Abstract
Purpose
This study examined the associations of device-measured moderate-to-vigorous physical activity (MVPA) and sedentary time as well as self-reported MVPA with health-related quality of life (HRQoL) in patients with localized renal cell cancer (RCC) in the recovery phase after surgery.
Methods
At 3 months post-surgery, 341 patients with stage I-III RCC participating in the ReLife study wore an ActivPAL3 device to determine MVPA and sedentary time. The SQUASH questionnaire was used for assessing self-reported MVPA, and the EORTC QLQ-C30 for assessing HRQoL (range 0–100). Multivariable linear regression models were used to examine the cross-sectional associations of MVPA and sedentary time with HRQoL.
Results
The highest (≥ 6.7 h/week) versus lowest (≤ 2.7 h/week) quartile of MVPA was associated with a better global health status (β, 10.2; 95% CI, 5.1, 15.3), summary score (β, 4.6; 95% CI, 1.1, 8.1), physical (β, 7.7; 95% CI, 3.8, 11.6), role (β, 12.4; 95% CI, 4.7, 20.2), and social functioning (β, 7.3; 95% CI, 0.2, 14.4), and lower fatigue (β, − 11.2; 95% CI, − 18.1, − 4.2). Results for self-reported MVPA were in the same direction but weaker. The lowest (≤ 8.8 h/day) versus highest (≥ 11.5 h/day) quartile of sedentary time was associated with better physical functioning (β, 4.6; 95% CI, 0.8, 8.5).
Conclusions
In patients with localized RCC, higher MVPA 3 months post-surgery was associated with better HRQoL outcomes including less fatigue whereas lower sedentary time was only associated with better physical functioning. This information can contribute to the development of physical activity guidelines and interventions to improve HRQoL.
Keywords: Physical activity, Sedentary time, Self-reported, Accelerometry, Quality of life, Renal cell cancer
Introduction
Kidney cancer is the 14th most common cancer worldwide and about 90% of patients with kidney cancer are diagnosed with renal cell cancer (RCC) [1]. The majority of patients present with localized RCC, which is primarily treated with radical or partial nephrectomy [2]. In contrast to most of the other cancer types, radiotherapy, chemotherapy, and immunotherapy are usually not given since effectiveness has not been proven [2]. Overall health status including pain, gastrointestinal function, cognition, and activity as well as physical quality of life are reduced at 2 weeks after RCC surgery compared to before surgery [3]. At 12 weeks after RCC surgery, about 70–80% of patients are likely to have fully recovered [3]. However, patients may still suffer from side effects such as irritability, pain, worry, sleep disturbance, and fatigue [4]. These side effects may have an important impact on patients’ health-related quality of life (HRQoL) [5]. Thus, it is critical to investigate how HRQoL could be improved.
There is increasing evidence in cancer survivors that higher physical activity is associated with better HRQoL [6] while associations for sedentary time are inconsistent [7]. According to the World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR), cancer survivors should engage in at least 150 min/week of moderate-to-vigorous physical activity (MVPA) and minimize their sedentary time [8], similar to the recommendations of the World Health Organization [9]. To date, only one cross-sectional study investigated the association of physical activity with HRQoL among patients with RCC [10]. Significant dose–response associations of MVPA with HRQoL were observed from completely sedentary to 150–290 min/week of MVPA, with no further improvements in HRQoL for ≥ 300 min/week of MVPA. However, physical activity and HRQoL were assessed on average 6 years after diagnosis, which does not reflect the recovery phase after surgery. Also, physical activity was self-reported instead of being objectively measured via an accelerometer. The correlation of self-reported with device-measured MVPA [11] and sedentary time [12] has been reported to be low, and self-reported physical activity measures often result in overestimation of MVPA [13] and underestimation of sedentary time [14]. Questionnaires and accelerometers measure different aspects of physical activity and sedentary time [11–14]. Device-measured physical activity may provide a better understanding of how MVPA and sedentary time are related to HRQoL in patients with localized RCC. This information is important since it may contribute to informing future physical activity guidelines and interventions to improve HRQoL in the first months after surgery.
The main aim of this study was to investigate the association of device-measured MVPA and sedentary time with HRQoL in patients with localized RCC at 3 months after surgery. In addition, we assessed the correlation and bias between self-reported and device-measured MVPA, as well as the association of self-reported MVPA with HRQoL, We hypothesized that higher MVPA and lower sedentary time are associated with better HRQoL. We further postulated that the correlation between device-measured and self-reported MVPA is low, but that associations for device-measured and self-reported MVPA with HRQoL are similar.
Methods
Study design and population
The current cross-sectional analysis was performed using data from the Dutch multicenter cohort study ReLife (Renal Cell Cancer: Lifestyle, Prognosis, and Quality of Life) which has been described in detail elsewhere [15]. ReLife includes patients between the ages of 18 and 75 years who were newly diagnosed with a pathology-confirmed primary stage I, II, or III RCC tumor without lymph node or distant metastases and treated with surgery or ablation. Patients were recruited from January 2018 to June 2021 and requested to wear an accelerometer and to complete questionnaires at 3 months, 1 year, and 2 years after surgery. For this analysis, only data collected at 3 months after surgery were used, reflecting the recovery phase after surgery. A total of 882 patients were invited, of whom 46 appeared to be ineligible (reasons: deceased (n = 1), invitation letter could not be delivered (n = 3), did not fulfill the inclusion criteria due to stage IV disease (n = 16), previous tumor within past 5 years (n = 10), no RCC (n = 6), no surgery (n = 7), other reasons (n = 3)). In total, 368 of the 836 eligible patients agreed to participate (response rate 44%) [15]. Relative to non-responders, participants were more likely to be female (23% vs. 30%) but were similar regarding age, tumor stage, tumor grade, tumor morphology, and type of treatment [15]. After the exclusion of participants who did not wear the accelerometer at 3 months after surgery (n = 22), did not wear it for at least 3 consecutive days (n = 2), or did not complete the first questionnaire (n = 3), 341 participants were included in the current analysis. The study was in line with the principles of the Declaration of Helsinki. The study was approved by the Committee for Human Research region Arnhem-Nijmegen (CMO 2016–3078). Patients who agreed to participate provided written informed consent.
Physical activity and sedentary behavior
Objective data on MVPA and sedentary time were collected using the validated ActivPAL3 accelerometer (PAL Technologies Ltd., Glasgow, UK) [16]. Patients attached the ActivPAL3 with a waterproof sleeve to the center of the right thigh 10 cm above the knee using Tegaderm film (Tegaderm, 3 M Medical) according to written instructions. Participants were instructed to wear the activPAL3 for seven consecutive days, 24 h per day. The raw acceleration data from the accelerometers was transferred to the associated activPAL software version 8. Subsequently, the data was transformed into analyzable data using a customized algorithm written in MATLAB R2018b (MathWorks. Natick, MA, USA) [17]. The time spent in MVPA was estimated by summing the time spent in cadences (i.e., steps per minute) of ≥ 100 and expressed as h/week [18]. Additionally, sedentary time was estimated by summing the time spent in sitting, lying, and reclining posture, excluding algorithm-based sleeping time, and expressed as hours/day.
Self-reported data on MVPA was derived from the validated short questionnaire to assess health-enhancing physical activity (SQUASH) [19]. The reference period was a normal week during the past 3 months. Participants reported the average time spent on commuting, work, household, and leisure-time activities (walking, cycling, gardening, odd jobs, and up to four sports). The weekly time spent on MVPA was calculated by summing all activities with a metabolic equivalent of task value ≥ 3, based on the Ainsworth compendium of physical activities [20].
Covariates
Socio-demographic and lifestyle factors were collected via paper-and-pencil- or web-based questionnaires, as previously described [15]. These factors included among others age, biological sex, educational level (categorized as low, intermediate, or high), body weight, height, and cigarette smoking status (never, former, current). Body mass index (BMI in kg/m2) was calculated by dividing self-reported body weight by height squared and categorized according to the WHO classification [21] into underweight (BMI < 18.50), normal weight (BMI 18.50–24.99), overweight (BMI 25.00–29.99), and obesity (BMI ≥ 30.00). Information on 14 comorbidities was collected using an adapted version of the comorbidity questionnaire [22], and categorized as having 0, 1, or ≥ 2 comorbidities. Information on having any difficulties with walking in the previous week was also obtained by questionnaire and defined as mobility limitation (no, yes). Clinical factors were retrieved from the medical records by data managers of the Netherlands Comprehensive Cancer Organisation (IKNL) and included clinical and post-surgical TNM stage, tumor grade, type of surgery or ablation, and postoperative grade ≥ 2 complications graded using the Clavien-Dindo classification [23].
Health-related quality of life
Data on HRQoL was acquired using the validated cancer-specific European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 (EORTC QLQ-C30, version 3.0) [24]. The EORTC QLQ-C30 includes five functioning scales (i.e., physical, role, emotional, cognitive, social), three symptom scales (i.e., fatigue, nausea/vomiting, and pain), six single items (i.e., dyspnea, insomnia, loss of appetite, constipation, diarrhea, and financial difficulties), all scored from 1 (not at all) to 4 (very much), and a global health status scale ranging from 1 (very poor) to 7 (excellent). A summary score was calculated using all scales except global quality of life and financial difficulties [25]. All scales were linearly transformed to 0–100 scales. Higher scores indicated better HRQoL, except for the symptom scales and single items where higher scores implied worse HRQoL. The current analysis was focused on global health status, the five functioning scales, the summary score, and fatigue, since these were considered most likely to be influenced by MVPA and sedentary time [6].
Statistical analysis
Descriptive characteristics were calculated for the total study population and stratified by device-measured MVPA and sedentary time quartiles, respectively. Spearman’s correlation coefficient was calculated to assess convergent validity, i.e., the degree to which device-measured and self-reported MVPA are related. A Bland–Altman plot was used to evaluate bias, with 95% limits of agreement for the total error between the two methods. Agreement on classifying participants as adhering (≥ 150 min/week) and non-adhering (< 150 min MVPA/week) to the WCRF/AICR physical activity guidelines [8] was assessed using Cohen’s Kappa statistic.
To examine the association of device-measured and self-reported MVPA and device-measured sedentary time with HRQoL, MVPA and sedentary time were analyzed as quartiles and as continuous variables. In addition, adherence to the WCRF/AICR physical activity guidelines was examined. Multivariable linear regression models were constructed to estimate regression coefficients (β) and corresponding 95% confidence intervals (CIs). The least beneficial group (i.e., low MVPA or high sedentary time) was used as the reference. All models were adjusted for age, sex, BMI, comorbidities, tumor stage, smoking status, education level, and mobility limitation. These variables were selected a priori based on literature [7, 10, 26]. Furthermore, models for device-measured MVPA and sedentary time were mutually adjusted. Multicollinearity was investigated but none was found. The clinical relevance of the differences in HRQoL scales was assessed using the guidelines for cross-sectional differences, published by Cocks et al. [27].
R version 4.1.3 (R Foundation for Statistical Computing, Vienna, Austria) was used for all calculations and analyses. P-values < 0.05 were considered statistically significant.
Results
Participant characteristics
The study population predominantly consisted of males (71.3%) and had a mean age of 62.8 (SD 8.9) years (Table 1). The majority of participants were overweight or obese (69.3%), had stage I RCC (64.6%), were treated with radical nephrectomy (57.8%), and reported at least two comorbidities (61.7%). Participants with the lowest (versus highest) device-measured MVPA and participants with the highest (versus lowest) sedentary time more frequently reported to be male, to have a higher BMI, and to be current smokers. Also, they are more frequently reported to have two or more comorbidities, a grade 2 or higher complication, and a mobility limitation.
Table 1.
Descriptive characteristics for the total study population of patients with localized RCC and by quartiles of device-measured moderate-to-vigorous activity (MVPA) and sedentary time
| MVPA quartiles (h/week) | Sedentary time quartiles (h/day) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | 0–2.7 | 2.8–4.2 | 4.2–6.7 | ≥ 6.7 | ≥ 11.4 | 10.2–11.4 | 8.8–10.2 | 0–8.8 | |
| Number of participants, n | 341 | 85 | 85 | 85 | 86 | 86 | 85 | 85 | 85 |
| Age (years), mean (SD) | 62.8 (8.9) | 66.5 (6.5) | 61.4 (9.3) | 62.0 (10.6) | 61.4 (7.7) | 62.9 (8.7) | 64.7 (8.4) | 61.4 (9.0) | 62.2 (9.1) |
| Gender (male), n (%) | 243 (71.3%) | 65 (76.5%) | 58 (68.2%) | 60 (70.6%) | 60 (69.8%) | 68 (79.1%) | 66 (77.6%) | 61 (71.8%) | 48 (56.5%) |
| Body mass index (kg/m2), mean (SD) | 27.5 (4.6) | 28.9 (5.2) | 27.8 (4.4) | 27.3 (4.7) | 25.9 (3.5) | 28.6 (4.8) | 27.8 (4.5) | 27.3 (4.4) | 26.2 (4.4) |
| Body mass index categories (kg/m2), n (%) | |||||||||
| < 25a | 104 (30.5%) | 14 (16.5%) | 27 (31.8%) | 27 (31.8%) | 36 (41.9%) | 16 (18.6%) | 25 (29.4%) | 28 (32.9%) | 35 (41.2%) |
| 25–30 | 156 (45.7%) | 39 (45.9%) | 38 (44.7%) | 42 (49.4%) | 37 (43.0%) | 43 (50.0%) | 36 (42.4%) | 42 (49.4%) | 35 (41.2%) |
| ≥ 30 | 81 (23.8%) | 32 (37.6%) | 20 (23.5%) | 16 (18.8%) | 13 (15.1%) | 27 (31.4%) | 24 (28.2%) | 15 (17.6%) | 15 (17.6%) |
| Education, n (%) | |||||||||
| Low | 139 (40.8%) | 40 (47.1%) | 34 (40.0%) | 35 (41.2%) | 30 (34.9%) | 37 (43.0%) | 27 (31.8%) | 29 (34.1%) | 46 (54.1%) |
| Medium | 109 (32.0%) | 26 (30.6%) | 25 (29.4%) | 29 (34.1%) | 29 (33.7%) | 26 (30.2%) | 24 (28.2%) | 34 (40.0%) | 25 (29.4%) |
| High | 93 (27.3%) | 19 (22.4%) | 26 (30.6%) | 21 (24.7%) | 27 (31.4%) | 23 (26.7%) | 34 (40.0%) | 22 (25.9%) | 14 (16.5%) |
| Cigarette smoking status, n (%) | |||||||||
| Never | 129 (37.8%) | 17 (20.0%) | 40 (47.1%) | 44 (51.8%) | 28 (32.6%) | 35 (40.7%) | 30 (35.3%) | 29 (34.1%) | 35 (41.2%) |
| Former | 170 (49.9%) | 50 (58.8%) | 30 (35.3%) | 37 (43.5%) | 53 (61.6%) | 34 (39.5%) | 46 (54.1%) | 46 (54.1%) | 44 (51.8%) |
| Current | 42 (12.3%) | 18 (21.2%) | 15 (17.6%) | 4 (4.7%) | 5 (5.8%) | 17 (19.8%) | 9 (10.6%) | 10 (11.8%) | 6 (7.1%) |
| Comorbidities, n (%) | |||||||||
| 0 | 51 (15.0%) | 7 (8.2%) | 15 (17.6%) | 16 (18.8%) | 13 (15.1%) | 8 (9.3%) | 7 (8.2%) | 18 (21.2%) | 18 (21.2%) |
| 1 | 80 (23.5%) | 19 (22.4%) | 22 (25.9%) | 20 (23.5%) | 19 (22.1%) | 23 (26.7%) | 23 (27.1%) | 17 (20.0%) | 17 (20.0%) |
| ≥ 2 | 210 (61.6%) | 59 (69.4%) | 48 (56.5%) | 49 (57.6%) | 54 (62.8%) | 55 (64.0%) | 55 (64.7%) | 50 (58.8%) | 50 (58.8%) |
| Tumor stage, n (%) | |||||||||
| I | 220 (64.5%) | 49 (57.6%) | 58 (68.2%) | 62 (72.9%) | 51 (59.3%) | 57 (66.3%) | 51 (60.0%) | 53 (62.4%) | 59 (69.4%) |
| II | 53 (15.5%) | 17 (20.0%) | 11 (12.9%) | 7 (8.2%) | 18 (20.9%) | 9 (10.5%) | 17 (20.0%) | 14 (16.5%) | 13 (15.3%) |
| III | 68 (19.9%) | 19 (22.4%) | 16 (18.8%) | 16 (18.8%) | 17 (19.8%) | 20 (23.3%) | 17 (20.0%) | 18 (21.2%) | 13 (15.3%) |
| Tumor grade, n (%) | |||||||||
| 1 | 44 (14.5%) | 13 (16.5%) | 9 (12.0%) | 14 (17.9%) | 8 (11.1%) | 12 (15.0%) | 8 (10.1%) | 14 (19.2%) | 10 (13.9%) |
| 2 | 174 (57.2%) | 46 (58.2%) | 48 (64.0%) | 44 (56.4%) | 36 (50.0%) | 48 (60.0%) | 43 (54.4%) | 36 (49.3%) | 47 (65.3%) |
| 3 | 66 (21.7%) | 16 (20.3%) | 14 (18.7%) | 16 (20.5%) | 20 (27.8%) | 18 (22.5%) | 22 (27.8%) | 18 (24.7%) | 8 (11.1%) |
| 4 | 20 (6.6%) | 4 (5.1%) | 4 (5.3%) | 4 (5.1%) | 8 (11.1%) | 2 (2.5%) | 6 (7.6%) | 5 (6.8%) | 7 (9.7%) |
| Missing | 37 | 6 | 10 | 7 | 14 | 6 | 6 | 12 | 13 |
| Type of surgery, n (%) | |||||||||
| Radical nephrectomy | 197 (57.8%) | 58 (68.2%) | 46 (54.1%) | 42 (49.4%) | 51 (59.3%) | 45 (52.3%) | 58 (68.2%) | 51 (60.0%) | 43 (50.6%) |
| Partial nephrectomy | 140 (41.1%) | 24 (28.2%) | 39 (45.9%) | 43 (50.6%) | 34 (39.5%) | 38 (44.2%) | 26 (30.6%) | 34 (40.0%) | 42 (49.4%) |
| Other | 4 (1.2%) | 3 (2.6%) | 0 (0.0%) | 0 (0.0%) | 1 (2.6%) | 3 (2.6%) | 1 (1.2%) | 0 (0.0%) | 0 (0.0%) |
| Grade ≥ 2 postoperative complication, n (%) | 64 (18.8%) | 23 (27.1%) | 13 (15.3%) | 14 (16.5%) | 14 (16.3%) | 17 (19.8%) | 16 (18.8%) | 19 (22.4%) | 12 (14.1%) |
| MVPA (h/week), mean (SD) | 4.8 (2.9) | 1.8 (0.7) | 3.5 (0.5) | 5.1 (0.6) | 9.0 (2.1) | 3.4 (2.2) | 4.7 (2.7) | 5.0 (2.6) | 6.2 (3.3) |
| Sedentary time (h/day), mean (SD) | 10.1 (1.7) | 10.9 (1.8) | 10.5 (1.6) | 9.8 (1.4) | 9.3 (1.7) | 12.3 (0.8) | 10.7 (0.3) | 9.6 (0.4) | 7.9 (0.9) |
| Mobility limitation, n (%) | |||||||||
| No | 252 (73.9%) | 45 (52.9%) | 62 (72.9%) | 73 (85.9%) | 72 (83.7%) | 61 (70.9%) | 64 (75.3%) | 60 (70.6%) | 67 (78.8%) |
| Yes | 89 (25.5%) | 40 (47.1%) | 23 (27.1%) | 12 (14.1%) | 14 (16.3%) | 25 (29.1%) | 21 (24.7%) | 25 (29.4%) | 18 (21.2%) |
aIncluding 1 patient with underweight (BMI < 18.5 kg/m2)
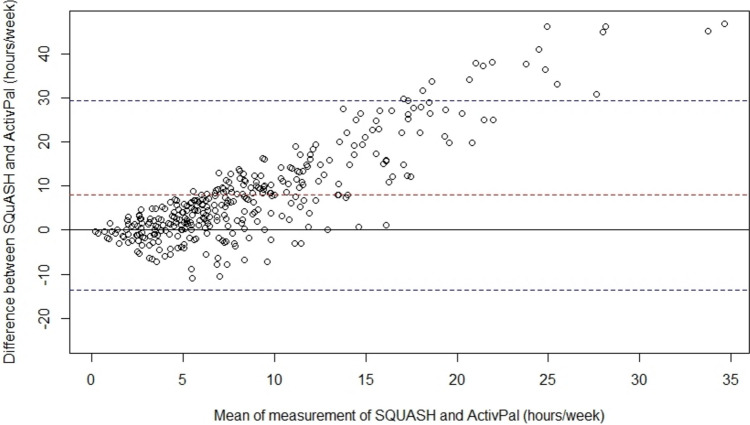
Descriptive statistics for the device-measured MVPA and sedentary time data and HRQoL measures are shown in Table 2. The device and questionnaire data were collected on average 101 (SD 29) and 99 (SD 30) days after surgery, respectively. Mean wearing time was 6 (SD 0.6) days, and mean time spent on MVPA and sedentary time was 4.8 (SD 2.9) h/week and 10.1 (SD 1.7) h/day, respectively. The mean self-reported MVPA was 12.7 (SD 11.1) h/week. Adherence to the WCRF/AICR recommendations was 77% for device-measured MVPA, while it was 88% for self-reported MVPA. The convergent validity of self-reported MVPA and device-measured MVPA was weak (Spearman’s Rho = 0.19). Bland–Altman analysis showed that self-reported MVPA assessed by SQUASH was systematically higher when compared to device-measured MVPA assessed by ActivPAL with a mean difference of 7.91 ± 10.97 h/week, and the 95% limits of agreement ranging between − 13.59 and 29.41 h/week (Fig. 1). Agreement on classifying participants as meeting the WCRF/AICR recommendations on MVPA using these methods (kappa = 0.01) was poor.
Table 2.
Descriptive statistics for ActivPal-measured physical activity, sedentary time, and health-related quality of life of patients with localized RCC
| Variable | Mean (SD) | Median (IQR) |
|---|---|---|
| Physical activity and sedentary behavior | ||
| Time between treatment and ActivPal measurement, days | 101 (29) | 94 (86, 105) |
| ActivPal valid days | 6.0 (0.6) | 6 (6, 6) |
| ActivPal valid week days | 4.4 (0.6) | 4 (4,5) |
| ActivPal valid weekend days | 1.6 (0.5) | 2 (1, 2) |
| Moderate-to-vigorous physical activity, h/week | 4.8 (2.9) | 4.2 (2.7, 6.4) |
| Sedentary time, h/day | 10.1 (1.7) | 10.2 (8.8, 11.4) |
| Health-related quality of life and fatigue | ||
| Time between treatment and questionnaire, days | 99 (30) | 91 (84, 102) |
| Global quality of life | 76.5 (17.3) | 79.2 (66.7, 83.3) |
| Physical functioning | 87.1 (14.6) | 93.3 (80.0, 100) |
| Role functioning | 80.6 (26.3) | 100 (66.7, 100) |
| Emotional functioning | 84.0 (18.9) | 75 (66.7, 91.7) |
| Cognitive functioning | 85.6 (20.1) | 100 (83.3, 100) |
| Social functioning | 83.7 (22.1) | 100 (66.7, 100) |
| QLQ C-30 summary score | 86.2 (12.0) | 89.7 (79.4, 95.5) |
| Fatigue | 26.5 (22.3) | 22.2 (11.1, 33.3) |
Fig. 1.
Bland–Altman plot for the agreement of moderate-to-vigorous physical activity of SQUASH and ActivPal. The dashed red line represents the mean bias (7.91, SD = 10.97 h/week), the dashed blue lines the upper (29.41 h/week) and lower (− 13.59 h/week) 95% limit of agreement, and the solid line perfect agreement
MVPA and HRQoL
The highest (≥ 6.7 h/week) versus the lowest quartile (≤ 2.7 h/week) of device-measured MVPA was significantly associated with better HRQoL scores for global health status (β, 10.2; 95% CI, 5.1, 15.3), physical (β, 7.7; 95% CI, 3.8, 11.6), role (β, 12.4; 95% CI, 4.7, 20.2), and social (β, 7.3; 95% CI, 0.2, 14.4) functioning, and the summary score (β, 4.6; 95% CI, 1.1, 8.1), and with lower fatigue (β, − 11.2; 95% CI, − 18.1, − 4.2) (Table 3). These differences were of small clinical relevance, except for the difference in global health status which was of medium clinical relevance [27]. Most second and third quartiles of MVPA were also associated with better scores on the same HRQoL domains, but effect estimates were smaller. Individuals adhering to the WCRF/AICR recommendations of 150 min/week MVPA compared to those not adhering had significantly better HRQoL scores for the same outcomes, except for social functioning (Table 3).
Table 3.
The association of device-measured and self-reported moderate-to-vigorous physical activity (MVPA) and device-measured sedentary time with health-related quality of life (HRQoL)
| Global health status | Physical functioning | Role functioning | Emotional functioning | Cognitive functioning | Social functioning | Summary score | Fatigue | |
|---|---|---|---|---|---|---|---|---|
| β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | |
| Device-measured MVPA in quartiles (h/week)a,b | ||||||||
| 0–2.7 | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| 2.8–4.2 | 7.7 (2.9, 12.5)* | 7.6 (3.9, 11.2)* | 8.2 (1.0, 15.5)* | 0.2 (− 5.6, 6.0) | − 0.8 (− 7.2, 5.7) | 2.6 (− 4.1, 9.3) | 2.8 (− 0.5, 6.1) | − 5.9 (− 12.4, 0.6) |
| 4.2–6.7 | 5.9 (0.8, 11.0)* | 5.8 (1.9, 9.6)* | 9.3 (1.6, 17.0)* | 0.2 (− 5.9, 6.3) | − 0.6 (− 7.5, 6.2) | 1.9 (− 5.1, 8.9) | 3.7 (0.2, 7.1)* | − 7.8 (− 14.6, − 0.9)* |
| ≥ 6.7 | 10.2 (5.1, 15.3)* | 7.7 (3.8, 11.6)* | 12.4 (4.7, 20.2)* | 1.7 (− 4.5, 7.9) | 0.6 (− 6.3, 7.5) | 7.3 (0.2, 14.4)* | 4.6 (1.1, 8.1)* | − 11.2 (− 18.1, − 4.2)* |
| p-trend | 0.001 | 0.001 | 0.003 | 0.60 | 0.83 | 0.06 | 0.01 | 0.002 |
| Device-measured MVPA continuous (h/week)a,b | ||||||||
| 1 unit increase | 1.1 (0.4, 1.7)* | 0.7 (0.2, 1.2)* | 1.3 (0.3, 2.2)* | 0.2 (− 0.5, 1.0) | − 0.1 (− 0.9, 0.8) | 0.8 (− 0.1, 1.6) | 0.5 (0.0, 0.9)* | − 1.3 (− 2.2, − 0.5)* |
| Device-measured MVPA in categories of WCRF/AICR recommendations (min/week)a,b,c | ||||||||
| < 150 | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| ≥ 150 | 7.9 (3.6, 12.3)* | 8.0 (4.7 (11.3)* | 11.0 (4.5, 17.5)* | 0.5 (− 4.8, 5.7) | − 0.0 (− 5.8, 5.8) | 3.9 (− 2.1, 9.9) | 3.6 (0.6, 6.5)* | − 7.5 (− 13.4, − 1.6)* |
| Self-reported MVPA in quartiles (h/week)a | ||||||||
| 0–4.8 | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| 5.0–9.5 | 2.5 (− 2.1, 7.1) | 5.5 (2.0, 9.0)* | 7.8 (0.9, 14.7)* | 0.4 (− 5.1, 5.8) | 0.4 (− 5.7, 6.5) | 3.3 (− 3.1, 9.6) | 1.3 (− 1.8, 4.5) | − 5.8 (− 12.0, 0.3) |
| 9.5–16.8 | 6.6 (1.8, 11.3)* | 7.6 (4.0, 11.2)* | 9.0 (1.9, 16.1)* | 0.5 (− 5.1, 6.2) | 0.7 (− 5.6, 7.0) | 3.6 (− 2.9, 10.1) | 2.5 (− 0.7, 5.7) | − 6.2 (− 12.6, 0.1) |
| ≥ 17.0 | 5.2 (0.5, 10.0)* | 8.4 (4.8, 11.9)* | 9.4 (2.4, 16.5)* | − 1.8 (− 7.4, 3.8) | 1.6 (− 4.7, 7.8) | 4.9 (− 1.5, 11.4) | 2.3 (− 0.9, 5.5) | − 5.8 (− 12.2, 0.5) |
| p-trend | 0.01 | < 0.001 | 0.01 | 0.55 | 0.61 | 0.15 | 0.12 | 0.09 |
| Self-reported MVPA continuous (h/week)a | ||||||||
| 1 unit increase | 0.1 (− 0.0, 0.3) | 0.2 (0.1, 0.3)* | 0.3 (0.1, 0.5)* | − 0.1 (− 0.3, 0.1) | 0.0 (− 0.2, 0.2) | 0.1 (− 0.1, 0.3) | 0.1 (− 0.0, 0.2) | − 0.1 (− 0.3, 0.1) |
| Self-reported MVPA in categories of WCRF/AICR recommendations (min/week)a,d | ||||||||
| < 150 | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| ≥ 150 | 2.8 (− 2.4, 8.0) | 4.4 (0.4, 8.3)* | 4.9 (− 2.9, 12.6) | − 2.3 (− 8.4, 3.8) | − 1.2 (− 8.0, 5.6) | − 1.4 (− 8.5, 5.6) | 0.2 (− 3.3, 3.7) | − 2.3 (− 9.3, 4.6) |
| Device-measured sedentary time in quartiles (h/day)a,e | ||||||||
| ≥ 11.4 | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| 10.2–11.4 | − 1.4 (− 6.1, 3.3) | 0.2 (− 3.4, 3.7) | − 6.1 (− 13.1, 0.9) | − 4.0 (− 9.6, 1.6) | − 5.7 (− 11.9, 0.5) | − 5.8 (− 12.3, 0.6) | − 1.9 (− 5.1, 1.3) | 4.4 (− 1.9, 10.6) |
| 8.8–10.2 | 0.8 (− 3.9, 5.6) | 3.8 (0.2, 7.4)* | 0.7 (− 6.4, 7.8) | − 1.7 (− 7.4, 3.9) | − 3.0 (− 9.3, 3.3) | − 0.1 (− 6.5, 6.4) | 1.4 (− 1.9, 4.6) | − 0.5 (− 6.8, 5.8) |
| 0–8.8 | 1.3 (− 3.7, 6.3) | 4.6 (0.8, 8.5)* | − 2.3 (− 9.8, 5.2) | − 4.9 (− 10.9, 1.1) | − 3.8 (− 10.5, 2.9) | − 2.2 (− 9.0, 4.7) | − 0.9 (− 4.3, 2.6) | 1.3 (− 5.4, 8.0) |
| p-trend | 0.46 | 0.005 | 1.00 | 0.20 | 0.40 | 0.94 | 0.89 | 0.93 |
| Device-measured sedentary time continuous (h/day)a,e | ||||||||
| 1 unit decrease | 0.5 (− 0.6, 1.5) | 1.2 (0.4, 2.0)* | 0.5 (− 1.1, 2.1) | 0.7 (− 1.9, 0.6) | − 0.3 (− 1.7, 1.0) | 0.2 (− 1.2, 1.7) | 0.2 (− 0.5, 0.9) | − 0.2 (− 1.6, 1.2) |
*p < 0.05
aModel is adjusted for age (years), sex (male, female), BMI (kg/m2), comorbidities (0, 1, ≥ 2), tumor stage (I, II, III), smoking status (never, former, current), education level (low, medium, high), and mobility limitation (yes, no)
bModel for device-measured MVPA is also adjusted for sedentary time (h/day)
c0–150 (n = 77), ≥ 150 (n = 264)
d0–150 (n = 40); ≥ 150 (n = 300)
eModel for sedentary time is also adjusted for MVPA (h/week)
For the highest (≥ 17.0 h/week) versus the lowest quartile (≤ 2.7 h/week) of self-reported MVPA, similar associations were found for global health status (β, 5.2; 95% CI, 0.5, 10.0), physical (β, 8.4; 95% CI, 4.8, 11.9), and role (β 9.4; 95% CI, 2.4, 16.5) functioning. Associations for social functioning, the summary score, and fatigue were in the same direction but not statistically significant (Table 3).
Sedentary time and HRQoL
The lowest (≤ 8.8 h/day; β, 4.6; 95% CI, 0.8, 8.5) and second (8.8–10.2 h/day; β, 3.8; 95% CI, 0.2, 7.4) versus the highest quartile (≥ 11.5 h/day) of sedentary time was associated with better physical functioning (Table 3). Differences were below the 5-point threshold for a small clinically relevant difference. No significant associations between sedentary time and the other HRQoL domains were observed.
Discussion
In this cross-sectional study, we investigated the association between device-measured MVPA and sedentary time and self-reported MVPA with HRQoL at 3 months after surgery in patients with localized RCC. Higher device-measured MVPA and meeting the WCRF/AICR physical activity recommendations were significantly associated with better global quality of life, physical, role, and social functioning, and the summary score, and with less fatigue. Associations for self-reported MVPA were in the same direction but weaker. Lower sedentary time was significantly associated with better physical functioning but not with other domains of HRQoL.
We found that higher device-measured MVPA was associated with higher scores on several HRQoL domains including less fatigue. Patients with localized RCC are an understudied population with respect to MVPA and sedentary time in relation to HRQoL including fatigue. Although primary treatment mainly involves surgery, patients’ HRQoL can still be affected by side effects [4]. Indeed, HRQoL levels in our study were lower and fatigue levels were higher compared to published values of a Dutch normative population [28]. The results of studies conducted on other cancer survivors were mixed. For example, some studies in patients with breast, prostate, or lung cancer showed no clear associations of device-measured MVPA with HRQoL or fatigue [29–31] while other studies in patients with breast and colorectal cancer showed similar associations as in our study [32–35]. These mixed results may be explained by differences in patient characteristics, cancer type, stage, and (timing after) treatment.
We showed that lower device-measured sedentary time was only associated with higher physical functioning and not with other HRQoL outcomes or fatigue. A systematic review on sedentary time and health outcomes among cancer survivors including ten studies with data on device-measured sedentary time and HRQoL or fatigue did not support a clear association either [7]. Only four cross-sectional studies among patients with breast [29], colorectal [36], lung cancer [31], or mixed cancers [37] found an association of sedentary time with HRQoL [31], physical functioning [31, 36], or fatigue [29, 31, 36]. These primarily null observations have been suggested to be due to the high HRQoL and the low fatigue levels in the included study populations [7]. However, three more recent studies among breast [34, 35] and colorectal survivors [38] did observe an association of higher sedentary time with lower HRQoL and/or higher fatigue outcomes.
The convergent validity between device-measured and self-reported MVPA in our study was low and the bias was high, as also described in previous studies comparing device-measured and self-reported physical activity in adults [11, 13, 39]. This can be explained by the fact that both measures capture different aspects of physical activity as well as by recall and social desirability bias inherent to self-report [11, 13]. Despite this low correlation, we found that associations with HRQoL were in the same direction but slightly stronger for device-measured compared to self-reported MVPA, underscoring the robustness of our findings. Interestingly, a study among 1348 survivors of breast, prostate, and colorectal cancer also found similar associations for device-measured and self-reported MVPA with fatigue while only self-reported MVPA was found to be associated with HRQoL [40].
Our results for self-reported MVPA are consistent with findings from one previous cross-sectional study among 463 Canadian patients with RCC reporting an association between self-reported physical activity and HRQoL at 6 years after diagnosis [10]. In contrast to our study, only 26% of patients reported to be moderately to vigorously active for at least 150 min/week. Numerous cross-sectional studies in other cancer populations also reported associations between higher self-reported MVPA with better HRQoL outcomes [41–43].
According to the guidelines for the interpretation of the EORTC QLQ-C30 effect estimates [27], the mean differences for HRQoL outcomes between the highest vs. lowest quartiles of device-measured and self-reported MVPA were of small clinical relevance, which is considered subtle but nevertheless clinically relevant [27]. A medium clinically relevant difference, considered likely to be clinically relevant [27], was only found for device-measured MVPA and global quality of life. These results are in line with an observational study among patients with breast cancer showing small effect sizes for device-measured MVPA and HRQoL and fatigue 3 months after surgery before the start of adjuvant therapy [35].
Although our results indicate that more than three quarters of our study population adhered to the WCRF/AICR recommendations for cancer prevention to engage in at least 150 min/week of MVPA, there is still room for improvement. It is currently unclear whether the relation of MVPA with HRQoL and fatigue is causal as patients may also have less MVPA because of a lower HRQoL or more fatigue (i.e. reverse causality). Thus, based on our results we can only suggest that increasing MVPA might be an intervention target to improve HRQoL and reduce fatigue in patients with localized RCC after surgery. Future longitudinal studies and intervention studies should be conducted to obtain better insight into the causality of our observed associations. Until more evidence becomes available, following the WCRF/AICR recommendations to engage in at least 150 min/week of MVPA and to minimize the sedentary time is advisable, also because of the potential beneficial effects on other health outcomes.
This study had several strengths. To the best of our knowledge, this is the first study to investigate the association between device-measured MVPA and sedentary time in patients with localized RCC. The validated activPAL3 monitors, which are a highly accurate and valid tool for measuring sedentary, standing, and stepping time [16], were worn for 24 h/day for an average of 6 days, providing an accurate reflection of MVPA and sedentary time during the post-surgery recovery phase. Also, extensive data on potential confounders, such as BMI, smoking, and mobility limitation, were collected and taken into account in the analyses.
There are also limitations to consider. First, our cross-sectional study design limits the ability to draw conclusions regarding causality, and we need to be aware that findings may also be explained by reverse causality. Second, the use of activPAL3 accelerometers to calculate MVPA does not account for physical activity other than stepping movements. As a result, not all movements (e.g., upper body movements; push and pull exercises which are frequently performed during fitness) were captured in the total time spent in MVPA. Future studies might benefit from combining thigh-worn accelerometers with chest-mounted tri-axial accelerometers measuring free weight exercises [44]. Third, the activPAL3 monitor does not provide information on the context within which physical activity and sedentary behavior are occurring. Different domains of physical activity (e.g. commuting, recreational, sports) [45] but possibly also sedentary behavior (e.g. office work, watching television) may be differently related to HRQoL, and accelerometers cannot take this context into account. Fourth, the response rate in our study was 44% and we cannot rule out that healthier patients or those with a healthier lifestyle were more likely to participate, also because activity levels in our population were relatively high. This may affect the generalizability of our findings. However, except for being slightly more likely to be female, participants of this study were comparable to invited non-participants with respect to age, tumor stage, tumor grade, morphology, and type of treatment while other (lifestyle-related) characteristics of non-participants were not available [15]. Lastly, residual confounding by unmeasured factors cannot be excluded.
In conclusion, both higher device-measured and self-reported MVPA were independently associated with significantly better HRQoL outcomes including less fatigue among patients with localized RCC, with differences being of small clinical relevance. Lower sedentary time was only associated with better physical functioning. Our findings add to the current evidence that advice on increasing MVPA may also be effective for patients with localized RCC to improve HRQoL including fatigue outcomes while decreasing sedentary time seems less relevant. Future longitudinal studies and intervention studies should provide insight into whether the observed associations are causal. This information can contribute to the development of targeted MVPA guidelines and interventions to improve health outcomes in this patient group.
Acknowledgements
We are grateful to all the patients who participate in this study and we thank the following hospitals for their involvement in recruitment for the ReLife study: Amphia Ziekenhuis, Breda/Oosterhout (D.K.E. van der Schoot); Ziekenhuis Bernhoven, Uden (A.Q.H.J. Niemer); Canisius-Wilhelmina Ziekenhuis, Nijmegen (D.M. Somford); Catharina Ziekenhuis, Eindhoven (W.A. Scheepens); Elisabeth-TweeSteden Ziekenhuis, Tilburg/Waalwijk (P.J.M. Kil and B.P. Wijsman); Elkerliek Ziekenhuis, Helmond (P.J. van Hest); Gelre Ziekenhuizen, Apeldoorn/Zutphen (D.M. Bochove-Overgaauw); Jeroen Bosch Ziekenhuis, ‘s-Hertogenbosch (S. van der Meer); Maasziekenhuis Pantein, Boxmeer (E. van Boven); Maxima Medisch Centrum, Veldhoven/Eindhoven (L.M.C.L. Fossion and K. de Laet); Meander Medisch Centrum, Amersfoort (F.S. van Rey); Radboudumc, Nijmegen; Rijnstate, Arnhem/Velp/Zevenaar (G.A.H.J. Smits); Slingeland Ziekenhuis, Doetinchem (A.D.H. Geboers); St Jansdal Ziekenhuis, Harderwijk (W.J. Kniestedt); UMC Utrecht (R.P. Meijer); Ziekenhuis Gelderse Vallei, Ede (M.D.H. Kortleve) and Ziekenhuisgroep Twente, Almelo/Hengelo (S. Stomps). In addition, we thank Ms. Ivy Beeren, Ms. Monique Eijgenberger, Ms. Jolanda van Haren and Ms. Ursula Oldenhof for their assistance in data collection. We also thank the data managers of the Netherlands Cancer Registry held by the Netherlands Comprehensive Cancer Organisation (IKNL) for inviting patients and collecting the clinical data.
Author contributions
A.V.: Conceptualization, data curation, formal analysis, funding acquisition, methodology, project administration, software, supervision, writing-original draft, and writing-review and editing.
J.S.F.M.: Investigation, writing-review and editing.
J.G.: Data curation, formal analysis, methodology, software, writing-original draft, writing-review and editing.
L.M.B.: Writing-review and editing.
K.K.H.A.: Investigation, resources, writing-review and editing.
J.P.M.S.: Resources, writing-review and editing.
E.A.B.: Supervision, writing-review and editing.
L.A.L.M. K.: Writing-review and editing.
Funding
This project is funded by the Dutch Cancer Society (KUN 2015–7948). Sponsors were not involved in the study design; the collection, analysis, and interpretation of data; or the publications that will result from this study.
Data availability
The data that support the findings of this study are available upon request from the corresponding author.
Declarations
Ethics approval and consent to participate
This study involves human participants and was approved by the Committee for Human Research region Arnhem-Nijmegen (CMO 2016–3078). The study was in line with the principles of the Declaration of Helsinki. Participants gave informed consent to participate in the study before taking part.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bukavina L, Bensalah K, Bray F, Carlo M, Challacombe B, Karam JA et al (2022) Epidemiology of renal cell carcinoma: 2022 update. Eur Urol 82(5):529–542. 10.1016/j.eururo.2022.08.019 [DOI] [PubMed] [Google Scholar]
- 2.Ljungberg B, Albiges L, Abu-Ghanem Y, Bedke J, Capitanio U, Dabestani S et al (2022) European Association of Urology guidelines on renal cell carcinoma: the 2022 update. Eur Urol 82(4):399–410. 10.1016/j.eururo.2022.03.006 [DOI] [PubMed] [Google Scholar]
- 3.Althaus AB, Chang P, Mao J, Olugbade K, Taylor K, Dewey L et al (2020) Patient-reported quality of life and convalescence after minimally invasive kidney cancer surgery. Urology 144:123–129. 10.1016/j.urology.2020.06.020 [DOI] [PubMed] [Google Scholar]
- 4.Harding G, Cella D, Robinson D Jr, Mahadevia PJ, Clark J, Revicki DA (2007) Symptom burden among patients with renal cell carcinoma (RCC): content for a symptom index. Health Qual Life Outcomes 5:34. 10.1186/1477-7525-5-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parker PA, Swartz R, Fellman B, Urbauer D, Li Y, Pisters LL et al (2012) Comprehensive assessment of quality of life and psychosocial adjustment in patients with renal tumors undergoing open, laparoscopic and nephron sparing surgery. J Urol 187(3):822–826. 10.1016/j.juro.2011.10.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell KL, Winters-Stone KM, Wiskemann J, May AM, Schwartz AL, Courneya KS et al (2019) Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc 51(11):2375–2390. 10.1249/MSS.0000000000002116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swain CTV, Nguyen NH, Eagles T, Vallance JK, Boyle T, Lahart IM, Lynch BM (2020) Postdiagnosis sedentary behavior and health outcomes in cancer survivors: a systematic review and meta-analysis. Cancer 126(4):861–869. 10.1002/cncr.32578 [DOI] [PubMed] [Google Scholar]
- 8.World Cancer Research Fund/American Institute for Cancer Research. Continuous update project expert report 2018. Diet, nutrition, physical activity and kidney cancer. Available at dietandcancerreport.org.
- 9.Bull FC, Al-Ansari SS, Biddle S, Borodulin K, Buman MP, Cardon G et al (2020) World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med 54(24):1451–1462. 10.1136/bjsports-2020-102955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trinh L, Plotnikoff RC, Rhodes RE, North S, Courneya KS (2011) Associations between physical activity and quality of life in a population-based sample of kidney cancer survivors. Cancer Epidemiol Biomarkers Prev 20(5):859–868. 10.1158/1055-9965.EPI-10-1319 [DOI] [PubMed] [Google Scholar]
- 11.Prince SA, Adamo KB, Hamel ME, Hardt J, Connor Gorber S, Tremblay M (2008) A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act 5:56. 10.1186/1479-5868-5-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chastin SFM, Dontje ML, Skelton DA, Cukic I, Shaw RJ, Gill JMR et al (2018) Systematic comparative validation of self-report measures of sedentary time against an objective measure of postural sitting (activPAL). Int J Behav Nutr Phys Act 15(1):21. 10.1186/s12966-018-0652-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colley RC, Butler G, Garriguet D, Prince SA, Roberts KC (2018) Comparison of self-reported and accelerometer-measured physical activity in Canadian adults. Health Rep 29(12):3–15 [PubMed] [Google Scholar]
- 14.Prince SA, Cardilli L, Reed JL, Saunders TJ, Kite C, Douillette K et al (2020) A comparison of self-reported and device measured sedentary behaviour in adults: a systematic review and meta-analysis. Int J Behav Nutr Phys Act 17(1):31. 10.1186/s12966-020-00938-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maurits JSF, Sedelaar JPM, Aben KKH, Kampman E, ReLife study group, Kiemeney L, Vrieling A (2023) Cohort profile - the renal cell cancer: lifestyle, prognosis and quality of life (ReLife) study in the Netherlands. BMJ Open 13(3):e066909. 10.1136/bmjopen-2022-066909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bourke AK, Ihlen EAF, Helbostad JL (2019) Validation of the activPAL3 in free-living and laboratory scenarios for the measurement of physical activity, stepping, and transitions in older adults. J Meas Phys Behav 2(2):58–65. 10.1123/jmpb.2018-0056 [Google Scholar]
- 17.van der Berg JD, Willems PJ, van der Velde JH, Savelberg HH, Schaper NC, Schram MT et al (2016) Identifying waking time in 24-h accelerometry data in adults using an automated algorithm. J Sports Sci 34(19):1867–1873. 10.1080/02640414.2016.1140908 [DOI] [PubMed] [Google Scholar]
- 18.Tudor-Locke C, Mora-Gonzalez J, Ducharme SW, Aguiar EJ, Schuna JM Jr, Barreira TV, Moore CC et al (2021) Walking cadence (steps/min) and intensity in 61–85-year-old adults: the CADENCE-adults study. Int J Behav Nutr Phys Act 18(1):129. 10.1186/s12966-021-01199-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wendel-Vos GC, Schuit AJ, Saris WH, Kromhout D (2003) Reproducibility and relative validity of the short questionnaire to assess health-enhancing physical activity. J Clin Epidemiol 56(12):1163–1169 [DOI] [PubMed] [Google Scholar]
- 20.Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR Jr, Tudor-Locke C et al (2011) 2011 compendium of physical activities: a second update of codes and MET values. Med Sci Sports Exerc 43(8):1575–1581. 10.1249/MSS.0b013e31821ece12 [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization (2000) Obesity: preventing and managing the global epidemic. Report of a WHO consultation. Tech Rep Ser 894:i-xii, 1–253 [PubMed]
- 22.Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN (2003) The self-administered comorbidity questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum 49(2):156–163. 10.1002/art.10993 [DOI] [PubMed] [Google Scholar]
- 23.Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205–213. 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ et al (1993) The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85(5):365–376 [DOI] [PubMed] [Google Scholar]
- 25.Giesinger JM, Kieffer JM, Fayers PM, Groenvold M, Petersen MA, Scott NW et al (2016) Replication and validation of higher order models demonstrated that a summary score for the EORTC QLQ-C30 is robust. J Clin Epidemiol 69:79–88. 10.1016/j.jclinepi.2015.08.007 [DOI] [PubMed] [Google Scholar]
- 26.Sweegers MG, Boyle T, Vallance JK, Chinapaw MJ, Brug J, Aaronson NK et al (2019) Which cancer survivors are at risk for a physically inactive and sedentary lifestyle? Results from pooled accelerometer data of 1447 cancer survivors. Int J Behav Nutr Phys Act 16(1):66. 10.1186/s12966-019-0820-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cocks K, King MT, Velikova G, Martyn St-James M, Fayers PM, Brown JM (2011) Evidence-based guidelines for determination of sample size and interpretation of the European Organisation for the Research and Treatment of Cancer quality of life questionnaire core 30. J Clin Oncol 29(1):89–96. 10.1200/JCO.2010.28.0107 [DOI] [PubMed] [Google Scholar]
- 28.van de Poll-Franse LV, Mols F, Gundy CM, Creutzberg CL, Nout RA, Verdonck-de Leeuw IM et al (2011) Normative data for the EORTC QLQ-C30 and EORTC-sexuality items in the general Dutch population. Eur J Cancer 47(5):667–675. 10.1016/j.ejca.2010.11.004 [DOI] [PubMed] [Google Scholar]
- 29.Trinh L, Amireault S, Lacombe J, Sabiston CM (2015) Physical and psychological health among breast cancer survivors: interactions with sedentary behavior and physical activity. Psychooncology 24(10):1279–1285. 10.1002/pon.3872 [DOI] [PubMed] [Google Scholar]
- 30.Gaskin CJ, Craike M, Mohebbi M, Salmon J, Courneya KS, Broadbent S, Livingston PM (2016) Associations of objectively measured moderate-to-vigorous physical activity and sedentary behavior with quality of life and psychological well-being in prostate cancer survivors. Cancer causes control 27(9):1093–1103. 10.1007/s10552-016-0787-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.D’Silva A, Gardiner PA, Boyle T, Bebb DG, Johnson ST, Vallance JK (2018) Associations of objectively assessed physical activity and sedentary time with health-related quality of life among lung cancer survivors: a quantile regression approach. Lung Cancer 119:78–84. 10.1016/j.lungcan.2018.03.010 [DOI] [PubMed] [Google Scholar]
- 32.Vallance JK, Boyle T, Courneya KS, Lynch BM (2014) Associations of objectively assessed physical activity and sedentary time with health-related quality of life among colon cancer survivors. Cancer 120(18):2919–2926. 10.1002/cncr.28779 [DOI] [PubMed] [Google Scholar]
- 33.Phillips SM, Awick EA, Conroy DE, Pellegrini CA, Mailey EL, McAuley E (2015) Objectively measured physical activity and sedentary behavior and quality of life indicators in survivors of breast cancer. Cancer 121(22):4044–4052. 10.1002/cncr.29620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nurnazahiah A, Shahril MR, Nor Syamimi Z, Ahmad A, Sulaiman S, Lua PL (2020) Relationship of objectively measured physical activity and sedentary behaviour with health-related quality of life among breast cancer survivors. Health Qual Life Outcomes 18(1):222. 10.1186/s12955-020-01478-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vallance JK, Friedenreich CM, Wang Q, Matthews CE, Yang L, McNeely ML et al (2023) Associations of device-measured physical activity and sedentary time with quality of life and fatigue in newly diagnosed breast cancer patients: baseline results from the AMBER cohort study. Cancer 129(2):296–306. 10.1002/cncr.34531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Roekel EH, Winkler EA, Bours MJ, Lynch BM, Willems PJ, Meijer K et al (2016) Associations of sedentary time and patterns of sedentary time accumulation with health-related quality of life in colorectal cancer survivors. Prev Med Rep 4:262–269. 10.1016/j.pmedr.2016.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.George SM, Alfano CM, Groves J, Karabulut Z, Haman KL, Murphy BA, Matthews CE (2014) Objectively measured sedentary time is related to quality of life among cancer survivors. PLoS one 9(2):e87937. 10.1371/journal.pone.0087937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kenkhuis MF, VAN R EH, Breedveld-Peters JJL, Breukink SO, Janssen-Heijnen MLG, Keulen ETP et al (2021) Longitudinal associations of sedentary behavior and physical activity with quality of life in colorectal cancer survivors. Med Sci Sports Exerc 53(11):2298–308. 10.1249/MSS.0000000000002703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Terpstra SES, Hoogervorst LA, van der Velde J, Mutsert R, van de Stadt LA, Rosendaal FR, Kloppenburg M (2024) Validation of the SQUASH physical activity questionnaire using accelerometry: the NEO study. Osteoarthr Cartil Open 6(2):100462. 10.1016/j.ocarto.2024.100462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lally P, Miller NE, Lawrence C, Beeken RJ, Fisher A (2023) Associations of self-reported and device-assessed physical activity with fatigue, quality of life, and sleep quality in adults living with and beyond cancer. J Sport Health Sci 12(6):664–673. 10.1016/j.jshs.2023.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Babatunde OA, Adams SA, Orekoya O, Basen-Engquist K, Steck SE (2016) Effect of physical activity on quality of life as perceived by endometrial cancer survivors: a systematic review. Int J Gynecol Cancer 26(9):1727–1740. 10.1097/igc.0000000000000821 [DOI] [PubMed] [Google Scholar]
- 42.Teba PP, Esther MG, Raquel SG (2022) Association between physical activity and patient-reported outcome measures in patients with lung cancer: a systematic review and meta-analysis. Qual Life Res 31(7):1963–1976. 10.1007/s11136-021-03053-3 [DOI] [PubMed] [Google Scholar]
- 43.Matias M, Baciarello G, Neji M, Di Meglio A, Michiels S, Partridge AH et al (2019) Fatigue and physical activity in cancer survivors: a cross-sectional population-based study. Cancer Med 8(5):2535–2544. 10.1002/cam4.2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hussain A, Zafar K, Baig AR, Almakki R, AlSuwaidan L, Khan S (2022) Sensor-based gym physical exercise recognition: data acquisition and experiments. Sensors (Basel) 22(7): 2489. 10.3390/s22072489 [DOI] [PMC free article] [PubMed]
- 45.Ribeiro FE, Tebar WR, Vanderlei LCM, Fregonesi C, Caldeira DT, Tosello G et al (2021) Physical activity domains are differently related with quality of life in breast cancer survivors: a cross-sectional study. Menopause 28(11):1233–1238. 10.1097/GME.0000000000001837 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available upon request from the corresponding author.