blood mononuclear cell solid-phase cytokine release assay [Data on File, OBI Pharma Inc.]. The levels of inflammatory cytokines, such as interleukin (IL)-2, IL-4, IL-6, IL-10, IL-17, tumor necrosis factor-α, and interferon-γ, were not significantly higher than those with trastuzumab treatment, demonstrating that OBI-888 has a minimal effect on nonspecific lymphocyte activation.
Here, we report the results of a first-in-human study of OBI-888 in patients with advanced solid tumors. We evaluated the safety, tolerability, pharmacokinetics, pharmacodynamics, and preliminary antitumor activity of OBI-888 as a single agent. On the basis of the 28-day rat and monkey toxicity studies (200 mg/kg/week), a starting dose of 5 mg/kg was chosen for this study. At the expected maximum dose of 20 mg/kg/week, the safety margin was approximately 10-fold.
Patients and methods
Patients
Eligible patients were ≥ 18 years of age and had histologically and/or cytologically confirmed advanced solid tumors that had been previously treated with standard-of-care therapy, and either their physicians had determined that such therapy was no longer effective, or patients had declined to receive further non-investigational treatments. Other inclusion criteria were measurable disease according to the Response Evaluation Criteria in Solid Tumors version 1.1. (RECIST v1.1); Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1; and adequate hepatic, renal, and hematologic function. Patients in Part B had to have a documented Globo H H-score of ≥ 100, as determined by a validated immunohistochemical assay (NeoGenomics®, San Diego, California, USA) approved by the US Food and Drug Administration (FDA) for use in clinical trials.
Exclusion criteria included a period of less than 3 weeks from prior cytotoxic chemotherapy or radiation therapy; primary immunodeficiency, systemic steroids (prednisone > 10 mg/day or equivalent) or other immunosuppressive agents within the past 14 days; or an active infection requiring systemic therapy, including known infection with human immunodeficiency virus (HIV) or active infection with hepatitis B or C virus; known untreated CNS metastases; or a history of solid organ transplantation. The complete list of eligibility criteria is provided in the Supplementary file.
The study was conducted at multiple centers in the United States and in Taiwan. The study was approved by the institutional review boards of these institutions, and all patients provided written informed consent. The study was conducted in accordance with the ethical principles of the International Council on Harmonization for Good Clinical Practice guidelines, the Declaration of Helsinki, and applicable local regulations and was registered at www.clinicaltrials.gov (NCT03573544).
Study design
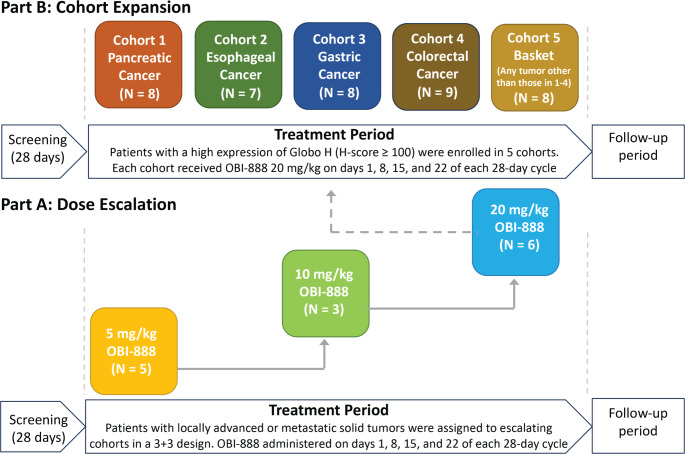
This study was conducted in two parts: Part A (dose escalation) and Part B (dose expansion). The primary objectives of Part A were to establish the maximum tolerated dose (MTD) and recommended phase II dose (RP2D) of OBI-888 and of Part B (cohort expansion) to further characterize the safety and clinical activity of the RP2D dose of OBI-888 administered as monotherapy. The secondary endpoints included the characterization of the PK/PD profiles of OBI-888.
This was an open-label study with eligible patients assigned to a dose level in the order of study entry according to the 3 + 3 design in Part A or to a cohort according to tumor type in Part B (cohort 1: pancreatic adenocarcinoma; cohort 2: esophageal cancer; cohort 3: gastric cancer; cohort 4: colorectal cancer; cohort 5: basket [any solid tumor type other than those included in cohorts 1 through 4]) (Fig. 2).
Fig. 2.
Study design: A. Dose escalation; B. Expansion part of the study
Treatment
OBI-888 was administered via intravenous (IV) infusion at doses of 5, 10, or 20 mg/kg in Part A (dose escalation) and at the RP2D of 20 mg/kg in Part B (cohort expansion), on days 1, 8, 15, and 22 of each 28-day cycle in both parts of the study (Fig. 2). The infusion was administered for a duration of approximately 90 min (± 10 min) for cycles 1 and 2, with the option of reducing the infusion time of cycle 3 to 60 or 30 min at the discretion of the investigator, provided that no infusion-related AEs occurred at prior dose levels. DLTs were defined as any of the following that occurred within 28 days of starting the study treatment: grade 4 neutropenia, grade ≥ 3 febrile neutropenia with or without infection, grade 4 thrombocytopenia or grade 3 thrombocytopenia with bleeding, grade ≥ 3 nausea and vomiting or diarrhea for more than 72 h despite optimal supportive care, or any other grade ≥ 3 non-hematological AE that did not resolve before the next infusion. Treatment was continued until progressive disease, unacceptable toxicity, or a decision by the investigator or patient to discontinue treatment.
Patient monitoring
Safety assessments, including vital signs, physical examinations, electrocardiography recordings, AEs, and clinical laboratory tests (routine hematology, serum chemistry, coagulation, and urinalysis), were performed at protocol-specific visits (on cycle 1; days 1, 8, 15, and 22; and on day 1 of each subsequent treatment cycle).
A treatment-emergent adverse event (TEAE) was defined as an AE with an onset date on or after the start of treatment with the study drug or an AE that was present prior to the receipt of the study treatment and worsened in severity or increased in frequency, on or after the first dose. TEAEs were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE), version 4.03. AEs having both onset and end dates missing were defined as TEAEs, and events reported as “unrelated” or “unlikely related” were defined as unrelated to the study drug. Events reported as “possibly related,” “probably related,” or “definitely related,” as well as events with no reported relationship information, were defined as drug related.
Computed tomography or magnetic resonance imaging evaluations were performed during screening and every 8 weeks (± 1 week) for the first 6 months of the study, and then every 12 weeks (± 1 week) thereafter and at the termination visit. The tumor response was measured using RECIST v1.1 [11]. Stable disease lasting ≥ 4 months was considered clinical benefit [12, 13].
Pharmacokinetics
Blood samples for the PK analysis of OBI-888 levels were collected immediately prior to infusion, immediately following the end of the 90-min infusion, and 1, 4, and 8 h after the end of the infusion during cycle 1 on days 1, 8, 15, and 22 in Part A. Samples were collected prior to infusion during cycle 1, days 1, 8, and 15; immediately after infusion during cycle 1, days 1 and 15; and 1, 3, and 6 h after the end of infusion during cycle 1, and day 1 in Part B.
The concentration of the study drug was determined from the serum samples using a validated ELISA method. PK parameters were calculated using a non-compartmental method (Phoenix WinNonlin Software, v8.3) from blood samples collected during cycle 1, dose 1 and included Cmax, total exposure (AUC), half-life (t1/2), clearance, and volume of distribution (Vd). To assess the attainment of the steady state, trough (Cmin) concentrations and peak concentrations (end of infusion) of each dose were obtained directly from the analytical data.
Pharmacodynamics and biomarkers
Tumor tissue samples were collected from all the patients at the time of screening for entry in both parts of the study. While attempts were made to obtain fresh biopsy specimens, archival samples were retained along with the histology or pathology report when possible. All fresh biopsy specimens or archival samples were sent to the central laboratory (NeoGenomics, Aliso Viejo, California, USA) for Globo H expression analysis using a validated immunohistochemical assay (NeoGenomics) approved by the US FDA for use in clinical studies.
Tumor biopsy samples mandated at baseline were also used to examine the expression of immune markers, such as tumor-infiltrating lymphocytes, including natural killer cells, and immune checkpoints, such as PD-L1, using immunohistochemistry, as well as to evaluate the expression of additional tumor-associated glycans. Blood samples used for PK and antidrug antibody (ADA) assessments were pooled and used for serum glycan analysis. Blood samples were collected during cycle 1, days 1 and 15; cycle 2, day 22; and cycle 4, day 1 for ADCC analysis (serum samples were analyzed using the ADCC Reporter Bioassay [Promega, Madison, Wisconsin, USA] established on Globo H-expressing MCF-7 cells); cycle 1, days 1, 8, and 15 for CDC/ADCC analysis; and cycle 1, day 1 for KIR, HLA, and FcγR genotyping.
Immunogenicity
ADAs against OBI-888 were assessed in serum samples obtained prior to infusion during cycles 1 and 2, days 1, 8, 15, and 22; every two cycles beginning with cycle 3; and at the end of the study/early termination using a series of validated ELISA-based assays (Syneos Health, Morrisville, North Carolina, USA). For patients with persistent antibodies at the end of the study, an additional ADA sample was collected four months after the end-of-study visit, if possible.
Statistical analysis
A “3 + 3” design was used in the dose-escalation part of the study (Part A). The cohort-expansion phase (Part B) was designed to enroll up to 150 total patients based on Simon’s 2-stage design [14], with up to 9 patients in each cohort recruited in the first stage. If at least one objective response was observed within the first six cycles of therapy, up to 21 additional patients were enrolled in that cohort, for a total of up to 30 patients per cohort. If at least four objective responses were observed within the first six cycles of therapy in these 30 patients, then OBI-888 was considered worthy of further evaluation. This design was based on the assumption that an overall response rate of 5% or lower would result in a low level of interest in further development of the treatment, whereas an overall response rate of 25% or higher would elicit a high level of interest in the treatment. The sample size was based on a one-sided alpha of 0.05 and 90% power. The 95% confidence interval (CI) was estimated using the exact binomial distribution.
Results
Patient demographics
The patient demographics are detailed in Table 1. Fourteen patients were enrolled in Part A. Their mean age was 58.2 years. Eleven patients were women and three men. Thirteen patients had an ECOG score of 1 and one patient had a score of 0. Their median number of prior therapies was three (range, 1–9). All patients treated in Part A were included in the safety and PK study.
Table 1.
Baseline patient demographics
| Variable | Part A: Dose Escalation | Part B: Cohort Expansion | Total Part A and Part B | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cohort 1 5 mg/kg (N = 5) |
Cohort 2 10 mg/kg (N = 3) |
Cohort 3 4.0 mg/kg (N = 6) |
Total Part A (N = 14) |
Cohort 1 Pancreatic (N = 8) |
Cohort 2 Esophageal (N = 7) |
Cohort 3 Gastric (N = 8) |
Cohort 4 Colorectal (N = 9) |
Cohort 5 Basket (N = 8) |
Total Part B (N = 40) |
(N = 54) | |
| Sex, n (%) | |||||||||||
| Females | 5 (100) | 3 (100) | 3 (50.0) | 11 (78.6) | 6 (75.0) | 0 | 3 (37.5) | 2 (22.2) | 5 (62.5) | 16 (40.0) | 27 (50.0) |
| Males | 0 | 0 | 3 (50.0) | 3 (21.4) | 2 (25.0) | 7 (100) | 5 (62.5) | 7 (77.8) | 3 (37.5) | 24 (60.0) | 27 (50.0) |
| Age, years | |||||||||||
| Mean | 51.0 | 57.7 | 64.5 | 58.2 | 63.8 | 57.4 | 64.9 | 52.9 | 60.6 | 59.8 | 59.4 |
| SD | 12.9 | 9.5 | 12.1 | 12.6 | 13.3 | 9.4 | 9.7 | 14.8 | 8.1 | 11.8 | 11.9 |
| Median | 54.0 | 61.0 | 67.0 | 58.5 | 70.0 | 61.0 | 63.5 | 54.0 | 60.5 | 61.0 | 61.0 |
| Min – Max | 38/69 | 47/65 | 45/76 | 38/76 | 47/77 | 42/68 | 52/78 | 21/68 | 48/74 | 21/78 | 21/78 |
| Race (%) | |||||||||||
| Asian | 0 | 0 | 1 (16.7) | 1 (7.1) | 3 (37.5) | 6 (85.7) | 7 (87.5) | 0 | 4 (50.0) | 20 (50.0) | 21 (38.9) |
| Black | 1 (20.0) | 1 (33.3) | 0 | 2 (14.3) | 0 | 0 | 0 | 0 | 0 | 0 | 2 (3.7) |
| Caucasian | 3 (60.0) | 1 (33.3) | 5 (83.3) | 9 (64.3) | 5 (62.5) | 1 (14.3) | 1 (12.5) | 9 (100) | 4 (50.0) | 20 (50.0) | 29 (53.7) |
| Native American | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pacific Islander | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Unknown | 1 (20.0) | 1 (33.3) | 0 | 2 (14.3) | 0 | 0 | 0 | 0 | 0 | 0 | 2 (3.7) |
| Other | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ethnicity, n (%) | |||||||||||
| Hispanic or Latino | 1 (20.0) | 0 | 1 (16.7) | 2 (14.3) | 0 | 0 | 0 | 0 | 0 | 0 | 2 (3.7) |
| East Asian | 0 | 0 | 0 | 0 | 3 (37.5) | 6 (85.7) | 7 (87.5) | 0 | 3 (37.5) | 19 (47.5) | 19 (35.2) |
| Not reported | 2 (40.0) | 0 | 1 (16.7) | 3 (21.4) | 1 (12.5) | 0 | 0 | 0 | 1 (12.5) | 2 (5.0) | 5 (9.3) |
| Other | 2 (40.0) | 2 (66.7) | 3 (50.0) | 7 (50.0) | 4 (50.0) | 1 (14.3) | 1 (12.5) | 8 (88.9) | 3 (37.5) | 17 (42.5) | 24 (44.4) |
| Unknown | 0 | 1 (33.3) | 1 (16.7) | 2 (14.3) | 0 | 0 | 0 | 1 (11.1) | 1 (12.5) | 2 (5.0) | 4 (7.4) |
| ECOG performance status, n (%) | |||||||||||
| 0 | 0 | 0 | 1 (16.7) | 1 (7.1) | 1 (12.5) | 0 | 6 (75.0) | 3 (33.3) | 4 (50.0) | 14 (35.0) | 15 (27.8) |
| 1 | 5 (100) | 3 (100) | 5 (83.3) | 13 (92.9) | 7 (87.5) | 7 (100) | 2 (25.0) | 6 (66.7) | 4 (50.0) | 26 (65.0) | 39 (72.2) |
| Time since initial diagnosis (months) | |||||||||||
| n | 5 | 3 | 6 | 14 | 8 | 7 | 8 | 9 | 8 | 40 | 54 |
| Mean | 49.7 | 25.8 | 55.7 | 47.1 | 48.6 | 17.3 | 48.7 | 50.3 | 44.2 | 42.7 | 43.8 |
| SD | 38.7 | 13.7 | 46.7 | 38.4 | 74.8 | 9.8 | 101.2 | 28.1 | 36.3 | 58.3 | 53.6 |
| Median | 69.7 | 23.7 | 32.3 | 32.3 | 18.6 | 13.2 | 12.7 | 46.5 | 37.7 | 23.4 | 25.6 |
| TNM stage at diagnosis, n (%) | |||||||||||
| Stage 1 | 0 | 0 | 1 (16.7) | 1 (7.1) | 0 | 0 | 0 | 1 (11.1) | 0 | 1 (2.5) | 2 (3.7) |
| Stage 2 | 0 | 0 | 0 | 0 | 2 (25.0) | 1 (14.3) | 0 | 0 | 1 (12.5) | 4 (10.0) | 4 (7.4) |
| Stage 3 | 1 (20.0) | 0 | 0 | 1 (7.1) | 2 (25.0) | 1 (14.3) | 1 (12.5) | 2 (22.2) | 2 (25.0) | 8 (20.0) | 9 (16.7) |
| Stage 4 | 0 | 0 | 2 (33.3) | 2 (14.3) | 1 (12.5) | 5 (71.4) | 7 (87.5) | 5 (55.6) | 1 (12.5) | 19 (47.5) | 21 (38.9) |
| Others | 4 (80.0) | 0 | 1 (16.7) | 5 (35.7) | 0 | 0 | 0 | 0 | 1 (12.5) | 1 (2.5) | 6 (11.1) |
| Not reported | 1 (12.5) | 0 | 0 | 1 (11.1) | 1 (12.5) | 3 (7.5) | 3 (5.6) | ||||
| Not available | 3 (100) | 2 (33.3) | 5 (35.7) | 2 (25.0) | 0 | 0 | 0 | 2 (25.0) | 4 (10) | 9 (16.7) | |
| Presence of metastasis, n (%) | |||||||||||
| Yes | 4 (80.0) | 2 (66.7) | 6 (100) | 12 (85.7) | 3 (37.5) | 7 (100) | 7 (87.5) | 9 (100) | 6 (75.0) | 32 (80.0) | 44 (81.5) |
| No | 1 (20.0) | 1 (33.3) | 0 | 2 (14.3) | 5 (62.5) | 0 | 1 (12.5) | 0 | 2 (25.0) | 8 (20.0) | 10 (18.5) |
| Location of metastases, n (%) | |||||||||||
| Bone | 0 | 0 | 1 (16.7) | 1 (7.1) | 0 | 0 | 1 (12.5) | 1 (11.1) | 0 | 2 (5.0) | 3 (5.6) |
| Left kidney | 1 (20.0) | 0 | 0 | 1 (7.1) | 0 | 0 | 0 | 0 | 0 | 0 | 1 (1.9) |
| Liver | 0 | 1 (33.3) | 4 (66.7) | 5 (35.7) | 1 (12.5) | 1 (14.3) | 2 (25.0) | 6 (66.7) | 2 (25.0) | 12 (30.0) | 17 (31.5) |
| Liver and lung | 0 | 0 | 2 (33.3) | 2 (14.3) | 0 | 0 | 0 | 0 | 0 | 0 | 2 (3.7) |
| Liver and lymph nodes | 0 | 0 | 1 (16.7) | 1 (7.1) | 0 | 0 | 0 | 0 | 0 | 0 | 1 (1.9) |
| Lung | 3 (60.0) | 1 (33.3) | 2 (33.3) | 6 (43.6) | 1 (12.5) | 2 (28.6) | 1 (12.5) | 4 (44.4) | 2 (25.0) | 10 (25.0) | 16 (29.6) |
| Lymph nodes | 0 | 1 (33.3) | 1 (16.7) | 2 (14.3) | 3 (37.5) | 6 (87.5) | 5 (62.5) | 2 (22.2) | 4 (50.0) | 20 (50.0) | 22 (40.7) |
| Omentum /liver | 1 (20.0) | 0 | 0 | 1 (7.1) | 0 | 0 | 0 | 0 | 0 | 0 | 1 (1.9) |
| Peritoneum | 0 | 0 | 0 | 0 | 1 (12.5) | 0 | 3 (37.5) | 1 (11.1) | 1 (12.5) | 6 (15.0) | 6 (11.1) |
| Other | 0 | 0 | 0 | 0 | 3 (37.5) | 1 (14.3) | 2 (25.0) | 4 (44.4) | 3 (37.5) | 13 (32.5) | 13 (24.1) |
| Globo H H-score, n | 8 | 7 | 8 | 9 | 8 | 40 | 40 | ||||
| Mean | 210.6 | 195.0 | 186.9 | 167.2 | 215.0 | 194.3 | 194.3 | ||||
| SD | 82.3 | 86.7 | 75.2 | 55.6 | 83.5 | 74.8 | 74.8 | ||||
| Median | 217.5 | 240.0 | 172.5 | 150.0 | 232.5 | 180.0 | 180.0 | ||||
| Min/Max | 100/300 | 100/300 | 100/300 | 100/285 | 100/300 | 100/300 | 100/300 | ||||
| Globo H H-score ≥ 100, n (%) | 8 (100) | 7 100) | 8 (100) | 9 (100) | 8 (100) | 40 (100) | 40 (74.1) | ||||
Forty patients were enrolled in Part B. Their mean age was 59.8 years. Twenty-four patients were men and 16 were women. Twenty-six (65%) of 40 patients had an ECOG score of 1 and 14 had a score of 0. Their median number of prior therapies was four (range, 1–9). All patients treated in Part B were included in the safety study population and 16 patients (16/40, 40.0%) were included in the PK study population.
Safety and tolerability
The median duration of treatment with OBI-888 was 7.1 weeks (range, 0.1–39.1). The median number of doses given was 8.0 (range, 1–36). In Part A, the median duration of treatment with OBI-888 was 6.9 weeks (range, 1.1–27.1). The median number of doses given was 8.0 (range, 2–26). All 14 patients received at least two OBI-888 doses, with 4/14 patients (28.6%) receiving six doses and 3/14 patients (21.4%) receiving eight doses, with a mean extent of exposure of 67.0 days (SD = 51.8). Thirteen patients (13/14, 92.9%) experienced TEAEs during Part A (Supplementary Table 1A), with 50% of the TEAEs attributed to OBI-888. Two patients (2/14, 14.3%) reported three serious adverse events (SAEs) that were unrelated to OBI-888. There were no DLTs or TEAEs leading to discontinuation of OBI-888 treatment, and no deaths were reported in Part A of the study. TEAEs related to OBI-888 in Part A by system organ class and preferred terms are shown in Supplementary Table 1B.
The MTD was not achieved at the maximum dose tested (20 mg/kg) in Part A; therefore, 20 mg/kg was determined as the RP2D, and it was used in Part B of the study. In Part B, the median number of doses given was 8.0 (range, 1–36). Thirteen of 40 (32.5%) patients received ≥8 doses of OBI-888, 7/40 patients (17.5%) received 4 doses, and the remainder of the patients received 1–3 doses, with mean extent of exposure being 57.5 (SD = 55.7) days. Thirty-nine patients (39/40, 97.5%) experienced TEAEs in Part B, and 47.5% of these TEAEs were attributed to OBI-888 (55 drug-related TEAEs) (Table 2). Twenty-two patients (22/40, 55%) experienced grade ≥ 3 TEAEs and 17 patients (17/40, 42.5%) reported SAEs (only one SAE was determined to be OBI-888 related). TEAEs led to the discontinuation of OBI-888 in five patients (5/40, 12.5%). The TEAEs in four of the five patients were not serious, but the fifth patient had a serious infusion-related reaction. Two patients (2/40, 5.0%) died during Part B: one patient from cohort 1 (pancreatic cancer) died following the development of sepsis and one patient from cohort 4 (colorectal cancer) died from progressive disease (the study drug was discontinued because of disease progression two weeks prior to the patient’s assessment for abdominal pain that was attributed to disease progression). Deaths were not attributable to OBI-888. TEAEs related to OBI-888 in Part B according to system organ class and preferred terms are shown in Supplementary Table 2.
Table 2.
Summary of TEAEs
| Part A Total (N = 14) n (%); no. of events |
Cohort 1 - Pancreatic (N = 8) n (%); no. of events |
Cohort 2 - Esophageal (N = 7) n (%); no. of events |
Cohort 3 - Gastric (N = 8) n (%); no. of events |
Cohort 4 - Colorectal (N = 9) n (%); no. of events |
Cohort 5 - Basket (N = 8) n (%); no. of events |
Part B Total (N = 40) n (%); no. of events |
Study Total (Parts A and B) (N = 54) n (%); no. of events |
|
|---|---|---|---|---|---|---|---|---|
| TEAEs | 13 (92.9); 87 | 8 (100); 79 | 6 (85.7); 24 | 8 (100); 57 | 9 (100); 53 | 8 (100); 65 | 39 (97.5); 278 | 52 (96.3); 365 |
| TEAEs grade ≥ 3 | 3 (21.4); 5 | 6 (75.0); 23 | 3 (42.9); 6 | 5 (62.5); 15 | 4 (44.4); 6 | 4 (50.0); 13 | 22 (55.0); 63 | 25 (46.3); 68 |
| TEAEs related to OBI-888 | 7 (50.0); 24 | 5 (62.5); 17 | 2 (28.6); 3 | 2 (25.0); 2 | 5 (55.6); 26 | 5 (62.5); 7 | 19 (47.5); 55 | 26 (48.1); 79 |
| TEAEs leading to discontinuation of OBI-888 | 0 | 2 (25.0); 7 | 1 (14.3); 1 | 0 | 2 (22.2); 3 | 0 | 5 (12.5); 11 | 5 (9.3); 11 |
| TEAEs leading to death | 0 | 1 (12.5); 1 | 0 | 0 | 1 (11.1); 1 | 0 | 2 (5.0); 2 | 2 (3.7); 2 |
| Serious TEAEs | 2 (14.3); 3 | 4 (50.0); 9 | 2 (28.6); 2 | 5 (62.5); 11 | 4 (44.4); 5 | 2 (25.0); 12 | 17 (42.5); 39 | 19 (35.2); 42 |
| Serious TEAEs related to OBI-888 | 0 | 1 (12.5); 1 | 0 | 0 | 0 | 0 | 1 (2.5); 1 | 1 (1.9); 1 |
| Serious TEAEs leading to discontinuation of OBI-888 | 0 | 2 (25.0); 4 | 1 (14.3); 1 | 0 | 1 (11.1); 1 | 0 | 4 (10.0); 6 | 4 (7.4); 6 |
TEAE, treatment-emergent adverse event
Laboratory assessments, vital signs, echocardiography, and physical examinations did not reveal any safety concerns for OBI-888.
Immunogenicity
A positive result for anti–OBI-888 neutralizing antibodies was observed at baseline (prior to cycle 1, day 1 dose) in 6 patients (the signal disappeared at subsequent time points in one patient, suggesting that it was not induced by OBI-888). ADA induction at week 21 was observed in 5 patients and at the end of the study in 4 patients.
Tumor biopsies and Globo H assessment in Part B
Of the 40 patients who were enrolled in Part B, 5 (12.5%) patients underwent a fresh tumor biopsy, and for the remaining 35 (87.5%) patients archival tumor tissue was used for assessment of Globo H results. Among the 35 archival biopsies, 42.9% were collected within 1 year, 31.4% were collected 1–2 years prior to enrollment, and the remaining 25.7% were collected > 2 years and within 5 years from enrollment on the study. The source of the biopsy was from the primary tumor in 25 (62.5%) patients and from metastatic sites in 15 (37.5%) patients.
Tumor biopsy samples were also used to evaluate the percentages of lymphocytes and macrophages within the intertumoral and peritumoral area, based on the cell morphology under H&E staining. The correlation between tumor-infiltrating lymphocytes (TILs) and tumor- infiltrating macrophages (TIMs) levels with the Globo H score is shown in the scatter plot of the Supplementary Fig. 2.
Pharmacokinetics and pharmacodynamics
Pharmacokinetics
While 14 patients were enrolled in Part A and 40 patients were enrolled in Part B, PK sampling was sparse in Part B, with samples collected only at the end of infusion and 72 and 168 h after the end of infusion of the first dose (cycle 1, day 1). Therefore, PK parameters cannot be determined using non-compartmental analyses.
The mean PK parameters of OBI-888 in both studies are presented in Table 3. The maximum concentration of OBI-888 was reached shortly after the end of infusion, followed by a bi-exponential decline. OBI-888 exposure and Cmax generally increased with increasing dose during the dose-escalation phase (Part A). The PK profile of OBI-888 at a 20 mg/kg dose following single-dose administration in Part B was similar to that of the 20 mg/kg dose in Part A (Supplementary Fig. 3 [A]).
Table 3.
OBI-888 pharmacokinetic parameters in part A (N = 14) and part B (N = 40)
| Co-hort | Dose,mg/kg | N | Cancer types | tmax (day) |
Cmax (ug/mL) |
AUC0-t (day*ug/mL) | AUCINF (day*ug/mL) | CL (L/day) |
Vz (L) |
MRTlast (day) |
t1/2 (day) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 5 | 5 | Various | 0.077 | (28.54) | 118.7 | (19.65) | 146.5 | (22.6) | 154.1 | (21.72) | 3.19 | (10.12) | 5.58 | (7.54) | 1.38 | (16.85) | 1.21 | (8.88) |
| 10 | 3 | Various | 0.064 | (3.12) | 192.6 | (5.84) | 194.0 | (16.6) | 205.7 | (14.03) | 3.52 | (5.06) | 7.92 | (77.05) | 1.384 | (20.38) | 1.56 | (70.21) | |
| 20 | 6 | Various | 0.073 | (19.35) | 357 | (18.86) | 465.7 | (35.1) | 477.6 | (35.06) | 2.76 | (28.01) | 4.94 | (27.01) | 1.496 | (13.71) | 1.24 | (10.61) | |
| B | 20 | 2a | Pancreatic cancer | 0.137 | (156.83) | 321 | (16.42) | 580.5 | - | 629.0 | - | 2.63 | (44.56) | 4.31 | (10.45) | 1.604 | - | 1.65 | - |
| 20 | 3 | Esophageal cancer | 0.066 | (0.6) | 327.4 | (14.71) | 406.4 | (28.5) | 418.7 | (28.03) | 3.34 | (36.4) | 5.95 | (41.6) | 1.475 | (14.8) | 1.24 | (17.56) | |
| 20 | 3b,c | Colorectal cancer | 0.063 | (3.94) | 315.7 | (11.69) | 327.0 | (66.0) | 403.8 | (34.14) | 3.53 | (81.8) | 5.74 | (40.5) | 1.013 | (93.17) | 1.13 | (33.96) | |
| 20 | 3 | Gastric cancer | 0.127 | (96.61) | 362.8 | (12.19) | 423.0 | (14.2) | 447.4 | (9.03) | 3.23 | (17.42) | 5.13 | (35.38) | 1.09 | (18.61) | 1.10 | (18.26) | |
| 20 | 3 | Basket | 0.088 | (28.0) | 412.8 | (22.91) | 593.6 | (39.9) | 616.0 | (41.07) | 2.49 | (39.95) | 4.55 | (26.97) | 1.538 | (16.48) | 1.27 | (17.23) | |
Note: Data expressed as geometric mean (GCV%)
a Excluded 1 subject from the descriptive statistics of half-lives and AUCinf: (Span < 1.5, extrapolated AUC > 20%)
b Excluded 1 subject from the descriptive statistics of half-lives and AUCint: (Span < 1.5, extrapolated AUC > 20%)
c Excluded 2 subjects from the statistical analysis of PK parameters due to samples exceeding the validated long-term stability period
Although AUCinf in the pancreatic and basket cohorts was slightly higher than that in the other cohorts (likely attributable to the small sample sizes), exposure to OBI-888 across all cohorts in Part B was generally similar (Supplementary Fig. 3 [B]). The mean volume of distribution (Vd) ranged from 4.3 to 7.9 L across the treatment cohorts, consistent with the expected distribution volume of large-protein therapeutics. The half-lives (t1/2) ranged from 1.1 to 1.6 days across all cohorts.
ADCC activity
ADCC was induced after each OBI-888 infusion, and cytotoxicity levels dropped back to baseline within 7 days. The average ADCC induction levels in the patient cohorts in Part A were 3.0-fold to 4.4-fold higher than baseline, with cohort 3 (20 mg/kg) having the highest ADCC induction level and the smallest SD of 0.6-fold (Supplementary Table 4), which supported the decision to use the 20 mg/kg dose in Part B of the study.
Serum samples across cohorts in Part B demonstrated a similar pattern of ADCC induction (average cohort increases of 3.7-fold to 5.3-fold). Patients with pancreatic cancer had significantly lower ADCC induction levels than those with esophageal cancer, gastric cancer, and basket cohorts (Supplementary Table 4). These results provide evidence that ADCC induction is a potential mechanism of OBI-888 action.
CDC Activity
All serum samples were analyzed using a chemiluminescence-based CDC assay, and CDC activity was expressed as the percentage of lysis for each sample. Serum samples from patients in Part A demonstrated weak or no CDC induction after OBI-888 infusion in Cohort 1 (5 mg/kg), 9% CDC activity in Cohort 2 (10 mg/kg), and 11% CDC activity in Cohort 3 (20 mg/kg). CDC activity was significantly higher in cohort 3 than in cohort 1 (p = 0.007). This observation supports the decision to use a 20 mg/kg dose in Part B of the study.
Serum samples from patients across all cohorts in Part B demonstrated CDC activity of 0–10%. These data suggest that OBI-888 has low potential to induce CDC; therefore, CDC is not a major mechanism of action of OBI-888. Similarly, it has been previously reported that CDC does not play a major role in mediating the effects of the anti-HER2 antibody trastuzumab [15].
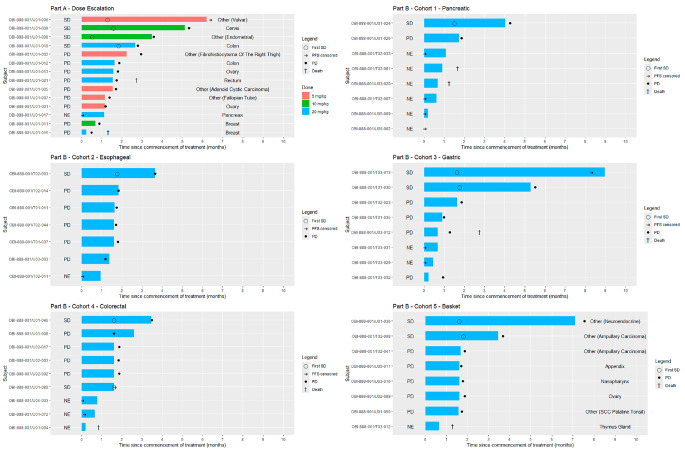
Response and PFS
The time of treatment and course of the disease for each patient are shown in the swimmer plot (Fig. 3). In Part A, of the 14 patients who were evaluable for response, 28.6% had stable disease by RECIST and 14.3% had stable disease lasting ≥ 4 months (95%CI, 1.8 − 42.8%). In Part B, of the 40 patients who were evaluable for response, 20% had stable disease by RECIST (ranging from 12.5 to 25.0% in the individual cohorts), and 10% had stable disease lasting ≥ 4 months (95%CI, 2.8 − 23.7%). The median PFS was 1.8 months (95% CI, 1.18–2.96) in Part A, and 1.8 months (95% CI, 1.71–1.87) in Part B respectively (Supplementary Table 3). Overall, three patients had stable disease for 6 + months (adenoid cystic carcinoma of the Bartholin gland), 7 + months (well-differentiated neuroendocrine carcinoma), and 9 months (gastric cancer).
Fig. 3.
Swimmer plot shows time on treatment and course of the disease for each patient
For patients who discontinued treatment, the primary reason was disease progression in 41 (75.9%) patients; adverse events in six (11.1%), and consent withdrawal in seven (13.0%) patients, including four patients who had clinical progression.
Discussion
This is the first Phase I–II study of OBI-888, a humanized monoclonal IgG1 antibody targeting the tumor-associated carbohydrate antigen Globo H, in patients with advanced solid tumors. Treatment with OBI-888 was associated with a favorable safety profile and a low incidence of adverse effects across all tested doses. The MTD of OBI-888 was not reached, and no DLTs were noted up at the maximum dose level tested. Overall, 28.6% and 20% in Parts A and B, respectively had disease stabilization, indicating a potentially cytostatic effect in heavily pretreated patients. The induction of ADCC after each OBI-888 dose, with an average increase of 3.8-fold in Part A and 4.7-fold in Part B, suggests that ADCC induction may be a potential mechanism of action of OBI-888. In contrast, the overall induction of CDC activity was low.
The data from the current study are consistent with our previously reported results of a dose-escalation study of OBI-999, a Globo H-targeting antibody-drug conjugate in heavily pretreated patients with advanced solid tumors [16]. The latter study identified 1.2 mg/kg as the MTD/RP2D for OBI-999. Stable disease was noted in selected patients treated with OBI-999, indicating that targeting Globo H could lead to disease stabilization but it was inadequate to induce objective responses [16]. In total, the findings from both studies indicate that targeting Globo H with OBI-888 and OBI-999 was safe, but it was associated with limited antitumor activity. This is likely attributed to the complexity associated with targeting Globo H in patients with advanced metastatic tumors, whose disease is characterized by complex molecular abnormalities that cannot be inhibited by a single agent. Notably, it is plausible that the nature (fresh or archival), timing (pretreatment or within 5 years) and site (primary or metastatic) of the biopsy affected the Globo H results [17]. Refinement of patient selection criteria to pinpoint subpopulations and combination strategies involving OBI-888 alongside other treatment modalities, such as chemotherapy, immunotherapy, and/or targeted agents, could have potentiated its effectiveness.
In summary, OBI-888 was safe and well-tolerated across the studied dose range, with a low incidence of OBI-888-related TEAEs and SAEs. Given the limited antitumor activity noted despite the prolonged disease stabilization in three patients, no further development of OBI-888 is planned.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the investigators, coordinators, and study site personnel, as well as the patients and their families, for their participation in this study. We also acknowledge Evelyn Albu, PhD, and Neil Matheson of the E4 Health Group for their partial assistance in writing part of this manuscript, which was funded by OBI Pharma USA, Inc.
Author contributions
Conceptualization, methodology, validation, formal analysis, investigation, resources, data curation, writing—original draft preparation, writing—review and editing, visualization, supervision, project administration, and funding acquisition: A.M.T., A.G., D.S.S., H.J.L., H.S.H., Y.C., L.Y.B., C-J.L.Y., D.X, M.W.S. All authors have read and approved the manuscript.
Funding
This study was funded by OBI Pharma, Inc. (Taipei, Taiwan). This work was supported in part by funds from Mr. and Mrs. Steven McKenzie’s Endowment, Katherine Russell Dixie Distinguished Endowment, and Jamie’s Hope for Dr. Tsimberidou’s Personalized Medicine Program at the University of Texas MD Anderson Cancer Center. This work was also supported in part by the NIH National Cancer Institute award number P30 CA016672 (to The University of Texas MD Anderson Cancer Center).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
Apostolia Maria Tsimberidou: Clinical Trial Research Funding (received through the institution): OBI Pharma, Agenus, Vividion, Macrogenics, AbbVie, IMMATICS, Novocure, Tachyon, Parker Institute for Cancer Immunotherapy, Tempus, and Tvardi; fees for consulting or advisory roles for Avstera Therapeutics, Bioeclipse, BrYet, Diaccurate, Macrogenics, NEX-I, and VinceRx. Dong Xu and M. Wayne Saville are employees of OBI Pharma USA, Inc. The remaining authors declare no relevant conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Menard S, Tagliabue E, Canevari S, Fossati G, Colnaghi MI (1983) Generation of monoclonal antibodies reacting with normal and cancer cells of human breast. Cancer Res 43(3):1295–1300 [PubMed] [Google Scholar]
- 2.Zhang S, Cordon-Cardo C, Zhang HS, Reuter VE, Adluri S, Hamilton WB, Lloyd KO, Livingston PO (1997) Selection of tumor antigens as targets for immune attack using immunohistochemistry: I. Focus on gangliosides. Int J Cancer 73(1):42–49. 10.1002/(sici)1097-0215(19970926)73:1%3C42::aid-ijc8%3E3.0.co;2-1 [DOI] [PubMed] [Google Scholar]
- 3.Yu AL, Hung JT, Ho MY, Yu J (2016) Alterations of Glycosphingolipids in embryonic stem cell differentiation and development of glycan-targeting Cancer Immunotherapy. Stem Cells Dev 25(20):1532–1548. 10.1089/scd.2016.0138 [DOI] [PubMed] [Google Scholar]
- 4.Chang WW, Lee CH, Lee P, Lin J, Hsu CW, Hung JT, Lin JJ, Yu JC, Shao LE, Yu J, Wong CH, Yu AL (2008) Expression of Globo H and SSEA3 in breast cancer stem cells and the involvement of fucosyl transferases 1 and 2 in Globo H synthesis. Proc Natl Acad Sci U S A 105(33):11667–11672. 10.1073/pnas.0804979105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng JY, Wang SH, Lin J, Tsai YC, Yu J, Wu JC, Hung JT, Lin JJ, Wu YY, Yeh KT, Yu AL (2014) Globo-H ceramide shed from cancer cells triggers translin-associated factor X-dependent angiogenesis. Cancer Res 74(23):6856–6866. 10.1158/0008-5472.CAN-14-1651 [DOI] [PubMed] [Google Scholar]
- 6.Kuo T-M, Tsai Y-C, Lee C-C, Lai J-S (2020) The role of Globo H in cancer cell survival. Cancer Res 80(16Supplement):2934 [Google Scholar]
- 7.Chuang P-K, Hsiao M, Hsu T-L, Chang C-F, Wu C-Y, Chen B-R, Huang H-W, Liao K-S, Chen C-C, Chen C-L (2019) Signaling pathway of globo-series glycosphingolipids and β1, 3-galactosyltransferase V (β3GalT5) in breast cancer. Proceedings of the National Academy of Sciences 116(9):3518–3523 [DOI] [PMC free article] [PubMed]
- 8.Tsai Y, Huang J, Cheng J, Lin J, Hung J, Wu Y, Yeh K, Yu A (2013) A prevalent cancer associated glycan, globo H ceramide, induces immunosuppression by reducing Notch1 signaling. J Cancer Sci Ther 5(7):264–270 [Google Scholar]
- 9.Chen I-J, Yang M-C, Chen Y-J (2020) The prevalence of Globo H in different tumor types: breast, pancreatic, lung, gastric, colorectal, liver, and esophageal cancers. Cancer Res 80(16_Supplement):2946 [Google Scholar]
- 10.Chen Y-C, Yang M-C, Shia C-S, Tsao C-Y, Lai J-S, Chen I-J (2019) Specificity, biodistribution, tumor targeting, and pharmacokinetics of a novel humanized anti-globo H antibody, OBI-888, for cancer immunotherapy. Cancer Res 79(13_Supplement):481431431463 [Google Scholar]
- 11.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 12.Tolcher AW (2009) Stable disease is a valid end point in clinical trials. Cancer J 15(5):374–378. 10.1097/PPO.0b013e3181bdbb05 [DOI] [PubMed] [Google Scholar]
- 13.Villaruz LC, Socinski MA (2013) The clinical viewpoint: definitions, limitations of RECIST, practical considerations of measurement. Clin Cancer Res 19(10):2629–2636. 10.1158/1078-0432.CCR-12-2935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simon R (1989) Optimal two-stage designs for phase II clinical trials. Control Clin Trials 10(1):1–10 [DOI] [PubMed] [Google Scholar]
- 15.Liu M, Yang Y-J, Zheng H, Zhong X-R, Wang Y, Wang Z, Wang Y-G, Wang Y-P (2014) Membrane-bound complement regulatory proteins are prognostic factors of operable breast cancer treated with adjuvant trastuzumab: a retrospective study. Oncol Rep 32(6):2619–2627. 10.3892/or.2014.3496 [DOI] [PubMed] [Google Scholar]
- 16.Tsimberidou AM, Vo HH, Beck J, Shia CS, Hsu P, Pearce TE (2023) First-in-human study of OBI-999, a Globo H-Targeting antibody-drug Conjugate, in patients with Advanced Solid tumors. JCO Precis Oncol 7:e2200496. 10.1200/PO.22.00496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hung JT, Chen IJ, Ueng SH, Huang CS, Chen SC, Chen MY, Lin YC, Lin CY, Campbell MJ, Rugo HS, Yu AL (2022) The clinical relevance of humoral immune responses to Globo H-KLH vaccine adagloxad simolenin (OBI-822)/OBI-821 and expression of Globo H in metastatic breast cancer. J Immunother Cancer 10(6). 10.1136/jitc-2021-004312 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.