Abstract
Introduction
Recent studies have suggested that ambulatory management is feasible for acute uncomplicated diverticulitis (AUD); however, there is still no consensus regarding the most appropriate management settings. This study presents a multi-centre experience of managing patients presenting with AUD, specifically focusing on clinical outcomes and comparing ambulatory treatment with in-patient management.
Methods
A retrospective multi-centre study was conducted across four hospitals in the UK and included all adult patients with computed tomography (CT) confirmed (Hinchey grade 1a) acute diverticulitis over a 12-month period (January – December 2022). Patient medical records were followed up for 1-year post-index episode, and outcomes were compared between those treated through the ambulatory pathway versus inpatient treatment using 1:1 propensity score matching (PSM). All statistical analysis was performed using the R Foundation for Statistical Computing, version 4.4.
Results
A total of 348 patients with Hinchey 1a acute diverticulitis were included (260 in-patients; 88 ambulatory pathway), of which nearly a third (31.3%) had a recurrent disease. Inpatient management was dominant (74.7%), with a median of 3 days of hospital stay. PSM resulted in 172 patients equally divided between the two care settings. Ambulatory management was associated with a lower readmission rate (P = 0.02 before PSM, P = 0.08 after PSM), comparable surgical (P = 0.57 before PSM, 0% in both groups after PSM) and radiological interventions (P = 0.99 before and after PSM) within one year. In both matched and non-matched groups, a strong association between readmissions and inpatient management was noted in univariate analysis (P = 0.03 before PSM, P = 0.04 after PSM) and multivariate analysis (P = 0.02 before PSM, P = 0.03 after PSM).
Conclusion
Our study supports the safety and efficacy of managing patients with AUD through a well-designed ambulatory care pathway. In particular, hospital re-admission rates are lower and other outcomes are non-inferior to in-patient treatment. This has implications for substantial cost-savings and better utilisation of limited healthcare resources.
Supplementary information
The online version contains supplementary material available at 10.1007/s00384-024-04759-9.
Keywords: Uncomplicated diverticulitis, Hinchey 1a, Ambulatory management
Introduction
Diverticular disease is common, with an estimated prevalence of up to 85% in patients aged 50 years and above and a 4% lifetime risk of developing acute diverticulitis [1, 2]. The increasing occurrence within Western populations incurs a significant burden on patient quality of life and the economy worldwide. In the United States, the disease is thought to adversely impact the economy to an estimated two billion dollars per annum in direct and indirect costs [3]. In the United Kingdom, studies have reported up to 5% consumption of annual general surgical budgets being utilised for investigations and management of this condition [4].
A significant contribution to cost and quality of life is the in-patient management of diverticular disease. This remains a popular choice amongst clinicians despite 80% of patients presenting with uncomplicated diverticulitis [5]. Traditionally, hospitalisation offers bed rest, analgesia, replacement of electrolytes, and treatment with intravenous fluid and antibacterials.
Recent prospective trials have suggested that ambulatory management is non-inferior to in-patient admission and treatment of uncomplicated diverticulitis with regard to re-admission and recurrence rates, with substantial cost savings for healthcare providers (approximately three times lower) [6]. In such cases (Hinchey grade 1a), the adoption of a no-antibiotic strategy provides a shorter duration of treatment time and a lower disease recurrence rate. Moreover, no difference was observed in short-term morbidity and mortality and the need for elective or emergency resections between the in-patient and ambulatory cohorts [5, 7]. Additionally, the rate of treatment failure in the outpatient setting appears not to be influenced by previous episodes of acute diverticulitis, the type of antibiotic treatment prescribed or the presence of co-morbidities [8].
The present literature and reviews have some methodological limitations, with low-quality studies and few prospective, randomised trials included. There remains no consensus on the in-patient versus ambulatory management of acute uncomplicated diverticulitis (AUD) [9].
This study aims to report primary and secondary outcomes of four hospitals’ experiences in managing AUD in an ambulatory setting compared with in-patient treatment. To our knowledge, this is the first study to explore ambulatory treatment for AUD at a multicentre level in the UK.
Methods
Study design and setting
This was a retrospective multicentre study conducted across four hospitals in the United Kingdom (Queen’s Hospital Burton, Sandwell General Hospital, Peterborough City Hospital, and Royal Shrewsbury Hospital). The study was approved by the local audit department of each participating hospital and was conducted and reported in accordance with the Declaration of Helsinki and STROBE recommendations, respectively (Appendix 1). All patients presenting with computed tomography (CT) confirmed acute diverticulitis from 01/01/2022 till 31/12/2022 were considered against the study inclusion and exclusion criteria. All included patients had a follow-up period of at least 12 months.
Patient selection criteria
Eligible participants were identified through each hospital’s electronic medical records. A modified Hinchey classification system was used to grade the severity of diverticulitis [10]. Our inclusion criteria were adult patients (aged ≥ 18 years) presenting with abdominal pain and a CT scan showing colonic Hinchey Ia acute diverticulitis. Hinchey Ia was defined as confined/localised pericolic inflammation (phlegmon).
Patients with diverticulitis in the gastrointestinal tract other than the colon, cases of complicated diverticulitis (Hinchey grade Ib: confined pericolic abscess, grade II: pelvic, intraabdominal, or retroperitoneal abscess; grade III: generalised purulent peritonitis; and grade IV: faecal peritonitis) and patients diagnosed clinically in the absence of radiological confirmation (no CT scan) were excluded. Moreover, patients with incomplete datasets or equivocal CT findings were also excluded.
Data collection
Data were extracted from all eligible patients’ electronic and (where necessary) archived medical case notes and stored on an encrypted, password-protected computer. Data included demographics (age, gender, comorbidities (including previous episodes of diverticulitis), and steroid use), vital signs scores (temperature (°C)), respiratory rate, blood pressure (mmHg), and levels of consciousness assessed using the Glasgow Coma Scale (GCS), inflammatory markers on presentation (White Blood Cell Count (WCC, x 10*9/L) and C-Reactive Protein (CRP, mg/L)), antibiotic prescription, treatment setting (ambulatory vs. inpatient), length of hospital stay (LOS) and follow-up colonic investigation(s).
Additionally, all patients were followed up for 12 months after the index AUD presentation, and any surgical or radiological intervention(s) were recorded. In this study, ambulatory care was defined as < 24-hour hospital stay. Patients staying in the hospital for ≥ 24 h were categorised as in-patients.
Outcomes
Our primary outcome of interest was hospital admission with acute diverticulitis within 12 months of the index presentation. This was defined as patients presenting with symptoms typical of acute diverticulitis necessitating admission to an in-patient bed for further observation and treatment. These included either patients presenting with new symptoms (recurrent disease/flare-up) or treatment failure of the initial episode.
Our analysed secondary outcomes were as follows: any surgical or radiological intervention for diverticulitis during the follow-up period, LOS (in days), post-recovery colonic investigation(s) (colonoscopy, flexible sigmoidoscopy, CT colonography), and a diagnosis of colorectal cancer (CRC) within the first year.
Statistical analysis
The overall unmatched and matched data were analysed. Two propensity score-matched (PSM) groups of near-equal size for ambulatory versus inpatient management of Hinchey 1a diverticulitis were formed. PSM was performed using one-on-one near-neighbour matching with a calliper of 0.1. The variables used for matching were age, gender, comorbidities (previous diverticulitis diagnosis, diabetes mellitus, and steroid usage), inflammatory markers on presentation (WCC and CRP), and antibiotic treatment. A standardised mean difference (SMD) of less than 0.1 was deemed negligible, indicating appropriate matching (Appendix 2).
Data are summarised using median and interquartile range (IQR) for continuous variables and number and percentage for categorical data. Variables are compared between ambulatory-care and inpatient-care groups using the Mann-Whitney U tests for continuous variables and Fisher’s exact analysis for categorical variables. Odds ratio (OR) and 95% confidence intervals (95% CI) were calculated for patient factors and outcomes associated with the ambulatory care of Hinchey 1a diverticulitis, using both univariate and multivariate binomial logistic regression (adjusting for age, gender and comorbidities). P-values of < 0.05 were considered statistically significant. Statistical analysis was performed using R version 4.4 (R Foundation for Statistical Computing, Vienna, Austria) utilising the matchit, haven and cobalt packages.
Results
Patient characteristics
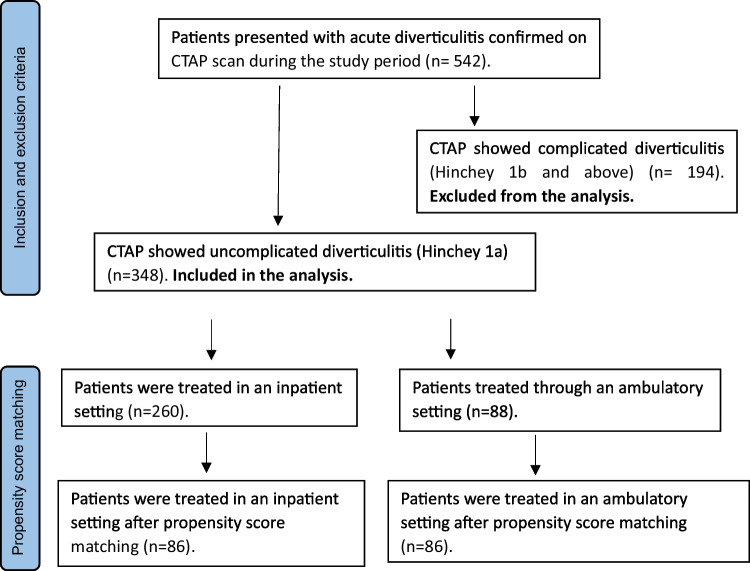
A total of 348 patients with Hinchey 1a acute diverticulitis presenting during the study period were included. The majority of these were treated as in-patients [n = 260/348, (74.7%)], and the remainder [n = 88/348, (25.3%)] in an ambulatory setting. Figure 1 demonstrates the study flow diagram, and patient characteristics are summarised in Table 1.
Fig. 1.
Study flow diagram. CTAP: computed tomography of abdomen and pelvis, n: total number of patients
Table 1.
Comparison of patient’s characteristics and treatment outcomes between ambulatory and inpatient groups before and after propensity score matching (PSM)
| Characteristic | Before PSM matching | After PSM matching | ||||||
|---|---|---|---|---|---|---|---|---|
| All
(N = 348) |
Ambulatory
(n = 88) |
Inpatient
(n = 260) |
P-value | All
(N = 172) |
Ambulatory
(n = 86) |
Inpatient
(n = 86) |
P-value | |
| Age, median (IQR) years | 62 (51–74) | 59 (50–72) | 62 (51–75) | 0.153 | 62 (52–72) | 59 (51–72) | 63 (53–73) | 0.307 |
| Female gender, n (%) | 228 (65.5) | 58 (65.9) | 170 (65.4) | > 0.999 | 113 (65.7) | 57 (66.3) | 56 (65.1) | > 0.999 |
| Diabetic, n (%) | 40 (11.5) | 6 (6.8) | 34 (13.1) | 0.125 | 14 (8.1) | 6 (7.0) | 8 (9.3) | 0.782 |
| Use of steroids, n (%) | 14 (4.0) | 1 (1.1) | 13 (5.0) | 0.205 | 2 (1.2) | 1 (1.2) | 1 (1.2) | > 0.999 |
| Previous diverticulitis, n (%) | 109 (31.3) | 23 (26.1) | 86 (33.1) | 0.235 | 45 (26.2) | 23 (26.7) | 22 (25.0) | > 0.999 |
| Complicated | 23 (6.6) | 4 (4.6) | 19 (7.3) | 0.463 | 5 (2.9) | 4 (4.7) | 1 (1.2) | 0.368 |
| Vital signs, n (%) | ||||||||
| Temperature ≥ 38 °C | 18 (5.2) | 1 (1.1) | 17 (6.5) | 0.052 | 5 (2.9) | 1 (1.2) | 4 (4.7) | 0.368 |
| Elevated RR ≥ 20 | 14 (4.0) | 1 (1.1) | 13 (5.0) | 0.205 | 5 (2.9) | 1 (1.2) | 4 (4.7) | 0.368 |
| Low SBP ≤ 90 mmHg | 19 (5.5) | 2 (2.3) | 17 (6.5) | 0.176 | 9 (5.2) | 2 (2.3) | 7 (8.1) | 0.168 |
| Altered GCS < 15 | 1 (0.3) | 0 (0.0) | 1 (0.4) | > 0.999 | 1 (0.6) | 0 (0.0) | 1 (1.2) | > 0.999 |
| Inflammatory markers, n (%) | ||||||||
| WCC (x 10*9/L) | ||||||||
| <10 | 119 (34.2) | 32 (36.4) | 87 (33.5) | 0.697 | 65 (37.8) | 31 (36.1) | 34 (39.5) | 0.753 |
| 10–15 | 160 (46.0) | 50 (56.8) | 110 (42.3) | 0.019* | 88 (51.2) | 49 (57.0) | 39 (45.4) | 0.170 |
| 15–20 | 57 (16.4) | 6 (6.8) | 51 (19.6) | 0.004* | 18 (10.5) | 6 (7.0) | 12 (14.0) | 0.212 |
| > 20 | 12 (3.4) | 0 (0.0) | 12 (4.6) | 0.042* | 1 (0.6) | 0 (0.0) | 1 (1.2) | > 0.999 |
| CRP (mg/L) | ||||||||
| < 50 | 155 (44.5) | 49 (55.7) | 106 (40.8) | 0.018* | 95 (55.2) | 47 (54.7) | 48 (55.8) | > 0.999 |
| 50–100 | 72 (20.7) | 14 (15.9) | 58 (22.3) | 0.226 | 37 (21.5) | 14 (16.3) | 23 (26.7) | 0.137 |
| 100–200 | 101 (29.0) | 24 (27.3) | 77 (29.6) | 0.786 | 37 (21.5) | 24 (27.9) | 13 (15.1) | 0.063 |
| 200–300 | 14 (4.0) | 0 (0.0) | 14 (5.4) | 0.025* | 2 (1.2) | 0 (0.0) | 2 (2.3) | 0.497 |
| >300 | 6 (1.7) | 1 (1.1) | 5 (1.9) | > 0.999 | 1 (0.6) | 1 (1.2) | 0 (0.0) | > 0.999 |
| Antibiotics prescription n (%) | 337 (96.8) | 83 (94.3) | 254 (97.7) | 0.154 | 164 (95.3) | 83 (96.5) | 81 (94.2) | 0.720 |
| Length of stay, median (IQR) days | 2 (0–3) | 0 (0–0) | 3 (2–4) | < 0.001† | 0.5 (0–2) | 0 (0–0) | 2 (1–4) | < 0.001† |
| Re-admission within 1-year, n (%) | 63 (18.1) | 9 (10.2) | 54 (20.8) | 0.026* | 25 (14.5) | 8 (9.3) | 17 (19.8) | 0.082 |
| IR within 1-year, n (%) | 1 (0.3) | 0 (0.0) | 1 (0.4) | > 0.999 | 1 (0.6) | 0 (0.0) | 1 (1.2) | > 0.999 |
| Surgery within 1-year, n (%) | 3 (0.9) | 0 (0.0) | 3 (1.2) | 0.575 | 0 (0.0) | 0 (0.0) | 0 (0.0) | - |
| CRC within 1-year, n (%) | 5 (1.4) | 2 (2.3) | 3 (1.2) | 0.604 | 2 (1.2) | 2 (2.3) | 0 (0.0) | 0.497 |
| 1-year follow up, n (%) | 188 (54.0) | 51 (58.0) | 137 (52.7) | 0.458 | 97 (56.4) | 50 (58.1) | 47 (54.7) | 0.759 |
| Endoscopy, n (%) | 176 (50.6) | 46 (52.3) | 130 (50.0) | 0.805 | 89 (51.7) | 45 (52.3) | 44 (51.2) | > 0.999 |
| Weeks post-review, median (IQR) | 7 (6–11) | 6 (5.8–8) | 8 (6–12) | 0.055 | 7 (6–11) | 6 (6–8) | 8 (6–12) | 0.089 |
| Flexible sigmoidoscopy, n (%) | 86 (24.7) | 23 (26.1) | 63 (24.2) | 0.775 | 41 (23.8) | 23 (26.7) | 18 (20.9) | 0.474 |
| Colonoscopy, n (%) | 90 (25.9) | 23 (26.1) | 67 (25.8) | > 0.999 | 48 (27.9) | 22 (25.6) | 26 (30.2) | 0.610 |
| CTC, n (%) | 15 (4.3) | 7 (8.0) | 8 (3.1) | 0.067 | 10 (5.8) | 7 (8.1) | 3 (3.5) | 0.329 |
| Weeks before CTC | 9.5 (6–27.3) | 8.5 (5.5–24.8) | 9.5 (6–46) | 0.423 | 2 (1–2) | 2 (1–2) | 2 (1–4) | 0.617 |
*Indicates statistically significant using Fisher’s exact analysis
†Indicates statistically significant using Mann-Whitney U-test
IQR interquartile range, RR Respiratory rate, SBP Systolic blood pressure, GCS Glasgow coma scale, WCC White cell count, CRP C-reactive protein, IR Interventional radiology, CRC Colorectal cancer, CTC Computed tomography colonography
The median age of our cohort was 62 years (51–74), and 228 (65.5%) were female. Approximately one-third (n = 109, (31.3%)) had a previous diagnosis of diverticulitis, and of these patients, 23 (6.6%) were graded as having complicated disease. Forty patients (11.5%) were co-morbid with diabetes mellitus, and 14 (4.0%) were using long-term steroids.
On presentation, 18 (5.2%) patients had a fever (temperature ≥ 38 °C), 14 (4.0%) were tachypnoeic (RR ≥ 20), 19 (5.5%) were hypotensive (systolic blood pressure ≤ 90 mmHg), and one (0.3%) had reduced level of consciousness (GCS < 15).
One-to-one PSM yielded a cohort of 172 patients, of which half (n = 86) were treated in the ambulatory setting and the other half (n = 86) as in-patients. The median age of this group was 62 years (52–72), and 113 (65.7%) were female.
Of the total cohort, 45 (26.2%) patients had a previous diagnosis of diverticulitis, and 5 (2.9%) of these were classified as complicated disease. On presentation, 5 (2.9%) patients had a fever (temperature ≥ 38 °C), 5 (2.9%) were tachypnoeic (RR ≥ 20), 9 (5.2%) were hypotensive (systolic blood pressure ≤ 90 mmHg), and one (0.6%) had an altered level of consciousness (GCS < 15).
Patients presenting with a raised temperature (≥ 38 °C) were significantly less likely to be managed in the ambulatory setting in adjusted models for the unmatched data [OR 0.16 (0.02, 1.25), P = 0.023], becoming less significant on matched data [OR 0.22 (0.02, 1.98), P = 0.128] (Table 2).
Table 2.
Patient factors associated with ambulatory care using univariate and multivariate binomial logistic regression modelling for the unmatched data
| Characteristic | Univariate OR (95% CI) |
p-value | Multivariate OR (95% CI) |
p-value |
|---|---|---|---|---|
| Age | 1.00 (0.97, 1.03) | 0.871 | 1.00 (0.96, 1.04) | 0.871 |
| Female gender | 0.72 (0.27, 1.92) | 0.548 | 0.73 (0.26, 2.04) | 0.551 |
| Diabetic | 0.76 (0.15, 3.75) | 0.751 | 0.77 (0.15, 3.84) | 0.745 |
| Steroid use | 0.98 (0.10, 9.20) | 0.951 | 1.07 (0.11, 10.83) | 0.952 |
| Previous diverticulitis | 0.72 (0.42, 1.23) | 0.312 | 0.75 (0.44, 1.30) | 0.307 |
| Complicated | 0.78 (0.24, 2.59) | 0.665 | 0.76 (0.22, 2.60) | 0.660 |
| Temperature > 38 °C | 0.16 (0.02, 1.25) | 0.081 | 0.16 (0.02, 1.25) | 0.023* |
| Elevated RR | 0.22 (0.03, 1.69) | 0.161 | 0.23 (0.03, 1.80) | 0.088 |
| Low SBP | 0.33 (0.08, 1.47) | 0.223 | 0.39 (0.09, 1.77) | 0.176 |
| Altered GCS | 0.00 (0.00, INF) | 0.988 | 0.00 (0.00, INF) | 0.419 |
| WCC 10–15 (x 10*9/L) | 1.79 (1.1, 2.92) | 0.032* | 1.71 (1.05, 2.80) | 0.031* |
| WCC > 15 (x 10*9/L) | 0.23 (0.10, 0.55) | 0.003* | 0.27 (0.11, 0.64) | < 0.001* |
| CRP < 50 (mg/L) | 1.83 (1.12, 2.97) | 0.043* | 1.67 (1.02, 2.73) | 0.042* |
| CRP > 200 (mg/L) | 0.15 (0.02, 1.11) | 0.105 | 0.18 (0.02, 1.42) | 0.040* |
*Indicates statistically significant
95% CI 95% confidence interval, RR Respiratory rate, SBP Systolic blood pressure, GCS Glasgow coma scale, WCC White cell count, CRP C-reactive protein
Inflammatory markers and management decision
In unmatched data, patients were more likely to be treated in the ambulatory care setting if their WCC was in the range of 10–15 × 10*9/L and a CRP of less than 50 mg/L. Patients were more likely to be admitted to a hospital bed if their WCC was more than 15 × 10*9/L and CRP was greater than 200 mg/L (Table 1).
These findings were also valid in the multivariate adjusted models associated with ambulatory care for the unmatched data [WCC 10–15 × 10*9/L: OR 1.71 (1.05, 2.80), P = 0.031], [CRP < 50 mg/L, OR 1.67 (1.02, 2.73), P = 0.042], [WCC > 15 × 10*9/L: OR 0.27 (0.11, 0.64), P < 0.001], [CRP > 200 mg/L: OR 0.18 (0.02, 1.42), P = 0.040] (Table 2). The statistical significance of these parameters was lost in the matched data (Table 1).
However, in the multivariate adjusted models associated with ambulatory care for the matched data, CRP of < 200 mg/L remained significantly associated with treatment in the ambulatory setting [CRP < 200 mg/L, OR2.72 (1.21, 6.12), P = 0.012] (Table 3).
Table 3.
Patient factors associated with ambulatory care using univariate and multivariate binomial logistic regression modelling for the matched data
| Characteristic | Univariate OR (95% CI) |
p-value | Multivariate OR (95% CI) |
p-value |
|---|---|---|---|---|
| Age | 0.99 (0.97, 1.01) | 0.349 | 0.99 (0.97,1.01) | 0.348 |
| Female gender | 1.05 (0.56, 1.98) | 0.856 | 1.06 (0.56, 2.01) | 0.856 |
| Diabetic | 0.73 (0.24, 2.20) | 0.714 | 0.81 (0.26, 2.52) | 0.713 |
| Steroid use | 1.00 (0.06, 16.25) | 0.969 | 0.95 (0.06, 15.51) | 0.969 |
| Previous diverticulitis | 1.06 (0.54, 2.10) | 0.702 | 1.15 (0.57, 2.32) | 0.702 |
| Complicated | 4.67 (0.48, 45.62) | 0.200 | 4.67 (0.44, 49.21) | 0.161 |
| Temperature > 38 °C | 0.24 (0.03, 2.20) | 0.175 | 0.22 (0.02,1.98) | 0.128 |
| Elevated RR | 0.24 (0.03, 2.20) | 0.238 | 0.26 (0.03,2.44) | 0.194 |
| Low SBP | 0.27 (0.05, 1.33) | 0.116 | 0.27 (0.05, 1.38) | 0.088 |
| Altered GCS | 0.00 (0.00, INF) | 0.987 | 0.00 (0.00, INF) | 0.216 |
| WCC 10–15 (x 10*9/L) | 1.6 (0.87, 2.91) | 0.139 | 1.58 (0.86, 2.89) | 0.138 |
| WCC > 15 (x 10*9/L) | 0.42 (0.15, 1.17) | 0.148 | 0.47 (0.17, 1.31) | 0.138 |
| CRP < 200 (mg/L) | 2.17 (1.02, 4.63) | 0.016* | 2.72 (1.21, 6.12) | 0.012* |
*Indicates statistically significant
95% CI: 95% confidence interval, RR Respiratory rate, SBP Systolic blood pressure, GCS Glasgow coma scale, WCC White cell count, CRP C-reactive protein
Antibiotic usage
Antibiotics were used in the treatment of 337/348 (96.8%) patients (Table 1). Antibiotics were unlikely to be prescribed for patients with a WCC < 10 × 10*9/L and CRP < 50 mg/L in multivariate adjusted models for both the unmatched and matched data [OR 0.15 (0.04, 0.53), P = 0.003], [OR 0.10 (0.02, 0.54), P = 0.003], respectively. The likelihood of antibiotics not being prescribed dissipates with increasing WCC or CRP from the above levels.
Length of hospital stay
Patients admitted into the hospital stayed a median of 3 (2–4) days in the unmatched cohort and 2 (1–2) days in the matched cohort, in comparison to zero days (P < 0.001) for corresponding patients treated via ambulatory care (Table 1). This significant difference remained in multivariate-adjusted models (Table 4 and 5).
Table 4.
Outcomes associated with ambulatory care using univariate and multivariate binomial logistic regression models for the unmatched data
| Characteristic | Univariate OR (95% CI) |
p-value | Multivariate OR (95% CI) |
p-value |
|---|---|---|---|---|
| Length of stay | 0.00 (0.00, INF) | 0.987 | 0.00 (0.00, INF) | < 0.001* |
| IR drainage | 0.00 (0.00, INF) | 0.988 | 0.00 (0.00, INF) | 0.390 |
| Surgery | 0.00 (0.00, INF) | 0.986 | 0.00 (0.00, INF) | 0.144 |
| Readmission | 0.43 (0.20, 0.92) | 0.039* | 0.45 (0.21, 0.96) | 0.028* |
| Follow up | 1.21 (0.74, 1.98) | 0.589 | 1.15 (0.70, 1.88) | 0.589 |
| Endoscopy (≤ 8 weeks) | 2.40 (1.10, 5.25) | 0.040* | 2.31 (1.04, 5.12) | 0.032* |
| Flexible sigmoidoscopy | 1.06 (0.54, 2.08) | 0.931 | 1.03 (0.51, 2.09) | 0.931 |
| Colonoscopy | 0.94 (0.48, 1.84) | 0.931 | 0.97 (0.48, 1.97) | 0.931 |
| CTC (≤ 8 weeks) | 1.00 (0.12, 8.31) | 0.864 | 1.22 (0.13, 11.34) | 0.864 |
| Cancer with one year | 1.99 (0.33, 12.12) | 0.376 | 2.35 (0.35, 15.62) | 0.390 |
*Indicates statistically significant
95% CI: 95% confidence interval, IR Interventional radiology, CTC Computed tomography colonography
Table 5.
Outcomes associated with ambulatory care using univariate and multivariate binomial logistic regression models for the matched data
| Characteristic | Univariate OR (95% CI) |
p-value | Multivariate OR (95% CI) |
p-value |
|---|---|---|---|---|
| Length of stay | 0.00 (0.00, INF) | 0.999 | 0.00 (0.00, INF) | < 0.001* |
| IR drainage | 0.00 (0.00, INF) | 0.987 | 0.00 (0.00, INF) | 0.232 |
| Surgery | 0.00 (0.00, INF) | 0.986 | 0.00 (0.00, INF) | 0.239 |
| Readmission | 0.42 (0.17, 1.02) | 0.044* | 0.39 (0.16, 0.97) | 0.037* |
| Follow up | 1.15 (0.63, 2.11) | 0.655 | 1.15 (0.62, 2.12) | 0.655 |
| Endoscopy (≤ 8 weeks) | 2.42 (0.96, 6.11) | 0.051 | 2.52 (1.00, 6.37) | 0.047* |
| Flexible sigmoidoscopy | 1.51 (0.65, 3.49) | 0.419 | 1.42 (0.60, 3.35) | 0.418 |
| Colonoscopy | 0.66 (0.29, 1.53) | 0.469 | 0.72 (0.30, 1.75) | 0.469 |
| CTC (≤ 8 weeks) | 0.50 (0.03, 8.95) | 0.621 | 0.46 (0.02, 9.91) | 0.614 |
| Cancer with one year | > 100 (0.00, INF) | 0.988 | > 100 (0.00, INF) | 0.108 |
*Indicates statistically significant
95% CI: 95% confidence interval, IR Interventional radiology, CTC Computed tomography colonography
Re-admission with acute diverticulitis during the follow-up period (12 months)
In the unmatched cohort, patients requiring in-patient treatment were more likely to be re-admitted with diverticulitis within the first year from the index presentation (20.8% vs. 10.2%, P = 0.026) (Table 1). The level of significance was not equivalent in the matched cohort (19.8% vs. 9.3%, P = 0.082) (Table 1); however, the association of reduced readmission and prior ambulatory treatment was maintained in both unmatched and matched cohorts in adjusted models [OR 0.45 (0.21, 0.96, p = 0.028] (Table 4), [OR 0.39 (0.16, 0.97), P = 0.037] (Table 5), respectively.
Surgical and radiological intervention during the follow-up period (12 months)
There was a comparable frequency in the surgical and radiological interventions required by patients in both the ambulatory and in-patient groups within the first year. This was observed for both the unmatched and matched cohorts (Tables 1 and 4, 5).
Follow-up investigations during the follow-up period (12 months)
No significant difference was detected in the number of follow-up investigations (flexible sigmoidoscopy, colonoscopy and CT colonography) performed for patients in both the ambulatory and in-patient groups within the first year. This was true for both the unmatched and matched cohorts (Tables 1 and 4, 5). However, endoscopic investigations were more likely to be performed earlier (≤ 8 weeks) for patients treated via the ambulatory care pathway, and this was seen in both unmatched and matched cohorts [OR 2.31 (1.04, 5.12), P = 0.032] (Table 4), [OR 2.52 (1.00, 6.37), P = 0.047], respectively (Table 5).
Colorectal cancer diagnosis during the follow-up period (12 months)
There was no significant difference in the incidence of CRC diagnosis in patients in either treatment group within the first year; before PSM (ambulatory 2.3% vs. in-patient 1.2%, P = 0.60) and after PSM (ambulatory 2.3% vs. 0.0% in-patient, P = 0.49) (Table 1). This was also true in the adjusted models for unmatched and matched cohorts (Table 4 and 5).
Discussion
Literature and medical societies’ guidelines suggest that ambulatory treatment of AUD is safe and efficacious in more than 90% of patients presenting to hospital emergency departments [1, 6, 7, 11]. Despite these findings, in-patient management remains popular in treating this condition.
In the present study, 75% of patients with AUD were admitted to the hospital, in keeping with previous reports [6, 12]. Multivariate analysis demonstrated that abnormal vital signs and raised inflammatory markers were the main factors influencing this decision. This high inpatient management rate could be due to a lack of established hospital protocols and pathways advocating ambulatory management.
The antibiotic protocol used was similar across the four centres. First line for severe cases requiring IV antibiotics were co-amoxiclav, metronidazole, ± gentamycin. Oral co-amoxiclav was used when stepping down antibacterial therapy and in the ambulatory setting.
The overall re-admission rate for diverticulitis during the 12-month follow-up period was 18.1%. This was similar to the recurrence rate reported by the DIABOLO trial [13] and Strate et al. [14], 16.4% and 20%, respectively. A significantly higher recurrence rate was reported in the DIRECT trial (30%) [15] and the LASER trial (61%) [16]. These variations reported in the literature could be due to the length of follow-up (being five years in both the DIRECT and LASER trials) and the inclusion criteria adopted by these studies.
In this study, the ambulatory group was associated with a lower risk of re-admission with diverticulitis compared with the in-patient group. Several factors may explain this finding, including the nature of hospital treatment, often involving a period of intravenous antibiotics that may exacerbate gastrointestinal symptoms (abdominal cramps, nausea, diarrhoea), as well as the psychological trauma and emotional impact of hospitalisation influencing subsequent patient behaviour and choices. Different antibiotic regimens have been used for the included population, depending on local hospital protocols.
Since inception, the Hinchey classification and its various modifications and alterations have been used for treating acute diverticulitis based on CTAP findings [10, 17]. It grades the degree of inflammation and associated abscess formation and colonic perforation into four types to guide surgical management.
More recently the original classification system has been questioned for its application in modern day-to-day practice, especially with the introduction and availability of advanced CT imaging. Consequently, Sartelli et al. have suggested a simplified classification system grading acute diverticulitis into complicated and uncomplicated based on radiological (CT) findings [18].
The uncomplicated group includes localised colonic inflammation only (not extending to the peritoneum). The complicated diverticulitis group is subdivided into pericolic air bubbles or fluid, diverticular abscess formation, and free peritoneal fluid and widespread pneumoperitoneum. In addition to the simplified grading of disease, the Sartelli et al. classification also provides recommendations on management.
The evidence in the literature concerning patients’ role in treatment and management remains limited [19, 20]. Rate of hospital re-admission/re-attendance may be influenced by an individual’s past experience [21]. Patients with previous episodes of in-patient care may re-present to acute units whenever they experience symptom flare-up as opposed to those managed successfully in an ambulatory setting seeking medical help only when absolutely necessary. The PSM analysis used in our study aimed to neutralise biases of patient-related factors such as co-morbidities, age and gender. However, other factors, such as BMI and physical activity levels, were not considered.
By definition, there was a difference in LOS between our two groups. Patients managed as in-patients had a median LOS of 3 (unmatched cohort) and 2 (matched cohort) days, respectively. This is similar to previous studies [13, 22]; however, a longer LOS of six days was reported in two studies that reported data before 2013 [23, 24]. This reflects the recent paradigm shift toward earlier patient discharge.
The American Society of Colon and Rectal Surgeons (ASCRS) suggests that patients presenting with signs of peritonitis, are immunocompromised, of advanced age, or are unable to tolerate oral intake should be admitted for inpatient treatment [25]. Otherwise, acute diverticulitis can be managed on an outpatient basis, provided appropriate follow-up has been arranged. We would advocate for the same approach, especially in patients with AUD. Avoiding unnecessary hospitalisation allows for increased capacity and better utilisation of finite resources. Moreover, this approach is supported by our finding that no significant difference in surgical and/or radiological interventions is required within the first 12 months of index presentation between the two groups.
Following recovery from an episode of acute diverticulitis, patients must be followed up to exclude colonic malignancy [26, 27]. Several options with varying risks and benefits are available, including radiological (CT colonography, barium enema) and endoscopic (flexible sigmoidoscopy, colonoscopy) investigations. In this study, no significant difference was detected in the number of follow-up investigations performed between the two groups over the 12-month follow-up period. Moreover, luminal investigations were more likely to be performed earlier in patients managed through the ambulatory setting, with no significant differences in colorectal cancer detection rates.
Mortality rates in AUD are negligible with appropriate management [14]. Based on our study findings, and with the correct dietary advice, patient education, analgesia, and follow-up, AUD can be managed safely in an outpatient environment. The advantages of freeing up limited resources associated with this are clear and numerous. Although the present study does not address cost-savings directly, we would suggest the potential for significant economic benefits when adopting an ambulatory approach. An estimated 60–80% cost-saving per patient per episode has been reported in the literature [28, 29], - further emphasising the adoption of an ambulatory care strategy in cases of AUD. The DIVER trial, an RCT conducted in five Spanish tertiary care facilities, reported cost savings of €1,124 per patient [6].
The shift towards an ambulatory care model, not only for AUD but for other pathologies historically managed in an in-patient setting, can potentially alleviate significant pressures on stretched in-patient facilities, allowing optimal resource allocation and utilisation. Additionally, ambulatory care allows patients to maintain their daily routines, improves quality of life parameters and psychosocial well-being, and reduces the risk of ‘institutionalisation’ [30, 31]. However, patient selection is an integral and critical part of choosing patients to be managed on ambulatory pathways.
In two previous reports, surgeons and emergency physicians reported being uncomfortable using a no-antibiotic and ambulatory management strategy for AUD. Cited factors were a lack of defined hospital pathway/protocol, surgeon concerns about treatment failure, and follow-up logistics [9, 32].
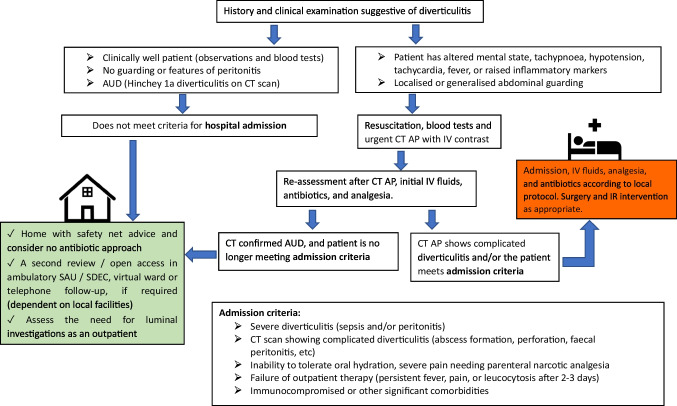
Figure 2 shows a simple algorithm for the ambulatory management of uncomplicated diverticulitis. This pathway is currently used in one of the participating centres in the study, and was drawn from the Getting It Right First Time (GIRFT) programme. It can be adopted and tailored according to the individual hospitals’ available facilities [33].
Fig. 2.
Algorithm for the management of acute uncomplicated diverticulitis (AUD) CT AP, computed tomography scan of abdomen and pelvis; IV, intravenous; SAU, surgical assessment unit; SDEC, same-day emergency care
This study provides some useful insights; however, it is essential to interpret the findings in the context of its limitations. Firstly, as a retrospective and non-randomised study, it inherently carries a risk of selection bias. The PSM analysis helps to mitigate this issue but does not completely eliminate it [34]. The other drawback of the retrospective nature of this study is the reliance on pre-existing records, which may not fully represent the entire patient population and miss relevant information. The non-standardised data collection methods used in the original records also contribute to the variability and potential errors in data collection and analysis.
Additionally, the sample size was relatively small, which limited the findings’ statistical power and may have affected the robustness of the conclusions drawn from the analysis. The effects and indications of antibiotic prescription and follow-up investigations have not been investigated thoroughly in this study due to the lack of detailed treatment protocols in most of the centres included. Lastly, the multi-centre setting of this study aids in enhancing the generalisability of the findings across different populations and settings, but it also introduces variability in the management protocols used. Different centres might have distinct treatment protocols, patient management pathways, and data recording methods, which can affect the uniformity of the data and the study’s outcomes.
Conclusion
Our study provides evidence for the safety and effectiveness of ambulatory care in managing patients with AUD. Compared with in-patient treatment, the ambulatory pathway seems to be associated with significantly reduced hospital readmissions for AUD, with other outcomes being non-inferior. Appropriate patient selection is critical, as well as well-designed ambulatory pathways incorporating safety-netting mechanisms. This model of care has important implications for substantial cost-savings, the wider health economy, and better utilisation of limited healthcare resources.
Supplementary Information
Below is the link to the electronic supplementary material.
(DOCX 32.2 KB)
(DOCX 164 KB)
Author contributions
Conception and study design: AYM, MI, MH, NH. Data collection, analysis and interpretation: AYM, MH, MI, MA, ES. Writing article and critical revision: All authors. Supervision: SB, DS, WB, PT, NH. Final approval: All authors.
Funding
No funding was required for this study.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethical approval
The audit departments in the included centres approved this study (Queen’s Hospital Burton approval number UHDB5415, Sandwell General Hospital approval number 2583, Peterborough City Hospital approval number 3817, and Royal Shrewsbury Hospital approval number 5971).
Informed consent
Considering the nature of this study, informed consent was not required.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sartelli M, Weber DG, Kluger Y et al (2020) 2020 update of the WSES guidelines for the management of acute colonic diverticulitis in the emergency setting. World J Emerg Surg 15(1):32. 10.1186/s13017-020-00313-4. (Published 2020 May 7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller AS, Boyce K, Box B et al (2021) The association of coloproctology of Great Britain and Ireland consensus guidelines in emergency colorectal surgery. Color Dis 23(2):476–547. 10.1111/codi.15503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peery AF, Dellon ES, Lund J, Crockett SD, McGowan CE, Bulsiewicz WJ et al (2012) Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology 143(5):1179–1187e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Papagrigoriadis S, Debrah S, Koreli A, Husain A (2004) Impact of diverticular disease on hospital costs and activity. Colorectal Dis 6(2):81–4. 10.1111/j.1463-1318.2004.00532.x [DOI] [PubMed] [Google Scholar]
- 5.Teke E, Ciyiltepe H, Bulut NE, Gunes Y, Fersahoglu MM, Ergin A et al (2022) Management of Acute Uncomplicated diverticulitis: Inpatient or Outpatient. Med Bull Sisli Etfal Hosp 56(4):503–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biondo S, Golda T, Kreisler E, Espin E, Vallribera F, Oteiza F et al (2014) Outpatient versus hospitalization management for uncomplicated diverticulitis: a prospective, multicenter randomized clinical trial (DIVER Trial). Ann Surg 259(1):38 [DOI] [PubMed] [Google Scholar]
- 7.Mohamedahmed AY, Zaman S, Das N, Kakaniaris G, Vakis S, Eccersley J et al (2024) Systematic review and meta-analysis of the management of acute uncomplicated diverticulitis: time to change traditional practice. Int J Color Dis 39(1):47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cirocchi R, Randolph JJ, Binda GA, Gioia S, Henry BM, Tomaszewski KA et al (2019) Is the outpatient management of acute diverticulitis safe and effective? a systematic review and meta-analysis. Tech Coloproctology 23(2):87–100 [DOI] [PubMed] [Google Scholar]
- 9.Correa Bonito A, Cerdán Santacruz C, Pellino G et al (2024) Results of a national survey about the management of patients with acute uncomplicated diverticulitis. Cir Esp (Engl Ed) 102(4):202–208. 10.1016/j.cireng.2023.11.023 [DOI] [PubMed] [Google Scholar]
- 10.Wasvary H, Turfah F, Kadro O, Beauregard W (1999) Same hospitalization resection for acute diverticulitis. Am Surg 65(7):632–636 [PubMed] [Google Scholar]
- 11.Pelaez N, Pera M, Courtier R et al (2006) Applicability, safety and efficacy of an ambulatory treatment protocol implicated acute diverticulitis. Cir Esp 80:369–372 [DOI] [PubMed] [Google Scholar]
- 12.van de Wall BJ, Draaisma WA, van der Kaaij RT, Consten EC, Wiezer MJ, Broeders IA (2013) The value of inflammation markers and body temperature in acute diverticulitis. Colorectal Dis 15(5):621–6. 10.1111/codi.12072 [DOI] [PubMed] [Google Scholar]
- 13.Unlü C, de Korte N, Daniels L et al (2010) A multicenter randomised clinical trial investigating the cost-effectiveness of treatment strategies with or without antibiotics for uncomplicated acute diverticulitis (DIABOLO trial). BMC Surg. 10:23. 10.1186/1471-2482-10-23 [DOI] [PMC free article] [PubMed]
- 14.Strate LL, Morris AM (2019) Epidemiology, pathophysiology, and treatment of diverticulitis. Gastroenterology 156(5):1282-1298e1. 10.1053/j.gastro.2018.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bolkenstein HE, Consten ECJ, van der Palen J et al (2019) Long-term outcome of surgery versus conservative management for recurrent and ongoing complaints after an episode of diverticulitis: 5-year follow-up results of a multicenter randomized controlled trial (DIRECT-Trial). Ann Surg 269(4):612–620. 10.1097/SLA.0000000000003033 [DOI] [PubMed] [Google Scholar]
- 16.Santos A, Mentula P, Pinta T et al (2023) Quality-of-life and recurrence outcomes following laparoscopic elective sigmoid resection vs conservative treatment following diverticulitis: prespecified 2-Year analysis of the LASER randomized clinical trial. JAMA Surg 158(6):593–601. 10.1001/jamasurg.2023.0466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hinchey EJ, Schaal PG, Richards GK (1978) Treatment of perforated diverticular disease of the colon. Adv Surg 12:85–109 [PubMed] [Google Scholar]
- 18.Sartelli M, Moore FA, Ansaloni L, Di Saverio S, Coccolini F et al (2015) A proposal for a CT driven classification of left colon acute diverticulitis. World J Emerg Surg 10:3. 10.1186/1749-7922-10-3. [DOI] [PMC free article] [PubMed]
- 19.Schneeberger AR, Werthmueller S, Barco S, Heuss SC (2023) Patients’ preference regarding inpatient versus outpatient setting - a systematic review. Int J Health Plann Manage 38(5):1409–1419 [DOI] [PubMed] [Google Scholar]
- 20.Coulter A (2017) Measuring what matters to patients. BMJ 356:j816. 10.1136/bmj.j816 [DOI] [PubMed]
- 21.Wong ELY, Poon CM, Cheung AWL, Chen FY, Yeoh EK (2022) Relationship between patient experience and hospital readmission: system-level survey with deterministic data linkage method. BMC Med Res Methodol 22:197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Azhar N, Aref H, Brorsson A, Lydrup ML, Jörgren F, Schultz JK, Buchwald P (2022) Management of acute uncomplicated diverticulitis without antibiotics: compliance and outcomes -a retrospective cohort study. BMC Emerg Med 22(1):28. 10.1186/s12873-022-00584-x. (PMID: 35189812; PMCID: PMC8862329) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wheat CL, Strate LL (2016) Trends in hospitalization for diverticulitis and diverticular bleeding in the United States from 2000 to 2010. Clin Gastroenterol Hepatol 14:96–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lorente L, Cots F, Alonso S, Pascual M, Salvans S, Courtier R et al (2013) [Outpatient treatment of uncomplicated acute diverticulitis: impact on healthcare costs. Cir Esp 91:504–509 [DOI] [PubMed] [Google Scholar]
- 25.Hall J, Hardiman K, Lee S et al (2020) The American society of colon and rectal surgeons clinical practice guidelines for the treatment of left-sided colonic diverticulitis. Dis Colon Rectum 63(6):728–747. 10.1097/DCR.0000000000001679 [DOI] [PubMed] [Google Scholar]
- 26.Zaman S, Chapman W, Mohammed I, Gill K, Ward ST (2017) Patients with computed tomography-proven acute diverticulitis require follow-up to exclude colorectal cancer. Intest Res 15(2):195–202. 10.5217/ir.2017.15.2.195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meireles LC, Fernandes SR, Ribeiro LC, Velosa J (2015) Role of endoscopy after an acute episode of diverticulitis: analysis of a cohort of Portuguese patients from a tertiary referral center. Eur J Gastroenterol Hepatol. 27(12):1429-32. 10.1097/MEG.0000000000000474. [DOI] [PubMed]
- 28.Mizuki A, Nagata H, Tatemichi M, Kaneda S, Tsukada N, Ishii H (2005) The outpatient management of patients with acute mild.tomoderate colonic diverticulitis. Aliment Pharmacol Ther 21:889–897 [DOI] [PubMed] [Google Scholar]
- 29.Moya P, Arroyo A, Perez-Legaz J et al (2012) Applicability, safety and efficiency of outpatient treatment in uncomplicated diverticulitis. Tech Coloproctol 16:301–307 [DOI] [PubMed] [Google Scholar]
- 30.Mattila K, Lahtela M, Hynynen M (2012) Health-related quality of life following ambulatory surgery procedures: assessment by RAND-36. BMC Anesthesiol 12:30.10.1186/1471-2253-12-30 > [DOI] [PMC free article] [PubMed]
- 31.Schneeberger AR, Werthmueller S, Barco S, Heuss SC (2023) Patients’ preference regarding inpatient versus outpatient setting - a systematic review. Int J Health Plann Manage 38(5):1409–1419. 10.1002/hpm.3669 [DOI] [PubMed] [Google Scholar]
- 32.Brière R, Benhamed A, Émond M, Blanchard PG, Drolet S (2023) Evaluation of physicians’ current practices and awareness regarding the treatment of acute uncomplicated diverticulitis: results of a provincial survey. CJEM 25(12):968–975. 10.1007/s43678-023-00606-y [DOI] [PubMed] [Google Scholar]
- 33.Brière R, Benhamed A, Émond M, Blanchard PG, Drolet S https://gettingitrightfirsttime.co.uk/surgical_specialties/general-surgery/ [Accessed on 25/10/2024]. [DOI] [PubMed]
- 34.Liau MYQ, Toh EQ, Muhamed S, Selvakumar SV, Shelat VG (2024) Can propensity score matching replace randomized controlled trials? World J Methodol 14(1):90590. 10.5662/wjm.v14.i1.90590 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 32.2 KB)
(DOCX 164 KB)
Data Availability Statement
No datasets were generated or analysed during the current study.